Abstract
The Mycobacterium bovis bacillus Calmette-Guérin (BCG) cell wall skeleton (CWS) consists of mycolic acids, arabinogalactan, and peptidoglycan (PGN) and activates Toll-like receptor 2 (TLR2) and TLR4. Here we investigated the ability of the essential portion of highly purified BCG CWS to support the TLR agonist function by using the following criteria: myeloid dendritic cell (DC) maturation, i.e., tumor necrosis factor alpha (TNF-α) production and CD83/CD86 up-regulation. The purified PGN region was sufficient to activate TLR2 and TLR4 in mouse DCs and macrophages; in TLR2 and TLR4 double-knockout cells the BCG PGN-mediated TNF-α production ability was completely impaired. Likewise, stimulation with BCG CWS of HEK293 cells expressing either human TLR2 or TLR4, MD-2, and CD14 resulted in NF-κB activation as determined by a reporter assay. Notably, specific blockers of extracellular human TLR2 (an original cocktail of monoclonal antibodies TLR2.45 and TH2.1) and TLR4 (E5531) inhibited BCG CWS-mediated NF-κB activation by 80%. Using this human TLR blocking system, we tested whether human myeloid DC maturation was TLR2 and TLR4 dependent. BCG PGN-mediated DC maturation was blocked by 70% by suppression of both TLR2 and TLR4 and by 30 to 40% by suppression of either of these TLRs. Similar but less profound suppression of BCG CWS-mediated DC maturation was observed. Hence, the presence of BCG PGN is a minimal requirement for activation of both TLR2 and TLR4 in human DCs, unlike the presence of PGNs of gram-positive bacteria, which activate only TLR2. Unexpectedly, however, BCG PGN, unlike BCG CWS, barely activated NF-κB in HEK293 cells coexpressing TLR2 plus TLR1, TLR2 plus TLR4, TLR2 plus TLR6, or TLR2 plus TLR10, suggesting that PGN receptors other than TLR2 and TLR4 present on human DCs but not on HEK293 cells are involved in TLR signaling for DC activation.
Phagocytosis of Mycobacterium tuberculosis by antigen-presenting cells is usually accompanied by activation of the transcription factor NF-κB, secretion of inflammatory and initial cytokines, release of the reactive nitrites, including NO, and secretion of several chemokines (9, 16). These responses involve the outputs of the signaling of pattern recognition receptors for microbes (16, 34). More than 10 members of the mammalian Toll-like receptor (TLR) family in the innate immune system have been identified as representatives of such receptors that primarily respond to microbial constituents to elicit the immune response in macrophages and dendritic cells (DCs) (25, 34). M. tuberculosis-mediated adjuvant activity may be expressed through TLRs on DCs.
Two of the human TLRs, TLR2 and TLR4, are involved in M. tuberculosis-mediated intracellular signaling in vitro (22, 41). Means et al. (22) demonstrated that viable M. tuberculosis bacilli contain distinct ligands that activate cells via TLR2 and TLR4, while heat-killed M. tuberculosis fails to activate cells via TLR4. Several purified mycobacterial ligands have now been identified as TLR2 agonists. Underhill et al. (43) suggested that TLR2 is selectively associated with macrophage phagosomes that contain yeast particles or zymosan. By using mouse macrophages lacking TLR2 or TLR4, it has been shown that TLR2 and TLR4 act as receptors of the cell wall skeleton (CWS) of Mycobacterium bovis bacillus Calmette-Guérin (BCG) (41). Thus, some TLR ligands of M. tuberculosis, called pathogen-associated molecular patterns (PAMPs) (26), may interact with several different TLRs. Recent reports have suggested that TLR4 senses such patterns in association with CD14 and MD-2 (28) and that TLR2 recognizes microbial molecular patterns in combination with TLR1, TLR2, TLR6, and probably TLR10 (1, 32, 37). Thus, the primary recognition step of TLR signaling induced by M. tuberculosis may be more complicated than previously expected (22, 41, 43).
It is notable that these receptor repertoires of M. tuberculosis components have been determined by using the mouse knockout system (41) or overexpression analysis (43), which may not reflect physiological human cellular responses. Furthermore, in humans the functional roles of the TLRs in DC maturation have been difficult to analyze separately since no good system has been established to discriminate among the functions of various human TLRs. In humans, accordingly, the receptor usage of M. tuberculosis constituents has been defined only poorly. Since BCG CWS is an M. tuberculosis vaccine and its adjuvant activity has been studied in conjunction with its Th1-dominant therapeutic potential (12, 20), the downstream signaling of the TLRs for BCG CWS must differ from that of lipopolysaccharide (LPS), a representative toxic PAMP.
It has been demonstrated previously that human DCs mature in response to BCG CWS (41). The results are consistent with the reported immunopotentiating activity of BCG CWS, which acts as a potent immune adjuvant sufficient to promote antibody production, induce CTL proliferation, and exert antitumor activity in immunoadjuvant therapy (2, 47, 49). BCG CWS is a complex consisting of mycolic acids, arabinogalactan, and peptidoglycan (PGN) (2), and which part is responsible for BCG CWS activity has not been determined (Fig. 1A). E5531, a synthetic lipid A-like compound of a TLR4 antagonist (6), reportedly inhibited M. tuberculosis-mediated TLR4 activation (23). However, the functional repertoires of human TLR2 in the M. tuberculosis-dependent DC response have remained unclear because of the lack of an appropriate inhibitor of human TLR2.
FIG. 1.
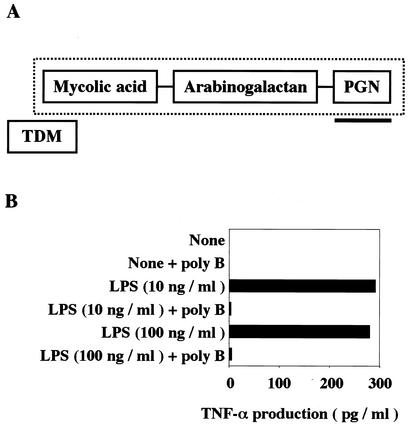
(A) Schematic representation of the putative structure of BCG CWS. The diagram was based on the findings of Besra and Chatterjee (4). The CWS portion is indicate by the dotted rectangle. The PGN portion of the BCG cell wall complex is underlined. TDM, trehalose dimycolate. (B) Polymyxin B blocks LPS-mediated TNF-α production in DCs. Human DCs were prepared as described in Materials and Methods and were treated with 10 or 100 ng of LPS per ml in the absence or presence of polymyxin B (poly B) (15 μg/ml). At timed intervals (typically 24 h), TNF-α levels in the media were measured by ELISA. The same experiments were performed three times, and representative results are shown.
In the present study, we established a human system for separate blocking of TLR2 and/or TLR4, which allowed us to determine the active center of BCG CWS and the degree of participation of TLR2 and TLR4 in human cell NF-κB activation and DC maturation induced by M. tuberculosis components.
MATERIALS AND METHODS
Reagents and antibodies.
Materials were obtained as indicated below. Fetal calf serum (FCS) was obtained from Bio Whittaker (Walkersville, Md.); Dulbecco modified Eagle medium and RPMI 1640 were obtained from Gibco BRL (Rockville, Md.); granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4) were obtained from Pepro Tech EC (London, United Kingdom); LPS (Escherichia coli O127:B8) was obtained from Difco Laboratories (Detroit, Mich.); mouse immunoglobulin G2b (IgG2b) was obtained from Sigma (St. Louis, Mo.); anti-CD83 monoclonal antibody (MAb) was obtained from Cosmo Bio (Tokyo, Japan); anti-CD80 MAb was obtained from Immunotech (Marseille, France); anti-CD86 MAb was obtained from Ancell (Bayport, Minn.); fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG F(ab′)2 was obtained from American Qualex Manufacturers (San Clemente, Calif.); and anti-TLR2 MAb TL2.1 was obtained from CASCADE Bioscience (Winchester, Mass.).
E5531, a well-characterized antagonist of LPS for TLR4 stimulation (19, 23), was a gift from Eisai Co., Ltd. (Tokyo, Japan). LPS, macrophage-activating lipopeptide 2 of mycoplasma origin (MALP-2), and polymyxin B were obtained as described previously (29). cDNAs for human TLR1, TLR2, TLR4, TLR6, and TLR10 were cloned in our laboratory by reverse transcription (RT)-PCR from mRNA of immature DCs (iDC) and were ligated at the cloning site of pEFBOS. The details of the constructs will be published elsewhere. cDNAs for MD-2 and CD14 were provided by K. Miyake and H. Nishimura, respectively.
