Abstract
CmeABC functions as a multidrug efflux pump contributing to the resistance of Campylobacter to a broad range of antimicrobials. In this study, we examined the role of CmeABC in bile resistance and its contribution to the adaptation of Campylobacter jejuni in the intestinal tract of the chicken, a natural host and a major reservoir for Campylobacter. Inactivation of cmeABC drastically decreased the resistance of Campylobacter to various bile salts. Addition of choleate (2 mM) in culture medium impaired the in vitro growth of the cmeABC mutants but had no effect on the growth of the wild-type strain. Bile concentration varied in the duodenum, jejunum, and cecum of chicken intestine, and the inhibitory effect of the intestinal extracts on the in vitro growth of Campylobacter was well correlated with the total bile concentration in the individual sections of chicken intestine. When inoculated into chickens, the wild-type strain colonized the birds as early as day 2 postinoculation with a density as high as 107 CFU/g of feces. In contrast, the cmeABC mutants failed to colonize any of the inoculated chickens throughout the study. The minimum infective dose for the cmeABC mutant was at least 2.6 × 104-fold higher than that of the wild-type strain. Complementation of the cmeABC mutants with a wild-type cmeABC allele in trans fully restored the in vitro growth in bile-containing media and the in vivo colonization to the levels of the wild-type strain. Immunoblotting analysis indicated that CmeABC is expressed and immunogenic in chickens experimentally infected with C. jejuni. Together, these findings provide compelling evidence that CmeABC, by mediating resistance to bile salts in the intestinal tract, is required for successful colonization of C. jejuni in chickens. Inhibition of CmeABC function may not only control antibiotic resistance but also prevent the in vivo colonization of pathogenic Campylobacter.
Bile is produced in the liver, stored in the gall bladder, and released into the small intestine for digestion of fats. Bile contains a group of detergent-like bile salts which not only play a role in fat digestion and absorption but also display potent bactericidal activity. Bile salts are amphipathic molecules which can kill bacteria by destroying the lipid bilayer of cell membrane (16, 20, 21). Thus, resistance to bile salts is essential for enteric bacteria to survive in the intestinal tract. To diminish the action of bile salts, enteric organisms (including pathogens and normal intestinal microflora) have evolved multiple mechanisms to resist the bactericidal effect of bile salts. Generally, enteric bacteria can resist bile salts by using efflux pumps, modulating synthesis of lipopolysaccharide and porins, or producing bile salt hydrolase (11, 16). Among these mechanisms, extrusion of bile salts from the bacterial cytoplasm directly out of the cell is considered a main mechanism of bile resistance in gram-negative bacteria and is mediated by multidrug resistance (MDR) efflux systems (16). Several MDR efflux systems in gram-negative bacteria have been demonstrated to confer resistance to bile salts in vitro (9, 12, 17, 32, 48). Despite these advances in understanding the role of MDR pumps in bile resistance, direct evidence that MDR efflux pumps are required for bacterial adaptation in animal intestines is still lacking. Thus, examining the role of MDR efflux pumps in bacterial survival and colonization in vivo will greatly improve our understanding of the pathophysiological functions of bacterial MDR efflux pumps.
Campylobacter jejuni is the leading bacterial cause of human enteritis in many industrialized countries (13). The majority of human infections result from consumption of undercooked poultry meat or other food products cross-contaminated with raw poultry meat during food preparation (47). As an enteric pathogen, C. jejuni enters the host intestine via oral ingestion and colonizes the distal ileum and colon. Once inside the intestine, C. jejuni is faced with multiple levels of stresses, such as the action of antimicrobial bile salts and peptides, starvation (e.g., iron limitation), competition with other residential flora, antibiotic treatments, and attack by host immune defenses. Campylobacter must counteract these harsh conditions in order to survive and multiply in an animal host. In the past decades, many efforts have been directed to understanding the virulence factors involved in Campylobacter adhesion, invasion, and cytotoxicity. Some known examples of putative virulence elements include CDT toxins (18, 19, 26), PEB1 (38), CadF (23), Fla (15, 35), JlpA (22), Cia proteins (24, 43), the pVir plasmid (4), and a phase-variable capsule (5), of which the motility-mediating flagellum (Fla) is the best-characterized virulence factor shown to be required for Campylobacter colonization in the gastrointestinal tract of birds and mammals (15, 34, 35, 37, 50). Despite these advances in understanding the pathobiology of Campylobacter pathogenesis, little is known about the mechanisms utilized by Campylobacter to adapt in the intestinal environment in the presence of various antimicrobial agents, such as bile salts. Understanding the adaptation mechanisms may facilitate the development of effective means to prevent and control Campylobacter infection in humans and animal reservoirs.
Recently, a Campylobacter multidrug efflux pump (named CmeABC) contributing to antimicrobial resistance was characterized (28, 31, 42). This efflux pump is chromosomally encoded by a three-gene operon (cmeABC) and shares significant sequence and structural homology with known tripartite multidrug efflux pumps in other gram-negative bacteria. Based on the sequence and structural homology with known bacterial efflux pumps, it was predicted that CmeA, CmeB, and CmeC are a periplasmic protein, an inner membrane drug transporter, and an outer membrane protein, respectively. It is believed that the three members function together and form a membrane channel for the extrusion of antimicrobials and other toxic compounds in Campylobacter (28). An insertional mutation in cmeB of various Campylobacter strains resulted in substantial decreases in Campylobacter resistance to various antimicrobials (28, 31, 42). Accumulation assays demonstrated that CmeABC functions as an energy-dependent efflux pump in C. jejuni. PCR and immunoblotting showed that cmeABC is broadly distributed and constitutively expressed in various Campylobacter isolates grown in Mueller-Hinton (MH) broth. Our previous findings (28) also suggested that CmeABC may be an important player in bile resistance, which prompted us to determine its role in the adaptation of Campylobacter to the intestinal environment of an animal host. Using both in vitro and in vivo systems, we demonstrated in this study that CmeABC, by mediating bile resistance, is essential for Campylobacter growth in bile-containing media and in colonization in animal intestinal tracts. These findings define a key natural function of a multidrug efflux pump in an enteric pathogen and open new avenues for the development of measures to control Campylobacter infection in humans and in animal reservoirs.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The various Campylobacter strains, mutants, and plasmids used in this study and their sources are listed in Table 1. These isolates were routinely grown in MH broth (Difco) or agar at 42°C under microaerophilic conditions, which were generated using a Campypak Plus (Becton Dickinson) gas pack in an enclosed jar. When needed, culture media were supplemented with kanamycin (30 μg/ml) or chloramphenicol (20 μg/ml).
TABLE 1.
