Abstract
Polymorphonuclear neutrophil granulocytes (PMN) have been implicated in the early inflammatory response against mycobacteria besides monocytes/macrophages. Yet, little is known about the interaction of mycobacteria with PMN. We investigated the potential of Mycobacterium bovis bacillus Calmette-Guérin (BCG) to stimulate and influence PMN phenotype, gene expression profile and spontaneous apoptosis. Flow cytometric analyses revealed an upregulation of the function-associated molecules Fcγ receptor III (FcγR III) and II (CD16 and CD32) as well as MAC-1 (CD11b and CD18) on BCG-stimulated PMN. As determined by cDNA microarrays and multiplex reverse transcriptase PCR, stimulation with BCG alters the expression of various genes for proinflammatory cytokines/chemokines or receptors in PMN. We detected an upregulation or de novo synthesis of interleukin 1α (IL-1α), IL-1β, IL-8, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, GRO-α, transforming growth factor β, MCP-1, IL-2 receptor γ (IL-2Rγ), IL-10Rα, and IL-6R. Genes for IL-9, IL-12α, IL-15, IL-5Rα, and IL-13Rα1 were found to be downregulated or switched off. Furthermore, Giemsa and annexin V-propidium iodide double staining demonstrated an inhibition of spontaneous PMN apoptosis following BCG stimulation. Changes in phenotype and inhibition of apoptosis did not depend on direct mycobacterial stimulation alone, but were a result of an autocrine-paracrine stimulation mechanism. Our findings support the hypothesis that PMN become activated at the site of mycobacterial infections and that this activation might set the stage for a subsequent antimycobacterial immune response.
Mycobacteria of the Mycobacterium tuberculosis complex such as M. tuberculosis, Mycobacterium bovis, or Mycobacterium africanum have a close phylogenetic and pathogenic relationship. They are the causative agents for tuberculosis in humans and animals (18). The early migration of activated monocytes/macrophages to the site of mycobacterial inflammations is an important step in the control of such infections. Phagocytosis and processing by these professional phagocytes initiates the activation of lymphocyte subpopulations such as NK cells and CD4+/CD8+ T cells, which are the main effector cells to eliminate the microorganisms (20, 21). However, polymorphonuclear neutrophil granulocytes (PMN) are the first subset of immune cells to leave the blood vessels and infiltrate tissue that has been invaded by mycobacteria (23). While, for a long time, little attention has been paid to a possible functional role of PMN in the inflammatory response against mycobacteria, recent evidence from in vivo studies suggests that PMN might indeed be involved in immune protection against mycobacterial infections (2, 6, 16, 32).
Circulating human PMN are short-lived with a half-life of only 6 to 10 h after being released from the bone marrow and undergo rapid apoptosis followed by elimination in the liver and spleen (31). Upon tissue injury they home to the site of infection and migrate from the bloodstream into the inflamed tissue. This complicated process involves several steps mediated by various adhesion molecules such as selectins, integrins and their respective ligands on endothelial cells and PMN (12). These activated, infiltrating PMN have a considerably longer life span than their circulating counterparts (11). They can phagocytose microorganisms, generate reactive oxygen intermediates and release lytic enzymes with antimicrobial potential (7). Although PMN have long been regarded as terminally differentiated cells with little capacity for protein synthesis, several in vitro studies have recently challenged this opinion about PMN. The expression and release of several cytokines and chemokines such as interleukin-1α/β (IL-1α/β), IL-8, transforming growth factor β (TGF-β), macrophage inflammatory protein 1α/β (MIP-1α/β), and GRO-α by stimulated PMN has been shown convincingly by various groups (7). Still, there is dissension about the functional in vivo relevance of this protein release and about the potential of PMN to produce additional cytokines/chemokines.
Little is known about the potential of mycobacteria to stimulate PMN and alter their basic biologic behavior. Two groups were able to demonstrate the release of IL-8, GRO-α and MIP-1α from PMN following stimulation with M. tuberculosis (19, 29). While some microbial pathogens such as Escherichia coli or Candida albicans reduce the life span of PMN, the intracellular parasite Leishmania major inhibits spontaneous apoptosis of these host cells (1). Whether mycobacterial stimulation has an influence on PMN survival has not yet been clarified.
To further define a potential role for PMN during mycobacterial infections we investigated the stimulatory effect of M. bovis bacillus Calmette-Guérin (BCG) on the phenotype, gene expression profile and survival of PMN. Mycobacterial stimulation of PMN induces an upregulation of function-associated surface molecules and upregulates mRNA for a set of cytokines/chemokines as well as cytokine/chemokine receptors. Furthermore, it inhibits spontaneous apoptosis of activated PMN. Our study supports the hypothesis that PMN play an important role in the early inflammatory host response during mycobacterial infections.
MATERIALS AND METHODS
Reagents, mycobacteria, and stimulation conditions.
Viable mycobacteria M. bovis BCG (Connaught substrain; kindly provided by Cytochemia) from the logarithmic growth phase were used for stimulation experiments at various concentrations as indicated in figure legends. The total number of CFU was routinely determined by incubation at 37°C for 4 weeks on Löwenstein-Jensen agar. Granulocyte-macrophage colony-stimulating factor (GM-CSF) with an activity of 10,000 U/μg was obtained from TEBU. Unless indicated otherwise, 106 PMN/ml were stimulated in RPMI 1640 culture medium (Biochrom) supplemented with 10% low-endotoxin fetal calf serum (FCS; Biochrom), penicillin (100 U/ml) and streptomycin (100 μg/ml), hereafter referred to as complete medium (CM).
In some experiments fresh conditioned supernatants of stimulated neutrophil granulocytes (24-h stimulation with various concentrations of BCG and GM-CSF) were used to stimulate allogeneic human PMN. Supernatants were harvested and further processed by centrifugation at 5,000 rpm (Hettich Rotanta 96R) for 15 min at 4°C. To gain particle-free supernatants they were additionally passed through a 0.45-μm-pore-size filter (Millipore) before use.
Preparation of PMN.
Peripheral blood was obtained from healthy donors. PMN were separated by Ficoll-Hypaque 1.077 (Biochrom) centrifugation followed by sedimentation in 1% polyvinyl alcohol (Merck) containing 0.9% NaCl. Remaining erythrocytes were removed by brief lysis in hypotonic saline. Recovered PMN were resuspended in CM. This cell preparation contained >99% PMN and >98% neutrophils with a viability of >98% as determined by Pappenheim staining and trypan blue dye exclusion. This preparation protocol was approved by the ethics committee of the Lübeck University Medical School (approval no. 02-137) after written informed consent from all individuals tested was obtained.
