Abstract
Gut-derived lymphocytes transiently migrate through the peripheral circulation before homing back to mucosal sites and can be detected using an ELISPOT-based antibody secreting cell (ASC) assay. Alternatively, transiently circulating lymphocytes may be cultured in vitro, and culture supernatants may be assayed for antigen-specific responses (antibody in lymphocyte supernatant [ALS] assay). The ALS assay has not been validated extensively in natural mucosal infection, nor has the ALS response been compared to the ASC assay and other cholera-specific immunological responses. Accordingly, we examined immune responses in 30 adult patients with acute cholera in Bangladesh, compared with 10 healthy controls, measuring ALS-immunoglobulin A (IgA), ASC-IgA, and serum and fecal IgA responses to two potent Vibrio cholerae immunogens, the nontoxic B subunit of cholera toxin (CtxB) and lipopolysaccharide (LPS) and a weaker V. cholerae immunogen, the mannose-sensitive hemagglutinin (MSHA). We found significant increases of anti-CtxB, anti-LPS, and anti-MSHA IgA in supernatants of lymphocytes cultured 7 days after onset of cholera using the ALS assay. We found that ALS and ASC responses correlated extremely well; both had comparable sensitivities as the vibriocidal responses, and both procedures were more sensitive than fecal IgA measurements. An advantage of the ALS assay for studying mucosal immune responses is the ability to freeze antibodies in supernatants for subsequent evaluation; like the ASC assay, the ALS assay can distinguish recent from remote mucosal infection, a distinction that may be difficult to make in endemic settings using other procedures.
Intestinal infections often prompt local mucosal immune responses, in large part comprised of secretory immunoglobulin A (IgA) responses (14, 21). Direct measurement of intestinal IgA in feces, however, can be problematic because of proteolytic degradation. Measuring IgA responses in samples collected via intestinal lavage or endoscopy may be more accurate, but such methods are often impractical. Surrogate markers of intestinal immune responses are often measured, therefore, as is done with the serum vibriocidal assay (10, 11) or the antibody secreting cell (ASC) assay, which takes advantage of the transient presence in peripheral blood of activated mucosal lymphocytes, peaking at approximately 1 week after intestinal presentation of antigen before rehoming to intestinal mucosal surfaces (6, 18). In the ASC assay, these lymphocytes are harvested, and specific IgA responses are detected as “spots” in an ELISPOT procedure (6, 18). ASC responses correspond well with subsequent mucosal antibody measurements. Based on a previously described technique, a new assay for measuring mucosal immune responses has recently been developed, the antibody in lymphocyte supernatant (ALS) assay (1, 3, 7-9, 22; E. R. Hall, H. Chang, R. McKenzie, F. Engstrom, P. Maples, C. Lee, M. Darsley, A. Turner, P. Bedford, S. Baqar, Z. Roberts, A. L. Bourgeios, and D. A. Sack, oral presentation, Vaccines Enteric Dis. meeting, Finland, 12 September 2001). In this assay, circulating lymphocytes collected 1 week after intestinal infection are cultured in vitro without stimulation, and antibodies produced by these lymphocytes and secreted to the culture supernatant may be assayed for specific antibody responses via enzyme-linked immunosorbent assay (ELISA). The ALS assay has been used in vaccine studies but has not previously been evaluated following natural mucosal infection. In order to do so, we measured ALS-IgA, ASC-IgA, vibriocidal, and serum and stool IgA antibody responses following intestinal infection with Vibrio cholerae O1. We evaluated immune responses to two potent V. cholerae immunogens, the nontoxic B subunit of cholera toxin (CtxB) and lipopolysaccharide (LPS), and a weaker V. cholerae immunogen, the mannose-sensitive hemagglutinin (MSHA), a type IV pilus antigen (18), in individuals with cholera in Bangladesh.
(Preliminary results from this study were presented at the 11th Annual Meeting of the International Centers for Tropical Disease Research, National Institute of Allergy and Infectious Diseases, Bethesda, Md., April 2002.)
