Abstract
Transmissible gastroenteritis virus (TGEV) is a porcine coronavirus that causes diarrhea, leading to near 100% mortality in neonatal piglets with corresponding devastating economic consequences. For the protection of neonatal and older animals, oral live vaccines present the attractive property of inducing desired mucosal immune responses, including colostral antibodies in sows—an effective means to passively protect suckling piglets. Newly attenuated Salmonella vaccine constructs expressing TGEV S protein epitopes were studied and evaluated for improved humoral immune response to TGEV. The macrophage-inducible Salmonella ssaH and spiC/ssaB promoters were compared for their ability to express the TGEV C and A epitopes in the context of the heterologous 987P fimbriae on Salmonella vaccines. Compared to the ssaH promoter, the Salmonella cya crp vector elicited significantly higher levels of mucosal and systemic antibodies in orally immunized mice when the chimeric fimbriae were expressed from the spiC promoter. The Salmonella spiC promoter construct induced the highest level of chimeric fimbriae after being taken up by the J774A.1 macrophagelike cells. The Salmonella cya crp vaccine vector was shown to incorporate into 987P partially degraded chimeric subunits lacking the TGEV epitopes. In contrast, its isogenic pgtE mutant produced fimbriae consisting exclusively of intact chimeric subunits. Mice immunized orally with the Salmonella pgtE vaccine expressing chimeric fimbriae from the spiC promoter elicited significantly higher systemic and mucosal antibody titers against the TGEV epitopes compared to the parental vaccine. This study indicates that the Salmonella cya crp pgtE vector and the spiC promoter can be used successfully to improve immune responses toward heterologous antigens.
Live vaccine vehicles offer a powerful approach for inducing protective immunity against pathogenic microorganisms. Genetically engineered and attenuated agents provide a method for delivering heterologous antigens derived from other pathogens. A variety of viruses, bacteria, and protozoans have been utilized successfully as vaccine delivery systems in several experimental models. Among them, attenuated Salmonella is being widely studied as an oral vaccine vehicle to induce mucosal as well as systemic immune responses to heterologous antigens in animals and humans (8, 55).
After oral ingestion, Salmonella initiates infection in the ileal mucosa by crossing epithelial cells or M cells to reach and enter macrophages and dendritic cells (7, 27, 44). A third route of infection involving direct uptake by CD18-expressing cells was recently proposed to be mediated by dendritic cells (13, 62). As a facultative intracellular pathogen, Salmonella has evolved to reside and replicate in dendritic cells (23) and macrophages in the Peyer's patches and other lymphoid tissues of the small intestine, where a local mucosal immune response is triggered. The Salmonella organisms are transported to the mesenteric lymph nodes by mononuclear phagocytes. Further destinations include the liver and spleen, where the Salmonella organisms induce systemic immune responses (62). A major hallmark of attenuated Salmonella organisms as live vectors is the stimulation of mucosal and systemic (including humoral and cellular) immune responses in animals and humans (34). The Salmonella vaccine strains developed so far were attenuated either in metabolic pathways (aro, pur), or in regulatory genes that have pleiotropic effects (cya crp, phoP, or phoPc) (55). It is crucial that these attenuated Salmonella strains cross the epithelial layers and reach the appropriate local or regional lymphoid cells and tissues for triggering the necessary signals leading to a desired immune response. It is as important that expression of the heterologous antigen is vigorously maintained or activated upon the interaction of a Salmonella vector with antigen-presenting cells (18, 35). The use of in vivo-regulated promoters is of special interest to prevent undesirable responses, such as tolerance due to premature release of soluble antigens (55). Such promoters are also helpful to influence the nature of the immune reaction (55), such as the acquisition of cellular Th1 responses (60) toward the heterologous antigen (4, 5).
Transmissible gastroenteritis virus (TGEV) is a coronavirus that causes acute diarrhea in piglets, characterized by up to 100% mortality among neonatal pigs (52, 53). Mortality is lower in older animals, although morbidity is high in TGEV-infected seronegative swine. Maternal antibodies, passed to piglets in colostrum and milk, provide protection against infection. The gut-mammary link of lymphocyte trafficking results in local antibody production in the mammary gland after oral immunization (48). The TGEV spike (S) protein is the major inducer of TGEV-neutralizing antibodies. The relevant epitopes for neutralization were mapped to the N-terminal domain of S protein, and four antigenic sites (A to D) were identified (11, 26). Among them, sites C and A are especially attractive, since they not only are the major inducers of neutralizing antibodies but are also linear epitopes that are easily incorporated into carrier molecules to improve their immunogenicity. For example, both purified chimeric CS31 and 987P fimbriae carrying TGEV C and A epitopes have been developed and shown to be immunogenic (12, 45). As a proteinaceous appendage, the 987P fimbria play a critical role in the pathogenesis of porcine enterotoxigenic E. coli (ETEC) by mediating bacterial attachment to enterocytes, thus ensuring efficient enterotoxin delivery to the host. By inducing antiadhesin antibodies, 987P fimbriae also serve as a major antigen in commercially effective ETEC vaccines (40). Passive immunization of piglets with antifimbriae antibodies protects them by blocking fimbria-mediated enteroadhesion of ETEC (24, 41, 43). More recently, we have investigated the immunogenicity of chimeric 987P fimbriae expressed by attenuated live Salmonella vaccine vectors (5). After oral immunization, mice developed mucosal and systemic immune responses to both the fimbriae and the TGEV epitopes. The immune responses were improved by immunizing with Salmonella constructs expressing the chimeric fimbriae from in vivo-inducible promoters (4). However, only anti-TGEV C and not anti-TGEV A antibodies were detected. Because the chimeric fimbriae expressed on the Salmonella vector consisted of a mixture of two 987P major subunits (FasA) of different molecular mass, we suspected that some proteolytic activity on the TGEV epitopes (5) resulted in suboptimal TGEV antigen presentation. This possibility was evaluated here by studying a mutant that did not express the outer membrane protease PgtE. Moreover, the use of the ssaH and spiC promoters of the Salmonella pathogenicity island 2 for directing the expression of chimeric 987P fimbriae and for the induction of 987P- and TGEV-specific immune responses was studied.
MATERIALS AND METHODS
Mice.
Six-week-old female BALB/cByJ mice were obtained from the Jackson Laboratory and housed in filter-top cages in an air-conditioned animal facility. Water and food were provided ad libitum. Mice were allowed to adapt for a minimum of 1 week after arrival before being immunized.
Bacterial strains and plasmid constructs.