Preparation of MAbs against human TLR2.
BALB/c mice were immunized with Ba/F3 cells that stably expressed human Flag-tagged TLR2, and spleen cells were fused with NS-1 myeloma cells. The detailed methods used have been described previously (21). MAbs TLR2.45 and TLR2.524 (mouse IgG1) were successfully established and used for this study. These MAbs were shown to be specific for TLR2 in transfection experiments and did not react with Ba/F3 cells stably expressing human TLR3 or TLR4 (data not shown). The specificity of an MAb was further confirmed by immunoprecipitation studies (31). Additional information concerning these anti-human TLR2 MAbs will be presented elsewhere in a comparison of the characteristics of MAbs against human TLRs recently established in our laboratory.
BCG CWS and other BCG constituents.
BCG CWS was prepared as described previously (6, 47). The purity of the lot of BCG CWS used for this study was estimated by chemical analysis, the results of which are shown together with the results of an analysis of BCG PGN in Table 1. Since BCG CWS is insoluble in water and organic solvents, an oil-in-water emulsion form of BCG CWS particles was used throughout this study. Dried powder of BCG CWS was suspended at a concentration of 1 mg/ml in emulsion buffer (phosphate-buffered saline [PBS] containing 1% Drakeol and 1% Tween 80) with a Potter homogenizer and then sterilized by heating for 30 min at 60°C.
TABLE 1.
Constituents of BCG CWS and BCG PGN used in this studya
| Prepn | Concn (%, wt/wt) of:
|
Amino acid and amino sugar concn (μmol/mg)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total lipids | Neutral sugars | Total phosphorus | Amino acids and amino sugars | Ala | Glutamine- glutamic acid | Dimethyl pimeric acid | Glc- NH2 | Muramic acid | Gal-NH2 | Other amino acids | |
| BCG CWS | 34.2 | 38.6 | 0.11 | 20.7 | 0.32 | 0.29 | 0.18 | 0.19 | 0.18 | —b | 0.10 |
| BCG PGN | ND | <0.2 | NDc | 49.9 | 1.0 | 0.8 | 0.5 | 0.2 | 0.2 | 0.0 | 1.7 |
Chemical analysis was performed method. Briefly, sample was hydrolyzed with NaOH, and the lipid fraction was analyzed by mass spectrometry. For analysis of carbohydrates, the samples were hydrolyzed with HSO4. The carbohydrate contents in the samples were determined by gas-liquid chromatography. The molar ratios of amino acids and amino sugars in the samples were determined after acid (HCl) hydrolysis.
—, no data in reference 2.
ND, not determined.
For FITC labeling of BCG CWS, dried BCG CWS was suspended in 50 mM HEPES-buffered saline (pH 8.5) at a concentration of 1 mg/ml. After this, 10 ml of a 10-mg/ml solution of FITC in dimethyl sulfoxide was added to the suspension and incubated for 15 min at 37°C. FITC-labeled BCG CWS was collected by centrifugation (15,000 × g, 10 min) and washed once with HEPES-buffered saline (pH 7.0). FITC-labeled BCG CWS was resuspended in emulsion buffer at a concentration of 1 mg/ml with a homogenizer and sterilized by heating for 30 min at 60°C.
Arabinogalactan, mycolic acid, arabinose mycolic acid, and trehalose dimycolic acid were prepared in I. Azuma's laboratory as described previously (2, 47, 49). The purity of each of these reagents was >98%, as determined by physicochemical analysis in our institute (data not shown). The endotoxin concentrations in the samples were less than the detection limit (>0.2 pg/ml), as determined by an endotoxin assay kit (29). The disposition of these constituents in the cell wall complex of BCG is shown schematically in Fig. 1A.
Separation of BCG PGN.
The PGN portion of the CWS was isolated from purified BCG CWS by a previously described method (3), with the following slight modification. Briefly, BCG CWS (200 mg) was agitated with 0.1 N HCl (5 ml) for 12 h at 60°C. After centrifugation, the residue was washed with distilled water and dried by suction. The product was then extracted with acetone and ether to remove the bound lipid. Mycolic acids were dissolved in the solution and removed from the cell walls by centrifugation. The insoluble material was suspended in 5% trichloroacetic acid and allowed to stand for 24 h at 60°C. The cell walls were collected by centrifugation and then washed thoroughly with water, acetone, and ether. Suction to dryness was performed between steps. A portion of the resultant insoluble component was allowed to react with 0.05 M NaIO4 for 14 days at 4°C in a dark room. The arabinogalactan region was removed from the PGN portion by this procedure. Finally, suction to dryness was performed after centrifugation, and the precipitate was washed with distilled water, acetone, and ether. PGN was identified in the insoluble material obtained by this treatment method. The purity of the PGN preparation was examined by chemical analysis (Table 1), and the findings suggested that the PGN preparation contained virtually no neutral sugars, which constitute the structure of arabinogalactan, and the N-acetylmuramyl acid-GlcNAc structure was partially damaged. Although there were some contaminating proteins in the preparation, they did not affect TLR agonist activity (Fig. 2D). Using methods similar to those used for FITC-labeled BCG CWS (see above), we prepared FITC-labeled BCG PGN.
FIG. 2.
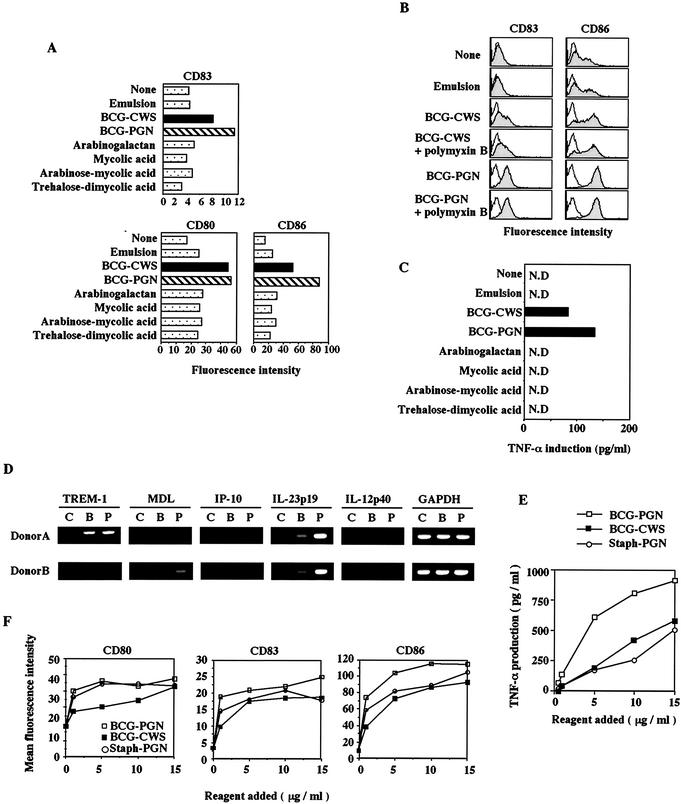
BCG CWS and BCG PGN up-regulate CD83, CD80, and CD86 expression and induce TNF-α in iDCs. (A) Monocyte-derived iDCs (1 × 106 cells/ml) were stimulated with GM-CSF alone (None), with emulsion buffer (15 μl/ml), with BCG CWS (15 μg/ml), with BCG PGN (15 μg/ml), with arabinogalactan (15 μg/ml), with mycolic acid (15 μg/ml), with arabinose-mycolic acid (15 μg/ml), or with trehalose-dimycolic acid (15 μg/ml). After 48 h, the cells were harvested, and the levels of expression of CD83, CD80, and CD86 were analyzed by flow cytometry. The data from one of three experiments in which similar results were obtained are shown. (B) iDCs were stimulated with polymyxin B-treated or untreated BCG CWS (15 μg/ml) or BCG PGN (15 μg/ml). Emulsion buffer was used as the control. After 48 h, the levels of expression of CD83 and CD86 were analyzed by flow cytometry. This experiment was repeated twice with similar results, and the results of a single representative experiment areshown. (C) BCG PGN induces cytokine production by human iDCs. iDCs were stimulated with BCG CWS, BCG PGN, or other components as described above, all of which had been treated with polymyxin B. After 48 h, the levels of TNF-α and IL-12 p40 (data not shown) in the supernatants were measured by ELISA. This experiment was repeated twice with similar results, and the results of a representative experiment are shown. Although data are not shown, IL-12 p40 was up-regulated only by BCG CWS and BCG PGN. N.D., not detected. (D) RT-PCR analysis of four BCG CWS-inducible genes (TREM-1, MDL, IL-23 p19, and IL-12 p40 genes) and one LPS-inducible gene (IP-10 gene). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as a control. iDCs were stimulated with BCG compounds or an oil emulsion only for 4 h, and their RNAs were extracted. RT-PCR was performed by using nonstimulated (lanes C), BCG CWS-stimulated (lanes B), and BCG PGN-stimulated (lanes P) DC RNAs. The template RNAs were obtained from two volunteers (donors A and B). The profiles for regulation of these genes by BCG CWS in human macrophages are described elsewhere (Begum et al., submitted). BCG PGN induced the IL-23 p19 mRNA more efficiently than BCG CWS induced this mRNA, although the reason for this is not known. An LPS-inducible IP-10 gene, representative of other interferon-inducible genes (data not shown), was barely up-regulated by stimulation with BCG CWS or BCG PGN. Similar results were obtained with iDCs stimulated for 8 h. The PCR conditions used are described elsewhere (Begum et al., submitted). (E) In terms of weight ratio, BCG PGN induces TNF-α more efficiently than BCG CWS induces TNF-α. BCG CWS, BCG PGN, or Staph PGN (1 to 15 μg/ml) was incubated with iDCs (1 × 105 cells/well) for 48 h, and the TNF-α liberated in the media was measured by ELISA. (F) Up-regulation of CD80, CD83, and CD86 on DCs stimulated with PAMPs. iDCs (1 × 105 cells/well) were incubated with different amounts of BCG CWS, BCG PGN, or Staph PGN for 24 h. Then the surface levels of CD80, CD83, and CD86 on DCs were assessed by flow cytometry, as shown on the y axis.