Thirty male and female adult patients with acute watery diarrhea caused by V. cholerae O1 presenting to the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B), Centre for Health and Population Research in Dhaka, Bangladesh, were enrolled in this study. Ten matched adults with no history of diarrhea during the previous 3 months were studied as controls. The study was approved by the Institutional Review Boards of ICDDR,B and Massachusetts General Hospital. Patients with confirmed V. cholerae O1 as the sole pathogen were recruited (2, 15, 17, 20). Stools of healthy controls were similarly screened. After rehydration of patients, feces and venous blood samples (30 ml) were collected on the second day of hospitalization (approximately 2 days after onset of diarrhea) as well as 5 and 19 days later during convalescence (approximately 7 and 21 days after onset of symptoms, respectively). Single blood and fecal samples were collected from healthy subjects. Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden) from venous blood (20). Serum was collected for antibody assays, aliquoted, and frozen. Fecal extracts were prepared (18), and aliquots were frozen at −70°C. Purified LPS of V. cholerae O1 (16) was used in assays, and MSHA purified from V. cholerae (13) and recombinant CtxB were used in the assays (both kindly provided by Ann Mari Svennerholm).
Wells of ELISPOT plates (Millititer HA; Millipore Corp., Bedford, Mass.) were coated with LPS, MSHA, and CtxB, and assays were carried out as described earlier (5, 16, 18). Affinity-purified goat anti-human immunoglobulin with IgA specificity, conjugated to horseradish peroxidase, was used at a dilution of 1:250 in 1% fetal bovine serum in phosphate-buffered saline (PBS)-Tween 20 (0.05%) as a secondary antibody (Southern Biotechnology Associates, Birmingham, Ala.). The number of lymphocytes expressing IgA reacting with antigens of interest was determined manually by counting positive spots under low-power microscopy (magnification, ×40). An ASC response greater than the geometric mean plus two standard deviations of the value observed for healthy controls was defined as a positive response (for CtxB: 22/107 PBMCs; LPS: 15/107 PBMCs; MSHA: 1/107 PBMCs, respectively).
Isolated PBMCs were also incubated in 24-well tissue culture plates at various concentrations (107 to 105/ml of RPMI medium with 10% fetal bovine serum, 1% glutamine, 1% sodium pyruvate and 1% penicillin-streptomycin) at 37°C in 5% CO2 for various periods (24 to 96 h). After incubation, plates were spun at 1,200 × g for 10 min and supernatant was collected. A protease inhibitor cocktail containing aprotinin (0.15 μM), leupeptin (10 μM), sodium azide (15 μM), and 4-(amino ethyl) benzene sulfonyl fluoride (0.2 μM) was added to the supernatant (10 μl/ml of supernatant), and sample aliquots were immediately frozen at −70°C until used as described below.
Serum samples (1:50 dilutions in PBS for testing anti-LPS and anti-MSHA responses; 1:200 dilution for testing anti-CtxB responses) collected from patients at acute (day 2) and convalescent (day 21) stages of infection were tested against LPS-coated (250 ng/well), CtxB-coated (50 ng/well), and MSHA-coated (100 ng/well) plates, utilizing previously described ELISA procedures (16, 18). Plates were developed using 10 mg of ortho-phenylene diamine (Sigma) in 10 ml of 0.1 M sodium citrate buffer (pH 4.5) to which 4 μl of 30% hydrogen peroxide was added just before use. Optical densities were measured kinetically at 450 nm. Plates were read for 5 min at 19-s intervals, and maximum slope for an optical density change of 0.2 U was expressed as milli-optical density absorbance units per min (mAB/min) (4, 12).
Total IgA content in fecal samples was determined by ELISA using pooled human Swedish milk with a known IgA concentration of 1 mg/ml as the standard and using affinity-purified goat antibodies to the F(ab′)2 fragment of human immunoglobulin as capture antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.), using methods described earlier (18, 21). Affinity-purified goat anti-human immunoglobulin with IgA specificity, conjugated to horseradish peroxidase, was used as a secondary antibody (Jackson). Anti-LPS, anti-CtxB, and anti-MSHA fecal IgA responses were detected with affinity-purified rabbit anti-human immunoglobulin with IgA specificity conjugated to horseradish peroxidase as a secondary antibody (Jackson). Responses were measured kinetically and expressed as mAB per minute per microgram of total IgA. For serum and stool responses, a twofold or greater increase from that seen at the acute stage (day 2) was defined as a positive response.
ALS specimens were assayed at dilutions varying from undiluted to 1:10 in PBS and detected with rabbit anti-human IgA-horseradish peroxidase conjugate. Responses were measured kinetically as previously described (4, 12).