Escherichia coli, Salmonella enterica serovar Typhimurium, and plasmids used in this study are listed in Table 1. Standard procedures were used to prepare new constructs. The Salmonella pgtE mutant CS14029 was constructed by allelic exchange as follows. First, the DNA sequences flanking pgtE were amplified by PCR using the upper primer 5′ GGGGTACCAGATTGCAGCCGAGCTTTACTAC and lower primer 5′ CGAGCTCCAGTCAGGCGGCGCTACACTTAC (the underlined bases represent the KpnI and SalI restriction sites) and cloned into the KpnI and SalI sites of suicide plasmid pDMS197 (15), thereby resulting in plasmid pCS188. A kanamycin resistance cassette (aphA gene), as a SmaI fragment of pBSL128 (1), was inserted into the blunted BstXI site within the pgtE gene of pCS188 to create plasmid pCS189, and the E. coli strain SM10λpir was transformed with pCS189. Second, allelic exchange was performed by conjugative transfer of pCS189 from SM10λpir into ATCC 14028, a wild-type strain of S enterica serovar Typhimurium. Single homologous recombination derivatives were screened by growth on minimal medium plates containing tetracycline to select for the Salmonella transconjugants carrying chromosomally inserted pCS189. Subculturing colonies on nutrient agar with 5% sucrose and kanamycin selected for bacteria that had lost plasmid DNA but kept the aphA gene by a second recombination event. The presence of pgtE::aphA on the chromosome of the resultant Salmonella strain, designated CS14029, was confirmed by PCR amplification with the primers used to clone pgtE, as described above. Allelic gene transfer between Salmonella strains was done by generalized transduction with P22HTint. The chimeric fasA gene described in this study was the fasA Ω(6bp::TGEV-CA) gene construct, where the C and A epitopes of the TGEV S protein were inserted at the sixth base pair of the DNA encoding the processed FasA protein, as described previously (45) and designated here fasA99. The pagC promoter of plasmid pCS173 (pSC101 derivative with asd and the pagC promoter, pagCp expressing chimeric 987P fimbriae with the TGEV C and A epitopes) (4) was removed as a BamHI-containing fragment, resulting in plasmid pCS187. The DNA sequences containing the ssaH promoter (ssaHp) or the spiC promoter (spiCp) region of Salmonella χ4550 were amplified by PCR, using the upper primer 5′ GGGGATCCTCGTTTTCAGGTATATACCGGATGT and lower primer 5′ GGGGATCCGATTTCCAGCAGCAACCGTCGAACA, or the upper primer 5′ GGGGATCCAATGCTTCCCTCCAGTTGCCTGTT and lower primer 5′ GGGGATCCAAATGGGAGTTTCTATCAAATTC, respectively (the underlined bases represent the recognition sequences of BamHI). The 349-bp DNA fragment encompassing the ssaH promoter and the 399-bp fragment encompassing the spiC promoter were inserted into the BamHI site of pCS187 to create plasmids pCS191 and pCS192, respectively (Fig. 1B). Correct orientation of the ssaH and spiC promoter regions in the latter plasmids was confirmed by PCR and sequencing.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli | ||
| 987 | O9:K103:987P | 25 |
| SE5000 | MC4100 recA56 (Fim−) | 54 |
| SM10 λpir | thi thr leu tonA supE recA λpir | 38 |
| S. enterica serovar Typhimurium | ||
| ATCC 14028 | Wild-type | ATCC |
| ATCC 14028 phoP | phoP::cat (Cmr) | J. Mekalanos; 37 |
| CS14029 | ATCC 14028 pgtE::aphA | This study |
| χ4550 | gyrA1816 ΔasdA1 Δ(zhf-4::Tn10) Δcrp-1 Δcya-1 | 51 |
| CS4551 | χ4550 phoP::cat | This study |
| CS4552 | χ4550 pgtE::aphA | This study |
| Plasmid | ||
| pDMS197 | oriT oriV sacB TcR | 15 |
| pBSL128 | plasmid with aphA (KanR cassette) | 1 |
| pCS154 | pSC101-derivative with asd and the tetA promoter expressing fasA*BCDEFG (fasA*: chimeric fasA) | 5 |
| pCS173 | pCS154 with the pagC promoter replacing the tetA promoter | 4 |
| pCS187 | pCS173 ΔpagC promoter | This study |
| pCS189 | pDMS197 pgtE::aphA | This study |
| pCS191 | pCS187 with the ssaH promoter | This study |
| pCS192 | pCS187 with the spiC promoter | This study |
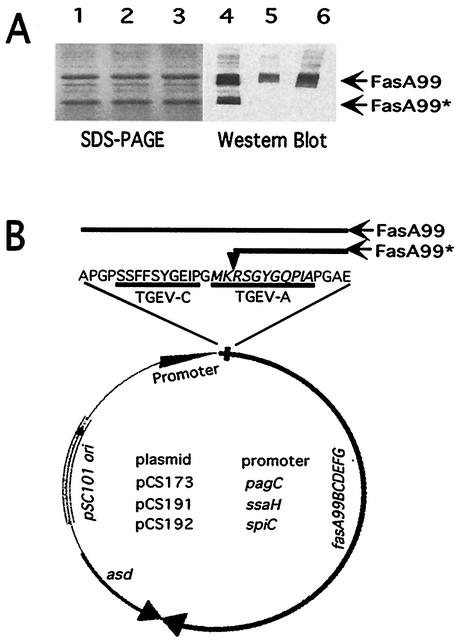
FIG. 1.
Cleavage of chimeric FasA and schematic representation of the plasmids. (A) SDS-PAGE (lanes 1 to 3) and Western blot (lanes 4 to 6) of chimeric 987P fimbriae isolated from S. enterica serovar Typhimurium vaccine strain χ4550. The antibodies used for Western blotting were anti-987P fimbriae (lane 4), anti-TGEV C epitope (lane 5), and anti-TGEV A epitope (lane 6) antibodies, respectively. (B) The expression plasmids were all constructed on the backbone of the low-copy-number plasmid pLG339 (pSC101 derivative) carrying asd for plasmid stabilization and maintenance in Salmonella asd strains. Expression of chimeric 987P fimbriae (fasA99BCDEFG) is driven by the pagC (pCS173), ssaH (pCS191), or spiC promoter (pCS192). The N-terminal amino acid sequence of FasA99 (chimeric FasA) with the TGEV C and A epitopes (in bold and underlined) is shown above the physical map. The cleavage site for the PgtE protease within the TGEV A epitope is indicated by a vertical arrow. Intact (FasA99) and cleaved chimeric FasA (FasA99*) are shown in panel A and represented as lines of different lengths above the sequence in panel B.
Media and reagents.
Wild-type E. coli strain 987 was grown in minimal medium E supplemented with pantothenic acid and glycerol (16). Strains SE5000, SM10 λpir, ATCC 14028, and CS14029 were grown in Luria-Bertani (LB) medium, whereas strains χ4550, CS 4551, and CS4552 were grown in LB medium with 50 μg of dl-α,∋-diaminopimelic acid (DAP; Sigma, St. Louis, Mo.) per ml. When necessary, media were supplemented with the following antibiotics: chloramphenicol (30 μg/ml), tetracycline (10 μg/ml), or kanamycin (45 μg/ml). Culture media were purchased from Difco (Detroit, Mich.). Restriction and modification enzymes were from New England Biolabs, Inc. (Beverly, Mass.). Unless specified otherwise, reagents were purchased from Sigma.
Peptides, fimbriae, and antibodies.