Preparation of human iDCs.
Human peripheral blood mononuclear cells were prepared from 400 ml of citrate-phosphate-dextrose-supplemented blood by methylcellulose sedimentation and density gradient centrifugation with Ficoll-Paque (Amersham Pharmacia Biotech AB, Piscataway, N.J.) (41). Monocytes were isolated from peripheral blood mononuclear cells with a magnetic cell sorting system by using anti-CD14-coated microbeads (MiltenyiBiotec, Gladbach, Germany) (18, 41). iDCs were generated from monocytes (5 × 105 cells/ml) by culturing for 6 days in RPMI 1640 (10 ml) supplemented with 10% heat-inactivated FCS in the presence of 500 IU of human GM-CSF per ml and 100 IU of human IL-4 per ml (23, 41). Preparations of iDCs were checked for the surface markers CD14−, CD40+, CD83−, CD80low, CD86low, and CD1a+.
DC maturation.
iDCs were prepared as described above. These cells were then cultured (5 × 105 cells/ml) for 2 days in RPMI 1640 containing 10% heat-inactivated FCS with 500 IU of GM-CSF per ml, 100 IU of IL-4 per ml, and either 10 ng of LPS per ml, 15 μl of emulsion buffer (vehicle for BCG CWS) per ml, 15 μg of BCG CWS per ml, or 15 μg of BCG PGN per ml. After 2 days, the adherent cells were collected at 4°C by gentle pipetting with PBS containing 10 mM EDTA. DC maturation was checked by analyzing the surface markers CD40+, CD83+, CD80high, CD86high, and CD1a+. In some cases, CD150 was used as a marker (17).
Appropriate doses of polymyxin B for blocking LPS activity were checked by measuring tumor necrosis factor alpha (TNF-α) induction by human DCs (Fig. 1B). Based on the results, 15 μg of polymyxin B per ml was used throughout the experiments; this amount sufficiently blocked the activity of 100 ng of the LPS per ml (Fig. 1B). Thus, in the following experiments we could exclude the possibility that contaminating LPS or an LPS-like product in the BCG preparations induced DC maturation. The lack of contaminating LPS in the BCG preparations was further verified by using an endotoxin detection kit (29) (data not shown).
Preparation of TLR-deficient macrophages and MyD88-deficient mouse DCs.
TLR2-, TLR4-, and MyD88-deficient mice were kindly donated by S. Akira, and mouse macrophages and DCs were prepared by using the protocol of the research group of this researcher (15, 37).
Briefly, peritoneal macrophages were prepared as described previously (41). Mice (F2 interbred from 129/Ola × C57BL/6) were intraperitoneally injected with 2 ml of 4% thioglycolate medium (Difco). Three days later, peritoneal exudate cells were isolated from the peritoneal cavity by washing with ice-cold Hanks buffered salt solution. Adherent monolayer cells were used as peritoneal macrophages.
Mouse myeloid DCs were prepared from the bone marrow of C57BL/6 or MyD88−/− mice (6 weeks, male C57BL/6) by treatment with GM-CSF for 6 days as described previously (15). Cells were then stimulated with appropriate PAMPs.
RT-PCR analysis.
BCG CWS- or BCG PGN-treated DCs were used as sources of RNA. RNA was extracted with a Qiagen kit. The practical method used for RT-PCR has been described previously (18). Briefly, all PCRs were performed by using a Takara Taq polymerase PCR kit and a Perkin-Elmer PCR machine (model 9600). DNase I-treated total RNA (2 μg) was used for synthesis of cDNA by using a First-strand cDNA synthesis kit (Life Technologies-BRL) with oligo-d(T) as the primer. The cDNA reaction product was diluted 1:25 for the PCR with various primer sets, and 10-μl aliquots of the product were analyzed by electrophoresis on gels. Two sets of such RNA samples were then chosen for subsequent analysis of BCG regulatory genes. The primer pairs used for each gene amplification will be described elsewhere (N. A. Begum, M. Kobayashi, Y. Moriwaki, M. Matsumoto, I. Azuma, K. Toyoshima, and T. Seya, submitted for publication).
Flow cytometric analysis of cell surface antigens.
Cells were suspended in PBS containing 0.1% sodium azide and 0.1% bovine serum albumin and then incubated for 30 min at 4°C with 5-μg portions of MAbs. The cells were washed and counterstained with FITC-conjugated goat anti-mouse IgG F(ab′)2 for 30 min at 4°C. Fluorescence intensity and mean fluorescence shifts were then determined by flow cytometry by using an attached computer (FACSCalibur; Becton-Dickinson).
ELISA.
Human iDCs (5.0 × 104 cells/well) were plated in duplicate wells in 96-well flat-bottom plates in the absence or presence of anti-TLR2 MAbs (Table 2) and/or E5531 (10 μM) before addition of stimulating compounds. iDCs were stimulated with polymyxin B-treated BCG CWS (15 μg/ml), BCG PGN (15 μg/ml), Staphylococcus aureus PGN (Staph PGN) (15 μg/ml), or polymyxin B-free LPS (100 ng/ml) for 16 h at 37°C. The appropriate amounts of these PAMPs were determined based on the levels of surface markers indicating the degree of DC maturation. The levels of human TNF-α in culture supernatants were measured by an enzyme-linked immunosorbent assay (ELISA) (Amersham Pharmacia Biotech AB).
TABLE 2.
Blocking of TLR2-mediated NF-κB activation by MAbs
| MAb | % Inhibition by MAbsa
|
|||
|---|---|---|---|---|
| TH2.1 | TLR2.45 | TLR2.524 | 5 μg | 10 μg |
| − | − | − | 0.0 | 0.0 |
| + | − | − | 12.8 | 26.1 |
| − | + | − | 10.7 | 20.9 |
| − | − | + | 12.8 | 24.0 |
| + | + | − | 57.5 | 81.6 |
| + | − | + | 2.2 | 6.3 |
| − | + | + | 14.9 | 27.2 |
| + | + | + | 38.3 | 66.3 |
A 5 or 10-μg portion of each mAb was used for blocking experiments. BCG-CWS and TLR2-expressing HEK 293 cells were used HEk293 were used as described in Materials and Methods. Two experiments were performed independently.