Serum samples collected at early (day 2) and convalescent (day 7 and 21) phases of cholera infection were tested for vibriocidal antibody response against the homologous serotype of bacteria using strain V. cholerae O1 El Tor Ogawa (strain 25049) or Inaba (strain 19479) as the target organism in procedures previously optimized (19). A fourfold or greater increase in titer between early and convalescent-phase samples was used to signify seroconversion. A reference pooled serum specimen from V. cholerae O1-infected patients was included in each test to control for variation between analyses (19).
The Wilcoxon signed rank test and the Mann-Whitney U test were used where applicable for statistical analysis. Nonparametric data are expressed as median and 25, 75 centiles; normally distributed data are expressed as geometric means ± standard errors of the mean. The relationship between immunological parameters was assessed using linear regression analysis. A two-sided P value of ≤0.05 was considered a significant difference. The F test was used to assess the contribution of an independent variable (ALS response) in predicting a dependent variable (ASC response). Analyses were carried out using SigmaStat (Jandel Scientific, San Rafael, Calif.). The sensitivity of the ALS, ASC, and antibody determinations were calculated with a four-field table analysis, using the vibriocidal response as a reference (11).
The clinical and epidemiological features of the cholera and control patients are listed in Table 1. No intestinal pathogens were identified in the stool of cholera patients except for V. cholerae O1. No intestinal pathogens were isolated from the stool of healthy controls.
TABLE 1.
Clinical features of study patients
| Parameter | Value for patients (n = 30) | Value for healthy controls (n = 10) |
|---|---|---|
| Age in yr, median (25,75 centile) | 28 (23-35) | 29 (27-31) |
| Gender, no. (%) | ||
| Male | 17 (57) | 6 (60) |
| Female | 13 (43) | 4 (40) |
| No. (%) with infecting serotype of V. cholerae O1 | ||
| Ogawa | 10 (33) | NAa |
| Inaba | 20 (67) | NA |
| No. (%) in blood group: | ||
| A | 7 (23) | 3 (30) |
| B | 6 (20) | 1 (10) |
| AB | 5 (17) | |
| O | 12 (40) | 6 (60) |
| Duration of illness, at presentation, h [median (25,75 centile)] | 24 (12,24) | NA |
| % of patients with level of dehydration at presentation | ||
| Severe | 93 | NA |
| Mild | 7 | NA |
NA, not applicable.
Compared with day 2 responses, 97% of cholera patients increased their titer of vibriocidal antibody in serum by fourfold or greater by day 7 (P < 0.001) and 100% of cholera patients increased their titer of vibriocidal antibody in serum by fourfold or greater by day 21 (P < 0.001) (Table 2). As expected, cholera patients also developed serum IgA antibodies to all three cholera-specific antigens (Table 2), with the highest-magnitude response being directed against CtxB and the lowest-magnitude response against MSHA. Serum antibodies to CtxB, LPS, and MSHA were also present at low levels in healthy controls.
TABLE 2.
Vibriocidal (sera) and specific IgA antibody responses (sera and feces) of patients with cholera
| Antibody response or parameter | Value for response ina:
|
|||||
|---|---|---|---|---|---|---|
| Serum
|
Feces
|
|||||
| Day 2 | Day 7 | Day 21 | Day 2 | Day 7 | Day 21 | |
| Vibriocidal | ||||||
| Median | 40 | 5,117 | 2,559 | NA | NA | NA |
| 25, 75 centile | 20, 160 | 2,559, 20,464 | 1,811, 14,489 | NA | NA | NA |
| P value compared to day 2 | NA | <0.001 | <0.001 | NA | NA | NA |
| Response rate, compared to day 2 (%) | NA | 97 | 100 | NA | NA | NA |
| Anti-CtxB-IgA | ||||||
| Median | 13 | 56 | 36 | 14.8 | 17.5 | 11.5 |
| 25, 75 centile | 6, 31 | 37, 94 | 19, 58 | 9, 18.5 | 9, 33 | 4.8, 29.7 |
| P value compared to day 2 | NA | <0.001 | <0.001 | NA | 0.37 | 0.69 |
| P value compared to healthy controls | 0.03 | <0.001 | <0.001 | NA | NA | NA |
| Response rate, compared to day 2 (%) | NA | 81 | 59 | NA | 48 | 35 |
