Abstract
Recent progress has been made regarding the prevention of hearing loss. However, the complete protection of both hair cells and spiral ganglion neurons, with restored function, has not yet been achieved. It has been shown that spiral ganglion neuronal loss can be prevented by neurotrophin 3 (NT3) and hair cell damage by N-methyl-d-aspartate (NMDA) receptor antagonists. Here we demonstrate that the combined treatment with MK801, a NMDA antagonist, and NT3 protect both cochlear morphology and physiology from injury. Pretreatment with MK801 prevented hearing loss and the dendrites of the spiral ganglion neurons from swelling after noise-induced damage. The acute phase of insult with the aminoglycoside antibiotic amikacin resulted in swollen afferent dendrites beneath the inner hair cells. The chronic phase resulted in complete hair cell loss and near-complete loss of spiral ganglion neurons. This damage caused a near-complete loss of hearing sensitivity as displayed by elevated (>90-dB sound pressure levels) auditory brainstem response thresholds. The treatment of amikacin-exposed animals with MK801 gave only a partial protection of hearing. However, the combined treatment with NT3 and MK801 in the amikacin-comprised ear resulted in improved mean hearing within 20 dB of normal. Furthermore, hair cell loss was prevented in these animals and spiral ganglion neurons were completely protected. These results suggest that the NMDA antagonist MK801 protects against noise-induced excitotoxicity in the cochlea whereas the combined treatment of NT3 and MK801 has a potent effect on preserving both auditory physiology and morphology against aminoglycoside toxicity.
One in 10 individuals is affected by hearing disorders. Hearing impairments can be caused by a variety of factors including noise, ototoxic substances such as aminoglycosides, and aging. These factors affect the hair cells in the mammalian organ of Corti that transduce auditory signals to the brain via the spiral ganglion neurons. Hearing deficiencies are caused by loss of hair cells or spiral ganglion neurons and these changes are often permanent. Sensory neurons and hair cells are not capable of postembryonic cellular mitosis to produce new hair cells and neurons. It is therefore necessary to develop pharmacological strategies aimed at preventing or repairing damaged hair cells or spiral ganglion neurons.
The most important afferent neurotransmitter in the peripheral auditory system is the excitatory amino acid glutamate (1, 2). When glutamate is released from the inner hair cells it binds to N-methyl-d-aspartate (NMDA) receptors located on the terminals of the spiral ganglion neurons. NMDA receptors are expressed in the auditory neurons and little or no mRNA for the receptor subunits has been detected in other areas of the cochlea (3). It has been shown that NMDA receptors play a key role in excitotoxic cell death after ischemic, stroke, hypoglycemia, alcohol intoxication, and epilepsy (4). Cell death caused by excitotoxicity has been suggested to be caused by cytoplasmic Ca2+ overload and can occur when glutamate receptors are overstimulated by excessive excitatory synaptic activation. Although the mechanism underlying hearing loss is not yet known, it is believed that NMDA receptors are involved (5, 6). Evidence has been obtained indicating that aminoglycosides can mimic the modulatory actions of polyamines on the NMDA receptor, causing excitotoxicity and cell death (7, 8). Recently, it has been shown that aminoglycoside-induced hair cell loss could be significantly reduced in the presence of NMDA antagonists (5). Pryer reflex measurements and distortion product otoacoustic emissions together with hair cell morphology were preserved in the animals receiving the combination of ototoxic antibiotic and an NMDA antagonist. In another study, protection of spiral ganglion neurons from aminoglycoside ototoxicity by neurotrophin 3 (NT3) has been demonstrated (9). Infusion of NT3 completely preserved the number of spiral ganglion neurons but was insufficient in protecting hair cells. In the present study, attempts were made to elucidate whether a NMDA receptor antagonist (MK801) and NT3 show complementary effects in the protection of inner ear morphology and function after injury.
Materials and Methods
General Experimental Paradigm.