Bacterial plasmids and strains used in this study
| Plasmid or strain | Description | Source or reference |
|---|---|---|
| Plasmids | ||
| pGEMT-Easy | PCR cloning vector, Ampr | Promega |
| pCMEC | pGEMT-Easy containing 1.5-kb cmeC fragment, Ampr | This study |
| pCMECK | pCMEC with kanamycin resistance cassette inserted in cmeC gene, Ampr Kanr | This study |
| pUOA18 | E. coli-C. jejuni shuttle vector, Cmr | 49 |
| pCME | pUOA18 derivative containing a wild-type cmeABC operon | This study |
| Strains | ||
| C. jejuni | ||
| 81-176 | Wild type; isolated from human | 7 |
| 21190 | Wild type; isolated from chicken | 25 |
| JL101 | 21190 derivative; cmeB::kan | 28 |
| JL102 | 21190 derivative; cmeC::kan | This study |
| JL103 | JL101/pCME, Kanr Cmr | This study |
| JL104 | JL102/pCME, Kanr Cmr | This study |
| E. coli JM109 | endA1 recA1 gyrA96 thi hsdR17 (rk− mk+) relA1 supE44 Δ(lac-proAB) [F′ traD36 lacIqZΔM15] | Promega |
Insertional mutation of cmeC.
An isogenic cmeC mutant of strain 21190 was constructed by insertional mutagenesis. According to the published complete sequence of the cmeABC operon in strain 81-176 (28), primers CF (5′-GGCTTATGAAATTACAGATGCAGA) and CR (5′-TCTTGGGAAAAAGAAAACAATAGC) were used to amplify a 1.5-kb fragment that spans from the 5′ end to 70 bp downstream of the stop codon of cmeC, which contains a unique BsrBRI restriction site in the middle of the open reading frame. The PCR product was cloned into pGEMT-Easy (Promega), resulting in the construct pCMEC. Primers KF (5′-AAAAGATACATATCGATGAATTGTGTCTCA) and KR (5′-AATTGATACATATCCAGTTGGTGATTTTG), which were designed with an attached BsrBRI restriction site (underlined in primers), were used to amplify the 1.1-kb kanamycin resistance cassette from the EZ::TN <KAN-2> Tnp transposon (Epicentre). The PCR product containing the kanamycin resistance cassette was digested by BsrBRI and ligated to the same enzyme-digested pCMEC to obtain the construct pCMECK. Sequencing of the construct indicated that the kanamycin resistance cassette was inserted in the BsrBRI site of cmeC and in the same orientation as the cmeC gene. The plasmid pCMECK, which served as a suicide vector, was electroporated into C. jejuni 81-176. Transformants were selected on MH agar containing 30 μg of kanamycin/ml. Inactivation of the cmeC gene in the transformants by insertion of the kanamycin resistance cassette was confirmed by PCR and immunoblotting using anti-CmeC antibodies as described previously (28). To create the isogenic cmeC mutant in strain 21190, the insertional mutation in cmeC of 81-176 was transferred into strain 21190 by natural transformation as described previously (28). The cmeC mutation in 21190 was further confirmed by PCR and immunoblotting (see Fig. 1). Sequence data showed that the kanamycin resistance cassette was inserted within the codon encoding amino acid 154 of CmeC in the same direction as the transcription of cmeC. The cmeC mutant of 21190 was named JL102 in this study. Generation of the cmeB mutant of 21190, named JL101 in this paper (Table 1), was described in a previous study (28).
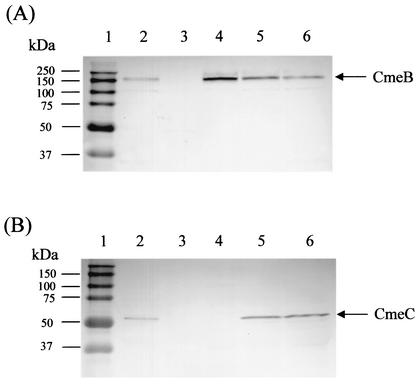
FIG. 1.
Immunoblot analysis of CmeB and CmeC expression in wild-type 21190 and various cmeABC mutant constructs. Cell envelopes prepared from C. jejuni 21190 (lane 2), JL101 (lane 3), JL102 (lane 4), JL103 (lane 5), and JL104 (lane 6) were blotted with specific antibodies against CmeB (A) and CmeC (B). Similar amounts of total proteins were loaded in each lane. Prestained molecular mass markers (lane 1; Bio-Rad) were coelectrophoresed and blotted to allow estimation of the sizes of the proteins.
Complementation of JL101 and JL102.
The 6-kb cmeABC operon was amplified from strain 81-176 by PCR using primers F and R, which span from the promoter region of cmeABC to 70 bp downstream of the stop codon of cmeC (28). PCR was performed with PfuTurbo DNA polymerase (Stratagene), using a 500 nM concentration of each primer. The annealing temperature used was 50°C. The blunt-ended PCR product was purified using a QIAquick PCR purification kit (Qiagen) and ligated to shuttle vector pUOA18 (49), which was digested with SmaI prior to ligation. The ligation mix was introduced into JL101 by electroporation. Transformants were selected on MH agar containing 20 μg of chloramphenicol/ml. One transformant harboring the cmeABC operon was identified, and the construct was designated pCME. Sequence analysis indicated that pCME contained intact cmeB and cmeC. However, the 5′-end 18 bp of cmeA were deleted. This deletion did not affect the expression of cmeB and cmeC from the plasmid, as shown in Fig. 1. Since the cmeB and cmeC mutations did not affect the expression of the chromosome copy of cmeA in JL101 (28) or JL102 (data not shown), pCME provided an ideal vector for the complementation of the mutants in this study. Electroporation of pCME into JL101 and JL102 created JL103 and JL104, respectively (Table 1). Expression of cmeB and cmeC in JL103 and JL104 was confirmed by immunoblotting using anti-CmeB and anti-CmeC antibodies as described previously (28).
Susceptibility tests.
The MICs of different bile salts, fatty acids, and detergents for C. jejuni 21190 and its cmeABC mutant constructs were determined using a microtiter broth dilution method as described in a previous publication (28). The compounds utilized in these assays were purchased from Sigma Chemical Co. (cholic acid, chenodeoxycholic acid, taurocholic acid, deoxycholic acid, dehydrocholic acid, glycocholic acid, taurodeoxyxholic acid, choleate, capric acid, butyric acid, palmitic acid, and Triton X-100), EM Science (sodium dodecyl sulfate [SDS]), Calbiochem (Empigen BB), and Amresco (Tween 20).
Preparation of chicken intestinal extracts.