Electron microscopic (EM) studies.
106 CFU/ml BCG were used to stimulate PMN for 2 h in CM at 37°C and 5%CO2. Cells were harvested and centrifuged, and the pellet was fixed in 0.1 M phosphate-buffered saline (PBS) with 3% glutaraldehyde. After being washed twice in 0.1 M PBS with 2% saccharose, the pellet was treated with 2% osmium tetroxide at 4°C for 1 h. Following dehydration in a graded series of alcohol, the pellet was embedded in epoxy resin and stained with uranyl acetate. Ultrathin sections (80 to 100 nm) were screened on a Zeiss EM910 (Zeiss) for neutrophils containing phagocytosed mycobacteria.
Flow cytometric analysis of PMN phenotype.
PMN were stimulated for 2 h in CM with various concentrations of BCG or GM-CSF as indicated in figure legends. Single-color immunofluorescence was performed on PMN either indirectly stained with monoclonal antibodies (MAb) (CD11b, clone ICRF44 [Pharmingen], and CD18, clone 7E4 [Immunotech]) and detected with a fluorescein isothiocyanate (FITC)-conjugated secondary MAb or directly stained with a FITC-conjugated primary MAb (CD16, clone DJ130c, and CD32, clone KB61; both DAKO). For each analysis 5 × 105 cells were incubated in washing buffer (PBS containing 0.1% sodium azide and 3% human AB serum) with 1 μg of the detection antibody/ml or an isotype control (clone DAK-GO1; DAKO) at 4°C for 30 min. After two washings, a goat anti-mouse FITC conjugate was added for another 30 min at 4°C in CD11b and CD18 stainings. Following two more washes in washing buffer, 1.5% formaldehyde was added and a total of 10,000 cells were analyzed on a FACScalibur (BD, Heidelberg, Germany). In all experiments, debris, nonviable and aggregated cells were gated according to forward/sideward scatter signals. Data were analyzed with WinMDI software.
Gene expression profiling by reverse transcriptase PCR (RT-PCR).
As indicated in figure legends, various concentrations of BCG were used to stimulate PMN for 3.5 h in CM. Cells were harvested and total RNA was prepared using an Invisorb RNA Kit II according to the manufacturer's instructions (Invitek). After cDNA with superscript RT was prepared according to the manufacturer's instructions (Gibco BRL), messages were amplified with the following PCR protocol: 15 s at 95°C, 30 s at 55°C, and 2 min at 72°C for 35 cycles with a 2-s extension for each cycle (Taq DNA polymerase from Gibco BRL). Primer pairs from four different multiplex RT-PCR kits were used to specifically detect messages. Human Inflammatory Cytokine Set I contained primers for tumor necrosis factor alpha (TNF-α), IL-1β, GM-CSF, IL-6, IL-8, and TGF-β; human Th1/Th2 Cytokine Set II contained primers for gamma interferon (IFN-γ), IL-2, IL-4, IL-5, IL-10, IL-12p40, and IL-13; and human Chemokine Set I and Set II contained primers for IL-8, IP-10, RANTES, MCP-1, MCP-2, MIP-1α, MIP-1β, eotaxin, ENA-78, and MIG [all from Biosource]). The sequences of GRO-α primers have been described elsewhere (29) and were as follows: sense 5′-TAG CCA CAC TCA AGA ATG GGC GGA AAG CTT GC-3′ antisense 5′-TGG CCA TTT GCT TGG ATC CGC CAG CCT-3′.
Gene expression profiling by cDNA microarray analysis.
PMN were stimulated for 1.5 or 3.5 h with 106 CFU of BCG/ml in CM. Total RNA was isolated with Trizol reagent (Invitrogen). The cDNA probes were synthesized with radiolabeled [α-32P]dCTP nucleotides (Amersham Bioscience), SuperScript II-Reverse Transcriptase (Invitrogen) and GeArray probe synthesis reagents (SuperArray) according to the manufacturer's instructions. Resulting cDNAs were hybridized on SuperArray nylon membrane microarrays (Human Inflammatory Cytokine/Receptor GeArray Q Series; HS-015). The hybridized membranes were analyzed with a PhosphorImager (Molecular Dynamics) and analyzed with Phoretix-Analyzing software (version 3.01).
Morphological assessment of PMN apoptosis.
PMN were cultured in CM for 24 h in the presence of various concentrations of BCG and GM-CSF. Giemsa-stained cytocentrifuge slides were prepared to analyze nuclear morphology by phase-contrast microscopy. A total of at least 200 cells were assessed for typical morphological criteria of granulocyte apoptosis. The respective figure shows the mean percentage of nonapoptotic cells ± standard deviation (SD).
Analysis of PMN apoptosis by annexin V-PI double staining.
Stimulated PMN (see stimulation conditions above) were harvested and double stained with annexin V-FITC and propidium iodide (PI) according to the manufacturer's instructions (Immunotech). A total of 10,000 cells were analyzed on a FACScalibur. Data were calculated with WinMDI software and depicted as two-dimensional density blots.
Detection of human IL-8 in PMN supernatants by ELISA.
PMN were cultured for 3.5 or 18 h in the presence of BCG (106 CFU/ml) or lipopolysaccharide (LPS) (0.1 μg/ml) as indicated in the figure legends. Supernatants were harvested, and IL-8 content was measured in a commercially available quantitative IL-8-(enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Biosource).
Experimental design and statistical analyses.
All experiments were performed at least in triplicate (unless otherwise denoted in figure legends) as independent experiments with PMN isolated from separate individuals. PMN from any individual were used only once for one single experiment. Significance of analyzed data was determined using a Mann-Whitney U test and given as P values. To correct for multiple testing hypotheses were ordered a priori. A P of ≤0.05 was considered significant. To interpret gene expression changes in RT PCR and cDNA microarray analyses, band and signal intensities were compared after the signals were normalized in relation to housekeeping gene signals. A greater-than-twofold increase or decrease in band intensity was considered relevant and used as the cutoff.
RESULTS
Phagocytosis and intracellular processing of BCG by PMN.