A total of 43 pigmented guinea pigs (250–400 g) without evidence of middle ear infection were used in this study. The care and use of animals was approved by the Ethical Committee at the Karolinska Institutet. For the noise study, five animals were noise-exposed [1-kHz tone at 105-dB sound pressure level (SPL) for 24 h]; another five animals were treated with MK801 (1 mg/kg, i.p.) (Tocris Cookson, Bristol, U.K.) just before noise trauma. Two animals were tested for determining the effects of maleate (0.5 mg/kg, i.p.), the salt used for preparing MK801, and were treated the same way as the animals receiving MK801. For the amikacin study 33 animals were implanted with osmotic pumps (Alzet, Palo Alto, CA, model 2ML2, 5 μl/h) filled with: artificial perilymph (n = 5) and amikacin (300 mM) for 24 h followed by perilymph infusion for 2 weeks (n = 15). Eight of these animals were injected with MK801 (1 mg/kg per day × 3, i.p.) before implanting the pump, amikacin (300 mM) and NT3 (300 ng/h) for 24 h followed by NT3 for the remaining 2 weeks. These animals were injected with MK801 (1 mg/kg per day × 3, i.p.) before implanting the osmotic pumps (n = 8). Another five animals were used for studying the acute affects of amikacin toxicity on cochlear morphology. Three animals were implanted with osmotic pumps filled with amikacin (300 mM) whereas two animals were implanted with pumps filled with artificial perilymph. After 6 h the animals were anesthetized with pentobarbital (30 mg/kg), then perfused intracardially with body temperature physiological saline followed by a solution of 5% glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer with 4 mM MgCl2. The cochleae were removed and postfixed with 2% osmium tetroxide in 0.1 M phosphate buffer, embedded in Agar 100 Resin. Sections were stained with toluidine blue and analysis of the afferent dendrite morphology was made. Analysis was performed with a Zeiss Axioscope microscope equipped with oil immersion objectives.
Auditory brainstem response (ABR) thresholds were measured before noise exposure and immediately after noise exposure. For the amikacin study, ABR thresholds were measured before implanting the osmotic pump, 24 h after as well as 1 and 2 weeks after implanting the pump. After the final auditory brainstem measurement the cochleae were removed for morphological analysis. A Student's t test was performed to determine the statistical significance of the data. Differences were considered statistically significant when the P value was 0.05 or below.
Implantation and Filling of the Osmotic Pump.
The microosmotic pump (Alzet, model 2ML2; 5 μl/h) was used according to the method described (9). Briefly, guinea pigs were anaesthetized with rompun (10 mg/kg, i.m.), and ketamine (50 mg/kg, i.m.) and 10% xylocaine containing adrenaline were applied locally. The right bulla was exposed postauricularly and a 2-mm hole was drilled through the bone of the bulla and a small hole (≈0.2 mm) was made to access the scala tympani in the basal turn. A steel needle (0.2 mm outer diameter) was connected to a plastic tube and was inserted into the hole and fixed with dental cement (Fuji I, Tokyo). A s.c. pocket was made to accommodate the pump in the back region and the catheter between the bulla and the microosmotic pump was fixed by superglue. The composition of the artificial perilymph was as follows: 137 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 12 mM NaHCO3, 11 mM glucose; the pH was adjusted if necessary to 7.4.
ABR.
After an i.m. injection of 50 mg ketamine and 10 mg rompun per kg body weight ABR responses were recorded s.c. with stainless-steel electrodes as the potential difference between an electrode on the vertex and an electrode on the mastoid, while the lower back served as ground. The body temperature of the animals was maintained at 38°C by using an isothermic heating pad. Stimulus intensity was calibrated with a one-quarter-inch condenser microphone (Bruel & Kjaer Instruments, Marlborough, MA, model 4135) and are expressed in peak SPL re: 20 μPa. The stimulus signal was generated through Tucker-Davis Technologies (Gainesville, FL) equipment controlled by computer and delivered by an earphone (Telephonics TDH 39, Farmingdale, NY). The stimuli were delivered through a closed acoustic system sealed into the external auditory meatus. The evoked response was amplified 100,000 times and averaged 2,048 sweeps in real time at a digital signal process (DSP32C) with a time-domain artificial rejection. Stimuli were presented at an intensity well above threshold and then decreased in 10-dB steps until the threshold was approached and then in 5-dB steps until the ABR wave disappeared. Threshold is defined as the lowest intensity at which a visible ABR wave was seen in two averaged runs.
Morphological Analysis of the Cochlea.