The TGEV C and A peptides of the TGEV spike protein, corresponding to amino acid residues 379 to 388 and 521 to 531, respectively, include a cysteine at their carboxy termini (SSFFSYGEIPC and MKRSGYGQPIAC). The cysteine served to cross-link the peptides to keyhole limpet hemocyanin (KLH). The antisera against 987P, TGEV C-KLH, and TGEV A-KLH were obtained by immunizing rabbits, as described previously (45, 49). Fimbriae expressed on the bacterial surface were prepared by heat extraction (29). Briefly, bacteria were pelleted by centrifugation, resuspended in 0.5 mM Tris-HCl (pH 7.4)-75 mM NaCl, and treated at 60°C for 30 min. After a subsequent centrifugation cleaning step, ammonium sulfate was added to the supernatant to a 20% final concentration to precipitate the fimbriae overnight on ice. The supernatant was centrifuged at 10,000 × g for 20 min, and the pellets were resuspended in phosphate-buffered saline (PBS; 10 mM NaHPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl [pH 7.5]) or Tris-buffered saline (10 mM Tris-HCl [pH 7.4], 154 mM NaCl). Excess ammonium sulfate was removed by dialysis against PBS.
Phagocytosis assay.
The murine macrophagelike cell line J774A.1 (ATCC TIB-67, a kind gift from Howard Goldfine) was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1.0 mM sodium pyruvate and 10% fetal bovine serum (i.e., DMEM-complete) at 37°C under an atmosphere of 5% CO2. To determine the expression of chimeric fimbriae in macrophages, the bacteria were opsonized in 10% fresh mouse serum in PBS for 20 min and added to cell cultures at a multiplicity of infection of 100. Internalization was allowed to proceed for 1 h. Nonphagocytosed bacteria were washed away with PBS. The infected macrophages were cultured in DMEM-complete containing 50 μg of gentamicin sulfate per ml for 24 h. The macrophages were washed three times with PBS and lysed with 0.1% Triton X-100 in PBS to release the intracellular bacteria. The bacteria were then collected by centrifugation (4,000 × g, 5 min) and solubilized in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and subjected to SDS-PAGE and Western blotting analysis.
SDS-PAGE and Western blotting.
Bacterial pellets, isolated fimbriae, or macrophage lysates were suspended in sample buffer and boiled for 5 min, and the proteins were separated by SDS-PAGE. Western blots were probed with rabbit anti-987P fimbriae, anti-TGEV C peptide, or anti-TGEV A peptide antibody, respectively, by using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence for detection (50).
N-terminal amino acid sequencing.
The chimeric 987P fimbriae were heat extracted from Salmonella χ4550/pCS173 grown in LB broth, separated with SDS-PAGE, and transferred onto a Sequiblot polyvinylidene difluoride membrane (Bio-Rad). Protein bands were visualized by reversible staining with Ponceau S. The intact and degraded chimeric FasA bands were excised from the membrane, and N-terminal amino acid sequencing was performed by the phenylthiohydantoin-derivatization procedure in a Beckman LH 2600 gas phase sequencer.
Immunization and sampling.
For each immunization, a single colony of Salmonella was grown in LB broth without antibiotics at 37°C on a rotary shaker at 150 rpm overnight. The bacterial cells were gently washed once and resuspended in sterile PBS (10 mM NaHPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl [pH 7.2]) at the concentration of (1 to 5) × 10 11 CFU/ml. Viable counts were performed on all the inocula. Before immunization, the mice were deprived of food and water for 4 h. The mice were administered 200 μl of bacterial suspensions orally with feeding needles and fasted for an additional 30 min. Mice were immunized at day 0 and at day 28. To evaluate the effect of a booster immunization on antibody titers, one group of mice was immunized only once, at day 0. During the immunization experiments, blood was collected from tail veins. At the end of the experiments, mice were anesthetized with Metofane (methoxyflurane; Mallinckrodt Veterinary, Inc., Mundelein, Ill.) and exsanguinated by heart punctures. Whole small intestines, from the duodenum to the ileo-cecal junction, were excised, and luminal contents were carefully collected with 3 ml of a cocktail solution of protease inhibitors (Complete; Roche, Indianapolis, Ind.) prepared according to the manufacturer's instructions. Recovered intestinal contents were vortexed vigorously for 5 min. After centrifugation at 13,000 × g for 15 min at 4°C, supernatants were collected and stored at −70°C.
ELISA.
Individual mouse sera were tested for immunoglobulin G (IgG) against 987P fimbriae or TGEV C or A peptides, and individual intestinal contents were tested for secretory IgA (sIgA) by using enzyme-linked immunosorbent assays (ELISA) (5). Briefly, 96-well ELISA plates (Immulon 4; Dynatech Laboratories, Inc., Chantilly, Va.) were coated with isolated 987P fimbriae (0.2 μg in 100 μl, 0.1 M carbonate buffer, pH 9.6, per well) overnight at 4°C. TGEV C or A peptide (1.0 μg in 100 μl 0.1 M carbonate buffer, pH 9.6, per well) were coated onto the plates by using a microwave oven as described previously (65) and were further coated overnight at 4°C. The plates were blocked with 0.5% bovine serum albumin in PBS at 37°C for 2 h, washed four times with PBS, and incubated with serial dilutions of body fluid samples in PBS-0.1% BSA-0.05% Tween 20 for 2 h at 37°C. After the second washing step, plates were incubated with horseradish peroxidase-conjugated anti-mouse IgG or IgA antibodies at 37°C for 1 h. After the last washing step, bound antibodies were detected by using o-phenylenediamine as the chromogenic substrate analogue and reading the absorbance at 450 nm.
Virus seroneutralization test.
TGEV neutralization was determined by using a limiting dilution microassay in 96-well plates (32). Briefly, 50 μl of serial twofold dilutions of antiserum was mixed with an equal volume of virus suspension containing 100 TCID50 of TGEV Purdue-115 strain (a kind gift from Linda J. Saif). After incubation at 37°C for 1 h, 4 × 104 trypsinized swine testis cells (ATCC CRL-1746) in 100 μl of DMEM supplemented with 10% fetal bovine serum and 0.1 mM nonessential amino acids were added to the antiserum-virus mixtures. Neutralization titers were determined 48 h later and calculated as the mean of the highest dilution that neutralized 100% of the cytopathic effect (cell rounding, detaching, and death) in duplicate experiments. Positive and negative reference sera were included in each experiment.
Statistical analysis.
Groups of log-transformed data were compared by using the unpaired Student's t test (39). Probability (P) values of less than 0.05 indicated that the groups were significantly different.
RESULTS
Degradation of TGEV epitopes.