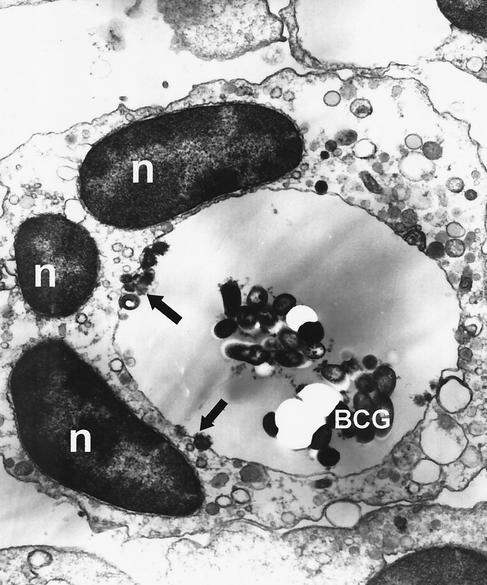
First, we wanted to assess the capacity of PMN to phagocytose and process mycobacteria in the absence of opsonizing factors. For this purpose, PMN were stimulated with 106 CFU of viable BCG/ml, which is analogous to a multiplicity of infection (MOI) of 1 for 2 h. Cells were harvested after removal of extracellular bacteria with the medium, fixed and examined by electron microscopy (Fig. 1). Ingested mycobacteria were found in large phagolysosomes/digestive vacuoles of various neutrophil granulocytes, but not within the cytoplasm. Additionally, phagocytosing PMN showed a large number of vacuoles and lysosomes. However, a thorough analysis of 100 ultrathin sections revealed that about 90% of PMN did not contain any microorganisms. This finding was confirmed by Ziehl-Neelsen staining (data not shown).
FIG. 1.
Phagocytosis and intracellular processing of BCG by neutrophil granulocytes. 106 of PMN/ml were stimulated with 106 CFU of viable mycobacteria/ml for 2 h. Cells were harvested and prepared for EM studies as described in Materials and Methods. The figure shows a human neutrophil granulocyte after phagocytosis of a cluster of BCG. The mycobacteria are stuck within a large phagolysosome. The arrows point to several lysosomes fusing with the digestive vacuole. Also note the polymorphous nucleus (n). Magnification, ×14,800.
Mycobacterial stimulation induces an upregulation of function-associated surface molecules on PMN.
The β2-integrin MAC-1, consisting of the 150-kDa α-chain CD11b and the 95-kDa β-chain CD18, plays a central role in neutrophil activation upon interaction with intercellular adhesion molecules, Sialyl-LewisX, and E-selectin on blood-vessel walls at sites of inflammation. The Fcγ receptors (FcγR) III and II, or CD16 and CD32, respectively, are of crucial importance for phagocytosis of opsonized particles, neutrophil chemotaxis, and ADCC (12). We examined the effect of mycobacterial stimulation on PMN surface expression of these function-associated molecules. PMN were stimulated with various concentrations of BCG for 2, 6, and 24 h, and changes in phenotype were determined by flow cytometry. After 2 h of stimulation, BCG induced an upregulation of CD11b (21%), CD16 (3.7%), CD18 (85.6%), and CD32 (6.1%) on PMN in a dose-dependent manner (Table 1). The most effective concentration was 106 CFU of viable mycobacteria/ml (MOI = 1). Higher concentrations of BCG led to PMN clustering and clumping, making an analysis impossible. The maximum increase in surface molecule expression was achieved after 2 h of stimulation. Prolonging the time of PMN stimulation gradually decreased the effect induced by BCG (data not shown).
TABLE 1.
BCG induces an upregulation of function-associated molecules on PMNa
| Stimulus | Δmean (% increase)
|
|||
|---|---|---|---|---|
| CD11b | CD16 | CD18 | CD32 | |
| None | 186.6 | 178.2 | 90.7 | 85.2 |
| BCG | ||||
| 105 CFU/ml | 193.3 (4) | 181.1 (1.6) | 112.8 (24.4) | 88.7 (4.1) |
| 106 CFU/ml | 225.9 (21) | 184.7 (3.7) | 168.3 (85.6) | 90.4 (6.1) |
PMN were stimulated with increasing concentrations of viable BCG for 2 h. Cells were harvested and analyzed by flow cytometry. Mean fluorescent intensities from isotype controls were subtracted from the mean of the respective specific stainings and are listed as Δmean. The percentages of change in surface molecule expression between stimulated and unstimulated cells are depicted in parentheses. The table shows the representative results of one of four independent experiments.
Next, we investigated whether the increase in surface molecule expression depends on direct interaction of the neutrophil with the pathogen or might be a result of an autocrine/paracrine stimulation by soluble factors released by stimulated PMN during coincubation with the mycobacteria. Supernatants from PMN after 24 h of coincubation with BCG were harvested and further processed as described in Materials and Methods. These supernatants were used to stimulate allogeneic PMN for 2 h, and surface molecule expression was determined. Supernatants of BCG-stimulated PMN also upregulated surface expression of CD11b, CD16, CD18, and CD32 on PMN (Table 2). PMN incubated in CM or in supernatants of BCG only did not show any changes in surface molecule expression.
TABLE 2.
Supernatants of BCG-stimulated PMN induce an upregulation of function-associated molecules on allogeneic PMNa
| Stimulus | Δmean (% increase)
|
|||
|---|---|---|---|---|
| CD11b | CD16 | CD18 | CD32 | |
| Supernatant of unstimulated PMN | 152.8 | 122.7 | 31.1 | 82.1 |
| Supernatant of PMN + 105 CFU of BCG/ml | 158.5 (3.8) | 127.2 (3.7) | 49.9 (60.5) | 84.1 (2.4) |
| Supernatant of PMN + 106 CFU of BCG/ml | 282.1 (84.6) | 155.6 (26.8) | 84.9 (173) | 105.8 (28.9) |
PMN were stimulated with increasing concentrations of viable BCG for 24 h. Supernatants were harvested and further processed as described in Materials and Methods. Allogeneic PMN were incubated in these supernatants for 2 h, harvested, and analyzed by flow cytometry. Mean fluorescent intensities from isotype controls were subtracted from the mean of the respective specific stainings and are listed as Δmean. The percentages of change in surface molecule expression are depicted in parentheses. The table shows the representative results of one of three independent experiments.
Mycobacterial stimulation inhibits spontaneous apoptosis of PMN.