| Response rate, compared to healthy controls (%) | 28 | 78 | 67 | NA | NA | NA |
| LPS-IgA | ||||||
| Median | 3 | 45 | 29 | 2 | 6.4 | 5 |
| 25, 75 centile | 1.3, 6 | 19.9, 92 | 8.3, 59.8 | 1, 4.5 | 3, 43.3 | 2, 12.9 |
| P value compared to day 2 | NA | <0.001 | <0.001 | NA | 0.002 | 0.003 |
| P value compared to healthy controls | 0.44 | <0.001 | <0.001 | NA | NA | NA |
| Response rate, compared to day 2 (%) | NA | 84 | 72 | NA | 61 | 52 |
| Response rate, compared to healthy controls (%) | 9 | 78 | 65 | NA | NA | NA |
| MSHA-IgA | ||||||
| Median | 2.5 | 6.5 | 2.5 | 1.9 | 6 | 3 |
| 25, 75 centile | 1.5, 5.3 | 3, 15 | 1.5, 5.3 | 1.0, 3.9 | 3, 10 | 1.5,5.1 |
| P value compared to day 2 | NA | <0.001 | 0.092 | NA | <0.001 | 0.038 |
| P value compared to healthy controls | 0.14 | <0.001 | 0.14 | NA | NA | NA |
| Response rate, compared to day 2 (%) | NA | 58 | 24 | NA | 58 | 48 |
| Response rate, compared to healthy controls (%) | 25 | 65 | 24 | NA | NA | NA |
For the vibriocidal response, a fourfold or greater increase in titer from day 2 samples was considered a significant increase. Serum anti-CtxB, anti-LPS, and anti-MSHA IgA responses are expressed as milli- absorbance units per min (mAB/min). Stool anti-CtxB, anti-LPS, and anti-MSHA IgA responses are expressed as mAB/min/μg of total IgA. For anti-CtxB, anti-LPS, and anti-MSHA IgA responses, a twofold or greater increase from day 2 samples was considered a significant increase. When comparing anti-CtxB, anti-LPS, and anti-MSHA IgA responses for cholera patients to those for healthy controls, a value ≥ the geometric mean + 2 standard deviations of the value in healthy controls was considered a significant increase (CtxB, 28.1; LPS, 13; MSHA, 5.3). NA, not applicable.
Cholera patients responded with IgA antibodies in stool specific for CtxB (responder frequency, 68%), LPS (responder frequency, 77%), and MSHA (responder frequency, 69%) by day 7 or day 21 post-onset of infection (Table 3).
TABLE 3.
Sensitivity of immunological assays, compared with the vibriocidal responsesa
| Response | Responder frequency (%)
|
|||
|---|---|---|---|---|
| Immunological assays
|
Fecal antibodies | Serum antibodies | ||
| ALS | ASC | |||
| Anti-CtxB IgA | 94 | 81 | 68 | 81 |
| Anti-LPS IgA | 88 | 94 | 77 | 88 |
| Anti-MSHA IgA | 69 | 77 | 69 | 69 |
Compared to day 2 values, all 30 patients developed a fourfold change in titers of vibriocidal antibody on day 7 or day 21. Fecal and serum antibody response frequencies represent comparisons of day 2 to either day 7 or day 21 values in individual patients.
To optimize the concentration of PBMCs appropriate for use in the ALS technique, various numbers of PBMCs obtained from 20 cholera patients on day 7 were used, ranging from 105 to 107 cells per well. For a strong immunogen, such as CtxB, 106 cells/well was sufficient to detect a significant response, compared to 105 cells (P = 0.003) or compared to healthy controls using 107 PBMC per well (P ≤ 0.001). To detect ALS IgA immune responses against LPS and MSHA (the latter a weak immunogen), at least 5 × 106 PBMCs were needed to generate a significantly elevated response compared to healthy controls (P ≤ 0.01).
PBMCs were also cultured for 24 to 96 h before recovering ALS supernatants (data not shown). A 48-h incubation period was optimal when either 1 × 107 or 5 × 106 PBMCs were used for detection of immune responses to any of the three evaluated antigens. When lower numbers of cells were used (106 or 105), a longer incubation time did not improve the response to the weaker immunogens, LPS and MSHA. Under no conditions was 105 PBMCs sufficient to demonstrate a significant immune response to LPS or MSHA. The optimal approach was to recover supernatants from 5 × 106 or 1 × 107 PBMCs after 48 h of culture and then use undiluted supernatants for ALS assays detecting immune responses to weaker immunogens and diluted supernatants for ALS assays detecting immune responses to strong immunogens.