Ten 21-day-old broiler chickens were used for the preparation of chicken intestinal extracts. All birds tested negative for Campylobacter by culturing cloacal swabs. Chicken intestinal extracts were prepared from three different sections of the intestine, including the duodenum, jejunum, and cecum. The intestinal contents from each section were pooled from the 10 chickens. Each pool of chicken intestinal contents was divided in half, and each half was resuspended in the same volume of MH broth or saline by vortex, followed by centrifugation at 10,000 × g at 4°C for 30 min. The supernatant from the MH or saline extraction was sterilized by successive filtration through a series of membrane filters (prefilter 1.2 μm and 0.45 μm; Millipore Co.). The filtered intestinal extracts were tested for sterility by plating 200 μl of the final filtrate on MH plates and incubating the plates under aerobic and microaerophilic conditions for 3 days, which showed there was no growth of any bacterial colony. The filtered intestinal extracts were used for measuring bile salt concentrations (saline-based extracts) and for in vitro growth inhibition assays (MH-based extracts).
Measurement of total bile salt concentrations.
Total bile concentrations in chicken duodenal, jejunal, and cecal extracts were measured using a total bile acids assay kit according to the procedure supplied by the manufacturer (Diazyme, San Diego, Calif.). The concentration (expressed as millimolar) of bile salts in each intestinal extract was recorded as the mean of four measurements.
In vitro growth assay.
To compare the growth characteristics of C. jejuni 21190 and its cmeABC mutants in the presence of bile salts and chicken intestinal extracts, an in vitro growth assay was performed in microtiter plates. Each well of the plate contained 225 μl of medium plus 25 μl of Campylobacter inoculum (approximately 5 × 106 CFU/ml). Several media were used for the assays, including MH, MH supplemented with sodium choleate (1 mg/ml; ∼2 mM bile salts), and MH-based chicken intestinal extracts. We chose sodium choleate (Sigma) for the in vitro assay because it is a crude ox bile extract that contains the sodium salts of cholic, glycocholic, deoxycholic, and taurocholic acids, which closely reflect the bile salt components in animal intestine. Triplicate wells were used for each strain and mutant. The cultures were incubated at 42°C under microaerophilic conditions for 48 h. During the incubation, samples were taken at different time points (1, 3, 7, 12, 24, and 48 h postinoculation), serially diluted, and plated on MH agar for enumeration of Campylobacter colonies in each sample. The log10-transformed CFU for each sample was used to compare the growth of various strains and mutants in different media.
Chicken colonization experiments.
Newly hatched day-old broiler chickens were obtained from a commercial hatchery. Prior to use, these chicks were screened for Campylobacter by culturing cloacal swabs, which were plated onto MH agar plates containing Campylobacter-specific growth supplements (SR084E and SR117E; Oxoid). All of the birds tested negative for Campylobacter. To compare the wild-type 21190 and its cmeABC mutant constructs, 48 2-day-old birds were assigned to four treatment groups (12 birds/group). Each group was inoculated with a similar dose (106 CFU/chicken, via oral gavage) of wild-type 21190, JL101, JL102, or JL103. Each group of chickens was maintained in a sanitized wire-floored cage and provided with unlimited access to feed and water. The feed (C-2-88; Ohio Agricultural Research and Development Center, Ohio State University) was manufactured on site and was Campylobacter-free and without any animal protein or antibiotic additives. The parent strain and cmeABC mutants showed similar growth patterns in the feed extract (data not shown). Thus, the feed should not be a factor influencing the growth of cmeABC mutants in the intestine. Because the chickens were given city water, which contained sodium hypochlorite at a concentration of 5 ppm, we also determined if CmeABC influences Campylobacter susceptibility to sodium hypochlorite. Wild-type 21190 and cmeABC mutants had the same MIC of 262 ppm for sodium hypochlorite, which is well above the chlorine concentration in the city water. In addition, both the wild type and the mutants showed similar survival patterns in fresh drinking water from the chicken house (data not shown). Thus, the feed and drinking water were not factors influencing the survival of cmeABC mutants in the chickens.
Fecal samples were collected using cloacal swabs, which were taken every 2 days and diluted in MH broth for enumeration of Campylobacter cells. For the groups inoculated with wild-type 21190 and JL103, each fecal suspension was plated onto MH plates containing Campylobacter-specific growth supplements (Oxoid) for counting Campylobacter colonies. Since inactivation of cmeABC makes Campylobacter become susceptible to rifampin (28), a selective agent present in the Campylobacter-specific growth supplement (SR117E; Oxoid), the standard selective medium was not suitable for the isolation of JL101 and JL102. Therefore, the selective medium was modified by replacing rifampin with kanamycin and retaining the other three selective agents present in the growth supplement, including trimethoprim (10 μg/ml), cycloheximide (100 μg/ml), and polymyxin B (1 μg/ml), which are not substrates of CmeABC (28). This modified selective medium fully supported the growth of JL101 and JL102 in pure culture or spiked fecal samples (data not shown). This modified medium was used for isolation of Campylobacter from chickens inoculated with JL101 or JL102.
To determine the minimum infective dose of C. jejuni 21190 and JL101, 40 2-day-old broilers were assigned to eight groups (five birds/group), four of which were inoculated with various doses of wild-type 21190 (1.2 × 104 to 1.2 × 107 CFU/chicken). The other four groups were inoculated with different doses of JL101 (3.2 × 106 to 3.2 ×109 CFU/chicken). After inoculation, cloacal swabs were collected from the chickens and cultured for Campylobacter. The minimum infective dose was defined as the lowest dose at which at least one chicken of the inoculated group was colonized within 2 weeks after inoculation.
Immunoblotting analysis of CmeABC expression.
Expression of cmeABC in JL101, JL102, JL103, and JL104 was examined using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting as described previously (28). Immunoblotting was performed to determine if CmeABC was expressed and immunogenic in vivo. The recombinant proteins for CmeA, CmeB, and CmeC were generated in a previous study (28). The recombinant proteins were separated by SDS-PAGE and were then electrophoretically transferred to a nitrocellulose membrane as described previously (28). The blots were incubated with chicken serum samples (1:100 dilution in blocking buffer), which included three negative control serum samples from 3-week-old Campylobacter-free broiler chickens and eight serum samples from 2-year-old specific-pathogen-free layers or 6-week-old broilers experimentally infected with C. jejuni (46). After incubation at 25°C for 1 h, the blots were washed three times with phosphate-buffered saline containing 0.05% Tween 20 and subsequently incubated with secondary antibodies (1:1,000 dilution of goat anti-chicken immunoglobulin G-horseradish peroxidase; Kirkegaard & Perry) at 25°C for 1 h. After washing, the blots were developed with the 4 CN membrane peroxidase substrate system (Kirkegaard & Perry).
RESULTS
Effects of cmeABC mutations on bile resistance.