We previously showed that E. coli strain SE5000 and S. enterica serovar Typhimurium χ4550 expressed chimeric fimbriae after being transformed with plasmid constructs harboring chimeric fasA genes with the other fas genes required for 987P biogenesis (4, 5, 45). It was also observed by SDS-PAGE that the chimeric fimbriae of these strains integrated two forms of FasA subunits of different sizes. It was postulated that these two forms represented intact and partially degraded chimeric FasA. To better characterize the two SDS-PAGE bands, fimbriae were isolated from the surface of Salmonella χ4550/pCS173 (pagCp) and analyzed by Western blotting with anti-987P fimbriae, anti-TGEV C, or anti-TGEV A epitope antibodies. Although the lower band was recognized by the anti-987P antibody, it did not react with the anti-TGEV C or the anti-TGEV A epitope antibodies, suggesting that the TGEV epitopes were missing. In contrast, the higher band was recognized by all three antibodies, confirming that this band represented the intact chimeric FasA subunit (Fig. 1A). This finding supported our previous studies by showing that the chimeric fimbriae expressed by the Salmonella vaccine strain χ4550 incorporate both intact and partially degraded FasA subunits, the latter subunits having lost the two TGEV epitopes. To further characterize the intact and partially degraded chimeric FasA subunits, isolated fimbriae from Salmonella χ4550/pCS173 (pagCp) were subjected to SDS-PAGE and blotted onto a polyvinylidene difluoride membrane. The two major bands, as located by Ponceau S staining, were cut from the membrane and subjected to N-terminal sequencing. The five first amino acid residues of the top band corresponded to the predicted N-terminal sequence of intact chimeric FasA with the TGEV epitopes (APGPS) (Fig. 1B). The N-terminal sequence of the bottom band was RSGYG (Fig. 1B). This sequence corresponded to the C-terminal portion of the TGEV A epitope, indicating that the smaller protein was an N-terminal truncate of chimeric FasA having lost the whole TGEV C epitope and part of the TGEV A epitope.
Expression of intact chimeric FasA on a pgtE mutant of Salmonella.
In preliminary studies, we observed that, in contrast to E. coli strain SE5000 and Salmonella strain χ4550, which displayed both intact and degraded chimeric major subunits, E. coli strain BL21 expressed essentially intact chimeric FasA (5). This strain does not express the Lon and OmpT proteases. OmpT cleaves proteins at specific sites between consecutive basic residues (10, 58). The protein sequence of the truncated chimeric FasA protein revealed a cleavage site located between a lysine and an arginine residue inside the TGEV A epitope (Fig. 1B). Thus, OmpT and PgtE, the homologous outer membrane proteases of Salmonella, were the proteases potentially responsible for the truncation of chimeric FasA. To test this possibility, we constructed an isogenic pgtE mutant of the Salmonella vaccine strain χ4550 by allelic exchange. The obtained mutant (CS4552) and its parental strain were transformed with three different plasmids for the expression of chimeric fimbriae. The resulting chimeric fimbriae were isolated and analyzed by SDS-PAGE. All the fimbriae produced by strain CS4552 (the pgtE mutant) were shown to consist mainly of intact chimeric FasA, whereas the parental strain χ4550 produced both intact and truncated subunits (Fig. 2). This result confirmed that PgtE was responsible for the partial removal of the N-terminal end of chimeric FasA. Interestingly, the concentration of Mg2+ ions in the medium modulated the relative amount of truncated subunits in the fimbriae (Fig. 2), consistent with the known regulation of pgtE transcription by the Mg2+-responsive phoP/Q regulatory system. Also, as expected, fimbriae produced from the phoP-independent promoter ssaH in the phoP mutant strain CS4551 harbored only intact chimeric subunits (Fig. 2). Thus, the Salmonella pgtE mutant strain might be a better vaccine vector than the parental strain by displaying higher levels of intact viral epitopes on chimeric fimbriae.
FIG. 2.
The regulation of the ssaH, spiC, and pagC promoters by Mg2+, as shown by SDS-PAGE of chimeric 987P fimbriae. Fimbriae were isolated from Salmonella strain χ4550, CS4551, or CS4552 with pCS173, pCS191, or pCS192 grown in LB containing different concentrations of Mg2+. The bands corresponding to the chimeric fimbrial subunit (FasA99) and to its cleavage product (FasA99*) are indicated at right, and strains are indicated at left. Bacteria were grown in LB (−) or LB supplemented with 10 mM Mg2+ (+). The presented data are representative of at least two separate reproducible experiments.
In vitro expression of chimeric fimbriae from SPI2 promoters.
The immunogenicity of chimeric 987P fimbriae expressed by the Salmonella crp cya vaccine strain χ4550 was previously shown to be improved by driving antigen expression from in vivo-inducible promoters, such as the nirB and pagC promoters (4). Since the ssaH and spiC promoters are turned on in Salmonella-containing vacuoles of antigen-presenting cells such as macrophages (6, 9, 59), the possibility that the specific spatial and temporal antigen display by these promoters is beneficial for the improvement of immune responses was explored. For this purpose, plasmids stabilized with the asd-balanced lethal system were engineered to transcribe the genes for chimeric fimbriae from the ssaH (pDM191) and spiC (pDMS192) promoters. Whether these plasmid constructs contained the sequences necessary to drive the expression of chimeric 987P fimbriae after induction by an appropriate environmental signal was determined in vitro in the context of the Salmonella vaccine strain χ4550 and of its isogenic phoP (CS4551) or pgtE (CS4552) mutants. The amounts of fimbriae isolated from the same numbers of bacteria were compared on SDS-polyacrylamide gels. Strains CS4552 and χ4550 with pCS173 (pagCp) expressed less fimbriae when MgCl2 was added to the LB medium (Fig. 2). LB by itself contains approximately 0.23 mM Mg2+ (Difco, typical analysis sheets); thus, fimbrial expression might be even higher with bacteria grown under the Mg2+-limiting conditions (10 to 75 μM) shown to best signal the induction of PhoP-activated SPI2 genes (9). As expected, the phoP-dependent pagC promoter was inactive in the phoP mutant strain CS4551. The ssaH promoter seemed less active in the presence of excess Mg2+ for both phoP+ strains (χ4550 and CS4552) (Fig. 2). However, expression of chimeric FasA by the ssaH promoter was independent of the medium concentration of Mg2+ for the phoP mutant strain CS4551, indicating that Mg2+ modulates the activation state of the ssaH promoter through PhoP and PhoQ. Adding Mg2+ ions to the growth medium had no significant effect on the activity of the spiC promoter (pCS192) in the two phoP+ strains (Fig. 2). In contrast, the spiC promoter was capable of driving expression of chimeric FasA in the phoP mutant strain only after supplementing the medium with Mg2+. This surprising result suggested that the spiC promoter in pCS192 is at least partially regulated by Mg2+ through a PhoP- and PhoQ-independent pathway.
Intravacuolar expression of intact chimeric fimbriae from SPI2 promoters.
Constructs with the different promoters were compared by analyzing the levels of intact subunits incorporated in chimeric fimbriae expressed on Salmonella contained in the macrophagelike cell line J774A.1. Western blots of expressed fimbriae from LB-grown or intracellular Salmonella χ4550 (pgtE+) probed with the anti-TGEV C antibody showed that, whereas the ssaH promoter in pCS191 was not efficiently induced in the macrophages, the spiC promoter in pCS192 was better at inducing the expression of intact chimeric fimbriae in the intracellular environment (Fig. 3). Although alternative explanations could involve reduced uptake or intracellular survival of the ssaH construct, both these possibilities would not be desirable for an effective attenuated vaccine designed to express heterologous antigens in an antigen-presenting cell. Similar to the spiC promoter, effective intracellular expression of fimbriae has been observed previously with the pagC promoter construct pCS173 (4). Comparisons of the levels of chimeric fimbriae expressed from LB-grown or intracellular Salmonella CS4552 (the pgtE mutant) demonstrated that both promoters were effective, the spiC promoter driving slightly more chimeric fimbriae on intracellular Salmonella than the pagC promoter. Better intravacuolar antigen expression from the spiC and pagC promoters in comparison to the ssaH promoter suggested that the former promoters might elicit better immune responses.