We also measured ALS and ASC responses 21 days after onset of diarrhea in five cholera patients (data not shown). Compared to day 7 responses, ALS and ASC responses were either absent or markedly reduced, suggesting that ALS and ASC responses are optimally measured approximately 1 week after intestinal presentation of antigen (3, 6, 16, 18).
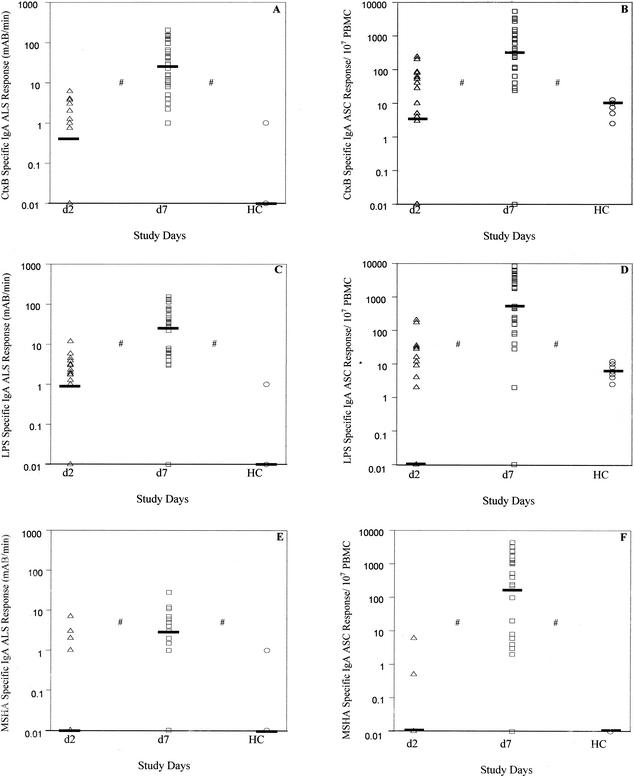
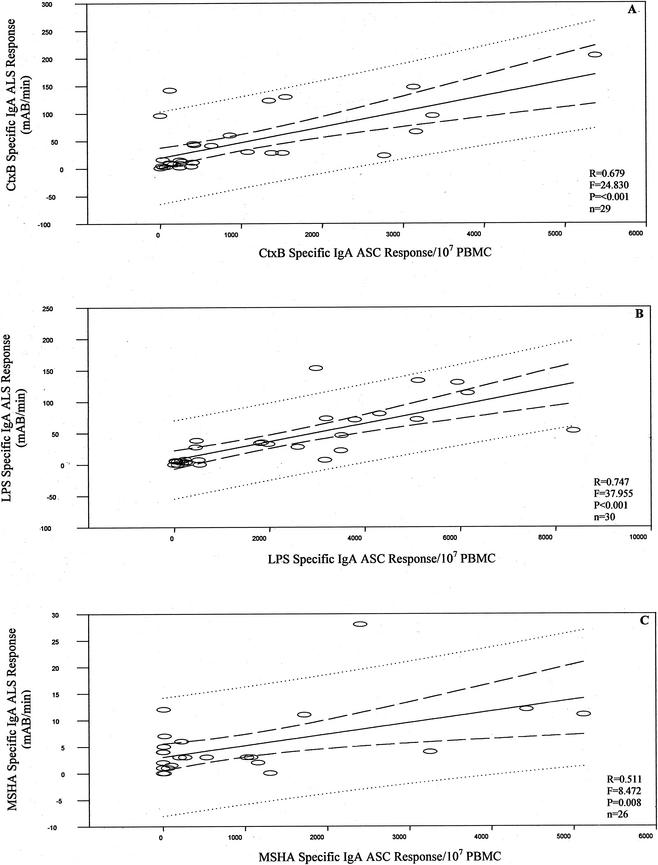
PBMCs from cholera patients were analyzed for both ASC and ALS responses, using blood samples collected on day 2 and day 7 after onset of illness. ASC-IgA and ALS-IgA responses to CtxB, LPS, and MSHA were determined (Fig. 1). For cholera patients, ALS IgA responses to all antigens were low at the acute stage of infection (day 2) but increased significantly by day 7 (P ≤ 0.001). ALS responses for cholera patients were also significantly elevated over those for healthy controls (P ≤ 0.001). ASC responses to all antigens were also low level on day 2, and they increased significantly by day 7 for cholera patients (Fig. 1). At the acute stage, 90% of patients had low ASC and low ALS responses. When comparing antigen-specific ALS and ASC responses 7 days after infection, we found a significant linear relationship between ALS and ASC responses to each of the antigens tested, with high correlation of ASC and ALS responses in individual patients (Fig. 2) (CtxB, P ≤ 0.001; LPS, P ≤ 0.001; MSHA, P = 0.008). Six patients with low ASC responses at day 7 (Ct × B-specific responses of <50 ASC/107 PBMC) also had low ALS titers (<5 mAB/min per 107 PBMC).
