Abstract
In this functional-MRI study we examined the hypothesis that the prefrontal cortex responds differently to the extent of competition during retrieval, whereas the parietal cortex is responsible for problem representation that should not be directly related to the competition. Participants mastered arbitrary person–location pairs, and their recognition memory was tested in a functional-MRI session. The pairs were constructed such that a person was associated with one, two, or three different locations and vice versa. The recognition time increased with the number of associations, reflecting increased competition. A confirmatory analysis of imaging data with prespecified prefrontal and parietal regions showed that, although both regions were highly involved during memory retrieval, only the prefrontal region responded to the levels of competition. This result was consistent with predictions of an information-processing model as well as with an exploratory identification of regions of interest.
Human memory has been regarded as an associative network of elementary concepts, and a piece of knowledge arises into awareness when an association is retrieved (1). Because the appropriateness of a particular association depends on a given context, competition is sometimes inevitable. Therefore, the ability to retrieve the correct association in the face of competing associations is critical to human cognition. The purpose of the current functional-MRI (fMRI) study is to examine neural mechanisms underlying memory retrieval from multiple associations. Particularly, our aim is to dissociate the roles served by the prefrontal and the parietal cortices during memory retrieval. A number of cognitive neuroimaging studies have found parietal as well as prefrontal activations highly involved in cognitive functions such as working memory maintenance (2, 3), task-switching (4–7), memory retrieval (8, 9), and arithmetic problem solving (10, 11). Considering the strong prefrontal–parietal interconnection (12, 13) and their functional coactivations (14), these two areas may serve at least complementary roles in the high-level cognition. However, although there is relative consensus that the prefrontal cortex directly controls processes involved in memory retrieval, it is not clear how the prefrontal function can be dissociated from the parietal function during memory retrieval.
Our hypothesis is that the prefrontal cortex increases its activity when retrieval of task-relevant information is more demanding and effortful. On the other hand, we hypothesize that the parietal cortex serves as an imaginal buffer that represents and holds task-relevant information by encoding stimuli and, if necessary, updating the changes in the stimulus representation (10). The prefrontal component of this hypothesis is consistent with the findings of greater prefrontal activation during memory retrieval compared with working memory maintenance (2, 9) or other control conditions (8). Although parietal activation has been found during memory-retrieval tasks (15–17), its exact role in relation to memory retrieval has not been well specified. The parietal component of our hypothesis is consistent with reports of posterior parietal activation in mental imagery tasks (18) as well as reports of parietal activation during encoding of verbal items (19, 20). Previously, we developed an information-processing model that successfully solved algebraic equations by appropriately scheduling retrieval operations and representation operations (10). The representation operations include stimulus encoding as well as updating of the mental image of an equation, because transformations are required to solve an equation. In this model, the prefrontal cortex is activated when there is direct retrieval of information. Although holding problem representation, the parietal cortex is activated only when the problem representation in the imaginal buffer changes through encoding and updating. The model specified how long both the retrieval operations and the representation operations should take. With this information, it was possible to predict blood oxygenation level-dependent (BOLD) functions specific to each component, as we will explain more fully later. The predicted BOLD signal based on the retrieval operations was best correlated with activities in the left prefrontal region in Brodmann area (BA) 44/45. In contrast, the predicted BOLD signal based on the representation operations was best correlated with activities in the left parietal region in BA 39/40. These results indicate that the prefrontal region may control memory retrieval more directly, whereas the parietal region may be related to the problem-representation component accompanying retrieval.
Our study of algebra problem solving (10) manipulated problem complexity. Unfortunately, problem complexity increases both the number of arithmetic facts to be retrieved and the number of changes made to the problem representation. Consequently, in that study predictions for one target region were correlated quite highly with the other target region. This confounding is not suitable for detecting the prefrontal–parietal differences. One way to dissociate them is to vary the cognitive load during memory retrieval (e.g., competition among associations to be retrieved), whereas the problem-representation requirement is controlled to be the same. To examine the involvement of the prefrontal and parietal regions during competitive memory retrieval, we took advantage of the fan effect (21). This effect refers to the phenomenon that as people study more facts or associations about a concept, the retrieval of any one of those facts about the concept takes longer. This increase is because the associative strength between memory probe and the target association becomes weaker as the probe affords more associations (high-fan condition) than fewer associations (low-fan condition). The increased retrieval time reflects competition among associations that share the same concept, demonstrating that more cognitive resources or more time has to be invested to retrieve a fact with many competitors than a fact with few or no competitors. A neural implication of the fan effect is that brain regions directly related to memory retrieval should be engaged longer in high-fan than in low-fan conditions.