After the final auditory brainstem measurement cochleae from the noise-exposed group (n = 5) and the noise group treated with MK801 (n = 5) were removed after cardiac perfusion with 4% paraformaldehyde and postfixed for 2 h in 4% paraformaldehyde. The cochleae were decalcified, embedded in paraffin, sectioned at 4 μm, and stained with toluidine blue. Dendrites under inner hair cells were visualized with an oil immersion microscope (Zeiss Axioscope under ×100 objectives). Noise-induced swelling of the afferent dendrites were identified as vacuoles at the base of the inner hair cell. These vacuoles could be as large as 10 μm in diameter and limited by a surrounding membrane. The number of swollen dendrites was counted in every 10th section throughout all cochlear turns.
For the quantification of spiral ganglion neurons after amikacin treatment, the cochleae were removed after cardiac perfusion with 4% paraformaldehyde, decalcified, embedded in paraffin, sectioned at 10 μm, and stained with cresyl violet. The number of neurons with cytoplasm, a clear nucleus, and nucleoli were counted in every 10th section.
To quantify the hair cell loss after amikacin damage cochleae were placed in 4% paraformaldehyde in PBS (pH 7.4) for 1–2 h. The cochleae were rinsed in PBS and the bone was dissected away. The tissue then was exposed to 0.3% Triton X-100 for 10 min, rinsed, and incubated in fluorescently labeled phalloidin (tetramethylrhodamine B isothiocyanate, TRITC) (1:100) (Molecular Probes) for 30 min and rinsed several times. The organ of Corti was dissected into ½-¾ coils and placed on a microscope slide in Citi-flour, and covered with a coverslip and sealed with nonfluorescent nail polish. All hair cells throughout the cochlea were examined by using a ×40 objective, and the percent hair cell loss per mm distance from the round window then was plotted on a cochleogram. An estimated frequency map also is indicated where the 9-mm distance from the round window represents the 8-kHz region, the 11-mm region represents the 4-kHz region, and the 13-mm region represents the 2-kHz region.
Results
MK801, an NMDA Antagonist, Prevents Loss of Auditory Threshold Sensitivity After Noise Exposure.
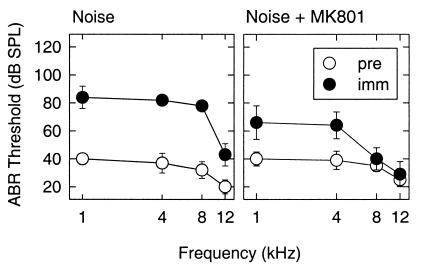
We have used a well-established means of causing hair cell loss that can lead to a permanent loss in hearing sensitivity by exposing guinea pigs to noise trauma. Hearing sensitivity was measured by determining the ABR threshold. ABR thresholds were determined before and immediately after noise exposure (1 kHz, 105 dB SPL, 24 h). This particular noise trauma causes a moderate loss of auditory sensitivity and a mild loss of hair cells (10). A threshold shift of approximately 45 dB at 1, 4, and 8 kHz, and 25 dB at 12 kHz was found for the untreated noise group (Fig. 1). Pretreatment with MK801 resulted in just a 25-dB threshold shift at 1 and 4 kHz. At 8 and 12 kHz the threshold shift was about 5 dB. Thus, MK801 caused statistically significantly reductions in ABR threshold shifts at all frequencies studied (P < 0.05 at 1 kHz; P < 0.01 at 4, 8, and 12 kHz). In comparison with the untreated noise group, MK801 resulted in 18 dB improved thresholds at 1 kHz, 20 dB at 4 kHz, 41 dB at 8 kHz, and 19 dB at 12 kHz. Thus, a complete protection was seen at 8 and 12 kHz and a partial protection at 1 and 4 kHz.
Figure 1.
ABR thresholds were determined before (pre) and immediately (imm) after noise exposure (1 kHz, 105 dB SPL, for 24 h) in a group exposed to noise (Left) and a group treated with MK801 before noise exposure (Right). A threshold shift of approximately 45 dB at 1, 4, and 8 kHz and 25 dB at 12 kHz was found for the untreated noise group, whereas pretreatment with MK801 resulted in a 25-dB threshold shift at 1 and 4 kHz. At 8 and 12 kHz the threshold shift was about 5 dB.