FIG. 1.
Comparison of ALS and ASC responses to CtxB (A and B), LPS (C and D), and MSHA (E and F). For ALS assays, cells were diluted to 107 PBMCs per ml and incubated for 48 h. Samples were collected on day 2 (d2) and day 7 (d7). Responses in 10 healthy controls (HC) are also represented. Bars represent median values. #, P ≤ 0.001, comparing d2 to d7 values and d7 to HC values. R, regression coefficient; F, measurement of the contribution of the independent variable in predicting the dependent variable.
FIG. 2.
Relationship of ALS and ASC responses in individual patients to CtxB (A), LPS (B), and MSHA (C). The number of patients compared for each assay is indicated (n).
In order to further evaluate the usefulness of ALS as an accurate marker of recent mucosal immune responses, we compared ALS responses to other immunological assays (Table 3). Relative to the vibriocidal response (100% response rate), ALS and ASC responses to CtxB, LPS, and MSHA were comparable, and both were more sensitive than fecal or serum IgA responses to these same antigens.
Acute watery diarrhea caused by V. cholerae induces mucosal and systemic antibody responses to cholera toxin and other cell surface components (11, 16, 18). Although the serum vibriocidal response best predicts protective immunity following cholera (10, 11), the reason that a serum, complement-binding antibody assay predicts protection following a noninvasive mucosal infection, such as that caused by V. cholerae, is currently unknown. The vibriocidal response may be a surrogate marker for an as yet undefined mucosal immune response following cholera. Directly measuring mucosal immune responses can, however, be problematic. Our results suggest that measuring specific, in vitro-produced antibodies secreted from intestinal lymphocytes transiently migrating in the peripheral blood may serve such a purpose. The ASC assay detects this migration of mucosal lymphocytes, and our finding that the ALS response similarly peaks on day 7 and is absent by day 21 suggests that the ALS assay also detects these transiently circulating mucosal lymphocytes (3, 6, 16, 18).
The ALS and ASC assays had similar sensitivity compared with the vibriocidal response, with the highest sensitivity associated with immune responses against the potent immunogen CtxB (ALS, 94%; ASC, 81%) and the lowest sensitivity associated with the weak immunogen MSHA (ALS, 69%; ASC, 77%). The ALS and ASC assays were also more sensitive than the fecal antibody assays at detecting recent mucosal infection, probably reflecting protease-driven degradation of antibodies in stool samples. Of note, in our patients, vibriocidal, serum IgA, and stool IgA immune responses against V. cholerae were already significantly increased by day 7. Among our study population, V. cholerae is an endemic infection, and this early increase in immune responses probably reflects an anamnestic response. The ALS responses were either absent or markedly reduced by late convalescence (day 21), while serum IgA responses remained quite high, suggesting that the ALS assay measures antibodies produced by transiently circulating mucosal lymphocytes.
Our study is the first analysis of the ALS assay following a natural intestinal infection. The ALS assay has a number of advantages over the ASC assay, including the ability to easily store supernatants (facilitating future analysis of samples) and the ability to use ALS supernatants in an ELISA format, eliminating subjective variability inherent in the counting of spots in the ASC assay. Although automated counters are now available for the ASC assay, such equipment is not available in most laboratories in the developing world. The ALS procedure may help distinguish recent from remote infection, a distinction that is sometimes difficult to make in individuals in endemic settings.
Acknowledgments
This research was supported by an International Collaborations in Infectious Disease Research Award from the National Institute of Child Health and Human Development, HD39165, grants from the Fogarty International Center, D43 TW05572, and National Institute of Allergy and Infectious Diseases, AI40725, and support from the ICDDR,B. The ICDDR,B is supported by countries and agencies which share its concern for the health problems of developing countries.