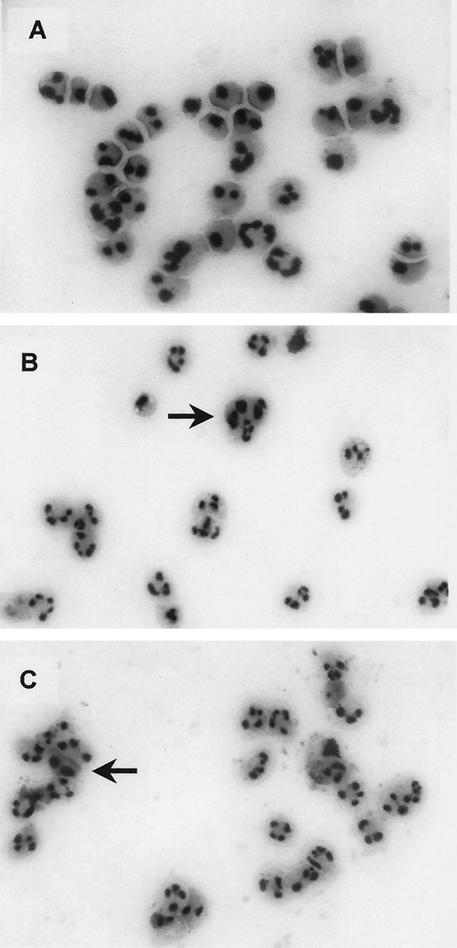
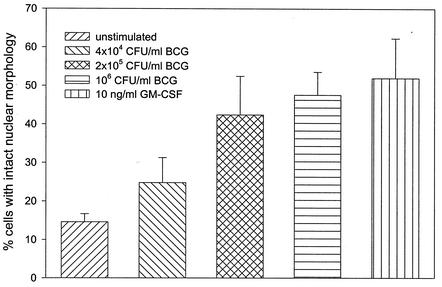
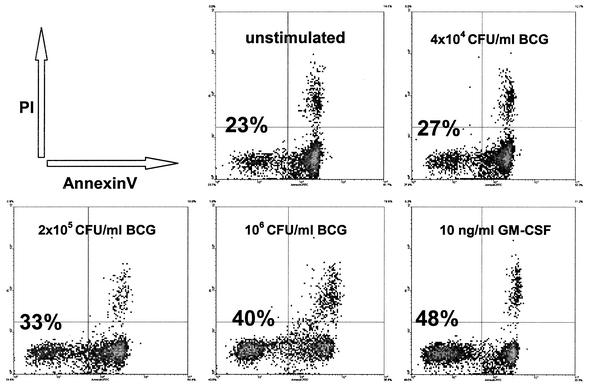
The effect of mycobacteria on the intrinsic process of PMN apoptosis is unclear. We investigated whether mycobacterial stimulation of PMN influences their spontaneous apoptotic potential. Apoptotic granulocytes exhibit morphological features such as cell shrinkage, cytoplasmic condensation and heterochromatin condensation (1, 28, 33). PMN were coincubated for 24 h with various concentrations of BCG and GM-CSF, and Giemsa-stained cytocentrifuge slides were assessed for changes in nuclear morphology using these criteria. The majority of unstimulated PMN showed morphological features of apoptosis after 24 h with only 15% (±2%) of cells remaining viable (Fig. 2A and 3). Coincubation of PMN with increasing concentrations of BCG induced a significant dose-dependent inhibition of apoptosis and an increasing percentage of viable PMN up to 48% (±6%; P < 0.001; Fig. 2B and 3). GM-CSF has been shown to inhibit granulocyte apoptosis (10) and was used as a positive control in our experiments (52% viable cells ±10%; P < 0.001; Fig. 2C and 3). In addition to assessing nuclear morphology we used annexin V-PI double staining to demonstrate the inhibitory effect of BCG mycobacteria on PMN apoptosis. The translocation of phosphatidylserine, which can be specifically detected with annexin V, to the outer leaflet of the cell membrane is one of the early events during the apoptotic process (34). The same experimental setup as described above was used to stimulate PMN. Harvested cells were double-stained with FITC-labeled annexin V and PI to detect early apoptotic and dead cells. The flow cytometric analysis supported the morphological results. Increasing concentrations of BCG and 10 ng of GM-CSF/ml inhibited the spontaneous apoptosis of stimulated PMN in a dose-dependent manner compared to that of unstimulated PMN (Fig. 4). The protection from apoptosis coincided with a reduction in the number of necrotic cells.
FIG. 2.
Morphological assessment of PMN apoptosis. PMN were cultured for 24 h in the absence (A) or presence of 106 CFU of BCG/ml (MOI = 1) (B) or 10 ng of GM-CSF/ml (C). Giemsa-stained cytocentrifuge slides were assessed for changes in nuclear morphology. The majority of unstimulated PMN shows apoptotic morphology with cell shrinkage, cytoplasmic and heterochromatin condensation. In contrast, most of the BCG- or GM-CSF-stimulated granulocytes exhibit typical polymorphonuclear morphology. Only occasional cells display the features of apoptosis (arrows). Magnification, ×40.
FIG. 3.
PMN apoptosis after stimulation with BCG or GM-CSF. PMN were cultured for 24 h in the presence of increasing concentrations of BCG or with GM-CSF. A total of ≥200 cells on three different Giemsa-stained cytocentrifuge slides were assessed for changes in nuclear morphology. The figure bars show the combined result of six independent experiments with the respective percentages of viable versus apoptotic cells given as mean ± SD (error bars) using morphological criteria.
FIG. 4.
Inhibition of PMN apoptosis after stimulation with BCG or GM-CSF. PMN were cultured for 24 h in the presence of increasing concentrations of BCG or with GM-CSF. Cells were double stained with FITC-labeled annexin V (x axis) and PI (y axis) to detect early apoptotic and dead cells. Events in the lower left quadrant represent viable, annexin V- and PI-negative PMN. Increasing concentrations of BCG induce an increase in the percentage of viable cells in a dose-dependent manner. Additionally, there is a slight decrease in the percentage of necrotic cells, represented by the annexin V- and PI-positive events in the upper right quadrant. The figure shows the representative result of one of three independent experiments.
In parallel to the experiments described above, we generated supernatants of BCG-stimulated granulocytes and tested their potential to inhibit the apoptosis of allogeneic PMN after 24 h using annexin V-PI double stainings. Compared to supernatants of unstimulated PMN (resulting in 17% viable cells), coincubation with supernatants of PMN stimulated with 105 and 106 CFU of BCG/ml conspicuously inhibited granulocyte apoptosis and prolonged survival (resulting in 30 and 47% viable cells, respectively). PMN incubated in CM or in supernatants of BCG only did not show any changes in spontaneous apoptosis.
Stimulation of PMN with mycobacteria alters their gene expression profile.