Our previous study showed that insertional mutation in cmeB impaired the expression of both cmeB and cmeC and increased the mutant's susceptibility to a few selected bile salts (28). To fully examine the substrate spectrum of CmeABC and the role of the individual member of the efflux pump in the efflux of bile salts, we constructed additional mutant constructs and tested their susceptibilities to various bile salts and detergents. In JL102, the insertional mutation in cmeC abolished the production of CmeC but did not affect the expression of cmeA (data not shown) and cmeB (Fig. 1). Similar to JL101, in which both cmeB and cmeC were not expressed, JL102 showed significantly increased susceptibilities to all bile salts tested in this study (Table 2). The MICs of bile salts for JL101 and JL102 decreased 128-fold for deoxycholic acid and taurodeoxycholic acid; 64-fold for chenodeoxycholic acid, taurocholic acid, and choleate; 32-fold for cholic acid; 8-fold for glycocholic acid; and 4-fold for dehydrocholic acid. JL101 and JL102 also showed increased susceptibilities to other detergents (Table 2). The same MIC changes for JL101 and JL102 indicated that inactivation of CmeC alone would cause malfunction of the CmeABC pump, further supporting the notion that the three members of CmeABC function together in the efflux of substrates. Notably, MICs of Triton X-100 and Tween 20 decreased more than 1,000-fold in these two mutants. The MIC of capric acid decreased eightfold in the mutants, but the MICs of butyric acid and palmitic acid had no changes, suggesting that CmeABC is not the main pump for the efflux of fatty acids.
TABLE 2.
Susceptibilities of strain 21190 and its mutant constructs to different bile salts, fatty acids, and detergents
| Antimicrobial | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| 21190 | JL101a | JL102a | JL103a | JL104a | |
| Cholic acid | 3,125 | 98 (32) | 98 (32) | 6,250 (1/2) | 6,250 (1/2) |
| Chenodeoxycholic acid | 1,250 | 19.5 (64) | 19.5 (64) | 1,250 (—) | 1,250 (—) |
| Taurocholic acid | 50,000 | 780 (64) | 780 (64) | 50,000 (—) | 50,000 (—) |
| Deoxycholic acid | 1,250 | 9.75 (128) | 9.75 (128) | 1,250 (—) | 1,250 (—) |
| Dehydrocholic acid | 10,000 | 2,500 (4) | 2,500 (4) | 10,000 (—) | 10,000 (—) |
| Glycocholic acid | 5,000 | 625 (8) | 625 (8) | 10,000 (1/2) | 10,000 (1/2) |
| Taurodeoxyxholic acid | 10,000 | 78 (128) | 78 (128) | 10,000 (—) | 10,000 (—) |
| Choleateb | 6,250 | 97 (64) | 97 (64) | 50,000 (1/8) | 50,000 (1/8) |
| SDS | 156 | 39 (4) | 39 (4) | 312 (1/2) | 312 (1/2) |
| Triton X-100 | 50,000 | 24.5 (2,040) | 24.5 (2,040) | 50,000 (—) | 50,000 (—) |
| Tween 20 | 50,000 | 49 (1,020) | 49 (1,020) | 50,000 (—) | 50,000 (—) |
| Empigen | 75 | 38 (2) | 38 (2) | 75 (—) | 75 (—) |
| Capric acid | 250 | 31 (8) | 31 (8) | 250 (—) | 250 (—) |
| Butyric acid | 5,000 | 5,000 (—) | 5,000 (—) | 5,000 (—) | 5,000 (—) |
| Palmitic acid | >400 | >400 (—) | >400 (—) | >400 (—) | >400 (—) |
The numbers in parentheses indicate the fold differences in MICs between 21190 and its mutant constructs. —, no MIC difference was observed.
Choleate (Sigma) is a crude ox bile extract which contains the sodium salts of taurocholic, glycocholic, deoxycholic, and cholic acids.
Complementation of JL101 and JL102 with pCME completely restored the production of cmeB and cmeC in JL103 and JL104 (Fig. 1), which showed the wild-type level of resistance to bile salts and other detergents (Table 2). These in vitro results indicate that CmeABC contributes significantly to bile resistance in Campylobacter.
Inhibition of Campylobacter growth by choleate.
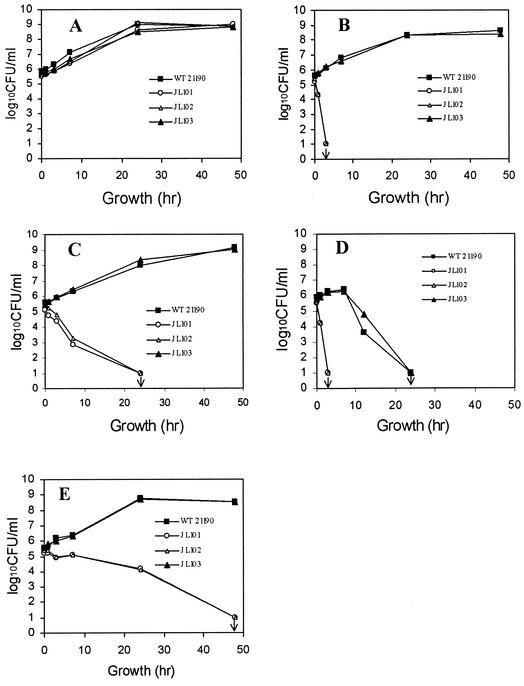
To further examine the contribution of CmeABC in bile resistance, we compared the in vitro growth characteristics of 21190 and its cmeABC mutants in MH broth or MH broth containing 1 mg of choleate/ml (∼2 mM bile salts). In conventional MH broth, both the wild-type strain and the mutants showed similar growth patterns (Fig. 2A). However, addition of choleate in MH broth greatly inhibited the growth of JL101 and JL102, but it did not have any effect on wild-type 21190 (Fig. 2B). In fact, no viable JL101 and JL102 cells were detected after 3 h of incubation in MH broth with choleate (Fig. 2B). Complementation of JL101 with pCME (JL103) completely restored the growth of the mutant to the wild-type level in MH broth supplemented with choleate (Fig. 2B). Together, these results indicate that the CmeABC pump is essential for Campylobacter growth in bile salts-containing medium in vitro.
FIG. 2.
Effects of bile salts and chicken intestinal extracts on the in vitro growth of C. jejuni 21190 and its isogenic cmeABC mutants. Bacteria were grown in MH broth (A), MH broth supplemented with sodium choleate (1 mg/ml) (B), chicken duodenal extract (C), chicken jejunal extract (D), and chicken cecal extract (E). The downward arrow indicates that no viable colonies were detected in the experiments, and the detection limit of the method was 10 CFU/ml. Each data point represents the mean value obtained from triplicate wells in the microtiter plate growth assay.
Inhibition of Campylobacter growth by chicken intestinal extracts.