In mouse experiments, peritoneal macrophages and DCs were prepared from wild-type (C57BL/6) mice, MyD88-deficient mice, TLR2-deficient mice, TLR4-deficient mice, and mice that were deficient in both TLR2 and TLR4 and were cultured (2.5 × 105 cells/ml) with BCG CWS (15 μg/ml) or BCG PGN (5 μg/ml) for 24 h at 37°C. The levels of liberated mouse TNF-α were measured by an ELISA (Genzyme) (41).
Luciferase reporter assay.
HEK293 cells (1 × 106 cells/well) were plated in six-well plates and 24 h later were transiently transfected by using Lipofectamine 2000 reagent (Gibco-BRL) and 1 μg of expression plasmid pEFBOS containing MD-2, CD14, and/or TLR cDNA, 1 μg of an NF-kB reporter plasmid encoding luciferase (Stratagene), or 1 μg of the pSV β-galactosidase control vector (Promega, Madison, Wis.) for normalization of transfection efficiency. In some experiments, cells were cotransfected with 1-μg portions of vectors containing cDNA of TLR2 and cDNA of either TLR1, TLR4, TLR6, or TLR10. At 24 h after transfection, cells were harvested, seeded into five wells of 24-well plates (2 × 105 cells/well), and stimulated with 2 μg of LPS, polymyxin B-treated BCG CWS (15 μg/ml), polymyxin B-treated BCG PGN (5 to 15 μg/ml), or 200 nM MALP-2 at 37°C for 6 h. The cells were lysed, and luciferase activity was measured by using the PicaGene protocols (Toyo Ink, Tokyo, Japan). The specific activity was calculated as follows: light intensity with stimulation/light intensity without stimulation.
Blocking of TLR2 or TLR4.
Blocking experiments for TLR4 with E5531 (EISAI Co., Tokyo, Japan) were performed as described previously (19, 23). Briefly, human iDCs (5.0 × 104 cells/well; 96-well plates) or HEK293 cells (2 × 105 cells/well; 24-well plates) were incubated with 10 μM E5531 for 30 min at 37°C. The E5531-treated DCs were then stimulated with PAMPs, and the effects on the cells were evaluated.
Blocking experiments for TLR2 were performed with anti-TLR2 MAbs (TL2.1, TLR2.45, TLR2.524). Briefly, cells (2 × 105 cells/well) were incubated with 5 to 10 μg of each MAb or combinations of MAbs for 30 min at 37°C. The cells were then stimulated with agonists, and the effects on the cells were evaluated. This method could be used for all of the cell types except macrophage-like cells that had high-affinity Fc receptors (data not shown). Since both purified MAbs and their F(ab′)2 fragments induced similar blocking profiles in DCs, we used the whole IgG molecules of MAbs and nonimmune IgG controls.
RESULTS
BCG PGN functions as a DC maturation inducer.
iDCs were prepared with GM-CSF and IL-4 and then incubated with various constituents of BCG CWS in an oil emulsion or with emulsion only (buffer control) for 48 h at 37°C. The surface levels of CD80, CD86, and CD83 on the affected DCs were measured by flow cytometry (Fig. 2A). BCG PGN had the ability to up-regulate CD80, CD86, and CD83 on the cell surface of iDCs, as did the starting material, BCG CWS. BCG PGN up-regulated the costimulators even after polymyxin B treatment, indicating that if there was any contaminating LPS, it did not have an effect (Fig. 2B). The other components of the BCG cell wall, including arabinogalactan, mycolic acid, arabinose-mycolic acid, and treharose-dimycolic acid, barely induced up-regulation of costimulators (Fig. 2A).
One of the main factors inducing DC maturation is TNF-α (39, 41), which is produced in DCs by BCG CWS stimulation and induces expression of many BCG regulatory genes via NF-κB (Begum et al., submitted). We next examined the levels of TNF-α induced in DCs by the stimulants. Only one of the constituents, BCG PGN, effectively induced TNF-α (Fig. 2C). The BCG PGN prepared in this study contained little arabinogalactan or lipids, as judged by physicochemical analysis (it contained no lipids and neutral sugars) (Table 1) and by functional analysis when we tested the gene regulatory profiles of the selected BCG CWS regulatory genes (Begum et al., submitted) and the LPS regulatory gene (Fig. 2D). Hence, BCG PGN induces TNF-α in DCs, which in turn induces up-regulation of costimulators.
BCG PGN appeared to induce CD83 and CD86 more effectively than CWS in terms of the weight ratio (15 μg/ml) (Fgi. 2A). The levels of inducible TNF-α and costimulators were evaluated with different doses of BCG CWS and BCG PGN (Fig. 2E and F). Staph PGN, a TLR2 agonist (38), was used as a control. TNF-α was incrementally generated in DCs as the doses of the stimulants increased (Fig. 2E). BCG PGN more effectively induced TNF-α in DCs than the other two inducers; thus, BCG PGN induced >600 pg of TNF-α per ml even at a low dose (5 μg/ml), whereas Staph PGN and BCG CWS induced <200 pg of TNF-α per ml at the same dose (Fig. 2E). We roughly determined that 5 μg of BCG PGN per ml is functionally equivalent to 15 μg of BCG CWS per ml for TNF-α induction (Fig. 2E). The relatively high activity of BCG PGN on a weight basis was probably due to the high molar ratio of the active center in the preparation of BCG PGN compared with that of BCG CWS. Based on these findings, we decided to use 5 μg of BCG PGN per ml and 15 μg of BCG CWS per ml throughout this study.
In the DCs, less than 5 μg of BCG PGN per ml sufficiently up-regulated costimulators, and this was followed by mild increases in their levels in a dose-dependent manner. At the same weight ratio, BCG PGN usually induced the up-regulation of costimulators more efficiently than BCG CWS did (Fig. 2F). Less than 5 μg of each of these reagents per ml was sufficient to up-regulate CD83 and CD86, both of which were more sensitive to BCG PGN than to Staph PGN or BCG CWS (Fig. 2F). CD80 was equally sensitive to Staph PGN and BCG PGN. Our findings thus indicated that the PGN portion of BCG CWS is the active center for inducing human DC maturation in terms of induction of TNF-α and up-regulation of costimulators.
TLR2 and TLR4 are receptors for BCG PGN in macrophages and DC.
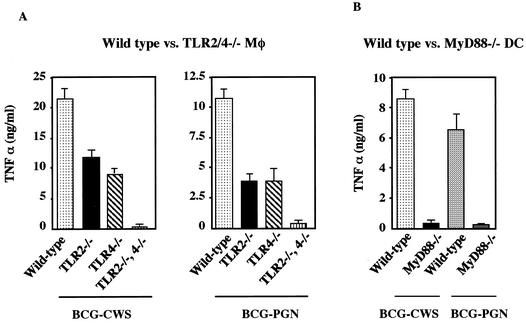
PGN of gram-positive bacteria usually serves as a ligand of TLR2 in mouse macrophages (38). We therefore examined the receptor usage of BCG PGN compared with that of BCG CWS using TLR gene-disrupted mouse macrophages (Fig. 3A). Control BCG CWS was an agonist for TLR2 and TLR4, as previously reported (41). BCG PGN induced TNF-α in wild-type macrophages with potency similar to that of BCG CWS (Fig. 3A). The TNF-α-inducing activity of BCG PGN was reduced by about 50% in TLR2−/− or TLR4−/− macrophages and was entirely eliminated in TLR2-TLR4 double-negative macrophages, a pattern similar to that seen with BCG CWS. These results were reproduced with MyD88-deficient DCs, which eliminated TLR2- and TLR4-mediated TNF-α-inducing pathways (Fig. 3B). Murine macrophages and DCs reportedly express sufficient MD-2 and CD14 to respond to LPS (15, 28). However, the BCG PGN response profiles were barely affected by the addition of polymyxin B (data not shown). Thus, in mouse macrophages, TLR2 and TLR4 contribute almost equally to TNF-α production in response to BCG PGN, as well as CWS. BCG PGN appears to be a distinct PAMP with respect to receptor usage compared to the previously described PGN of bacteria, which selectively activates TLR2 but not TLR4 (38).
FIG. 3.