In the current study, participants first memorized 28 person–location pairs. The pairs were constructed such that a person was associated with one, two, or three different locations and vice versa. After memorization, participants' recognition memory was tested. The load during memory retrieval was defined as the number of facts (fan) associated with a probe (a person–location pair). We expected that this memory-retrieval load would tap processes specific to the prefrontal cortex. However, the representation of the probe stimulus does not vary with retrieval load. Thus, unlike algebra problems, this recognition paradigm did not involve different numbers of problem states during task performance. We expected that the parietal cortex would be activated during recognition performance because the task requires attention to the probe. However, the parietal activation would not vary with the retrieval load, because the problem-representation requirement does not change with competition.
Alternatively, there is evidence that the parietal cortex may represent not only the currently presented stimulus but also the information that is strongly associated with the stimulus (22, 23). These studies adopted a stimulus–response compatibility (SRC) paradigm, in which participants respond to the target in the face of a distractor that affords either a compatible or incompatible response with the one associated with the target. Results showed that parietal activation was higher when the distractor was associated with a response than when the distractor was neutral even though the number of stimulus elements was controlled. This result suggests that the parietal cortex represents information that is activated by the presence of a stimulus, although there may be no need to update the stimulus representation. Further, it has been suggested that the parietal cortex may maintain this representation actively throughout problem solving (22, 23). If this applies to the recognition-memory paradigm, we should see greater activation in the parietal cortex with high-fan probes, because they may activate more associated facts than low-fan probes.
Methods
Behavioral Protocol. Participants were recruited locally and provided written informed consent in accordance with the guidelines at the University of Pittsburgh. Eight right-handed participants (three female and five male, 19–28 years of age with an average age of 23.75) completed the study of one session of behavioral practice and one scanning session. The design of the current study closely followed the original fan study (21). The E-PRIME (24) software package was used to present stimuli and collect behavioral performance.
On the practice day, 1 day before the scan, participants memorized 28 person–location associations in the form of sentences (e.g., “The hippie is in the park.”). Participants' memory of these associations was perfected through a two-pass dropout cued-recall procedure. In each pass, participants received in a random order all possible questions such as: “Where is the person?” or “Who is in the location?” Participants had to type in all locations associated with that person or all people associated with that location. If they recalled all the answers to a question, then the question was dropped out of the pass. If they failed, the correct answer was shown and the question was repeated after all the other questions had been asked. This process continued until all questions had been answered correctly. This whole procedure was repeated a second time so that each question would have been answered correctly twice. In the recognition test, after the memorization phase, a probe was presented on every trial in the form of a person–location pair (“hippie–park”). Participants made a judgment whether they studied probe or not. To create foils, we swapped person–location pairs from the same fan condition.
On the scanning day, participants received 56 “warm-up” trials, and then they judged 112 targets and 112 foils during an fMRI scanning. The fMRI trials were organized into 16 blocks of 14 trials each. A trial lasted for 18 sec: 2.4 sec of a warning signal, 2.4 sec of a recognition window, and 13.2 sec for a resting period. A response-button unit for the right hand was used to collect responses. The middle-finger button was assigned to the “yes” response, and the index-finger button was assigned to the “no” response. The same protocol was used for both the behavioral practice session outside the scanner and the actual performance during functional imaging.
fMRI Procedures. Event-related fMRI data were collected by using a single-shot spiral acquisition on a GE 3T scanner with a 1,200-msec repetition time, 18-msec echo time, 70° flip angle, 20-cm field of view, 21 axial slices per scan with 3.2 mm thickness, 64 × 64 matrix, and anterior commissure–posterior commissure at the bottom slice. For each trial, 15 scans were acquired. Images were motion-corrected by using the six-parameter rigid-body model of the AIR (25) program and then cross-registered to a common reference brain by minimizing signal-intensity differences. Then, functional images were set to a standard mean intensity, smoothed (8-mm full-width half-maximum 3D Gaussian kernel), and pooled across participants to improve the signal-to-noise ratio.