FIG. 3.
Expression of chimeric fimbriae by different vaccine constructs grown in LB broth (A) or after uptake and growth in macrophages (B). Western blots of chimeric fimbriae expressed by Salmonella strains strain χ4550, CS4551, or CS4552 with pCS191, pCS192, or pCS173. A total of 107 CFU of each construct grown for 16 h in LB broth were analyzed by Western blot (panel A). The same number of bacteria were used to infect J774A.1 macrophagelike cells for 1 h, followed by washing steps and 24 h incubation in gentamicin-containing DMEM; washed macrophages were lysed and analyzed by Western blot (panel B). The blots were probed with anti-TGEV C peptide antibodies.
Antibody responses against fimbriae with the new in vivo-inducible antigen system.
The immunogenicity of the chimeric 987P fimbriae expressed by the Salmonella vaccine strains χ4550 (pgtE+) or CS4552 (the pgtE mutant) hosting the newly constructed expression plasmids was evaluated after oral immunization of BALB/c mice. Note that 987P-specific serum IgG was detected in all the mice immunized with both vaccine strains, regardless of which promoter was used for antigen expression (Fig. 4A). Interestingly, both the systemic 987P-specific IgG and intestinal sIgA titers were significantly higher in the mice immunized with χ4550/pCS192 (pgtE+/spiCp) in comparison to those immunized with χ4550/pCS191 (pgtE+/ssaHp) (Fig. 4A and B). This result was consistent with the previous finding that the spiC promoter produced more intravacuolar fimbrial antigen than the ssaH promoter (Fig. 3). The anti-987P antibody titers were not significantly different between CS4552/pCS192 (pgtE mutant/spiCp) and χ4550/pCS192 (pgtE+/spiCp) (Fig. 4A), consistent with the earlier observation that the total amount of 987P antigen was not reduced by removal of the N-terminal end of chimeric FasA in the pgtE mutant.
FIG. 4.
Anti-987P fimbriae antibody responses. ELISA titers of 987P fimbriae-specific serum IgG (A) and intestinal sIgA (B) at the 8th week postimmunization. BALB/c mice were immunized orally with two doses (at a 28-day interval) of 987P-fimbriated S. enterica serovar Typhimurium vaccine strain. The columns represent the means of individually measured serum IgG or intestinal sIgA. Error bars represent standard errors of the means for 9 to 10 mice. Only statistically significant differences are marked by asterisks (*, P < 0.05).
Antibody responses against TGEV with the new in vivo-inducible antigen system.
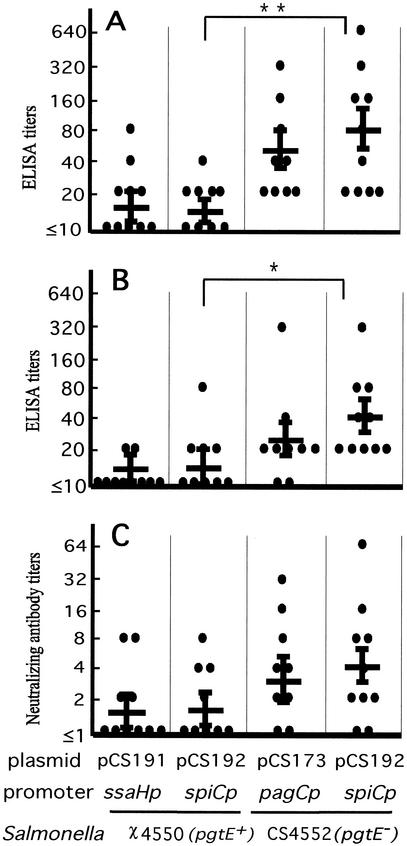
The pgtE mutant strain CS4552 expressing intact chimeric fimbriae from the spiC promoter induced significantly higher titers of anti-TGEV C epitope (Fig. 5A) (P = 0.007) and anti-TGEV A epitope (Fig. 5B) (P = 0.02) IgG than the parental strain χ4550 (pgtE+) with the same promoter. Although the mean neutralizing antibody titer for the CS4552/pCS192 (pgtE mutant/spiCp)-vaccinated mice (mean, 4.29) was higher than the mean for the χ4550/pCS192 (pgtE+/spiCp)-vaccinated mice (mean, 1.85), the statistical difference was not significant (Fig. 5C) (P = 0.11).
FIG. 5.
Anti-TGEV antibody responses. Anti-TGEV-C (A), anti-TGEV-A epitope-specific serum IgG (B), and TGEV neutralizing antibody responses (C) at the 8th week postimmunization. BALB/c mice were immunized orally with two doses (at a 28-day interval) of 987P-fimbriated S. enterica serovar Typhimurium vaccine strains. Geometric means and standard errors of the means for 9 to 10 mice are shown by a horizontal line with error bars. TGEV C and A epitope-specific antibody titers elicited by CS4552/pCS192 (pgtE mutant/spiCp) were significantly higher than the antibody titers induced by χ4550/pCS192 (pgtE+/spiCp) (**, P< 0.01; *, P< 0.05).
Booster immunization enhanced the immune responses against fimbriae.
Whether booster immunizations with attenuated Salmonella strains are immunosuppressive remains somewhat controversial. To examine how booster immunizations with Salmonella vectors modulate the immune responses against the chimeric fimbriae, groups of mice were immunized either once or twice with strain CS4552 (the pgtE mutant) expressing fimbriae from the pagC or spiC promoter. Mice were given ∼5 × 1010 CFU of Salmonella orally at day 0, and mice from the booster group received the same doses again at day 28. Not only was immunosuppression not detected, but the mice immunized twice with CS4552/pCS173 (pgtE mutant/pagCp) had significantly higher humoral (Fig. 6A) antibody responses against the chimeric fimbriae at day 56 as well as significantly higher levels of fimbriae-specific intestinal sIgA (Fig. 6C) after booster immunizations.
FIG. 6.
Effect of a booster immunization on 987P fimbriae-specific antibody responses. BALB/c mice were immunized orally with one dose (solid bars) or two doses (28-day interval, hatched bars) of 987P-fimbriated Salmonella enterica strain CS4552/pCS173 (pgtE mutant/pagCp) or CS4552/pCS192 (pgtE mutant/spiCp). The columns represent the means of individually measured serum IgG at day 56 (A) and at day 88 (B) and intestinal sIgA at day 88 (C). Error bars represent standard errors of the means for 9 to 10 mice. Only statistically significant differences are marked by asterisks (*, P < 0.05).