BCG PGN, like BCG CWS, induces TNF-α production by mouse macrophages and DCs through TLR2, TLR4, and MyD88. (A) Thioglycolate-elicited peritoneal macrophages from wild-type mice, TLR2−/− mice, TLR4−/− mice, and TLR2−/− TLR4−/− double-knockout mice were cultured with BCG CWS (15 μg/ml) or BCG PGN (5 μg/ml) for 24 h. The TNF-α secreted into the culture supernatants was measured by ELISA. The experiment was repeated twice with similar results, and the results of a representative experiment are shown. Mφ, macrophages. (B) Bone marrow-derived DCs were prepared from wild-type mice and MyD88−/− mice as described in Materials and Methods (15). Cells were cultured with BCG CWS (15 μg/ml) or BCG PGN (5 μg/ml) for 24 h. The concentrations of TNF-α in the culture supernatants were measured by ELISA.
Difference between BCG CWS and BCG PGN in NF-κB reporter assay.
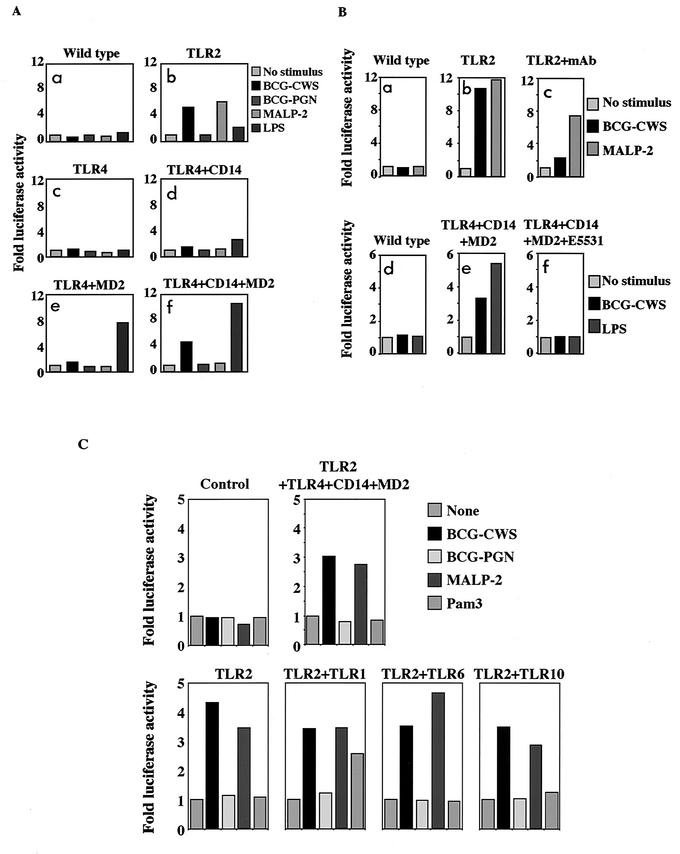
Using the HEK293 NF-κB-luciferase expression system, we next examined what factors participate in recognition of the BCG components in the human system (Fig. 4A). HEK293 cells expressed neither the mRNAs of TLR1 to TLR10 (except a trace of TLR5 mRNA) nor the mRNAs of CD14 and MD-2, as shown by RT-PCR (21). Constitutive expression of TLR2 alone conferred responsiveness to BCG CWS, as well as to the Mycoplasma lipopeptide MALP-2 (which was used as a positive control for TLR2 stimulation [29]), on HEK293 cells, as shown by an NF-κB reporter gene assay. Transient expression of MD-2 on TLR4-expressing cells gave rise to luciferase activity in response to control LPS, as reported previously (36). Coexpression of CD14 and MD-2 on TLR4-expressing cells conferred moderate BCG CWS responsiveness also (Fig. 4A).
FIG.4.
Reporter assay for determination of the receptor usage of BCG CWS and BCG PGN. (A) TLR2- and TLR4-mediated activation of NF-κB in HEK293 cells by BCG CWS but not by BCG PGN. Wild-type HEK293 cells with the NF-κB luciferase reporter plasmid were used as a control (panel a). HEK293 cells stably expressing TLR2 (panel b) or TLR4 (panels c to f) were transfected with the NF-κB luciferase reporter plasmid and cotransfected with empty vector (panel c) or other expression vectors (human MD-2, human CD14) (panels d, e, and f). After 24 h, cells were stimulated with BCG CWS (15 μg/ml), BCG PGN (5 μg/ml) (treated with polymyxin B), or positive controls (MALP-2 for TLR2 and LPS for TLR4) for 6 h, and NF-κB activation was determined by measuring the luciferase activity in the cell lysates. The specific activity was obtained by using the method described in Materials and Methods. The luciferase activity of the HEK293 transfectants was measured along with that of controls. Experiments were performed twice in triplicate, and the results are expressed as means. Statistical significance was tested by using the InStat program. (B) Inhibition of NF-κB activity induced by BCG CWS with a cocktail containing anti-TLR2 MAbs or E5531. HEK293 cells expressing no TLR (panels a and d), TLR2 (panels b and c), or TLR4, MD-2, and CD14 (panels e and f) were incubated with BCG CWS (15 μg/ml) or control PAMPs in the absence of inhibitors (panels b and e) or in the presence of inhibitors (panels c and f). The specific activities were determined as described above. (C) NF-kB activation in HEK293 cells with combination stimulation of TLRs. HEK293 cells were transfected with various combinations of TLRs, as indicated above each graph. Wild-type HEK293 cells were used as a control. Cells were stimulated with BCG CWS or BCG PGN. As positive controls, MALP-2, LPS (data not shown), and tripalmitoyl-Cys-Ser-Ser-Asn-Ala (PAM-3) were used as representative PAMPs of TLR2 plus TLR6, TLR4 plus MD-2 plus CD14, and TLR2 plus TLR1, respectively. The potencies of TLR-mediated NF-κB activation were determined by the luciferase reporter assay. Although the natural ligands for TLR2 plus TLR10 are not known, they responded to BCG CWS or MALP-2 but not to BCG PGN.
Next, inhibitor studies were performed to confirm the function of BCG CWS by using TLR-expressing HEK293 cells. E5531 was employed as a TLR4-specific inhibitor. Since no specific inhibitor is available for TLR2, we produced MAbs against human TLR2. BCG CWS-mediated TLR4 signaling was completely blocked in HEK293 cells by E5531, supporting the knockout mouse finding that TLR4 mediates the signaling of BCG CWS (41). BCG CWS-mediated TLR2 signaling was inhibited by ∼80% by the combination of the MAbs in the HEK293 system (Table 2); both of the MAbs inhibited the BCG CWS response more effectively than they inhibited the MALP-2-mediated response (Fig. 4B). Hence, BCG CWS-mediated NF-κB activation largely involves TLR2 and TLR4. The blockers of TLR2 and TLR4 used are known to interact with the extracellular domains of the relevant TLRs (Table 2) (21, 31). Altogether, these findings indicate that BCG CWS is recognized by the extracellular domains of both TLR2 and TLR4 and activates NF-κB through the intracellular adapters.
Nevertheless, no NF-κB activation by BCG PGN was observed with TLR2 and TLR4 even under these conditions. Likewise, constitutive expression of TLR2 and TLR4 and additional transient expression of CD14 and MD-2 in HEK293 cells (8) resulted in virtually no induction of luciferase activity in response to BCG PGN. No other BCG components induced NF-κB activation in this system either (data not shown).
To further confirm this unexpected discrepancy in the responsiveness of HEK293 transfectants to BCG CWS and BCG PGN, TLR combination studies were performed by using HEK293 cells expressing various combinations of TLRs (Fig. 4C). TLR2 is known to recognize microbial patterns in combination with TLR1 or TLR6 (1, 32, 37). Although the specific ligand recognized by the combination of TLR2 and TLR10 has not been identified, it is tentatively accepted that TLR2 and TLR10 combine to recognize some PAMP. BCG PGN did not activate NF-κB with any combination containing TLR2 or TLR2 plus TLR4 in addition to CD14 and MD-2 under the conditions where NF-κB was activated by positive controls (Fig. 4C). In contrast, BCG CWS activated NF-κB in cells expressing TLR2 and/or TLR4, but no additive effect was observed by expressing TLR1, TLR6, or TLR10 in addition to TLR2 (Fig. 4C). Collectively, for BCG CWS, TLR2 alone was sufficient for BCG CWS-mediated signaling, whereas CD14 and MD-2 were required in addition to TLR4 to sustain BCG CWS-mediated TLR4 signaling. Thus, the functional profile of BCG CWS encompassed receptors for representative bacterial patterns, TLR2 and TLR4. Collectively, the molecular assemblies including TLR2 or TLR4 do not explain the difference in responsiveness between BCG CWS and BCG PGN. Some additional cellular factors would be required in order for BCG PGN to activate the signaling of TLR2 and TLR4 in HEK293 cells.