Because MK801 is dissolved in maleate and maleate has been found to act as a mild antioxidant in some systems, a control group receiving maleate at the same concentration found in MK801 were included. No effect of maleate was found in ABR thresholds after noise trauma.
Quantification of Swollen Dendrites Under Inner Hair Cells After Noise Trauma.
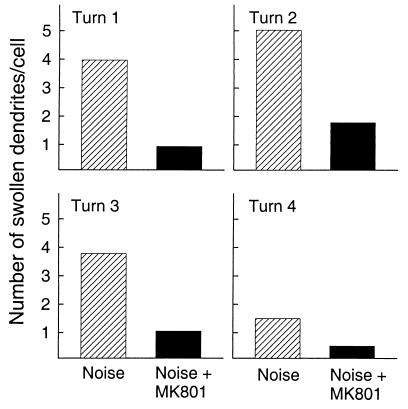
The acute effects of noise trauma caused swelling of the radial afferent dendrites under the inner hair cells. Quantification of the number of swollen dendrites per cell was made for the group exposed to noise and the group treated with MK801 before noise exposure (Fig. 2). Noise caused a marked swelling of the radial afferent dendrites under the inner hair cells, and this damage affected the entire cochlea when studied 2 h postexposure. The animals exposed to noise had swollen dendrites extending from turn 1 (basal turn, high frequencies) to turn 4 (apical turn, low frequencies) with turn 4 being the least affected. MK801 effectively prevented swelling of dendrites after noise. Significant differences were found between the two groups for turns 1, 2, and 3. These three cochlear turns approximately reflect the region of 8, 4, and 1 kHz in the cochlea. Note that these regions show the greatest ABR threshold shifts. The data for the control group (no noise exposure, no MK801 treatment) showed primarily intact dendrites, yet, on a rare occasion a slightly swollen dendrite could be observed. Table 1 shows the percent of inner hair cells demonstrating swollen dendrites for the different cochlea turns. The effect of MK801 on reducing the number of swollen dendrites under the inner hair cells was apparent throughout all of the cochlear turns. There is a near 2-fold decrease of inner hair cells showing swollen dendrites in the animals receiving MK801.
Figure 2.
Quantification of the number of swollen dendrites per cell for the untreated noise group and the noise-treated group (MK801). Noise caused a marked swelling of the radial afferent dendrites under the inner hair cells extending from turn 1 (basal turn, high frequencies) to turn 4 (apical turn, low frequencies). MK801 effectively prevented swelling of dendrites after noise.
Table 1.
Percentage of cells showing swollen dendrites
| Noise, % | MK 801 + noise, % | |
|---|---|---|
| Turn 1 | 72 | 42 |
| Turn 2 | 73 | 36 |
| Turn 3 | 54 | 21 |
| Turn 4 | 23 | 15 |
The Effect of MK801 and/or NT3 on ABR Thresholds After Amikacin Ototoxicity.
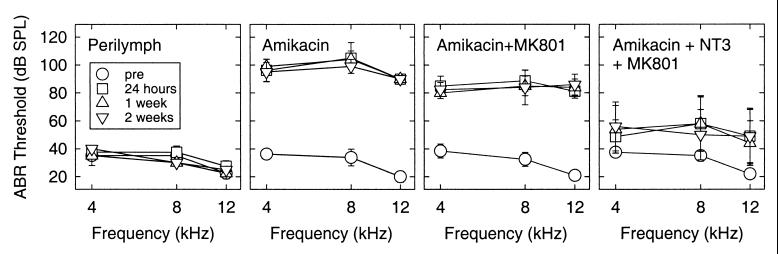
Noise trauma was shown to alter hair cell morphology, and these changes resulted in a loss of auditory sensitivity. We found that this injury could be prevented by pretreatment with the NMDA antagonist, MK801. Because MK801 had a potent protective effect on hair cell morphology and physiology after noise trauma, we tested the potential protective effect of MK801 in a model that causes injury to both the hair cells and the auditory neurons. The aminoglycoside antibiotic, amikacin, delivered by an osmotic pump at the concentration of 300 mM resulted in a rapid loss of hair cells and spiral ganglion neurons. Amikacin treatment resulted in ABR threshold shifts already after 24 h (Fig. 3). The loss in ABR sensitivity was still evident at 1 week and 2 weeks postamikacin exposure. At 2 weeks postamikacin treatment the mean ABR thresholds were 90 dB at 4 kHz, 105 dB at 8 kHz, and at 12 kHz was beyond the equipment limit (>90 dB SPL).