The production of various cytokines and chemokines by PMN in response to several stimuli has been convincingly demonstrated (7). However, until now there has been no comprehensive analysis available of cytokine and chemokine responses of PMN after mycobacterial stimulation. We investigated the potential of mycobacteria to influence PMN gene expression by using cDNA microarray analyses. PMN were stimulated for 1.5 or 3.5 h with 106 CFU of BCG/ml in CM. Total RNA was isolated and messages were specifically identified with cDNA probes as described in Materials and Methods. We were able to identify several regulated genes as summarized in Tables 3 and 4. The tables show the combined results of three independent experiments. Besides various housekeeping genes we found a considerable number of constitutively expressed genes in unstimulated as well as stimulated PMN. We detected an upregulation or de novo synthesis of several proinflammatory cytokines/chemokines as well as cytokine/chemokine receptors such as Il-1α, IL-1β, MIP-1α, MIP-1β, IL-2Rγ, IL-10Rα, and IL-6R. Genes coding for IL-9, IL-12α, IL-15, IL-5Rα, and IL-13Rα1 were found to be downregulated or switched off. Changes in gene expression were at a maximum after 3.5 h of stimulation. There were no messages for classic CD4+-T-cell-derived Th1/Th2 cytokines like IFN-γ or IL-2 and monocyte-derived cytokines like TNF-α or IL-6 excluding a contamination of PMN with these cell populations (8).
TABLE 3.
PMN gene expression profile before and after stimulation with BCGa
| Gene | Level of expression
|
Mode of regulation | ||
|---|---|---|---|---|
| Unstimulated | 1.5 h | 3.5 h | ||
| TNFR2 | ++++ | ++++ | ++++ | |
| MIF | ++++ | ++++ | ++++ | |
| β-Actin | ++++ | ++++ | ++++ | |
| MPIF-1 | ++++ | ++++ | +++ | |
| Ribosomal protein L13A | +++ | +++ | +++ | |
| IL-1R2 | +++ | +++ | +++ | |
| TARC | +++ | −/+++ | +++ | |
| SCYB13 | +++ | +++ | +++ | |
| MDC | +++ | +/+++ | +++ | |
| GAPDH | +++ | −/+++ | −/+++ | |
| TECK | +++ | −/+++ | +++ | |
| TGF-β3 | +++ | +/+++ | +++ | |
| SCYA19 | +++ | −/+++ | −/+++ | |
| Peptidylpropyl isomerase | +++ | +++ | ++ | |
| IL-21 | +++ | −/+++ | +++ | |
| IL-16 | +++ | ++ | +++ | |
| IL-17R | ++ | ++ | + | |
| CXCR4 | ++/++++ | ++/++++ | ++/++++ | |
| IL-1β | ++ | ++/++++ | +++ | Up |
| IL-2Rγ | ++ | +++ | +++ | Up |
| IL-11 | ++ | −/++ | ++ | |
| IL-1R1 | ++ | −/++ | +/+++ | |
| IL-10Rα | ++ | ++ | +++ | Up |
| IL-15 | ++ | −/++ | −/++ | Down |
| Eotaxin | ++ | −/++ | −/++ | |
| MIP-1β | ++ | +/++++ | +/++++ | Up |
| IL-10Rβ | ++ | −/++ | + | |
| IL-13Rα1 | +/+++ | +/+++ | −/++ | Down |
| IL-6R | +/+++ | ++ | +++ | Up |
| IL-25 | + | −/++ | + | |
| IL-5Rα | + | −/++ | − | |
| IL-9 | + | − | −/++ | Down |
| GCP-2 | −/++ | −/++ | −/++ | |
| CCXCR1 | −/++ | − | − | |
| IL-12α | −/++ | − | − | Off |
| IL-11Rα | −/++ | − | − | |
| IL-17 | −/++ | − | − | |
| SDF-2 | −/+++ | −/++ | − | |
| TGF-β1 | −/+++ | −/++ | −/++ | |
| MIP-1α | −/++ | −/+++ | −/++++ | De novo |
| I-309/SCYA1 | −/+++ | −/+++ | −/+++ | |
| MIP-1δ | −/++ | −/++ | −/++ | |
| CCR1 | −/++ | − | − | |
| CCR3 | −/++ | −/++ | −/++ | |
| IL-10 | − | − | − | |
| IL-12Rβ1 | − | − | − | |
| IL-15Rα | − | − | − | |
| CCR7 | − | − | − | |
| HCC-1/SCYA14 | − | − | − | |
| IL-18R1 | − | − | − | |
| CCR5 | − | − | − | |
| IL-2Rβ | − | −/++ | − | |
| MIP-3α | − | − | −/++ | |
| CCR2 | − | − | − | |
| IL-13 | − | − | − | |
| LTA | − | − | − | |
| RANTES | − | − | −/++ | |
| IL-1α | − | + | +/+++ | De novo |
| SCYC2 | − | −/++ | −/++ | |
| CCR4 | − | − | − | |
| IL-18 | − | − | − | |
| MCP-4 | − | − | − | |
| IL-13Rα2 | − | − | − | |
| IL-2Rα | − | − | − | |
| IL-12Rβ2 | − | − | − | |
| IL-6 | − | − | − | /PICK> |
| CCR6 | − | − | − | |
| blank | − | − | − | |
| Burkitt lymphoma R1 | − | − | − | |
| CCR8 | − | − | − | |
| CCR9 | − | − | − | |
| CX3CR1 | − | − | − | |
| IFN-γ | − | − | − | |
| IL-12β | − | − | − | |
| IL-2 | − | − | − | |
| IL-20 | − | − | − | |
| IL-4 | − | − | − | |
| IL-5 | − | − | − | |
| IL-6 signal transd./gp130 | − | − | − | |
| IL-9R | − | − | − | |
| Leptin | − | − | − | |
| Lymphotoxin β/LTB | − | − | − | |
| LTBR | − | − | − | |
| puc18 | − | − | − | |
| HCC-4/ SCYA16 | − | − | − | |
| PARC | − | − | −/+++ | |
| MCP-1 | − | − | − | |
| MIP-2 | − | − | − | |
| MPIF-2 | − | − | − | |
| MCP-3 | − | − | − | |
| MCP-2 | − | − | − | |
| P10/IP10 | − | − | − | |
| I-TAC/IP9 | − | − | − | |
| ENA-78/ SCYB5 | − | − | − | |
| Lymphotactin | − | − | − | |
| Fractalkine | − | − | − | |
| SCYE1 | − | − | − | |
| SDF-1 | − | − | − | |
| TGF-α | − | − | − | |
| TGF-β2 | − | − | − | |
| TNFα | − | − | − | |
| TNFR1 | − | − | − | |
PMN were stimulated with 106 CFU of viable BCG/ml for 1.5 or 3.5 h. Total RNA was prepared, and messages were specifically identified with radiolabeled cDNA probes as described in Materials and Methods. Resulting cDNAs were hybridized on SuperArray nylon membrane microarrays and analyzed with a PhosphorImager. The table shows the combined result of three independent experiments. Using Phoretix Analyzing software (Version 3.01), total values were converted and grouped into five different expression levels: −, no expression; +, low expression; ++, moderate expression; +++, high expression; ++++, very high expression. Where more than one result is given, results separated by the slash show that expression was variable between experiments. Equal expression levels of + or higher for unstimulated as well as stimulated PMN denote constitutive gene expression. A >2-fold change in total expression level (either up or down) was considered relevant and used as a cutoff.