Results
Both behavioral data and fMRI imaging data were analyzed in terms of the sum of the person fan (number of locations associated with the person) and the location fan of the probe. This combined fan ranged from 2 to 6. To increase the chance of detecting the neural changes due to the fan effect, analyses contrasted low-fan (2 and 3) and high-fan (5 or 6) probes and excluded the intermediate-fan probes (combined fan, 4). For latency and imaging data, only the correct trials were analyzed. The low-fan trials were responded to faster and more accurately (1,188 msec and 95% correct) than the high-fan trials (1,344 msec and 93%) [F(1,7) = 6.83, mean squared error (MSE) = 30,080, and P < 0.05 for latency and F(1,7) = 5.61, MSE = 0.001, and P < 0.05 for accuracy]. All the other effects or interactions were not significant for latency or accuracy (P > 0.30). The nonsignificant target–foil effect is surprising considering that this effect has been normally obtained (21, 26, 27). Although the target–foil effect was in the right direction (43 msec slower for foils), it was quite small compared with the fan effect (156 msec). Therefore, only the fan dimension was considered for imaging data.
The imaging data were analyzed in three stages. First, we prescribed prefrontal, parietal, and motor regions following our previous study (10) that showed a retrieval effect in the left prefrontal cortex and a problem-representation effect in the left posterior parietal cortex. In this analysis, we examined the fan effect in these regions during memory retrieval. Second, we took an existing information-processing model of the fan effect and used it to predict the BOLD signal. In this analysis, we examined the correspondence between the predictions of the model and the response patterns in the three prescribed regions. Third, we identified exploratory regions of interest (ROI) that responded to the fan effect and examined their consistency with the confirmatory analysis.
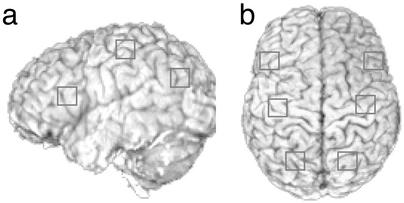
Confirmatory Analysis on Imaging Data. The six prespecified ROI are illustrated in Fig. 1. Each ROI was 5 voxels wide, 5 voxels long, and 4 voxels high on the x, y, and z coordinates, respectively, for a total of 100 voxels per region (voxel size is 3.125 × 3.125 × 3.2 mm3). The majority of the left prefrontal ROI, with its central voxel located at -44, 21, 21 in Talairach coordinates, was in left BA 44/45, and part of it was in left BA 9/46. The left posterior parietal ROI was in left BA 39/40, with its central voxel located at -24, -64, 34 in Talairach coordinates. The left motor ROI was in left BA 3/4 including both motor and somatosensory regions, with its central voxel located at -37, -24, 47 in Talairach coordinates. These three regions on the left hemisphere showed corresponding activity to retrieval, representation, and motor operations in the previous algebra study (10). Therefore, the current confirmatory analysis would provide a generalization of the previous findings to a different task and material. Also, to examine laterality, the right-hemisphere homologues of these regions were defined (i.e., x coordinates were positive).
Fig. 1.
Schematic view of three prespecified regions. (a) Regions in the left hemisphere. (b) Axial view of these three regions and their right-hemisphere homologues.
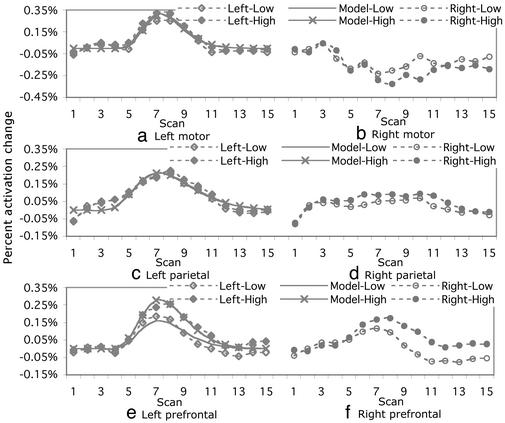
We conducted separate ANOVAs on the motor, parietal, and prefrontal regions with hemisphere, fan, and scan as factors. Fig. 2 shows within-trial percent activation changes in these regions. For the motor regions, there was a significant effect of hemisphere [F(1,7) = 13.80, MSE = 0.352, and P < 0.01] and a significant scan-by-hemisphere interaction [F(14,98) = 11.09, MSE = 0.024, and P < 0.0001]. Not surprisingly, the left hemisphere, which controls the right hand, shows a rise in its BOLD function with the emission of a response. In contrast, the right hemisphere shows a drop, presumably reflecting suppression of motor programming of the left hand. The fan effect and its interaction with the scan were not significant (P > 0.10).
Fig. 2.
Percent activation changes in the prespecified motor region (a and b), the prespecified parietal region (c and d), and the prespecified prefrontal regions (e and f). The baseline was the average of the first three scans. The solid lines indicate the predictions based on the model shown in Fig. 3.