Figure 3.
The effect of MK801 and/or NT3 on ABR thresholds after amikacin ototoxicity. Alterations in ABR thresholds were not found after the infusion of perilymph (Far Left). Exposure with amikacin resulted in significantly elevated ABR threshold shifts (>70 dB) at all postexposure times and frequencies (second from left). When guinea pigs were treated with amikacin and MK801, significant ABR threshold shifts at the different postexposure times were seen. However, 15-dB improvements in thresholds were found in the treated group compared with the group exposed to amikacin alone. The combined treatment of MK801 and NT3 resulted in a statistically significant attenuation of ABR threshold shifts by 25–40 dB compared with the group exposed only to amikacin (Far Right).
When guinea pigs were exposed with amikacin and treated with MK801, there were still significant ABR threshold shifts at the different postexposure times. However, 15-dB improvements in thresholds were found in the treated group compared with the group receiving amikacin alone (Fig. 3). These differences were significant at most of the frequencies and at the different exposure times (P < 0.01 at 4 kHz and 1 week postimplantation; P < 0.03 at 4 kHz and 2 weeks post). There were also significant differences at 24 h and 1 week postimplantation at 8 kHz but not at 2 weeks (P < 0.05 for 24 h, P < 0.02 at 1 week, P > 0.08 for 2 weeks). The ABR thresholds were beyond the equipment limit at 12 kHz after amikacin treatment so it was not reasonable to compare the two groups at 12 kHz. No alterations in ABR thresholds were found at any frequency and time in control animals after the infusion of perilymph, showing that our surgical procedure did not affect hearing.
Previously it has been shown that NT3 protects spiral ganglion neurons from amikacin damage but is ineffective in the protection of hair cells. In the present study, guinea pigs received the combined treatment of NT3 and MK801 while exposed to amikacin. This combined treatment resulted in a statistically significant attenuation of ABR threshold shifts, by 25–40 dB compared with the group exposed only to amikacin (Fig. 3). This was evident at all different postexposure times studied and at the different frequencies tested (P < 0.05 at the different postexposure times and the different frequencies). Thus, the combined treatment with MK801 and NT3 preserves auditory sensitivity as well as hair cell and spiral ganglion morphology (see below).
Gram-negative bacteria (Escherichia coli strain XL1blue) was subjected to the combination of NT3 and amikacin (at the concentrations used for the cochlea) to determine whether the NT interfered with the antibactierial effect of amikacin. The antibacterial effect of amikacin alone did not differ from the combined treatment of NT3 and amikacin.
Quantification of Hair Cell Loss After Amikacin Insult and the Combined Treatment with NT3 and MK801.
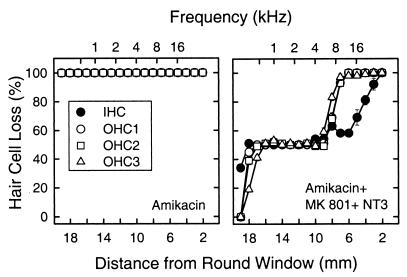
Hair cell loss was quantified on cochlear surface preparations from 19 mm to a 2-mm distance from the round window. The cochleae exposed to amikacin treatment had a 100% loss of inner and outer hair cells throughout the cochlea, and in addition, were completely deprived of supporting cells. The group receiving MK801 and amikacin had supporting cells (pillar, Deiters, and Hensen cells) remaining in the middle and apical cochlear turns. In addition, a limited number of scattered inner and outer hair cells remained in the more apical cochlear regions. When the cochleae were treated with the combination of NT3 and MK801 a significant protection of hair cells and supporting cells was found. Quantification of hair cell loss throughout the cochlea demonstrated a mean of 50% or more protection of both inner hair cells between 18 and 5 mm from the round window corresponding to approximately 500 Hz to 16 kHz, whereas the outer hair cells showed a 50% or more protection between 18 and 9 mm (500 Hz and 8 kHz). Regions more basal to 9 mm showed a mean between 50% and 100% loss for the outer hair cells whereas the inner hair cells displayed a more gradual loss from 5 mm (Fig. 4).