Blockage of DC maturation by TLR2 and TLR4 inhibitors.
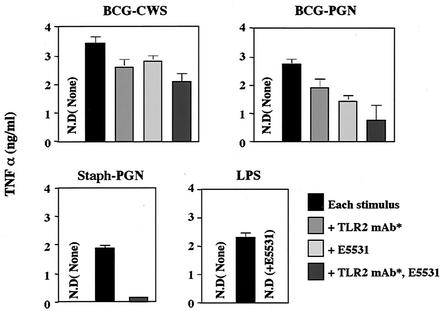
To test what additional factor(s) contributes to TLR2 and TLR4 activation by BCG PGN, function-blocking studies were performed with combinations of human DCs and BCG CWS or BCG PGN (Fig. 5). The TLR inhibitors (Fig. 4B) were used to test various TLR-responsive DC functions. TNF-α-mediated DC maturation was induced by BCG PGN or BCG CWS, while NF-κB in HEK293 cells was not activated by BCG PGN. As shown by the level of the TNF-α marker, BCG PGN-mediated DC maturation was reduced by blocking either TLR2 or TLR4 (Fig. 5) and was suppressed by ∼70% by function blocking of both TLR2 and TLR4 (Fig. 5). In contrast, CWS-mediated DC maturation was only slightly (∼15%) inhibited by either a TLR2 or TLR4 inhibitor. Even when both inhibitors were used, the maximal inhibition was less than 30% (Fig. 5). Under the same conditions, control stimulation with TLR2 (Staph PGN) and control stimulation with TLR4 (LPS) were much more markedly (85 and 98%, respectively) blocked (Fig. 5). Nonimmune mouse IgG, which was used instead of the MAb cocktail (5 μg each) as a control, induced virtually no inhibition of TNF-α production in this system.
FIG. 5.
Suppression of iDC-mediated TNF-α induction in response to BCG CWS or BCG PGN by inhibitors of TLR2 and/or TLR4. Human iDCs were prepared as described in the legend to Fig. 2 and were cultured in a 96-well plate (1 × 105 cells/well) with different TLR inhibitors and BCG derivatives for TLR agonists. After 48 h, the supernatants were harvested, and the levels of TNF-α were determined by ELISA. Staph PGN and LPS were used as representative TLR2 and TLR4 stimulators, respectively. The stimuli used are indicated above the graphs. An asterisk indicates the cocktail of MAbs against human TLR2. N.D., not detected.
CD80 and CD86 were up-regulated in response to low doses of BCG PGN or BCG CWS, and the up-regulation was barely affected by blocking of TLR2 and TLR4 (data not shown). CD83 and CD150 are maturation markers for DCs (17) and are up-regulated in response to BCG CWS or BCG PGN (27). In human DCs, TNF-α is induced secondary to BCG CWS and plays a crucial role in the up-regulation of these maturation markers. Therefore, we checked the levels of CD83 and CD150 by RT-PCR and flow cytometry in TLR2- and TLR4-blocked and unblocked BCG CWS-treated DCs. The levels of these markers were not changed on DCs irrespective of the antibody treatment (data not shown). TNF-α induction can be only partially blocked by TLR4 and/or TLR2 inhibitors, which may explain the failure of TLR inhibitors to suppress BCG-mediated DC maturation.
Binding of BCG CWS and BCG PGN to human DCs.
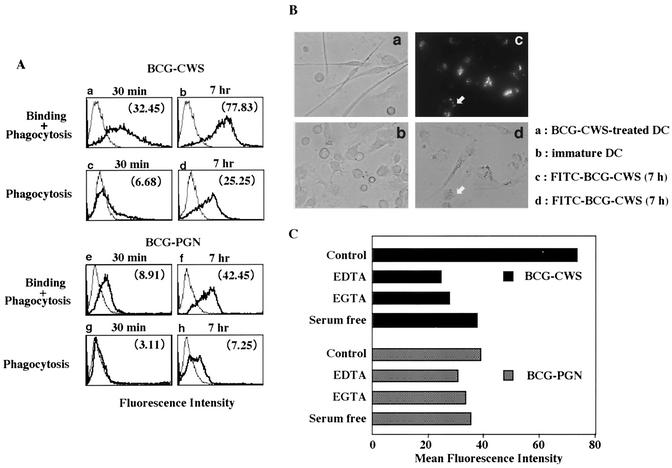
Binding of BCG CWS and BCG PGN to iDCs was examined by using the corresponding fluorescence-labeled molecules (Fig. 6). BCG CWS efficiently bound to the DC surface and was incorporated into endosomes in a time-dependent manner. BCG PGN was far less effectively bound and taken up by DCs (Fig. 6A and B). Thus, it is likely that BCG CWS uses a different receptor set than BCG PGN uses on human DCs. BCG CWS binding, but not BCG PGN binding, was blocked with EDTA, EGTA, or depletion of serum (Fig. 6C), suggesting that divalent metals (most likely Ca2+) support CWS binding. The portion present in BCG CWS but not BCG PGN is arabinogalactan and mycolic acids (Fig. 1A). Thus, the weak binding observed with BCG PGN should be due to interactions with PGN-binding receptors, whereas CWS binding should also be mediated by lectin-like receptors. Additional evidence indicating the difference in the receptors is that a divalent cation was essential for BCG CWS binding to DC but not for BCG PGN binding to DC (Fig. 6C). Testing of the blocking profiles of BCG CWS binding to DCs by using synthetic oligosaccharides that mimic the carbohydrate structure of CWS is in progress.
FIG. 6.
Binding of BCG CWS and BCG PGN to iDCs and ingestion. (A) Flow cytometric analysis of binding and phagocytic FITC-labeled BCG CWS or BCG PGN in iDCs. iDCs (5 × 105 cells/ml) were incubated for 30 min or 7 h with RPMI 1640 containing 10% FCS and FITC-labeled BCG CWS (15 μg/ml) (panels a to d) or FITC-labeled BCG PGN (5 μg/ml) (panels e to h). These cells were analyzed with a flow cytometer. The thin lines indicate self-fluorescence of cells, and the thick lines indicate fluorescence of FITC-labeled compounds which were bound and/or phagocytosed. The total fluorescence intensities (panels a, b, e, and f) reflect bound and phagocytosed FITC-labeled BCG compounds, and the fluorescence intensities quenched with trypan blue (panels c, d, g, and h) reflect phagocytosed FITC-labeled BCG compounds. The specific values for the mean fluorescence shift are indicated in parentheses. This experiment was repeated three times with similar results, and the results of a representative experiment are shown. (B) Microscopic analysis of bound and phagocytosed BCG compounds. iDCs treated with fluorescently labeled BCG CWS or BCG PGN (data not shown) for 7 h were examined with a fluorescence microscope (Olympus IX-70, BX-60). The arrows indicate bound BCG CWS. (C) Requirement of divalent cations for BCG CWS binding but not BCG PGN binding to iDCs. iDCs were treated with the reagents indicated and incubated for 4 h with FITC-labeled BCG compounds. EGTA (10 mM) was used for chelating divalent cations, most likely Ca2+. The results are expressed as mean fluorescence shift values as measured by flow cytometry. The results of one of three similar experiments are shown.
DISCUSSION
In the present study, we identified the functional properties of BCG PGN, which is responsible for DC-macrophage activation. The PGN portion of BCG CWS largely accounted for the TLR2- and TLR4-mediated maturation of human DCs. This BCG PGN activity was confirmed by using gene-disrupted mouse DCs and macrophages; mouse TLR2 and TLR4 contribute almost equally to the function of PGN, and MyD88 serves as a pivotal adapter for their signaling. Unlike BCG CWS activity, however, BCG PGN activity cannot be reproduced in the TLR overexpression system of HEK293 cells.
Functional differences of human TLRs, however, have not been clearly identified since no good system to do this has been available. Our findings demonstrate that specific blocking of the extracellular portion of human TLR2 or TLR4 by antibodies or the antagonist resulted in suppression of the PAMP-mediated TNF-α production in human DCs, suggesting that blocking of the PAMP-TLR association impairs activation of DCs. This conclusion is consistent with a previous finding about BCG CWS acting on human DCs (41) and findings obtained with TL2.1 (10, 45), which confers partial TLR2 inhibition. Hence, our system provides a tool for testing the relationship between DC activation and the degree of participation of TLR2 and TLR4 in humans.