For the posterior parietal regions, there was a significant effect of scan [F(14,98) = 5.02, MSE = 0.024, and P < 0.0001] and a significant scan-by-hemisphere interaction [F(14,98) = 4.82, MSE = 0.005, and P < 0.0001]. The response of the right hemisphere is much weaker than the left. There were no other significant effects of fan and its interaction with scan (P > 0.10). Thus, this research has confirmed the left lateralization of the parietal and motor regions reported in our earlier studies (10). Moreover, this research confirmed the hypothesis that the parietal cortex may not be directly related to the retrieval difficulty.
In the prefrontal regions, although there was a significant effect of scan [F(14,98) = 20.25, MSE = 0.008, and P < 0.0001], there was no significant effect of hemisphere (P > 0.30). More important, there was a significant fan-by-scan interaction [F(14,98) = 3.29, MSE = 0.005, and P < 0.0001], reflecting greater involvement of the prefrontal cortex in the high-fan condition than in the low-fan condition. The BOLD function rises higher in the high-fan condition for both the right and left hemispheres. The right-hemisphere functions begin to rise sooner than the left-hemisphere functions but do not peak as high as the left hemisphere. In general, however, we see relatively comparable responses between the right and left prefrontal regions.
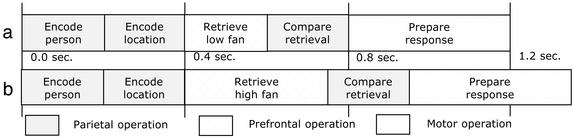
Modeling. To model BOLD signals specific to the prefrontal and parietal regions during memory retrieval, we took previously developed information-processing models for this task (26, 27). These models have been successful in predicting response latency. As illustrated in Fig. 3, at the beginning of a trial the person and location terms are encoded into the problem representation, then follows a variable-timed retrieval process, a comparison of the retrieved result with the problem representation, and finally the programming of the motor response. Our hypothesis is that the prefrontal cortex is involved in retrieval, the parietal cortex in the encoding and the comparison, and the motor cortex in response generation. The timings of the parietal and motor operations are constrained from the existing models (10): Each parietal operation takes 200 msec, and the motor programming takes 400 msec. The timing of the retrieval operation is a free parameter to be estimated to fit the latency data. On the basis of our behavioral results, we estimated the retrieval time as 200 msec in the low-fan condition and 350 msec in the high-fan condition. These times are comparable to estimates obtained in the earlier models.
Fig. 3.
Schematic model performance in the low-fan (a) and high-fan (b) conditions.
The key feature is that the performance of the model depends on the probe–target associative strength (28, 29). The facts associated with a probe would share the limited amount of cognitive resource to be invested for memory retrieval. Therefore, a target fact uniquely associated with the probe would require less retrieval time than one of many facts associated with a probe. Alternatively, other fMRI studies on memory retrieval imply that one could model this competition through active inhibition of alternative associations (30) or selection among multiple associations after retrieving and storing them in working memory (8, 31, 32). The current study is not designed to distinguish these possibilities, and our modeling effort does not imply any theoretical commitment to either possibility.
In work by Anderson et al. (10), a precise proposal was developed for how the length of activity in a region maps onto the predicted BOLD function. A number of researchers (33–35) have proposed that the BOLD response to an event varies according to the following function of time, t, since the event,
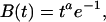 |
where estimates of the exponent, a, vary between 2 and 10. This is essentially a γ function that will reach maximum of a time units after the event. It was proposed that while a region is active it is constantly producing a change that will result in a BOLD response according the above-stated function. The observed fMRI response is integrated over the time that the region is active. Therefore, the observed BOLD response will vary with time as
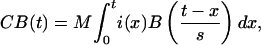 |
where M is the magnitude scale for response, s is the latency scale, and i(x) is 1 if the region is active at time x and 0 otherwise. For instance, in the low-fan condition in Fig. 3 the parietal region is active between 0 and 400 msec and between 600 and 800 msec. Note that because of the scaling factor, the prediction is that the BOLD function will reach maximum at roughly t = as sec. Also note that the area under the BOLD function is proportional to the time that the region is active.