Figure 4.
Cochleograms showing hair cell loss for different distances from the round window with an approximate corresponding frequency map. Quantification of hair cell loss after only amikacin exposure (Left) or the combined treatment with NT3 + MK801 (Right). In the amikacin treated cochleae (n = 4) there was total loss of inner and outer hair cells. With the combined treatment of NT3 + MK801 there was a significant protection of inner and outer hair cells.
Quantification of Spiral Ganglion Neuron Degeneration After Amikacin Insult.
The number of spiral ganglion neurons was assessed on cochlear sections stained with cresyl violet. The cochleae receiving only perilymph showed a similar number of neurons as compared with the cochleae obtained from the contralateral, unoperated side of the operated animals. These findings agree with our previous results (9) and imply that damage is not induced to the cochlea by implanting the osmotic pumps. Two weeks after amikacin treatment the number of spiral ganglion neurons was reduced to 10% of the control (perilymph) group. However, pretreatment with MK801 before amikacin delivery resulted in a 70% survival of spiral ganglion neurons. Thus spiral ganglion neurons were only partially protected from amikacin toxicity by MK801. Most notably, the combined treatment with both NT3 and MK801 resulted in complete protection of spiral ganglion neurons from amikacin ototoxicity (Table 2).
Table 2.
Spiral ganglion neuron counts after aminoglycoside toxicity
| Treatment | Spiral ganglion neuron counts, ±SEM | Mean of survival, % of control |
|---|---|---|
| Control (n = 5) | 37,160 ± 4,200 | 100 |
| Amikacin (n = 4) | 3,864 ± 680 | 10.4 |
| Amikacin+MK801 (n = 4) | 25,938 ± 3,500 | 69.8 |
| Amikacin+MK801+NT-3 (n = 4) | 38,279 ± 2,680 | 103 |
Analysis of Afferent Dendritic Morphology After Amikacin Insult.
The treatment of amikacin for 24 h resulted in complete loss of the inner and outer hair cells, and therefore a reduction in the infusion duration was required to be able to analyze the afferent dendrites. After 6 h of amikacin infusion, cochlea morphology demonstrated swollen afferent dendrites under the inner hair cells (Fig. 5). There were numerous large swollen dendrites evenly distributed on both sides of the inner hair cell. The inner hair cell body and stereocilia appeared normal. The outer hair cells showed signs of swelling. On the control side (untreated), there were normal-appearing afferent dendrites as well as, normal inner and outer hair cells. In the cochleae infused with perilymph for 6 h there were neither signs of swollen dendrites nor any pathology noted for the inner and outer hair cells.
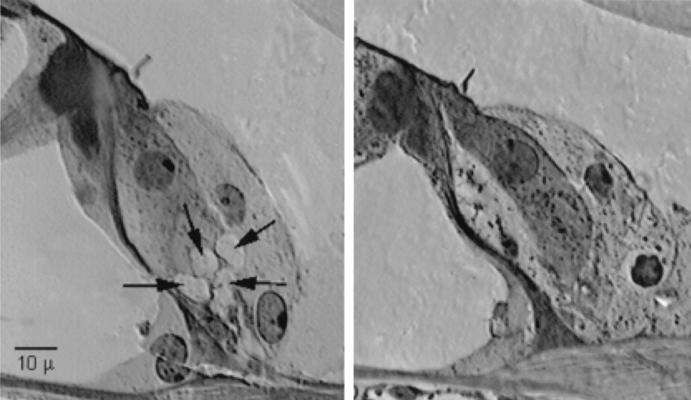
Figure 5.
Transverse light microscopic section of a normal inner hair cell (Left) and an amikacin-treated inner hair cell (Right). Note the swollen afferent dendrites beneath the inner hair cell of the amikacin-treated cochlea.