DISCUSSION
We describe a new vaccine vector system specialized for the expression of intact surface-exposed foreign antigens. The Salmonella vaccine vector described in this report is based on a pgtE mutation that was observed to abolish the proteolysis of a foreign epitope exposed on the bacterial surface. In our previous work, we found that chimeric 987P fimbriae with TGEV C and A epitopes on the surface of E. coli K-12 or Salmonella vaccine strain χ4550 were mainly composed of two proteins of different sizes. In addition to the intact chimeric FasA, a smaller form was present. That the latter form might have resulted from proteolytic activity on chimeric FasA was suggested by its absence in the OmpT and Lon protease mutant BL21 (5). The partially degraded chimeric FasA could be recognized by anti-987P antibody, but not by anti-TGEV C or anti-TGEV A antibodies, indicating that degradation affected the TGEV epitopes. Multimeric epitope expression on the Salmonella vaccine remained suboptimal, since approximately half of the major subunits in the fimbriae lacked the foreign epitopes. To identify the protease(s) responsible for the partial degradation of chimeric FasA, both the intact and partially degraded FasA from the Salmonella vaccine strain χ4550 were isolated and their N-terminal sequences were determined. The N-terminal end of the partially degraded FasA indicated that this protein had been cleaved within the TGEV A epitope between a lysine and an arginine residue, corresponding to the known cleavage pattern of OmpT in E. coli (10, 61). OmpT belongs to a family of outer membrane protease-designated omptins that includes the F plasmid-encoded OmpP of E. coli (20, 28, 36, 58), Pla of Yersinia pestis (56), Sop of Shigella flexneri (17), and PgtE of S. enterica serovar Typhimurium (64). That PgtE was effectively involved in partially degrading FasA was confirmed by constructing and investigating an isogenic pgtE mutant of the Salmonella cya crp asd vaccine strain. Unlike the parental strain, which produced both intact and degraded chimeric FasA, the pgtE mutant produced fimbriae that consisted almost exclusively of intact chimeric FasA. Moreover, chimeric FasA was not cleaved in the isogenic phoP mutant. This result was expected, since the expression of PgtE is activated by PhoP (21). The cleavage of chimeric FasA was also reduced in the parental strain under conditions that do not activate PhoP, such as high concentration of Mg2+. Most importantly, we showed that significant antibody titers against both TGEV epitopes were detectable only when mice were immunized with the pgtE mutant expressing the chimeric fimbriae. This result indicated that the pgtE mutant is an effective vector to display intact heterologous epitopes on its surface for delivery to a mammalian host.
In conjunction with the developed pgtE vaccine, two new potential in vivo-inducible promoters were studied for their use to express heterologous antigens. By investigating some of the various in vivo-inducible promoters that had been used for heterologous antigen expression in attenuated Salmonella strains (4, 5), we found that they could also be used to transcribe whole gene clusters for the expression of fimbriae. In agreement with others, it was observed that the pagC promoter seemed most effective for improving the immunogenicity of heterologous antigens expressed by Salmonella vectors (14, 47), including chimeric fimbriae (4). To identify additional in vivo-inducible promoters for multiple expression of heterologous antigens in Salmonella, the ssaH and the spiC promoters of SPI2 were studied. Both promoters are known to be activated in the phagocytic vacuoles of macrophages (6, 9, 22, 59). In comparison to the ssaH promoter, we found that the spiC promoter expressed significantly more chimeric fimbriae on intracellular Salmonella in macrophages. This correlated with a better systemic and mucosal antibody response in mice immunized with Salmonella vectors expressing chimeric fimbriae from the spiC promoter. In contrast to the ssaH promoter, the spiC promoter was found to be as strong as the pagC promoter in inducing immune responses against the chimeric fimbriae.
The pagC promoter is activated at a relatively low concentration of Mg2+, a condition which is found within Salmonella-containing vacuoles in macrophages (9, 19). The PhoP and PhoQ two-component system senses and regulates the cellular response to Mg2+ (19). Accordingly, our control construct with the pagC promoter was found to induce no fimbriae in the isogenic phoP mutant of Salmonella strain χ4550 (19). The ssaH promoter was previously shown to be regulated by OmpR and EnvZ through SsrB and SsrA—both protein pairs being two-component regulators—and not by PhoP and PhoQ (33, 59). In contrast, the activation of the spiC promoter was shown to require SsrB and to be modulated by PhoP (9). Consistent with a model of differential gene regulation within SPI2, our in vitro data indicated indirectly that the mechanism of regulation of the spiC and ssaH promoters differs. Alternatively, our observed differential regulation of SPI2 promoters might relate to promoter copy number effects, although the copy number of all plasmids used in this study is very low (∼4 to 6 copies for pSC101 derivatives). Use of a cya crp mutant strain could not explain our results, since carbon starvation does not activate SPI2 promoters (9).
As expected, the ssaH and pagC promoters were repressed when the bacteria were grown in media containing high concentrations of Mg2+ ions. Curiously, the SPI2 promoter spiCp was not as clearly repressed in such media. As anticipated, the activity of the ssaH promoter in the isogenic phoP mutant CS4551 was unaffected by Mg2+. However, induction of the activity of the spiC promoter in the phoP mutant by high concentration of Mg2+ was completely unexpected. One explanation is that the activity of the spiC promoter can be induced through both the PhoP and PhoQ protein pair and/or a second regulator. Hence, in the phoP+ strains, the spiC promoter would have been activated by both low and high concentrations of Mg2+, the former signal inducing through the PhoP and PhoQ system, the latter one activating directly or indirectly through the second regulator. In the phoP mutant, activation could only have occurred through the second regulator. That this regulation was mediated by OmpR and EnvZ is unlikely, since the amounts of Mg2+ used would not significantly affect the osmolarity or the pH of the medium. Moreover, it is unlikely that other known Mg2+-sensitive sensor-response regulators used by intravacuolar Salmonella—such as PmrA and PmrB or RcsA and YojN-RcsB—were involved, since these have been reported to be strictly PhoP dependent (42, 57). Taken together, our results strongly suggest that the ssaH and spiC promoters are not regulated in the same way by certain signals and that new regulatory mechanisms remain to be determined for these promoters.
In addition to the finding that the spiC promoter was an effective promoter for inducing anti-fimbriae antibodies, higher anti-TGEV antibodies were induced in mice immunized with the pgtE mutant CS4552 carrying the pagC or the spiC promoter for antigen expression. The pgtE mutation did not interfere with the induction of antibodies against the 987P antigen; strain χ4550 (pgtE+) or CS4552 (the pgtE mutant) with plasmid pCS192 (spiCp) elicited the same level of antibodies against the fimbriae. Although previous research demonstrated that PgtE contributes to Salmonella resistance to innate immunity (21), our data indicated that the addition of the pgtE mutation to the cya, crp, and asd mutations of strain χ4550 did not jeopardize its properties as a useful vaccine vector. On the contrary, the anti-TGEV C and A epitope antibody responses were significantly higher with the pgtE mutant strain CS4552. This result can be attributed to an increased level of TGEV epitopes in the chimeric fimbriae expressed on the Salmonella after uptake by professional antigen-presenting cells.