Bile salts are present in animal intestinal contents. To assess if CmeABC was required for the growth of C. jejuni in intestinal extracts, we determined the actual concentrations of bile salts in different sections of chicken intestinal tract and then measured their effects on Campylobacter growth. As determined with a commercial kit, total bile concentrations in chicken duodenum, jejunum, and cecum were 3.5 ± 0.2, 14.0 ± 0.3, 0.17 ± 0.02 mM, respectively. Based on the estimated average molecular mass of 500 Da for bile salts, the total bile salt concentrations in chicken duodenum, jejunum, and cecum were 1.75, 7, and 0.085 mg/ml, respectively. These measurements were comparable to the results reported in a previous study in which bile salt concentrations in chicken intestinal extract were determined using a procedure involving bile salt extraction followed by enzymatic analysis (14). Although varied in different locations of the intestine, bile salt concentrations, particularly in the duodenum and jejunum, are far above the MICs of bile salts for cmeABC mutants (Table 2).
To determine if chicken intestinal extracts inhibited the growth of Campylobacter, wild-type 21190, JL101, JL102, and JL103 cells were grown in MH-based intestinal extracts. As shown in Fig. 2, the growth of JL101 and JL102 was greatly inhibited compared with that of wild-type 21190, although each intestinal extract showed a unique inhibitory pattern on Campylobacter growth. The wild-type strain grew normally in chicken duodenal extract as in MH broth, but the mutants did not show any growth. In fact, no viable JL101 and JL102 cells were detected after 24 h of incubation (Fig. 2C). Unlike the duodenal extract, the jejunal extract not only showed a striking bactericidal effect on the mutants but also had strong growth inhibition for wild-type 21190 (Fig. 2D). However, the two mutants experienced much faster and greater growth reductions than the wild-type strain in the jejunal extract. After 3 h of incubation, both JL101 and JL102 were no longer detected, suggesting that the jejunal extract killed the mutants within 3 h. On the contrary, wild-type 21190 did not show any growth reduction for the first 6 h, had 1 log unit of growth reduction by 12 h of incubation, and was below the detection limit by 24 h of incubation (Fig. 2D). In the cecal extract, which had the lowest bile concentration, wild-type 21190 grew normally and reached stationary phase within 24 h (Fig. 2E). However, JL101 and JL102 failed to grow in the cecal extract, although both of them survived longer than in the duodenal extract. As shown with JL103, complementation of the cmeB mutant with pCME fully restored the growth of the mutant to the wild-type level in each of the three intestinal extracts (Fig. 2). Additional experiments conducted at different times also showed similar growth patterns for 21190 and its cmeABC mutants as described above (data not shown). In addition, the cmeABC mutants of strain 81-176 also showed growth defects in the chicken intestinal extracts (data not shown). The overall inhibitory effect of each intestinal extract on Campylobacter growth was well correlated with the bile concentration in each preparation. These results clearly demonstrated that CmeABC is essential for Campylobacter survival and growth in chicken intestinal extracts.
CmeABC is essential for the colonization of Campylobacter in chickens.
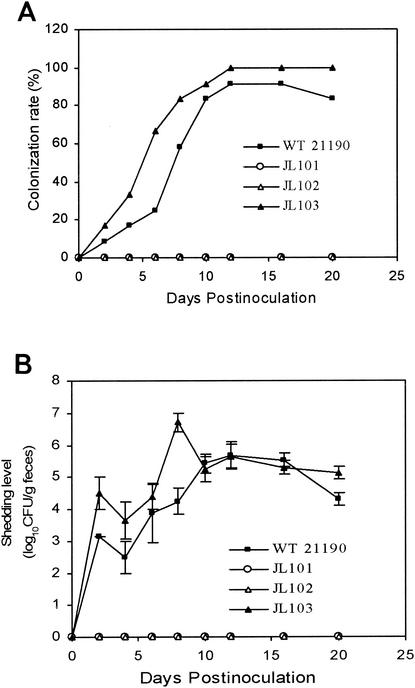
The inability of JL101 and JL102 to grow in the intestinal extracts suggested that CmeABC may be required for Campylobacter adaptation in chickens. To test this possibility, a typical chicken colonization model was used to determine the role of CmeABC in the colonization of C. jejuni. As shown in Fig. 3, wild-type 21190 colonized the chickens as early as 2 days postinoculation, and the majority of chickens were colonized at day 8 postinoculation as determined by culturing cloacal swabs. In individual chickens, the density of the organism was as high as 107 CFU/g of feces. In contrast, C. jejuni was not detected throughout the study in any of the cloacal samples collected from the chickens inoculated with JL101 or JL102 (Fig. 3). The construct JL103 displayed a colonization rate and shedding level comparable with those of wild-type 21190 (Fig. 3).
FIG. 3.
Colonization of C. jejuni 21190 and its isogenic cmeABC mutants in chickens. (A) Percentage of chickens colonized by C. jejuni after inoculation. (B) The shedding level of Campylobacter in chickens colonized by C. jejuni after inoculation. Each data point represents the mean CFU of the colonized chickens in each group. Standard errors are indicated by error bars.
To further compare the difference between wild-type 21190 and JL101 in colonization of chickens, the minimum infective dose was determined using multiple groups of chickens. As shown in Table 3, wild-type 21190 was able to colonize some of the chickens at a dose as low as 1.2 × 105 CFU/chicken, while JL101 failed to colonize the chickens even at a dose as high as 3.2 × 109 CFU/chicken (Table 3). At day 13 after the inoculation, the chickens inoculated with the highest dose of JL101 were necropsied and culturing of the cecal contents revealed no Campylobacter in the cecum, further confirming that JL101 failed to colonize the chicken intestinal tract. Thus, the minimum infective dose for JL101 was at least 2.6 × 104-fold higher than that of wild-type 21190. The difference between JL101 and 21190 in colonizing chickens was not attributable to the growth rate, because the in vitro growth characteristics of JL101 were quite similar to those of wild-type 21190 in conventional MH broth (Fig. 2A). Together, these results strongly indicated that CmeABC is essential for the in vivo adaptation of C. jejuni in the intestinal tract.
TABLE 3.
Determination of the minimum infective dose of 21190 and JL101 in broiler chickens
| Strain | Dose (CFU/chicken) | % Chickens colonized on day after inoculation:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 6 | 8 | 11 | 13 | ||
| WT 21190 | 1.2 × 104 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.2 × 105 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 20 | |
| 1.2 × 106 | 0 | 20 | 20 | 40 | 60 | 60 | 80 | 60 | |
| 1.2 × 107 | 0 | 40 | 60 | 60 | 80 | 80 | 100 | 100 | |
| JL101 | 3.2 × 106 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3.2 × 107 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3.2 × 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3.2 × 109 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0a | |
Chickens were necropsied and cecal contents were cultured for Campylobacter.