We used the hypothesized activity in Fig. 3 to generate predictions for each of the left regions in Fig. 1. We predicted separate BOLD functions for the high- and low-fan conditions for each region. To fit these data required estimating the basic parameters of the BOLD function which are M, s, and a. These parameters were estimated to minimize the χ2 deviation of the fit to the data defined as
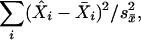 |
whereX̂i is the predicted mean, X̄i is the observed mean, and 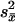 is the error of the mean estimated from the participants-by-condition interaction. The degrees of freedom for this statistic is the number of observations (30 data points for each region) minus the number of parameters (three for each region). These predictions are displayed along with the data in Fig. 2, and the parameter values are presented in Table 1. The predictions provide a good fit to the BOLD function in all three regions. The last two columns of Table 1 provide an alternative model fitting that predicts the prefrontal activity from the representation operation and the parietal activity with the retrieval operation. Although these alternative models provide a decent fit, they seem to deviate from the data more than the proposed model as indicated by the greater χ2 and lower correlations. If we take the χ2 difference (D) between two models fitting the same data, e-(D/2) is the relative likelihood of the data under the two models. It can be seen that data for both the prefrontal and parietal regions are >1,000 times more likely under the proposed model than under the alternative model.
is the error of the mean estimated from the participants-by-condition interaction. The degrees of freedom for this statistic is the number of observations (30 data points for each region) minus the number of parameters (three for each region). These predictions are displayed along with the data in Fig. 2, and the parameter values are presented in Table 1. The predictions provide a good fit to the BOLD function in all three regions. The last two columns of Table 1 provide an alternative model fitting that predicts the prefrontal activity from the representation operation and the parietal activity with the retrieval operation. Although these alternative models provide a decent fit, they seem to deviate from the data more than the proposed model as indicated by the greater χ2 and lower correlations. If we take the χ2 difference (D) between two models fitting the same data, e-(D/2) is the relative likelihood of the data under the two models. It can be seen that data for both the prefrontal and parietal regions are >1,000 times more likely under the proposed model than under the alternative model.
Table 1. Results of model fitting and parameters of the best-fitting BOLD predictions.
| Retrieval to prefrontal | Representation to parietal | Response to motor | Representation to prefrontal | Retrieval to parietal | |
|---|---|---|---|---|---|
| M | 0.04 | 0.024 | 0.033 | 0.018 | 0.463 |
| s | 0.74 | 1.160 | 0.59 | 0.65 | 1.23 |
| a | 7.92 | 5.44 | 8.60 | 8.91 | 5.21 |
| df | 27 | 27 | 27 | 27 | 27 |
| χ2 | 18.70 | 23.10 | 14.78 | 33.01 | 37.70 |
| R2 | 0.93 | 0.88 | 0.94 | 0.87 | 0.80 |
Of particular interest is the ability of the model to fit the low- and high-fan BOLD functions in the prefrontal regions. According to the model, the prefrontal region is active 75% longer in the high-fan than in the low-fan condition, predicting the higher BOLD function in the high-fan condition. The fact that the difference in the BOLD responses can be predicted from the difference in retrieval latencies confirms that this region does serve a retrieval function.
Exploratory ROI Analysis. To examine whether our confirmatory analysis missed out any important regions, in an exploratory analysis we conducted voxelwise fan-by-scan ANOVAs and searched for clusters of more than six contiguous voxels showing significant fan-by-scan interactions [F(14,98) = 2.46 and P < 0.001] (34). Note that this interaction is only significant when the low-fan and high-fan conditions result in different BOLD functions over scans. Because the retrieval-load effect is supposed to be specific to the prefrontal cortex, we expected that this exploratory ROI selection would not include parietal regions. As Table 2 shows, posterior parietal regions did not reach the selection criteria. Consistent with the confirmatory analysis, bilateral prefrontal activations were observed. The right prefrontal region constitutes a small section of the confirmatory prefrontal ROI discussed earlier. The left prefrontal region was located close to the confirmatory prefrontal region. Also, the exploratory prefrontal regions are primarily dorsolateral prefrontal cortices (DLPFCs), whereas the confirmatory prefrontal regions covered both DLPFC and ventrolateral prefrontal cortex (VLPFC). Other regions that showed a significant fan effect also included the precuneus and anterior cingulate regions, with higher activation increase in the high-fan than in the low-fan conditions. The left putamen showed a negative fan effect in the sense that the activation increase was lower in the high-fan condition. Last, a VLPFC region quite inferior to our confirmatory prefrontal region showed greater activation change in the low-fan than in the high-fan condition. In general, the exploratory analysis is consistent with the confirmatory analysis in that the DLPFC regions reached the significant level of the fan effect, whereas the parietal regions did not.