TABLE 4.
BCG-induced changes in PMN gene expressiona
| Mode of regulation | Regulated gene(s) (kinetics of regulation) |
|---|---|
| Up | IL-10Rα (4-fold after 3.5 h), IL-1β (5-fold after 1.5 h and 13-fold after 3.5 h), IL-2Rγ (2-fold after 3.5 h), IL-6R (3-fold after 1.5 h and 6.4-fold after 3.5 h), MIP-1β (12-fold after 3.5 h) |
| De novo | IL-1α (after 1.5 h and 3.5 h), MIP-1α (after 3.5 h) |
| Down | IL-13Rα1 (0.2-fold after 1.5 h), IL-15 (0.4-fold after 1.5 h), IL-9 (0.2-fold after 1.5 h) |
| Off | IL-12α (after 1.5 h), IL-5R (after 1.5 h) |
PMN were stimulated with 106 CFU of viable BCG/ml for 1.5 or 3.5 h. Total RNA was prepared, and messages were specifically identified with radiolabelled cDNA probes as described in Materials and Methods. Resulting cDNAs were hybridized on SuperArray nylon membrane microarrays and analyzed with a PhosphorImager. The table shows the combined results of three independent experiments. Using Phoretix Analyzing software (Version 3.01), total values were converted and grouped into four different modes of regulation (up, de novo synthesis, down, or switched off). A >2-fold change in total expression level (either up or down) was considered relevant and used as a cutoff.
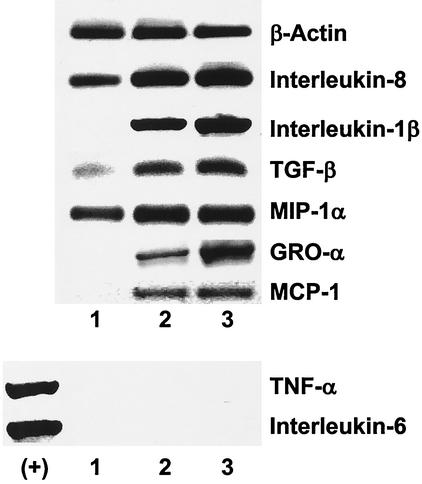
To validate these data, we performed parallel experiments and assessed PMN mRNA expression with RT-PCRs. PMN were stimulated with various concentrations of BCG for 3.5 h. Total RNA was prepared and analyzed as described in Materials and Methods. Using 4 different multiplex RT-PCR kits we were able to detect constitutive mRNA expressions in unstimulated PMN for IL-8, MIP-1α and also TGF-β, albeit on a low level (Fig. 5). Stimulation of PMN with BCG increased the level of expression for all three cytokines, with higher expression for an MOI of 1 than for an MOI of 0.1. Furthermore, BCG stimulation induced de novo expression of IL-1β, GRO-α and MCP-1. Again, we did not detect any mRNA for IL-6 or TNF-α, which strongly agues against monocyte or LPS contamination as the source of mRNA expression (8). Initial experiments demonstrated a maximum expression for most mRNAs after 3.5 h of stimulation. Analysis after 0.5, 6, or 18 h yielded lower expression levels compared to analysis at 3.5 h (data not shown).
FIG. 5.
Induction of cytokine/chemokine mRNA expression in BCG-stimulated PMN. PMN were coincubated for 3.5 h either in medium alone (lane 1) or with BCG (105 CFU/ml) (MOI = 0.1; lane 2) or BCG (106 CFU/ml) (MOI = 1; lane 3). Total RNA was prepared and reverse transcribed into cDNA as described in Materials and Methods. Primer pairs from four different commercially available multiplex RT-PCR kits and for GRO-α were used to amplify messages. The figure shows the representative result of one of three independent experiments. (+), positive controls for TNF-α and IL-6 RT-PCRs.
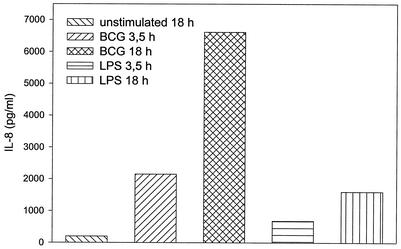
IL-8 is one of the most abundantly secreted cytokines produced by PMN (7). As IL-8 cDNA was not present on the commercially available microarrays used for this study, we performed IL-8 ELISAs of PMN supernatants to demonstrate that BCG stimulation actually results in IL-8 protein synthesis. PMN were stimulated with viable BCG at 106 CFU/ml or LPS at 0.1 μg/ml for 3.5 and 18 h, respectively. Supernatants were harvested and tested for their IL-8 content. Both stimuli induced a significant (P < 0.05) increase in IL-8 production in a time dependent fashion, corresponding to the increase in IL-8 mRNA expression (Fig. 6).
FIG. 6.
IL-8 synthesis by PMN stimulated with viable BCG or LPS. PMN were cultured for 3.5 or 18 h in the presence of BCG (106 CFU/ml) or LPS (0.1 μg/ml) as indicated in the figure legend. Supernatants were harvested, and IL-8 content was measured by ELISA. The figure bars show the combined results (mean values of duplicate samples) of three independent experiments (SDs < 20%).
DISCUSSION
In this study, we were able to demonstrate that mycobacterial stimulation of PMN induces various major changes in the basic biology of this subset of leukocytes. Stimulation of PMN with M. bovis BCG resulted in an increased expression of function-associated surface molecules. Furthermore, BCG stimulation resulted in an inhibition of spontaneous PMN apoptosis. We also provide the first large-scale gene expression analysis following mycobacterial activation of human PMN by using cDNA microarray analyses and multiplex RT-PCRs. PMN mRNA expression of several proinflammatory cytokines and chemokines as well as cytokine and chemokine receptors was altered in response to BCG. Furthermore, pretreatment of PMN with BCG induced the synthesis of the CXC chemokine IL-8. Our data emphasize that PMN have the potential to contribute to the early host response against mycobacterial infections.