One of the concerns of using live bacterial vectors such as Salmonella has been that induction of immunity to the bacterial vector may preclude its subsequent use for other vaccines or for boosting. Our system showed that booster immunization with the same vaccine regimen can enhance both systemic and mucosal immune response against the heterologous antigens delivered by live Salmonella. This was reflected by elevated serum and intestinal anti-987P fimbriae antibody titers for mice immunized twice rather than once. Similar results were previously demonstrated with some antigens (3, 30, 31, 63), although negative effects of preexisting immunity to Salmonella on the immune response to foreign antigens were also reported (2, 46). Some of these controversial results can be explained by the use of different genetic backgrounds of Salmonella, intervals for boosting, types of heterologous antigen expressed, and promoters. The results from this study demonstrated that with an appropriate immunization regimen, prior exposure to the pgtE vaccine strain did not interfere with the immune responses to the chimeric fimbriae, indicating that this live attenuated Salmonella strain can be used repeatedly.
In summary, we have demonstrated that a Salmonella cya crp asd pgtE can display intact TGEV epitopes on its surface in the context of multimeric fimbriae. Moreover, this bacterial vector display system was clearly found to elicit anti-TGEV-epitope antibodies, TGEV-neutralizing antibodies being detectable in a significant number of orally immunized animals. A booster immunization with the same vaccine regimens enhanced both the systemic and mucosal immune responses against the heterologous antigen. The systemic and mucosal immune responses to the heterologous antigens delivered by the Salmonella vaccine strains were optimal with the macrophage-inducible pagC and spiC promoters, opening new avenues for developing Salmonella vaccines expressing simultaneously different foreign antigens against the same or different pathogens.
Acknowledgments
We thank John Mekalanos, Sam Miller, and Roy Curtiss III for providing strains, and we thank Ron Harty for critically reading the manuscript and Jikang Fang for protein sequencing.
This work was supported by USDA grant number NRI 2001-2277.
Editor: B. B. Finlay
REFERENCES
- 1.Alexeyev, M. F., I. N. Shokolenko, and T. P. Croughan. 1995. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene 160:63-67. [DOI] [PubMed] [Google Scholar]
- 2.Attridge, S. R., R. Davies, and J. T. LaBrooy. 1997. Oral delivery of foreign antigens by attenuated Salmonella: consequences of prior exposure to the vector strain. Vaccine 15:155-162. [DOI] [PubMed] [Google Scholar]
- 3.Bao, J. X., and J. D. Clements. 1991. Prior immunologic experience potentiates the subsequent antibody response when Salmonella strains are used as vaccine carriers. Infect. Immun. 59:3841-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, H., and D. M. Schifferli. 2001. Enhanced immune responses to viral epitopes by combining macrophage-inducible expression with multimeric display on a Salmonella vector. Vaccine 19:3009-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, H., and D. M. Schifferli. 2000. Mucosal and systemic immune responses to chimeric fimbriae expressed by Salmonella enterica serovar Typhimurium vaccine strains. Infect. Immun. 68:3129-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 7.Clark, M. A., B. H. Hirst, and M. A. Jepson. 1998. Inoculum composition and Salmonella pathogenicity island 1 regulate M-cell invasion and epithelial destruction by Salmonella typhimurium. Infect. Immun. 66:724-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, L. V., and F. Schödel. 1997. Live bacterial vectors, p. 293-308. In P.-P. Pastoret, J. Blancou, P. Vannier, and C. Verschueren (ed.), Veterinary vaccinology. Elsevier Science, New York, N.Y.
- 9.Deiwick, J., T. Nikolaus, S. Erdogan, and M. Hensel. 1999. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol. Microbiol. 31:1759-1773. [DOI] [PubMed] [Google Scholar]
- 10.Dekker, N., R. C. Cox, R. A. Kramer, and M. R. Egmond. 2001. Substrate specificity of the integral membrane protease OmpT determined by spatially addressed peptide libraries. Biochemistry 40:1694-1701. [DOI] [PubMed] [Google Scholar]
- 11.Delmas, B., J. Gelfi, and H. Laude. 1986. Antigenic structure of transmissible gastroenteritis virus. II. Domains of the peplomer glycoprotein. J. Gen. Virol. 67:1405-1418. [DOI] [PubMed] [Google Scholar]
- 12.Der Vartanian, M., J.-P. Girardeau, C. Martin, E. Rousset, M. Chavarot, H. Laude, and M. Contrepois. 1997. An Escherichia coli CS31A fimbrillum chimera capable of inducing memory antibodies in outbred mice following booster immunization with the entero-pathogenic coronavirus gastroenteritis virus. Vaccine 15:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didierlaurent, A., J. C. Sirard, J. P. Kraehenbuhl, and M. R. Neutra. 2002. How the gut senses its content. Cell. Microbiol. 4:61-72. [DOI] [PubMed] [Google Scholar]
- 14.Dunstan, S. J., C. P. Simmons, and R. A. Strugnell. 1999. Use of in vivo-regulated promoters to deliver antigens from attenuated Salmonella enterica var. Typhimurium. Infect. Immun. 67:5133-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards, R. A., L. H. Keller, and D. M. Schifferli. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149-157. [DOI] [PubMed] [Google Scholar]
- 16.Edwards, R. A., and D. M. Schifferli. 1997. Differential regulation of fasA and fasH expression of Escherichia coli 987P fimbriae by environmental cues. Mol. Microbiol. 25:797-809. [DOI] [PubMed] [Google Scholar]
- 17.Egile, C., H. d'Hauteville, C. Parsot, and P. J. Sansonetti. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 23:1063-1073. [DOI] [PubMed] [Google Scholar]
- 18.Galen, J. E., and M. M. Levine. 2001. Can a “flawless” live vector vaccine strain be engineered? Trends Microbiol. 9:372-376. [DOI] [PubMed] [Google Scholar]
- 19.García Véscovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 20.Grodberg, J., and J. J. Dunn. 1989. Comparison of Escherichia coli K-12 outer membrane protease OmpT and Salmonella typhimurium E protein. J. Bacteriol. 171:2903-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guina, T., E. C. Yi, H. Wang, M. Hackett, and S. I. Miller. 2000. A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to α-helical antimicrobial peptides. J. Bacteriol. 182:4077-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensel, M. 2000. Salmonella pathogenicity island 2. Mol. Microbiol. 36:1015-1023. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins, S. A., F. Niedergang, I. E. Corthesy-Theulaz, and J.-P. Kraehenbuhl. 2000. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell. Microbiol. 2:59-68. [DOI] [PubMed] [Google Scholar]
- 24.Isaacson, R. E., E. A. Dean, R. L. Morgan, and H. W. Moon. 1980. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified K99 and 987P pili: antibody production in response to vaccination. Infect. Immun. 29:824-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaacson, R. E., B. Nagy, and H. W. Moon. 1977. Colonization of porcine small intestine by Escherichia coli: colonization and adhesion factors of pig enteropathogens that lack K88. J. Infect. Dis. 135:531-539. [DOI] [PubMed] [Google Scholar]
- 26.Jiménez, G., I. Correa, M. P. Melgosa, M. J. Bullido, and L. Enjuanes. 1986. Critical epitopes in transmissible gastroenteritis virus neutralization. J. Virol. 60:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufmann, A., Y. D. Stierhof, and U. Henning. 1994. New outer membrane-associated protease of Escherichia coli K-12. J. Bacteriol. 176:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan, A. S., and D. M. Schifferli. 1994. A minor 987P protein different from the structural fimbrial subunit is the adhesin. Infect. Immun. 62:4233-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler, J. J., L. B. Pathangey, and T. A. Brown. 1998. Oral immunization with recombinant Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin: effect of boosting on mucosal, systemic and immunoglobulin G subclass response. Oral Microbiol. Immunol. 13:81-88. [DOI] [PubMed] [Google Scholar]
- 31.Kohler, J. J., L. B. Pathangey, S. R. Gillespie, and T. A. Brown. 2000. Effect of preexisting immunity to Salmonella on the immune response to recombinant Salmonella enterica serovar Typhimurium expressing a Porphyromonas gingivalis hemagglutinin. Infect. Immun. 68:3116-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laude, H., J. M. Chapsal, J. Gelfi, S. Labiau, and J. Grosclaude. 1986. Antigenic structure of transmissible gastroenteritis virus. I. Properties of monoclonal antibodies directed against virion proteins. J. Gen. Virol. 67:119-130. [DOI] [PubMed] [Google Scholar]
- 33.Lee, A. K., C. S. Detweiler, and S. Falkow. 2000. OmpR regulates the two-component system SsrA-ssrB in Salmonella pathogenicity island 2. J. Bacteriol. 182:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinaro, M., H. Kiyono, J. L. VanCott, N. Okahashi, F. W. van Ginkel, D. W. Pascual, E. Ban, R. J. Jackson, H. F. Staats, and J. R. McGhee. 1996. Vaccines for selective induction of Th1- and Th2-cell responses and their roles in mucosal immunity, p. 461-475. In M. F. Kagnoff and H. Kiyono (ed.), Mucosal immunology. Academic Press, New York, N.Y.