We are grateful to Ann-Mari Svennerholm, Goteborg University, Sweden, for supplying MSHA and recombinant CtxB.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Angelakopoulos, H., and E. L. Hohmann. 2000. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 68:2135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benenson, A. S., M. R. Islam, and W. B. Greenough III. 1964. Rapid identification of Vibrio cholerae by darkfield microscopy. Bull. W. H. O. 30:827-831. [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, H. S., and D. A. Sack. 2001. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 8:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crean, T. I., M. John, S. B. Calderwood, and E. T. Ryan. 2000. Optimizing the germfree mouse model for in vivo evaluation of oral Vibrio cholerae vaccine and vector strains. Infect. Immun. 68:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czerkinsky, C., Z. Moldoveanu, J. Mestecky, L. A. Nilsson, and O. Ouchterlony. 1988. A novel two colour ELISPOT assay. I. Simultaneous detection of distinct types of antibody-secreting cells. J. Immunol. Methods 115:31-37. [DOI] [PubMed] [Google Scholar]
- 6.Czerkinsky, C., S. J. Prince, S. M. Michalek, S. Jackson, Z. Moldoveanu, M. W. Russell, J. R. McGhee, and J. Mestecky. 1987. Oral immunization with bacterial antigen induces IgA-secreting cells in peripheral blood in humans. Adv. Exp. Med. Biol. 216B:1709-1719. [PubMed] [Google Scholar]
- 7.DiPetrillo, M. D., T. Tibbetts, H. Kleanthous, K. P. Killeen, and E. L. Hohmann. 1999. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18:449-459. [DOI] [PubMed] [Google Scholar]
- 8.Forrest, B. D. 1988. Identification of an intestinal immune response using peripheral blood lymphocytes. Lancet i:81-83. [DOI] [PubMed] [Google Scholar]
- 9.Forrest, B. D. 1992. Indirect measurement of intestinal immune responses to an orally administered attenuated bacterial vaccine. Infect. Immun. 60:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass, R. I., A. M. Svennerholm, M. R. Khan, S. Huda, M. I. Huq, and J. Holmgren. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236-242. [DOI] [PubMed] [Google Scholar]
- 11.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J. Clin. Microbiol. 24:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John, M., T. I. Crean, S. B. Calderwood, and E. T. Ryan. 2000. In vitro and in vivo analyses of constitutive and in vivo-induced promoters in attenuated vaccine and vector strains of Vibrio cholerae. Infect. Immun. 68:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonson, G., M. Lebens, and J. Holmgren. 1994. Cloning and sequencing of Vibrio cholerae mannose-sensitive haemagglutinin pilin gene: localization of mshA within a cluster of type 4 pilin genes. Mol. Microbiol. 13:109-118. [DOI] [PubMed] [Google Scholar]
- 14.Levine, M. M., J. B. Kaper, R. E. Black, and M. L. Clements. 1983. New knowledge on pathogenesis of bacterial infections as applied to vaccine development. Microbiol. Rev. 47:510-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monsur, K. A. 1961. A highly selective gelatin-taurocholate tellurite medium for the isolation of Vibrio cholerae. Trans. R. Soc. Trop. Med. Hyg. 55:440-442. [DOI] [PubMed] [Google Scholar]
- 16.Qadri, F., F. Ahmed, M. M. Karim, C. Wenneras, Y. A. Begum, S. M. Abdus, M. J. Albert, and J. R. McGhee. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qadri, F., T. Azim, A. Chowdhury, J. Hossain, R. B. Sack, and M. J. Albert. 1994. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin. Diagn. Lab. Immunol. 1:51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadri, F., G. Jonson, Y. A. Begum, C. Wenneras, M. J. Albert, M. A. Salam, and A. M. Svennerholm. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 4:429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svennerholm, A. M., M. Jertborn, L. Gothefors, A. M. Karim, D. A. Sack, and J. Holmgren. 1984. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J. Infect. Dis. 149:884-893. [DOI] [PubMed] [Google Scholar]
- 22.Turner, A. K., T. D. Terry, D. A. Sack, P. Londono-Arcila, and M. J. Darsley. 2001. Construction and characterization of genetically defined aro omp mutants of enterotoxigenic Escherichia coli and preliminary studies of safety and immunogenicity in humans. Infect. Immun. 69:4969-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]