Discussion
The results presented here demonstrate that the combined treatment with MK801 and NT3 protects the morphology and physiology of both the cochlear hair cells and the spiral ganglion neurons from injury. First, the effects of noise trauma were significantly reduced by pretreatment with MK801. Pretreatment with MK801 protected the afferent dendrites from swelling under the inner hair cells after noise exposure. The afferent dendrites, originating from the type I spiral ganglion neurons, are sensitive to the excitotoxic actions of glutamate. Excessive osmotic imbalance from the influx of water causes dendritic swelling (11) and eventual loss of spiral ganglion neurons (12). Glutamate excitotoxicity leading to the degeneration of the afferent dendrites has been suggested as a mechanism underlying noise trauma. More specifically, it is known that noise exposure can induce the destruction of dendrites of the primary auditory neurons under the inner hair cells (11, 13, 14). The findings presented here afford a means of substantially limiting both hearing loss and swelling of dendrites under inner hair cells.
Second, by using an aminoglycoside toxicity model to cause injury to both the hair cells and the spiral ganglion neurons, we show that MK801 protects cochlear morphology and physiology. In the acute phase of amikacin toxicity there was a swelling of the afferent dendrites under the inner hair cells. This excitotoxic action of amikacin is presumably through the action of the NMDA receptors found on the afferent dendrites. Extending the duration of amikacin exposure (24 h) resulted in significantly elevated auditory thresholds, total hair cell, supporting cell loss, and an almost total loss of spiral ganglion neurons when analyzed 2 weeks postexposure. By treating the animals exposed to amikacin with MK801, a partial protection of the ABR (15 dB) and 70% survival of the spiral ganglion neurons was found. However, hair cell loss was not reduced by MK801. Basile et al. (5) proposed that aminoglycosides produce a polyamine-like enhancement of the glutamate NMDA receptor, resulting in excitotoxicity and eventual hair cell death. Those authors demonstrated that NMDA antagonists could prevent the loss of hair cells and hearing sensitivity. However, they did not analyze the spiral ganglion neurons, a critical relay station between the peripheral auditory organ and the central auditory system. This is of particular importance because NMDA receptors are expressed primarily by the spiral ganglion neurons and not by hair cells (3). The results shown in the present report demonstrate that NMDA antagonists can attenuate damage to spiral ganglion neurons and in that way minimize hair cell loss and thus can positively affect functional responses.
Third, the combined treatment of MK801 and NT3 on amikacin-exposed animals resulted in partial protection of hair cells and complete protection of the spiral ganglion neurons, and ABR thresholds were within 20 dB of normal values. In addition, it is important to note that this combined treatment resulted in complete protection of supporting cells (pillar, Deiters', and Hensen cells). NTs are known to play a critical role in the survival of auditory neurons, both during development (15), and in adults (16, 17), as well as after aminoglycoside-induced hearing loss (9, 18) and after chronic electrical stimulation (19). In our previous study (9) we showed that NT3 fully prevents spiral ganglion neuron loss from aminoglycoside ototoxicity; however, hair cell loss was not prevented by NT3. In the present study, we find that the protective role of MK801 for auditory physiology and morphology is crucial and that the combined treatment with NT3 rescues all components of the cochlea. Although several features of the injury induced by noise and aminoglycosides differ, our results suggest that both involve a general mechanism. At present it is not known whether the protective properties of NMDA antagonists are a direct action on hair cells or indirectly via the spiral ganglion neurons. Because NMDA receptors have not been identified on hair cells, it is more likely that the effect is indirect. Noise as well as aminoglycosides causes an excessive activation of NMDA receptors present on the auditory afferents, resulting in calcium influx. Elevated calcium levels in the brain has been shown through calmodulin to activate NO synthase, leading to elevated NO synthesis as well as release of excessive levels of NO. The participation of NO in central nervous system neurodegenerative phenomena is believed to occur through a nonenzymatic reaction with the superoxide anion to form the highly reactive and potent oxidizing molecule peroxynitrite (ONOO−) (20). Thus, the generation of reactive oxygen species is a direct consequence of glutamate receptor overstimulation (21, 22). Our results raise the possibility that similar mechanisms occur in the cochlea.