Mycobacterial infections such as tuberculosis are a serious global health threat. Approximately one-third to one-half of the world population are estimated to be infected with M. tuberculosis, and more than 2 million people die of this disease annually (21). The various subspecies of mycobacteria such as M tuberculosis, M. bovis or M. africanum share a close phylogenetic and pathogenic relationship and have therefore been termed M. tuberculosis complex (18). In immunocompetent hosts, infections with these pathogens are characterized by an early influx of professional phagocytes such as PMN and mononuclear phagocytes (MP). In the course of the inflammation typical granulomas consisting of matured MP (so-called epitheloid cells and giant cells), CD4+ T cells and a peripheral mantle of CD8+ T cells develop. MP are capable of controlling mycobacterial growth under the influence of T-cell-derived IFN-γ, TNF-α, and calcitriol (20, 21).
Even though PMN are the first subset of immune cells to arrive at the site of mycobacterial infections their role in the host defense against these microorganisms has often been neglected (23). In response to bacterial invasion PMN home to blood vessels of the inflamed tissues and leave the bloodstream. Homing involves the interaction of the β2-integrin MAC-1 (consisting of the α-chain CD11b and the β-chain CD18) on PMN with E-selectin and Sialyl-LewisX, which is upregulated on endothelial cells in response to IL-1, TNF-α and endotoxins (12). Our results demonstrate that mycobacterial stimulation with viable BCG induces an upregulation of CD11b and CD18 on PMN which might activate granulocytes and support trafficking to the site of infection. Similar priming and activation patterns have been shown for endotoxins from gram-positive and gram-negative bacteria such as phenol-soluble modulin, formyl-methionyl-leucyl-phenylalanine (fMLP) and LPS as well as for M. tuberculosis-derived 19-kDa lipoprotein (25, 27). All of these microbial substances induce an upregulation of MAC-1 on PMN which also facilitates pathogen binding and phagocytosis (17). Phagocytosis of opsonized particles also depends on recognition and binding through FcγR III and II, or CD16 and CD32 (5, 12). In our experiments BCG also enhanced the expression of CD16 and CD32 on granulocytes. Interestingly, supernatants of BCG-stimulated PMN also induced an upregulation of CD11b and CD18 as well as CD16 and CD32 on allogeneic granulocytes. Thus, PMN activation does not directly depend on BCG phagocytosis but might be the result of an autocrine/paracrine stimulation through soluble factors released by stimulated granulocytes.
Besides phenotypic changes, activation of PMN often involves variations in their constitutively short life span. Several proinflammatory cytokines including GM-CSF, G-CSF (10, 11), IL-1β (9), or IL-8 (22) have been shown to delay spontaneous apoptosis of mature neutrophils in vitro. Several microbial pathogens have also been shown to influence PMN survival which has since been discussed as a way to escape host defense mechanisms. While E. coli or C. albicans reduce the life span of PMN (30, 35), bacterial endotoxins such as LPS and fMLP (24) or, most noteworthy, the intracellular parasite L. major (1) inhibit spontaneous apoptosis of these host cells. Kasahara et al. studied the effect of M. tuberculosis on PMN survival in vitro. They found a slight induction of PMN apoptosis after stimulation with M. tuberculosis in combination with TNF-α but, however, no effect for M. tuberculosis alone (19). Our results clearly demonstrate an inhibition of PMN apoptosis following mycobacterial activation. Furthermore, we also observed prolonged PMN survival after stimulation with supernatants of BCG-activated granulocytes.
Immune cells need special guidance to find and reach sites of bacterial invasion. They move along a concentration gradient of certain chemotactic factors termed chemokines (3, 26). Together with different cytokines these leukocyte attractants are released in inflammatory foci and play a major role in directing the host response against the invading pathogens. Recently, the potential of PMN to synthesize a considerable amount of cytokines and chemokines in response to various stimuli has been described (7). Granulocytes have been reported to be the first subset of immune cells to arrive at sites of mycobacterial infection. Thus, it was tempting to speculate that they might act as a source of cytokines and/or chemokines that attract other cells of the immune system. The production of the chemokines IL-8, MIP-1α and GRO-α by PMN in response to stimulation with M. tuberculosis has been demonstrated by Riedel and Kaufman (29) and Kasahara et al. (19). Fessler et al. were able to show a broad activation of several cytokine and chemokine genes following LPS stimulation (15). We screened for changes in PMN gene expression to identify additional potential proinflammatory mediators that might be secreted after activation with mycobacteria. We were able to detect an upregulation of messages for the proinflammatory cytokines IL-1α, IL-1β and TGF-β, all of which are important multifunctional molecules during the early/acute phase of an inflammation (7). Furthermore, we found an increase in mRNA expression for chemokines of the CXC as well as CC subfamily. The chemokines we identified such as IL-8 or GRO-α (CXC) primarily act on PMN, whereas MIP-1α, MIP-1β and MCP-1 (CC) primarily act on monocytes (3, 4, 26). While we were able to detect strong signals for TGF-β and low levels of MCP-1 in RT-PCRs, expression of TGF-β was variable in cDNA microarrays and MCP-1 could not be detected at all. These differences can be explained with the lower sensitivity of the array system compared to RT-PCRs. PMN also show variations in mRNA expression for different cytokine or chemokine receptors in response to stimulation with BCG. The expression of IL-2Rγ, IL-10Rα and IL-6R was found to be increased after BCG pretreatment. In our experiments, PMN protein expression of IL-8 corresponded well with changes in mRNA expression observed in RT-PCRs. However, the correlation between variations in PMN mRNA expression and protein synthesis following BCG stimulation for other cytokines or cytokine receptors remains to be defined.
Our data draw a scenario in which PMN play an important inductive role in the early host response against mycobacterial infections. The changes in the expression of FcγR III and II as well as MAC-1 might facilitate pathogen binding, phagocytosis and granulocyte trafficking. Interestingly, these phenotypic changes do not only depend on direct mycobacterial stimulation. Once activated, PMN stimulate other granulocytes in an autocrine/paracrine fashion. At sites of mycobacterial invasion this might be part of a positive feedback loop that does not only affect PMN phenotype but also survival. The inhibition of apoptosis might enable granulocytes to cope with the invading pathogens and enhance as well as prolong the inflammatory response. Our study also identifies possible mediators of this autocrine-paracrine feedback loop. Proinflammatory cytokines or CXC chemokines such as IL-1β, IL-8, and GRO-α have all been described to inhibit PMN apoptosis (9, 13, 14, 22). These activated granulocytes cannot solely kill the pathogens but might temporarily control bacterial growth through the release of reactive oxygen intermediates until the initiation of a specific immune response. Additionally, PMN might contribute to the induction of this immune response against mycobacteria by attracting other immune cell subsets. The detection of enhanced levels of mRNAs for chemokines of the CC subfamily like MIP-1α, MIP-1β, and MCP-1 indicates that activated PMN might attract monocytes to the inflamed tissue. This finding is in accordance with the fact that neutrophils are found at sites of mycobacterial infections even before monocytes/macrophages (23). While the activity of the majority of genes analyzed in this study remained unaffected after mycobacterial stimulation, BCG induced changes only in a distinct subset of genes with proinflammatory and chemotactic potential. In summary, our study provides strong evidence to support the hypothesis that PMN are an indispensable link in the chain of events leading to the control of mycobacterial infections.