It is becoming clear that TLR2 and TLR4 recognize detailed molecular patterns mainly of bacterial origin through combination or complex formation with other proteins. TLR4 senses LPS in association with MD-2 and CD14 (28, 36). The same is true of BCG CWS recognition. On the other hand, TLR2 is a member of a subfamily of TLRs which consists of TLR1, TLR2, TLR6, and TLR10 (25). In the HEK293 expression system, BCG CWS was recognized through a multimolecular assembly including at least TLR2, like other PGNs that are recognized solely by TLR2. We concluded that TLR2 recognizes BCG CWS with no particular heterophilic combination and that TLR4 recognizes BCG CWS in combination with CD14 and MD-2, a complex similar to that used for LPS recognition.
Whether this conclusion is applicable to BCG PGN is the next question. In the HEK293 reporter system, no combination of expression of TLRs with TLR2 led to activation of NF-κB in response to BCG PGN. The possibility that combination expression of TLR2 and TLR4 led to recognition of BCG PGN was ruled out. However, in HEK293 cells expressing TLR2, TLR4 expression did not enhance NF-κB activation even in the presence of CD14 and MD-2. Thus, TLR2 and TLR4 respond independently to BCG PGN on DCs. Gene profiling analysis of BCG PGN-treated DCs supported this idea; part of the TLR4- and TLR2-specific genes are simultaneously regulated in BCG PGN-treated DCs (Begum et al., submitted).
In humans, TLR2 and TLR4 participate to similar extents in DC maturation by BCG PGN. TNF-α production is a major effect induced by NF-κB activation triggered by TLR2 and TLR4 agonists and is considered a major maturation inducer of human DCs (39, 41). In fact, costimulators (CD80 and CD86) and the DC maturation markers CD83 and CD150 were up-regulated by exogenously added TNF-α (41). Simultaneous blocking of TLR2 and TLR4 on DCs, however, did not result in effective suppression of the BCG PGN-mediated up-regulation of these markers and costimulators. We interpret this to mean that basal production of TNF-α leads to maturation of iDCs sufficient to elevate the levels of these markers (Fig. 2E). Alternatively, factors other than TNF-α may contribute to DC maturation, as judged by surface markers. The former interpretation is consistent with our finding that minute amounts of BCG PGN induce up-regulation of costimulators via induction of TNF-α (Fig. 2F). The latter interpretation partially matches the recent finding that mice in which the genes of MyD88 are disrupted retain the ability to mature DCs, while NF-κB activation and TNF-α production are impaired (15), and the finding that both TLR2 and TLR4 use the same set of adapters, Mal/TIRAP and MyD88 (14, 46).
A tantalizing point is that HEK293 cells expressing TLRs do not sense BCG PGN, while DCs induce TNF-α and maturation in response to BCG PGN. Since BCG CWS activates NF-κB in the same assay system, portions of the CWS molecule other than PGN participate in completion of the TLR signaling of both TLR2 and TLR4 in this expression system; both TLR2 and TLR4 require additional receptors for activation or other receptors expressed on DCs but not HEK293 cells, which may contribute to sustaining ligand binding and/or TLR signaling. The receptor sets responsible for the BCG CWS response and the BCG PGN response should be different. What receptors are responsible for DC maturation by BCG CWS or BCG PGN is the next question. Our current interpretation of this question is that iDCs provide uptake receptors in addition to signaling receptors (TLRs) for BCG CWS and BCG PGN. Indeed, BCG CWS and BCG PGN showed unequivocally distinct cation requirement profiles, internalizing efficacy profiles (Fig. 6), and profiles for blocking by monosaccharides (Uehori, Tsuji, and Seya, unpublished data). Furthermore, the inhibitor cocktails for TLR2 and TLR4 signaling suppressed TNF-α production mediated by BCG PGN more prominently than they suppressed TNF-α production mediated by BCG CWS. Thus, uptake receptors affect the TLR signaling functions, although it is not known whether membrane fixation or endosomal uptake of PAMPs is more critical for modulation of TLR function. In addition, recent muramyl dipeptide data suggested that intracellular NOD2 participates in recognition of MDP and induces NF-kB activation, while another signal is needed to translate relevant mRNAs to proteins (40, 45).
The ligand portions lost during the conversion from BCG CWS to BCG PGN must be a missing factor for completion of the TLR-stimulating activity of BCG PGN in HEK293 cells and probably all cells and tissues. One possible candidate for this missing factor is in a carbohydrate region responsible for binding to DCs and facilitating phagocytosis of CWS. Like C-type lectins, CWS binding requires cations, such as Ca2+ (Fig. 6). Indeed, no binding or phagocytosis of PGN was observed in HEK293 cells (data not shown), while subtle binding of PGN was observed in iDCs, paralleling the TLR-mediated NF-κB activation. By using confocal microscopy, BCG CWS and, to a lesser extent, BCG PGN were inserted into endosomes, where the intracellular pool of TLR2 accumulated. Similar findings with different approaches have been reported by several groups (44, 48). Thus, the carbohydrate-binding receptor for inducing phagocytosis may be important in TLR2 activation. Probably, the same is true for TLR4, although there is a relatively small pool of TLR4 in endosomes of human epithelial cells (13). Other receptors, presumably independent of TLR, participate in phagocytosis and NO production (23). Complement receptors CR3 and CR4 and CD46 may participate in endosomal uptake and NO production (18), and carbohydrate-recognizing receptors, including the macrophage mannose receptor and receptors for rhamnose-lectin or intelectin, are candidates for the TLR-independent cellular responses (42). Identification of these receptors will be important for testing this hypothesis.
Means et al. (23) demonstrated that the TLR4 antagonist E5531 largely suppressed M. tuberculosis-induced NF-κB activation and TNF-α production in a murine macrophage-like cell line, RAW264.7. In CHO cells expressing CD14 and TLR4, they observed complete blockade of NF-κB activation by E5531 (23). According to their results, TLR4 should have been a major factor for M. tuberculosis-mediated DC maturation. Underhill et al. (43), in contrast, showed that the cell wall components of M. tuberculosis were mainly engaged in TLR2-mediated TNF-α production by macrophages. Although the reagents used by the two groups were not the same, both TLR2 and TLR4 should be engaged in M. tuberculosis-mediated DC maturation, and the discrepancy in the findings may be explained by the difference in the predominant focus on TLR2 or TLR4. It is notable that almost all of these results were obtained with mouse macrophage cells and cell lines. Here, we determined the degree of participation of TLR2 and TLR4 in the human DC system, and our findings resulted in the interpretation that the cell wall complex of M. tuberculosis participates in TLR2 activation and TLR4 activation almost equally, followed by DC maturation in humans. In the tuberculin skin test and M. tuberculosis infection, the mouse model is not always a good model for human M. tuberculosis infection (5, 7). The present results offer evidence suggesting that the mouse repertoire of TLRs is similar for BCG CWS and BCG PGN to the repertoire of humans, at least when the antibody-blocking method and DCs are used. Some additional parts of the innate immune system other than TLR-dependent recognition of the M. tuberculosis cell wall complex must be different in humans and mice, leading to species-specific responses to M. tuberculosis.
Many M. tuberculosis components have been identified as TLR stimulators; these include lipoarabinomannan (24), a 19-kDa lipoprotein (30), the CpG repeat of nonmethylated DNA (11), and the PGN described here. It is notable that these components confer different activation profiles of TLRs; that is, lipoarabinomannan and the 19-kDa lipoprotein act on TLR2, CpG DNA acts on TLR9, and PGN acts on TLR2 and TLR4. The functional combination of these TLR ligands may explain the reported adjuvant-active profile of M. tuberculosis, and simultaneous activation of TLR2 and TLR4 by noninfectious BCG PGN may be important as a therapeutic approach in which the functional profile is not always consistent with those of other PGN species or LPS. Genechip analysis (33; Begum et al., submitted) should specify the genes regulated by BCG PGN and the properties of BCG PGN that are distinct from those of other PAMPs. Since live BCG and BCG derivatives have been applied to patients with cancer having a good prognosis (47), molecular remodeling of BCG PGN (35) may result in useful therapeutic reagents for DC maturation.