Table 2. Regions activated by fan-by-scan interaction.
| Regions | F* | Talairach coordinates |
|---|---|---|
| Anterior cingulate cortex (BA 32) | 3.79 | 11, 24, 34 |
| Anterior cingulate cortex (BA 24) | 3.68 | 19, 16, 34 |
| Left DLPFC (BA9) | 3.44 | -52, 12, 34 |
| Precuneus (BA 7) | 6.09 | 7, -70, 35 |
| Right DLPFC (BA 9) | 6.18 | 49, 27, 28 |
| Left putamen | 4.21 | -28, 2, 20 |
| Right VLPFC (BA 45) | 3.46 | 46, 34, 3 |
Local maximum
Discussion
Overall, this study confirmed the hypothesis that the prefrontal cortex directly contributes to memory retrieval, whereas the parietal cortex does not. One may have noticed that the behavioral fan effect (156 msec) is quite small compared with the temporal resolution adopted in the current study (repetition time = 1,200 msec). Although it is possible that the behavioral effect might not have been substantial enough to result in differences in the parietal cortex, three converging analyses demonstrate that the retrieval difficulty has greater impact on the prefrontal than the parietal cortex.
Prefrontal Cortex. The involvement of the prefrontal region in memory retrieval that we found is consistent with many other studies (2, 8–10). In the current paradigm, the probe–target associative strength is assumed to decrease with the fan or the number of associations from the terms in the probe, which is because distracting associations compete against each other for retrieval. There has been evidence that the prefrontal region responds to competition (22, 23). For example, in an SRC paradigm, prefrontal activation was greater when the target and the distractor were associated with incongruent responses than when they were associated with the same response. A further contribution of our study is to show that the prefrontal region does not simply respond to the existence of competition, but its involvement systematically increases with increasing competition. All of these studies point to the conclusion that the prefrontal region is crucial in retrieving information especially in the face of distracting information.
Anatomically, our prespecified prefrontal regions include both the DLPFC and VLPFC. Although both DLPFC and VLPFC seem to be involved in semantic/episodic retrieval, the VLPFC has been related mostly to maintaining information in working memory (9). Although our task does not allow functional distinction in the subregions of the prefrontal cortex, our exploratory analysis seems to have an implication for the existing dorsal–ventral distinction. In the exploratory analysis, retrieval-sensitive activations were found in predominantly dorsal stream of the prefrontal regions in both the right and left hemispheres. It is possible that our selection criterion may have left out the VLPFC region, but nonetheless it means that the activation change in the VLPFC was not as high as in the DLPFC.
Parietal Cortex. Although the posterior parietal activation has been frequently found along with the prefrontal activation during memory retrieval and other executive control tasks, the role of this region during memory retrieval has not been clear. In the current study, we manipulated the extent of competition during memory retrieval. The posterior parietal region did show activation increase during task performance, but this activation reflected nonretrieval processes because it was not related to the extent of competition. Our hypothesis is that the parietal region should be responsible for encoding the stimulus and updating the stimulus representations. Updating the stimulus representation was necessary in our previous algebra task but not in the current recognition-memory task. Therefore, unlike our algebra model, the model for this task predicted no effect of difficulty of conditions in the parietal region.
Although our hypothesis relates parietal activation to the number of items to be represented, an alternative proposal (22, 23) relates parietal activation to the number of stimulus–response mapping rules that a stimulus display affords. As mentioned earlier, in an SRC paradigm, parietal activation is greater when the distractor has a potential to evoke a response rather than when it is simply a neutral stimulus (22). This result has been attributed to the fact that the number of stimulus–response mappings is greater in the crosstalk condition than in the neutral condition. However, in our study, the number of associations to a probe did not affect the parietal activation. Besides the slight anatomical discrepancy (e.g., our confirmatory parietal regions are rather medial and posterior compared with the parietal regions in the above-mentioned studies), there are a few differences between a recognition-memory paradigm such as ours and the SRC paradigm. First, in the recognition-memory paradigm, the probe as a whole serves as a single retrieval cue for an association, whereas in the SRC paradigm a stimulus array consists of a target and a distractor. Second, in the recognition paradigm, the response differs depending on whether the appropriate association is retrieved successfully. In contrast, in the SRC paradigm, the associations with the stimulus elements already represent responses. Perhaps the parietal cortex may respond differently depending on the type of the display or the characteristics of the relevant associations.