To confirm that the isolates recovered from the experimental chickens were derived from the inoculated 21190 or its derivatives, the cmp gene encoding the major outer membrane protein was PCR amplified using primers F3 and R3 (52) from representative Campylobacter isolates obtained from the chickens. The sequence data showed that the cmp sequence was identical to that of 21190 (data not shown). In addition, the recovered JL103 isolates were confirmed by detecting the chloramphenicol resistance marker carried by pCME. These results indicated that no environmental contamination of the chickens by Campylobacter occurred in the experiments.
CmeABC is expressed and immunogenic in vivo.
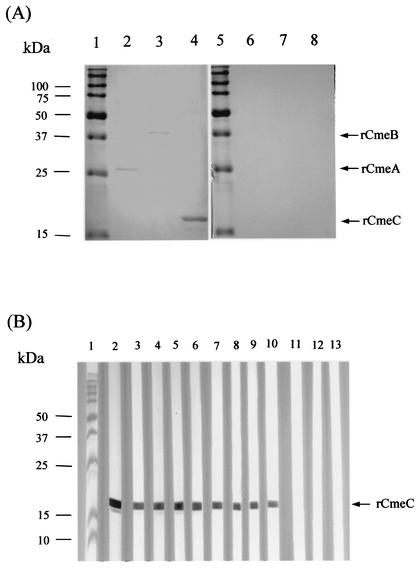
To determine if CmeABC is expressed and immunogenic in vivo, immunoblotting was performed using purified recombinant CmeABC peptides and serum samples from chickens infected with Campylobacter. As shown in Fig. 4A, the serum from a Campylobacter-infected chicken showed positive reaction to recombinant CmeA, CmeB, and CmeC, while the serum from a Campylobacter-free chicken did not react with any of the three recombinant proteins. Furthermore, multiple chicken sera were tested for reactivity with recombinant CmeC (Fig. 4B). All eight sera from Campylobacter-infected chickens showed a vivid antibody reactivity to recombinant CmeC (Fig. 4B), which is a predicted outer membrane component of the CmeABC efflux system. All the sera from Campylobacter-free chickens were negative for CmeC (Fig. 4B, lanes 11 to 13). These results suggest that CmeABC is expressed during Campylobacter infection of chickens and elicits a specific antibody response in the host.
FIG. 4.
Immunoblot analysis of in vivo antibody responses to CmeABC. (A) Purified recombinant CmeA (lanes 2 and 6), CmeB (lanes 3 and 7), and CmeC (lanes 4 and 8) peptides were blotted with serum from a chicken infected with C. jejuni (lanes 1 to 4) or serum from a Campylobacter-free chicken (lanes 5 to 8). (B) The recombinant CmeC was blotted with the rabbit anti-CmeC antibody (lane 2) or with individual chicken serum samples (lanes 3 to 13). Lanes 3 to 6, serum samples from 2-year-old Campylobacter-infected specific-pathogen-free layers; lanes 7 to 10, serum samples from 6-week-old broiler chickens infected with C. jejuni; lanes 11 to 13, serum samples from 3-week-old broiler chickens which were free of Campylobacter. Prestained molecule mass markers (lanes 1 and 5 in panel A and lane 1 in panel B; Bio-Rad) were coelectrophoresed and blotted to allow estimation of the sizes of the proteins.
DISCUSSION
The results of this study clearly demonstrated that CmeABC, by mediating bile resistance, plays a critical role in the in vivo adaptation of Campylobacter in chickens. This conclusion is supported by the following evidence. Firstly, CmeABC contributes significantly to Campylobacter resistance to various bile salts and detergents as determined using site-directed mutagenesis (Table 2). Secondly, mutations in cmeABC resulted in growth defects in bile salts-containing media and chicken intestinal extracts (Fig. 2). Thirdly, inactivation of cmeABC abolished the ability of Campylobacter to colonize chickens (Fig. 3). These phenotypic changes associated with the cmeABC mutants were directly linked to the inactivation of cmeABC, because the insertional mutation did not cause a polar effect on the gene (Cj0364) downstream of cmeABC (28) and complementation of the mutations with pCME fully restored the growth in bile-containing media and in vivo colonization (Fig. 2 and 3). These findings plus the fact that CmeABC is expressed during the course of Campylobacter colonization of the chicken host (Fig. 4) strongly indicate that CmeABC is essential for successful colonization of C. jejuni in the intestinal environment.
Bile salts are detergent-like amphipathic molecules normally present in animal intestinal tracts. As surface-active detergents, bile salts have potent antimicrobial activity (16, 20, 21). The major species of bile salts produced by the liver are the taurine and glycine conjugates of cholic acid and chenodeoxycholic acid, which are known as primary bile salts (2). These bile salts undergo further structural modifications (e.g., deconjugation and dehydroxylation) and result in various bile acid metabolites (e.g., cholic acid, chenodeoxycholic acid), which are known as secondary and tertiary bile salts (2). Under conditions existing in the gut of humans and animals, the bile concentration can be as high as 23 mM in jejunum, although a concentration of 2 mM is generally considered to be necessary for the formation of micelles for lipid digestion (10, 14, 51). The high concentration of bile salts in chicken small intestine (duodenum and jejunum) constitutes a formidable barrier for Campylobacter, and even the wild-type 21190 experienced a significant reduction in viability after 12 h of incubation in the jejunal extract (Fig. 2). Although the filtrates of chicken intestinal contents may not represent the microenvironment in which C. jejuni lives in the chicken intestinal tract, the inhibitory effect of the jejunal extract on Campylobacter growth is consistent with the fact that Campylobacter is rarely isolated from the small intestine of chickens (1, 6). In the cecum, the predominant site for Campylobacter colonization in chickens, the bile concentration is low enough to allow the growth of wild-type Campylobacter and high enough to inhibit the growth of cmeABC mutants (Fig. 2). This in vitro observation was consistent with the in vivo result that mutants JL101 and JL102 failed to colonize the intestinal tract (Fig. 3 and Table 3). Together, these findings strongly indicate that Campylobacter needs a functional CmeABC for growth in the gut.
To determine the substrate spectrum of the CmeABC system, we also investigated the role of CmeABC in the resistance to nonbile detergents as well as hydrophobic fatty acids. Strikingly, mutations in cmeABC resulted in much greater MIC reductions for nonionic detergents (Triton X-100 and Tween 20) than those for an ionic detergent (SDS) or zwitterionic detergent (Empigen). The susceptibility test conducted in this study revealed that CmeABC has a limited role in the efflux of fatty acids, suggesting that fatty acid resistance is an unlikely mechanism by which CmeABC contributes to Campylobacter adaptation in vivo. It should be pointed out that the MIC changes observed in JL101 and JL102 were not attributable to pH. All fatty acids and bile salts used in this study are sodium salts, and their pH is approximately 7.0 after solubilization in MH broth for the MIC test (data not shown). In addition, wild-type 21190 and JL101 grew similarly in acidic MH medium (pH 5.0), suggesting that CmeABC is not involved in resistance to acidic stress.