Acknowledgments
We are grateful to M. Tatsuta, K. Kodama, and H. Koyama (Osaka Medical Center for Cancer, Osaka, Japan) for support of this work and to H. Oshiumi, K. Hazeki, N. A. Begum, K. Shida, and S. Kikkawa in our laboratory for many useful discussions. We also thank M. Kurita-Taniguchi for completing the RT-PCR analysis.
This work was supported in part by Public Health Service grant AI41667 from the National Institutes of Health, by CREST, by JST (Japan Science and Technology Corporation), by grants-in-aid from the Ministry of Education, Science, and Culture (Specified Projects for Advanced Research), Ministry of Health and Welfare, Japan, and by the Takamatsunomiya Princess Memorial Foundation.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 2.Azuma, I., E. E. Ribi, T. J. Meyer, and B. Zbar. 1974. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J. Natl. Cancer Inst. 52:95-101. [DOI] [PubMed] [Google Scholar]
- 3.Azuma, I., S. Kishimoto, Y. Yamamura, and J. F. Petit. 1971. Adjuvanticity of mycobacterial cell walls. Jpn. J. Microbiol. 15:193-197. [DOI] [PubMed] [Google Scholar]
- 4.Besra, G. S., and D. Chatterjee. 1994. Lipids and carbohydrates of Mycobacterium tuberculosis, p. 285-306. In B. R. Bloom (ed.), Tuberculosis. American Society for Microbiology, Washington, D.C.
- 5.Bomford, R. 1980. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin. Exp. Immunol. 39:435-441. [PMC free article] [PubMed] [Google Scholar]
- 6.Christ, W. J., O. Asano, A. L. Robidoux, M. Perez, Y. Wang, G. R. Dubuc, W. E. Gavin, L. D. Hawkins, P. D. McGuinness, and M. A. Mullarkey. 1995. E5531, a pure endotoxin antagonist of high potency. Science 268:80-83. [DOI] [PubMed] [Google Scholar]
- 7.Collins, F. M., and V. Montalbine. 1973. Relative immunogenicity of streptomycin-sensitive and -resistant strains of BCG. Infect. Immun. 8:381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziarski, R., Q. Wang, K. Miyake, C. J. Kirschning, and D. Gupta. 2001. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J. Immunol. 166:1938-1944. [DOI] [PubMed] [Google Scholar]
- 9.Fenton, M. J. 1998. Macrophages and tuberculosis. Curr. Opin. Hematol. 1:72-78. [DOI] [PubMed] [Google Scholar]
- 10.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 13.Hornef, M. W., T. Frisan, A. Vandewalle, S. Normark, and A. Richter-Dahlfors. 2002. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 195:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horng, T., G. M. Barton, R. A. Flavell, and R. Medzhitov. 2002. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420:329-333. [DOI] [PubMed] [Google Scholar]
- 15.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688-5694. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 17.Kruse, M., E. Meinl, G. Henning, C. Kuhnt, S. Berchtold, T. Berger, G. Schuler, and A. Steinkasserer. 2001. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1 beta. J. Immunol. 167:1989-1995. [DOI] [PubMed] [Google Scholar]
- 18.Kurita-Taniguchi, M., A. Fukui, K. Hazeki, A. Hirano, S. Tsuji, M. Matsumoto, M. Watanabe, S. Ueda, and T. Seya. 2000. Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHP-1 to CD46. J. Immunol. 165:5143-5152. [DOI] [PubMed] [Google Scholar]
- 19.Lien, E., J. C. Chow, L. D. Hawkins, P. D. McGuinness, K. Miyake, T. Espevik, F. Gusovsky, and D. T. Golenbock. 2001. A novel synthetic acyclic lipid A-like agonist activates cells via the lipopolysaccharide/toll-like receptor 4 signaling pathway. J. Biol. Chem. 276:1873-1880. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto, M., T. Seya, S. Kikkawa, S. Tsuji, K. Shida, M. Nomura, M. Kurita-Taniguchi, M. Higashiyama, H. Ohigashi, H. Yokouchi, H. Takami, A. Hayashi, I. Azuma, T. Masaoka, K. Kodama, and K. Toyoshima. 2001. Interferon gamma-producing ability in blood lymphocytes of patients with lung cancer through activation of the innate immune system by BCG-cell wall skeleton. Int. Immunopharmacol. 1:1559-1569. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto, M., S. Kikkawa, M. Kohase, K. Miyake, and T. Seya. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 293:1364-1369. [DOI] [PubMed] [Google Scholar]
- 22.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 23.Means, T. K., B. W. Jones, A. B. Schromm, B. A. Shurtleff, J. A. Smith, J. Keane, D. T. Golenbock, S. N. Vogel, and M. J. Fenton. 2001. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J. Immunol. 166:4074-4082. [DOI] [PubMed] [Google Scholar]
- 24.Means, T. K., E. Lien, A. Yoshimura, S. Wang, D. T. Golenbock, and M. J. Fenton. 1999. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163:6748-6755. [PubMed] [Google Scholar]
- 25.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 27.Murabayashi, N., M. Kurita-Taniguchi, M. Ayata, H. Ogura, M. Matsumoto, and T. Seya. 2002. Susceptibility of human dendritic cells to measles virus depends on their activation stages in conjunction with the level of CDw150: role of Toll stimulators in DC maturation and MV amplification. Microbes Infect. 4:785-794. [DOI] [PubMed] [Google Scholar]
- 28.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3:667-672. [DOI] [PubMed] [Google Scholar]
- 29.Nishiguchi, M., M. Matsumoto, T. Takao, M. Hoshino, Y. Shimonishi, S. Tsuji, N. A. Begum, O. Takeuchi, S. Akira, K. Toyoshima, and T. Seya. 2001. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 166:2610-2616. [DOI] [PubMed] [Google Scholar]
- 30.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 31.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adapter molecule that participates in Toll-like receptor3-mediated interferon-β induction. Nat. Immunol. 4:161-167. [DOI] [PubMed] [Google Scholar]
- 32.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 34.Rescigno, M., F. Granucci, S. Citterio, M. Foti, and P. Ricciardi-Castagnoli. 1999. Coordinated events during bacteria-induced DC maturation. Immunol. Today 20:200-203. [DOI] [PubMed] [Google Scholar]
- 35.Seya, T., M. Matsumoto, S. Tsuji, N. A. Begum, I. Azuma, and K. Toyoshima. 2002. Structural-functional relationship of pathogen-associated molecular patterns: lessons from BCG-cell wall skeleton and Mycoplasma lipoprotein M161Ag. Microbes Infect. 4:955-961. [DOI] [PubMed] [Google Scholar]
- 36.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 39.Thurnher, M., R. Ramoner, G. Gastl, C. Radmayr, G. Bock, M. Herold, H. Klocker, and G. Bartsch. 1997. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int. J. Cancer 70:128-134. [DOI] [PubMed] [Google Scholar]
- 40.Tschopp, J., F. Martinon, and K. Burns. 2003. NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell Biol. 4:95-104. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect. Immun. 68:6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji, S., J. Uehori, M. Matsumoto, Y. Suzuki, A. Matsuhisa, K. Toyoshima, and T. Seya. 2001. Human intelectin is a novel soluble lectin that recognizes galactofranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 276:23456-23463. [DOI] [PubMed] [Google Scholar]
- 43.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 45.Wolfert, M. A., T. F. Murray, G.-J. Boons, and J. N. Moore. 2002. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J. Biol. Chem. 277:39179-39186. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto, M., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, K. Takeda, and S. Akira. 2002. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420:324-329. [DOI] [PubMed] [Google Scholar]
- 47.Yamamura, Y., I. Azuma, T. Taniyama, K. Sugimura, T. Hirao, R. Tokuzen, M. Okabe, W. Nakahara, K. Yasumoto, and M. Ohta. 1976. Immunotherapy of cancer with cell wall skeleton of Myocabacterium bovis-Bacillus Calmette-Guerin: experimental and clinical results. Ann. N.Y. Acad. Sci. 277:209-227. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 49.Zbar, B., E. Ribi, T. Meyer, I. Azuma, and H. J. Rapp. 1974. Immunotherapy of cancer: regression of established intradermal tumors after intralesional injection of mycobacterial cell walls attached to oil droplets. J. Natl. Cancer Inst. 52:1571-1577. [DOI] [PubMed] [Google Scholar]