Acknowledgments
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft to A. Böhle (SFB 367/C7).
We are indebted to G. Bentien for expert technical assistance. We also thank K. Marienfeld and I. Fricke for their help with the FACS analyzes and various imaging techniques, J. Galle and E. Vollmer for performing the EM studies and Ziehl-Neelsen staining, and E. Brandt for his critical review of the manuscript.
Editor: F. C. Fang
REFERENCES
- 1.Aga, E., D. M. Katschinski, G. van Zandbergen, H. Laufs, B. Hansen, K. Muller, W. Solbach, and T. Laskay. 2002. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169:898-905. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. G. Castro, S. Gomes, J. Pedrosa, and M. T. Silva. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature 392:565-568. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 5.Bredius, R. G., C. A. Fijen, M. De Haas, E. J. Kuijper, R. S. Weening, J. G. Van de Winkel, and T. A. Out. 1994. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology 83:624-630. [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, A. E., T. J. Holzer, and B. R. Andersen. 1987. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 156:985-989. [DOI] [PubMed] [Google Scholar]
- 7.Cassatella, M. A. 1999. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73:369-509. [DOI] [PubMed] [Google Scholar]
- 8.Cassatella, M. A., S. Gasperini, and M. P. Russo. 1997. Cytokine expression and release by neutrophils. Ann. N. Y. Acad. Sci. 832:233-242. [DOI] [PubMed] [Google Scholar]
- 9.Colotta, F., F. Re, N. Polentarutti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 10.Cox, G., J. Gauldie, and M. Jordana. 1992. Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am. J. Respir. Cell. Mol. Biol. 7:507-513. [DOI] [PubMed] [Google Scholar]
- 11.Coxon, A., T. Tang, and T. N. Mayadas. 1999. Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo. A role for granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 190:923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delves, P. J., and I. M. Roitt. 2000. The immune system. First of two parts. N. Engl. J. Med. 343:37-49. [DOI] [PubMed] [Google Scholar]
- 13.Dunican, A., P. Grutkoski, S. Leuenroth, A. Ayala, and H. H. Simms. 2000. Neutrophils regulate their own apoptosis via preservation of CXC receptors. J. Surg. Res. 90:32-38. [DOI] [PubMed] [Google Scholar]
- 14.Dunican, A. L., S. J. Leuenroth, A. Ayala, and H. H. Simms. 2000. CXC chemokine suppression of polymorphonuclear leukocytes apoptosis and preservation of function is oxidative stress independent. Shock 13:244-250. [DOI] [PubMed] [Google Scholar]
- 15.Fessler, M. B., K. C. Malcolm, M. W. Duncan, and G. S. Worthen. 2002. A genomic and proteomic analysis of activation of the human neutrophil by lipopolysaccharide and its mediation by p38 mitogen-activated protein kinase. J. Biol. Chem. 277:31291-31302. [DOI] [PubMed] [Google Scholar]
- 16.Fulton, S. A., S. M. Reba, T. D. Martin, and W. H. Boom. 2002. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect. Immun. 70:5322-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gbarah, A., C. G. Gahmberg, I. Ofek, U. Jacobi, and N. Sharon. 1991. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect. Immun. 59:4524-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin, J. F., C. G. Mackintosh, and G. S. Buchan. 1995. Animal models of protective immunity in tuberculosis to evaluate candidate vaccines. Trends Microbiol. 3:418-424. [DOI] [PubMed] [Google Scholar]
- 19.Kasahara, K., I. Sato, K. Ogura, H. Takeuchi, K. Kobayashi, and M. Adachi. 1998. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J. Infect. Dis. 178:127-137. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 22.Kettritz, R., M. L. Gaido, H. Haller, F. C. Luft, C. J. Jennette, and R. J. Falk. 1998. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha-mediated apoptosis of human neutrophils. Kidney Int. 53:84-91. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, K., C. Allred, R. Castriotta, and T. Yoshida. 1985. Strain variation of bacillus Calmette-Guerin-induced pulmonary granuloma formation is correlated with anergy and the local production of migration inhibition factor and interleukin 1. Am. J. Pathol. 119:223-235. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, A., M. K. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 25.Liles, W. C., A. R. Thomsen, D. S. O'Mahony, and S. J. Klebanoff. 2001. Stimulation of human neutrophils and monocytes by staphylococcal phenol-soluble modulin. J. Leukoc. Biol. 70:96-102. [PubMed] [Google Scholar]
- 26.Luster, A. D. 1998. Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 27.Neufert, C., R. K. Pai, E. H. Noss, M. Berger, W. H. Boom, and C. V. Harding. 2001. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J. Immunol. 167:1542-1549. [DOI] [PubMed] [Google Scholar]
- 28.Payne, C. M., L. Glasser, M. E. Tischler, D. Wyckoff, D. Cromey, R. Fiederlein, and O. Bohnert. 1994. Programmed cell death of the normal human neutrophil: an in vitro model of senescence. Microsc. Res. Tech. 28:327-344. [DOI] [PubMed] [Google Scholar]
- 29.Riedel, D. D., and S. H. Kaufmann. 1997. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65:4620-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotstein, D., J. Parodo, R. Taneja, and J. C. Marshall. 2000. Phagocytosis of Candida albicans induces apoptosis of human neutrophils. Shock 14:278-283. [DOI] [PubMed] [Google Scholar]
- 31.Savill, J. S., A. H. Wyllie, J. E. Henson, M. J. Walport, P. M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Investig. 83:865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva, M. T., M. N. Silva, and R. Appelberg. 1989. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb. Pathog. 6:369-380. [DOI] [PubMed] [Google Scholar]
- 33.Squier, M. K., A. J. Sehnert, and J. J. Cohen. 1995. Apoptosis in leukocytes. J. Leukoc. Biol. 57:2-10. [DOI] [PubMed] [Google Scholar]
- 34.Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 35.Watson, R. W., H. P. Redmond, J. H. Wang, C. Condron, and D. Bouchier-Hayes. 1996. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J. Immunol. 156:3986-3992. [PubMed] [Google Scholar]