Hemispheric Asymmetry. The motor and parietal regions showed clear left dominance during memory retrieval. The laterality in the motor region is obvious because participants made responses with their right hands. The parietal region often has been associated with attention (37), with the right parietal region showing greater activation particularly when attending to spatial characteristics of the stimulus. Perhaps the kind of processes incurred by our verbal material was more abstract, resulting in the left laterality. Also, the left parietal region has been postulated to be responsible for attention to local features as opposed to global features (38) and for object-based attention as opposed to location-based attention (39). These left–right distinctions in the parietal cortex are compatible with our finding, because our task involves attending to individual words in the probes.
The current study hardly showed any laterality in the prefrontal regions. There have been some efforts to make the left–right distinctions in the prefrontal regions in terms of episodic-semantic recognition as well as in terms of recall recognition. For example, greater activation was found in the left DLPFC with recall than with recognition and greater activation in the right DLPFC with recognition than with recall (40). We found retrieval-related activity in both DLPFCs, although strictly speaking, the current task can be classified as episodic recognition. However, it is also true that study-retrieval paradigms such as the one adopted here often evoke bilateral activation in the prefrontal region (2).
Conclusion
The purpose of the current study is to dissociate the prefrontal function from the parietal function during memory retrieval. In the fan paradigm adopted in this study, competition during memory retrieval increased with the number of items (fan) associated with a memory probe, but the representation of the probe did not change during memory retrieval. Our confirmatory analysis showed that the prefrontal region differentially responded as the retrieval load increased, whereas the parietal region responded to task performance but not to the retrieval load. This result was consistent with the predictions of our model as well as the exploratory analysis, supporting the proposed prefrontal–parietal dissociation during memory retrieval.
Acknowledgments
We thank Roberto Cabeza and Anthony Wagner for helpful comments on earlier drafts. This research was supported by National Science Foundation Grant BCS-9975220 (to J.R.A.) and National Institute of Mental Health Independent Scientist Award MH64190 (to C.S.C.).
Abbreviations: fMRI, functional MRI; BOLD, blood oxygenation level-dependent; BA, Brodmann area; SRC, stimulus–response compatibility; MSE, mean squared error; ROI, region(s) of interest; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex.
References
- 1.Collins, A. M. & Quillian, M. R. (1969) J. Verbal Learn. Verbal Behav. 8, 240-247. [Google Scholar]
- 2.Cabeza, R., Dolcos, F., Graham, R. & Nyberg, L. (2002) Neuroimage 16, 317-330. [DOI] [PubMed] [Google Scholar]
- 3.Postle, B., Berger, J. S. & D'Esposito, M. (1999) Proc. Natl. Acad. Sci. USA 96, 12959-12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dove, A., Pollmann, S., Schubert, T., Wiggins, C. J. & von Cramon, D. Y. (2000) Cognit. Brain Res. 9, 103-109. [DOI] [PubMed] [Google Scholar]
- 5.Kimberg, D. Y., Aquirre, G. K. & D'Esposito, M. (2000) Cognit. Brain Res. 10, 189-196. [DOI] [PubMed] [Google Scholar]
- 6.Konish, S., Nakajima, K., Uchida, I., Kaneyama, M., Nakahara, K., Sekihara, K. & Miyahsita, Y. (1998) Nat. Neurosci. 1, 80-84. [DOI] [PubMed] [Google Scholar]
- 7.Sohn, M.-H., Ursu, S., Anderson, J. R., Stenger, V. A. & Carter, C. S. (2000) Proc. Natl. Acad. Sci. USA 97, 13448-13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson-Schill, S. L., D'Esposito, M., Aguirre, G. K. & Farah, M. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14792-14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner, A. D., Maril, A. M., Bjork, R. A. & Schacter, D. L. (2001) Neuroimage 14, 1337-1347. [DOI] [PubMed] [Google Scholar]