The chicken is a natural host and major reservoir for C. jejuni. This organism is well adapted to the intestinal tract of poultry, which provides an excellent model for evaluating Campylobacter colonization (not a model for disease). Colonization of chickens by C. jejuni occurs mainly in lower intestines, where the organism mainly infects cecal and cloacal crypts (1, 6, 33). Unlike the infection in mammals (e.g., mice, swine, rabbits, monkeys, and humans), in which C. jejuni may invade intestinal epithelial cells and cause histopathologic changes (3, 8, 44, 45), infection of chickens by C. jejuni does not result in invasion of the intestinal epithelium and clinical disease under normal conditions (6, 33). Newly hatched chickens are usually Campylobacter-free, eliminating the potential contamination of experimental chickens by unknown Campylobacter strains. Using this chicken model, we demonstrated the essential role of CmeABC in in vivo colonization. To exclude the possibility that the colonization deficiency of cmeABC mutants was due to factors that are not related to bile resistance, we evaluated bacterial growth in feed extract and drinking water (detailed in Materials and Methods). Both wild-type 21190 and cmeABC mutants showed similar growth and survival patterns in the feed and drinking water. These observations further support our conclusion that the key role of CmeABC in Campylobacter colonization is related to bile resistance.
Efflux of antibiotics by MDR pumps is considered an opportunistic function of the pumps, and the natural functions of MDR efflux systems are still largely unknown (36, 39, 40). Previous studies using in vitro assays indicated that AcrAB of Escherichia coli (12, 32, 48), MtrCDE of Neisseria gonorrhoeae (17), and VceAB of Vibrio cholerae (9) are involved in the resistance to bile and/or fatty acids, suggesting that these MDR pumps may provide a protective role in the survival of pathogens in the gut or other mucous sites. However, these studies lack direct evidence showing the role of efflux pumps in the in vivo adaptation of these pathogenic bacteria. Work presented in this study provides direct evidence showing the in vivo protective function of CmeABC by mediating bile resistance. According to this observation plus our previous finding (28) that CmeABC is broadly distributed and expressed in Campylobacter strains from various sources, it can be concluded that bile resistance is an important natural function for CmeABC. Despite the constitutive expression of CmeABC in wild-type C. jejuni strains, it is possible that CmeABC is subject to regulation by other factors. This speculation is based on the finding of a putative transcriptional repressor gene occurring immediately upstream of cmeA and the presence of a putative operator in the intergenic region between cmeA and its upstream open reading frame (28). However, immunoblotting analysis of Campylobacter cells grown in the presence or absence of cholic acid did not reveal obvious differences in the expression of CmeABC (data not shown). At this stage, it is unknown if CmeABC could be regulated by other bile salts or other substrates. Investigation of the regulatory mechanism of CmeABC will further improve our understanding of the function of CmeABC in the adaptation of Campylobacter in various niches.
It has been proposed that inhibiting MDR efflux systems is one approach to enhance drug accumulation inside the bacterial cell, thereby increasing bacterial susceptibility to antimicrobials (30, 41). Recently, MDR efflux pump inhibitors have been developed and demonstrated to potentiate the activity of antimicrobial agents against a range of gram-negative bacteria (29, 30). The presence of such inhibitors also resulted in a decreased frequency of emergence of Pseudomonas aeruginosa strains that are highly resistant to fluoroquinolones (29). Based on the findings from this study, we speculate that inhibitors targeting the CmeABC efflux pump may not only control antibiotic resistance but also increase the susceptibility of C. jejuni to in vivo bile salts, consequently decreasing the colonization level of Campylobacter. Such pump inhibitors could be directly used as novel antimicrobials for therapeutic intervention of Campylobacter infection. Inhibiting bacterial efflux of bile salts with pump inhibitors in the gut may be a general approach for developing therapeutic measures for enteric pathogens. From the standpoint of vaccine development, the outer membrane components of MDR pumps of gram-negative bacteria may be exploited as the targets of immune interventions, preventing the development of antibiotic resistance and the establishment of in vivo infection (27). In this study, we demonstrated that the outer membrane component CmeC is expressed and immunogenic in vivo, supporting the feasibility of targeting CmeC for immune protection against Campylobacter colonization. This possibility remains to be examined in future studies.
Acknowledgments
This work was supported by USDA CSREE competitive grant 00-51110-9741 and National Institutes of Health grant DK063008.
DNA sequences were determined at the Molecular, Cellular, and Imaging Center of the Ohio Agricultural Research and Development Center at The Ohio State University.
Editor: D. L. Burns
REFERENCES
- 1.Achen, M., T. Y. Morishita, and E. C. Ley. 1998. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 42:732-737. [PubMed] [Google Scholar]
- 2.Agellon, L. B., and E. C. Torchia. 2000. Intracellular transport of bile acids. Biochim. Biophys. Acta 1486:198-209. [DOI] [PubMed] [Google Scholar]
- 3.Babakhani, F. K., and L. A. Joens. 1993. Primary swine intestinal cells as a model for studying Campylobacter jejuni invasiveness. Infect. Immun. 61:2723-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon, D. J., R. A. Alm, L. Hu, T. E. Hickey, C. P. Ewing, R. A. Batchelor, T. J. Trust, and P. Guerry. 2002. DNA sequence and mutational analyses of the pVir plasmid of Campylobacter jejuni 81-176. Infect. Immun. 70:6242-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. [DOI] [PubMed] [Google Scholar]
- 6.Beery, J. T., M. B. Hugdahl, and M. P. Doyle. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell, M. B., R. I. Walker, S. D. Stewart, and J. E. Rogers. 1983. Simple adult rabbit model for Campylobacter jejuni enteritis. Infect. Immun. 42:1176-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colmer, J. A., J. A. Fralick, and A. N. Hamood. 1998. Isolation and characterization of a putative multidrug resistance pump from Vibrio cholerae. Mol. Microbiol. 27:63-72. [DOI] [PubMed] [Google Scholar]
- 10.Dietschy, J. M. 1968. Mechanisms for the intestinal absorption of bile acids. J. Lipid Res. 9:297-309. [PubMed] [Google Scholar]
- 11.Dussurget, O., D. Cabanes, P. Dehoux, M. Lecuit, C. Buchrieser, P. Glaser, and P. Cossart. 2002. Listeria monocytogenes bile salt hydrolase is a virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 46:903.. [DOI] [PubMed] [Google Scholar]
- 12.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. American Society for Microbiology, Washington, D.C.