The ability of neurotrophic factors to block spiral ganglion neurons from aminoglycoside toxicity has been demonstrated (9). Neurotrophic factors have been shown to effectively regulate intracellular calcium levels and reduce oxidative damage (23). These observations strengthen the idea that oxidative stress may be a factor common to several auditory pathologies and that additional chemical substances directed at reducing activation of NMDA or its downstream effectors also may be beneficial for protecting the peripheral auditory system from different pathologies, as was previously suggested (24).
Acknowledgments
The technical assistance of Agneta Viberg and Jun Chen is gratefully acknowledged. This study was supported by grants from the Swedish Council for Work Life Research (96-0509), Medical Research Council (09476), AMF-trygghetsförsäkring, Stiftelsen Tysta Skolan, Svenska Sällskapet för Medicinsk Forskning, and the Karolinska Institute (B.C.) as well as The Swedish Medical Research Council (B.C. and P.E.), Capten Artur Erikssons Foundation (B.C. and P.E.), and Petrus and Augusta Hedlunds Foundation (P.E.). K.A. was supported by a fellowship from the Swedish National Network for Neuroscience.
Abbreviations
- NMDA
N-methyl-d-aspartate
- NT3
neurotrophin 3
- SPL
sound pressure level
- ABR
auditory brainstem response
Footnotes
See commentary on page 6939.
References
- 1.Bobbin R P. Exp Brain Res. 1979;34:389–393. doi: 10.1007/BF00235683. [DOI] [PubMed] [Google Scholar]
- 2.Eybalin M. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- 3.Niedzielski A S, Wenthold R J. J Neurosci. 1995;15:2338–2353. doi: 10.1523/JNEUROSCI.15-03-02338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muir K W, Lees K R. Stroke. 1995;26:503–513. doi: 10.1161/01.str.26.3.503. [DOI] [PubMed] [Google Scholar]
- 5.Basile A S, Huang J M, Xie C, Webster D, Berlin C, Skolnick P. Nat Med. 1996;2:1338–1343. doi: 10.1038/nm1296-1338. [DOI] [PubMed] [Google Scholar]
- 6.Puel J-L, Ladrech R, Chabert R, Pujol R, Eybalin M. Hear Res. 1991;51:255–264. doi: 10.1016/0378-5955(91)90042-8. [DOI] [PubMed] [Google Scholar]
- 7.Pullan L M, Stumpo R J, Powel R J, Paschetto K A, Britt M. J Neurochem. 1992;59:2087–2093. doi: 10.1111/j.1471-4159.1992.tb10099.x. [DOI] [PubMed] [Google Scholar]
- 8.Choi D W. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 9.Ernfors P, Duan M L, Elshamy W M, Canlon B. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 10.Duan M L, Canlon B. Audiol Neurootol. 1996;1:328–338. doi: 10.1159/000259217. [DOI] [PubMed] [Google Scholar]
- 11.Pujol R, Lenoir M, Robertson D, Eybalin M. Hear Res. 1985;18:145–151. doi: 10.1016/0378-5955(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 12.Juiz J M, Rueda J, Merchán J A, Sala M. Hear Res. 1989;40:65–74. doi: 10.1016/0378-5955(89)90100-7. [DOI] [PubMed] [Google Scholar]
- 13.Spoendlin H. Acta Otolaryngol. 1971;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- 14.Robertson D. Hear Res. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- 15.Ernfors P, Van DeWater T, Loring J, Jaenisch R. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 16.Merlio J P, Ernfors P, Jaber M, Persson H. Neuroscience. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- 17.Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumäe U, Saarma M. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 18.Staecker H, Kopke R, Malgrange B, Lefebrve P, Van de Water T R. NeuroReport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 19.Miller J M, Chi D H, O'Keeffe L J, Kruszka P, Raphael Y, Altschuler R A. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 20.Dawson V L, Dawson T M. J Chem Neuroanatom. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 21.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. Nature (London) 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds I J, Hastings T G. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattson M P, Ahang Y, Bose S. Exp Neurol. 1993;121:1–13. doi: 10.1006/exnr.1993.1066. [DOI] [PubMed] [Google Scholar]
- 24.Ernfors P, Canlon B. Nat Med. 1996;2:1313–1314. doi: 10.1038/nm1296-1313. [DOI] [PubMed] [Google Scholar]