- 35.Mastroeni, P., J. A. Chabalgoity, S. J. Dunstan, D. J. Maskell, and G. Dougan. 2001. Salmonella: immune responses and vaccines. Vet. J. 161:132-164. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo, E., G. Sampei, K. Mizobuchi, and K. Ito. 1999. The plasmid F OmpP protease, a homologue of OmpT, as a potential obstacle to E. coli-based protein production. FEBS Lett. 461:6-8. [DOI] [PubMed] [Google Scholar]
- 37.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittrücker, H. W., A. Köhler, T. W. Mak, and S. H. Kaufmann. 1999. Critical role of CD28 in protective immunity against Salmonella typhimurium. J. Immunol. 163:6769-6776. [PubMed] [Google Scholar]
- 40.Moon, H. W. 1990. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr. Top. Microbiol. Immunol. 151:147-165. [DOI] [PubMed] [Google Scholar]
- 41.Morgan, R. L., R. E. Isaacson, H. W. Moon, C. C. Brinton, and C.-C. To. 1978. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified 987 or K99 pili: protection correlates with pilus homology of vaccine and challenge. Infect. Immun. 227:771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouslim, C., and E. A. Groisman. 2003. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol. Microbiol. 47:335-344. [DOI] [PubMed] [Google Scholar]
- 43.Nagy, B., H. W. Moon, R. E. Isaacson, C.-C. To, and C. C. Brinton. 1978. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating dams with purified pili. Infect. Immun. 21:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neutra, M. R., A. Frey, and J. P. Kraehenbuhl. 1996. Epithelial M cells: gateways for mucosal infection and immunization. Cell 86:345-348. [DOI] [PubMed] [Google Scholar]
- 45.Rani, D. B. R., M. E. Bayer, and D. M. Schifferli. 1999. Polymeric display of immunogenic epitopes from herpes simplex virus and transmissible gastroenteritis virus surface proteins on an enteroadherent fimbria. Clin. Diagn. Lab. Immunol. 6:30-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts, M., A. Bacon, J. Li, and S. Chatfield. 1999. Prior immunity to homologous and heterologous Salmonella serotypes suppresses local and systemic anti-fragment C antibody responses and protection from tetanus toxin in mice immunized with Salmonella strains expressing fragment C. Infect. Immun. 67:3810-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts, M., J. Li, A. Bacon, and S. Chatfield. 1998. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect. Immun. 66:3080-3087. (Erratum, 67:468, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saif, L. J., J. L. van Cott, and T. A. Brim. 1994. Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet. Immunol. Immunopathol. 43:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schifferli, D. M., S. N. Abraham, and E. H. Beachey. 1987. Use of monoclonal antibodies to probe subunit- and polymer-specific epitopes of 987P fimbriae of Escherichia coli. Infect. Immun. 55:923-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schifferli, D. M., and M. Alrutz. 1994. Permissive linker insertion sites in the outer membrane protein of 987P fimbriae of Escherichia coli. J. Bacteriol. 176:1099-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schödel, F., S. M. Kelly, D. L. Peterson, D. R. Milich, and R. Curtiss III. 1994. Hybrid hepatitis B virus core-pre-S proteins synthesized in avirulent Salmonella typhimurium and Salmonella typhi for oral vaccination. Infect. Immun. 62:1669-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sestak, K., I. Lanza, S.-K. Park, P. A. Weilnau, and L. J. Saif. 1996. Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am. J. Vet. Res. 57:664-671. [PubMed] [Google Scholar]
- 53.Sestak, K., and L. J. Saif. 2002. Porcine coronaviruses, p. 321-330. In A. Morilla, K.-J. Yoon, and J. J. Zimmerman (ed.), Trends in emerging viral infections of swine. Iowa State Press, Ames, Iowa.
- 54.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusion. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 55.Sirard, J. C., F. Niedergang, and J. P. Kraehenbuhl. 1999. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol. Rev. 171:5-26. [DOI] [PubMed] [Google Scholar]
- 56.Sodeinde, O. A., and J. D. Goguen. 1989. Nucleotide sequence of the plasminogen activator gene of Yersinia pestis: relationship to ompT of Escherichia coli and gene E of Salmonella typhimurium. Infect. Immun. 57:1517-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soncini, F. C., and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugimura, K., and N. Higashi. 1988. A novel outer-membrane-associated protease in Escherichia coli. J. Bacteriol. 170:3650-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 60.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 156:1504-1514. [PubMed] [Google Scholar]
- 61.Vandeputte-Rutten, L., R. A. Kramer, J. Kroon, N. Dekker, M. R. Egmond, and P. Gros. 2001. Crystal structure of the outer membrane protease OmpT from Escherichia coli suggests a novel catalytic site. EMBO J. 20:5033-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 63.Whittle, B. L., and N. K. Verma. 1997. The immune response to a B-cell epitope delivered by Salmonella is enhanced by prior immunological experience. Vaccine 15:1737-1740. [DOI] [PubMed] [Google Scholar]
- 64.Yu, G. Q., and J. S. Hong. 1986. Identification and nucleotide sequence of the activator gene of the externally induced phosphoglycerate transport system of Salmonella typhimurium. Gene 45:51-57. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, L. Z., Y. F. Gong, Y. Fang, Y. S. Zhang, and F. S. Gu. 1993. Use of microwaves in immunoenzyme techniques. Clin. Chem. 39:2021.. [PubMed] [Google Scholar]