- 14.Green, J., and T. F. Kellogg. 1987. Bile acid concentrations in serum, bile, jejunal contents, and excreta of male broiler chicks during the first six weeks posthatch. Poultry Sci. 66:535-540. [DOI] [PubMed] [Google Scholar]
- 15.Guerry, P. 1997. Nonlipopolysaccharide surface antigens of Campylobacter species. J. Infect. Dis. 176(Suppl. 2):S122-S124. [DOI] [PubMed] [Google Scholar]
- 16.Gunn, J. S. 2000. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2:907-913. [DOI] [PubMed] [Google Scholar]
- 17.Hagman, K. E., C. E. Lucas, J. T. Balthazar, L. Snyder, M. Nilles, R. C. Judd, and W. M. Shafer. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117-2125. [DOI] [PubMed] [Google Scholar]
- 18.Hassane, D. C., R. B. Lee, M. D. Mendenhall, and C. L. Pickett. 2001. Cytolethal distending toxin demonstrates genotoxic activity in a yeast model. Infect. Immun. 69:5752-5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann, A. F., and K. J. Mysels. 1992. Bile acid solubility and precipitation in vitro and in vivo: the role of conjugation, pH, and Ca2+ ions. J. Lipid Res. 33:617-626. [PubMed] [Google Scholar]
- 21.Hofmann, A. F. 1998. Bile secretion and the enterohepatic circulation of bile acids, p. 937-948. In M. Feldman, B. F. Scharschmidt, and M. H. Sleisenger (ed.), Sleisenger and Fordtran's gastrointestinal and liver diseases. W. B. Saunders, Philadelphia, Pa.
- 22.Jin, S., A. Joe, J. Lynett, E. K. Hani, P. Sherman, and V. L. Chan. 2001. JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol. Microbiol. 39:1225-1236. [DOI] [PubMed] [Google Scholar]
- 23.Konkel, M. E., S. G. Garvis, S. L. Tipton, D. E. Anderson, Jr., and W. Cieplak, Jr. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24:953-963. [DOI] [PubMed] [Google Scholar]
- 24.Konkel, M. E., B. J. Kim, V. Rivera-Amill, and S. G. Garvis. 1999. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32:691-701. [DOI] [PubMed] [Google Scholar]
- 25.Lam, K. M., A. J. DaMassa, T. Y. Morishita, H. L. Shivaprasad, and A. A. Bickford. 1992. Pathogenicity of Campylobacter jejuni for turkeys and chickens. Avian Dis. 36:359-363. [PubMed] [Google Scholar]
- 26.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 27.Lin, J., S. Huang, and Q. Zhang. 2002. Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes Infect. 4:325-331. [DOI] [PubMed] [Google Scholar]
- 28.Lin, J., L. O. Michel, and Q. Zhang. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 46:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomovskaya, O., and W. Watkins. 2001. Inhibition of efflux pumps as a novel approach to combat drug resistance in bacteria. J. Mol. Microbiol. Biotechnol. 3:225-236. [PubMed] [Google Scholar]
- 31.Luo, N., O. Sahin, J. Lin, L. O. Michel, and Q. Zhang. 2003. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob. Agents Chemother. 47:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 33.Meinersmann, R. J., W. E. Rigsby, N. J. Stern, L. C. Kelley, J. E. Hill, and M. P. Doyle. 1991. Comparative study of colonizing and noncolonizing Campylobacter jejuni. Am. J. Vet. Res. 52:1518-1522. [PubMed] [Google Scholar]
- 34.Morooka, T., A. Umeda, and K. Amako. 1985. Motility as an intestinal colonization factor for Campylobacter jejuni. J. Gen. Microbiol. 131:1973-1980. [DOI] [PubMed] [Google Scholar]
- 35.Nachamkin, I., X. H. Yang, and N. J. Stern. 1993. Role of Campylobacter jejuni flagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl. Environ. Microbiol. 59:1269-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neyfakh, A. A. 1997. Natural functions of bacterial multidrug transporters. Trends Microbiol. 5:309-313. [DOI] [PubMed] [Google Scholar]
- 37.Pavlovskis, O. R., D. M. Rollins, R. L. Haberberger, Jr., A. E. Green, L. Habash, S. Strocko, and R. I. Walker. 1991. Significance of flagella in colonization resistance of rabbits immunized with Campylobacter spp. Infect. Immun. 59:2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pei, Z., and M. J. Blaser. 1993. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in gram-negative nutrient transport systems. J. Biol. Chem. 268:18717-18725. [PubMed] [Google Scholar]
- 39.Poole, K. 2000. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob. Agents Chemother. 44:2233-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole, K. 2001. Multidrug resistance in gram-negative bacteria. Curr. Opin. Microbiol. 4:500-508. [DOI] [PubMed] [Google Scholar]
- 41.Poole, K. 2001. Overcoming antimicrobial resistance by targeting resistance mechanisms. J. Pharm. Pharmacol. 53:283-294. [DOI] [PubMed] [Google Scholar]
- 42.Pumbwe, L., and L. J. Piddock. 2002. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol. Lett. 206:185-189. [DOI] [PubMed] [Google Scholar]
- 43.Rivera-Amill, V., B. J. Kim, J. Seshu, and M. E. Konkel. 2001. Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. J. Infect. Dis. 183:1607-1616. [DOI] [PubMed] [Google Scholar]
- 44.Russell, R. G., M. O'Donnoghue, D. C. Blake, Jr., J. Zulty, and L. J. DeTolla. 1993. Early colonic damage and invasion of Campylobacter jejuni in experimentally challenged infant Macaca mulatta. J. Infect. Dis. 168:210-215. [DOI] [PubMed] [Google Scholar]
- 45.Russell, R. G., J. I. Sarmiento, J. Fox, and P. Panigrahi. 1990. Evidence of reinfection with multiple strains of Campylobacter jejuni and Campylobacter coli in Macaca nemestrina housed under hyperendemic conditions. Infect. Immun. 58:2149-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahin, O., Q. Zhang, J. C. Meitzler, B. S. Harr, T. Y. Morishita, and R. Mohan. 2001. Prevalence, antigenic specificity, and bactericidal activity of poultry anti-Campylobacter maternal antibodies. Appl. Environ. Microbiol. 67:3951-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tauxe, R. V. 2002. Emerging foodborne pathogens. Int. J. Food Microbiol. 78:31-41. [DOI] [PubMed] [Google Scholar]
- 48.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]
- 50.Wassenaar, T. M., B. A. van der Zeijst, R. Ayling, and D. G. Newell. 1993. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 139:1171-1175. [DOI] [PubMed] [Google Scholar]
- 51.Watkins, J. B. 1975. Mechanisms of fat absorption and the development of gastrointestinal function. Pediatr. Clin. North Am. 22:721-730. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Q., J. C. Meitzler, S. Huang, and T. Morishita. 2000. Sequence polymorphism, predicted secondary structures, and surface-exposed conformational epitopes of Campylobacter major outer membrane protein. Infect. Immun. 68:5679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]