- 10.Anderson, J. R., Qin, Y., Sohn, M.-H., Stenger, V. A., Fissell, K. & Carter, C. S. (2003) Psychonom. Bull. Rev., in press. [DOI] [PubMed]
- 11.Dehaene, S., Spelke, E., Pinel, P., Stanesku, R. & Tsivkin, S. (1999) Science 284, 270-274. [DOI] [PubMed] [Google Scholar]
- 12.Petrides, M. & Pandya, D. N. (1984) J. Comp. Neurol. 228, 105-116. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz, M. L. & Golman-Rakic, P. C. (1984) J. Comp. Neurol. 226, 403-420. [DOI] [PubMed] [Google Scholar]
- 14.Cabeza, R. & Nyberg, L. (2000) J. Cognit. Neurosci. 12, 1-47. [DOI] [PubMed] [Google Scholar]
- 15.Mosovitch, M., Köhler, S. & Houle, S. (1995) Proc. Natl. Acad. Sci. USA 92, 3721-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tulving, E., Kapur, S., Craik, F. I. M., Moscovitch, M. & Houle, S. (1994) Proc. Natl. Acad. Sci. USA 91, 2012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabeza, R., Kapur, S., Craik, F. I. M., McIntosh, A. R., Houle, S. & Tuliving, E. (1997) J. Cognit. Neurosci. 9, 863-870. [DOI] [PubMed] [Google Scholar]
- 18.Zacks, J. M., Ollinger, J. M., Sheridan, M. A. & Tversky, B. (2002) Neuroimage 16, 857-872. [DOI] [PubMed] [Google Scholar]
- 19.Clark, D. & Wagner, A. D. (2003) Neuropsychologia 41, 304-317. [DOI] [PubMed] [Google Scholar]
- 20.Davachi, L., Maril, A. & Wagner, A. D. (2001) J Cognit. Neurosci. 13, 1059-1070. [DOI] [PubMed] [Google Scholar]
- 21.Anderson, J. R. (1974) Cognit. Psychol. 5, 451-474. [Google Scholar]
- 22.Bunge, S. A., Hazeltine, E., Scanlon, M. D., Rosen, A. R. & Gabireli, J. D. E. (2002) Neuroimage 17, 1562-1571. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher, E. H. & D'Esposito, M. (2002) Hum. Brain Mapp. 17, 193-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider, W., Eschman, A. & Zuccolotto, A. (2002) e-prime User's Guide (Psychology Software Tools, Pittsburgh).
- 25.Woods, R. P., Grafton, S. T., Holmes, C. J., Cherry, S. R. & Mazziotta, J. C. (1998) J. Comput. Assist. Tomogr. 22, 139-152. [DOI] [PubMed] [Google Scholar]
- 26.Anderson, J. R. & Lebiere, C. (1998) The Atomic Components of Thought (Erlbaum, Mahwah, NJ).
- 27.Anderson, J. R. & Reder, L. M. (1999) J. Exp. Psychol. Gen. 128, 186-197. [DOI] [PubMed] [Google Scholar]
- 28.Badre, D. & Wagner, A. D. (2002) Behav. Cognit. Neurosci. Rev. 1, 206-218. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, A. D., Paré-Blagoev, E. J., Clark, J. & Poldrack, R. A. (2001) Neuron 31, 329-338. [DOI] [PubMed] [Google Scholar]
- 30.Dreher, J. C. & Berman, K. F. (2002) Proc. Natl. Acad. Sci. USA 99, 14595-14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobbins, I. A., Foley, H., Schacter, D. L. & Wagner, A. D. (2002) Neuron 35, 989-996. [DOI] [PubMed] [Google Scholar]
- 32.Rowe, J. R., Toni, I., Josephs, O., Frackowiak, R. S. J. & Passingham, R. E. (2000) Science 288, 1656-1660. [DOI] [PubMed] [Google Scholar]
- 33.Boyton, G. M., Engel, S. A., Glover, G. H. & Heeger, D. J. (1996) J. Neurosci. 16, 4207-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen, M. S. (1997) Neuroimage 6, 93-103. [DOI] [PubMed] [Google Scholar]
- 35.Dale, A. M. & Buckner, R. L. (1997) Hum. Brain Mapp. 5, 329-340. [DOI] [PubMed] [Google Scholar]
- 36.Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A. & Noll, D. C. (1995) Magn. Reson. Med. 33, 636-647. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta, M., Kincade, J. M., Ollinger, J. M., McAvoy, M. P. & Shulman, G. L. (2000) Nat. Neurosci. 3, 292-297. [DOI] [PubMed] [Google Scholar]
- 38.Robertson, L. C. & Rafal, R. (2000) in The New Cognitive Neuroscience, ed. Gazzaniga, M. (MIT Press, Cambridge, MA), 2nd Ed.
- 39.Arrington, C. M., Mayer, A. R., Carr, T. H. & Rao, S. M. (2000) J. Cognit. Neurosci. 12, 106-117. [DOI] [PubMed] [Google Scholar]
- 40.Cabeza, R., Locantore, J. K. & Anderson, N. D. (2003) J. Cognit. Neurosci. 15, 249-259. [DOI] [PubMed] [Google Scholar]