Abstract
Mice deficient in complement component C3 (C3−/−) and syngeneic C57BL/6 control mice were challenged with Borrelia burgdorferi to determine the role of complement in immune clearance and joint histopathology during experimental Lyme borreliosis. Tibiotarsal joint, ear, and heart tissues were monitored for spirochete numbers at 2, 4, 8, and 12 weeks postinoculation with 105 B. burgdorferi B31 clone 5A4 by using quantitative real-time PCR. The spirochete load in joint and ear tissue remained higher in the C3−/− mice than in the wild-type counterparts throughout the 12-week study, whereas the numbers in heart tissue of both groups of mice decreased substantially at 8 to 12 weeks postinfection. Histopathology scores for joint tissue were generally higher in the C3−/− mice compared to C57BL/6 controls at 2 and 4 weeks postinfection, which may reflect the presence of higher numbers of bacteria in the joints at these early time points. Levels of anti-B. burgdorferi immunoglobulin G tended to be reduced in the C3−/− mice compared to control mice. Furthermore, a 5.5-fold-lower number of the complement-sensitive Borrelia garinii was needed to infect C3−/− mice compared to C57BL/6 mice, indicating that its sensitivity to complement is one barrier to infection of the mouse model by B. garinii. These results indicate that the complement system may be important in controlling the early dissemination and progression of B. burgdorferi infection.
Lyme borreliosis is a multisystemic infection caused by at least three Borrelia species: B. burgdorferi, B. garinii, and B. afzelii. In general, the disease progresses through three stages. Early Lyme disease can result in the development of erythema migrans at the site of infection and fever, fatigue, and other constitutional symptoms. The second stage, disseminated Lyme borreliosis, involves multiple organ systems and can include central and peripheral neuropathies and cardiac arrhythmias. The final stage is chronic Lyme borreliosis and is usually characterized by development of reoccurring arthritis in large joints as well as a continuation of neurologic manifestations (41, 47). The three Borrelia genospecies known to cause disease exhibit somewhat different manifestations; for example, B. burgdorferi tends to be associated with the development of arthritis, B. garinii tends to be associated with neuroborreliosis, and B. afzelii tends to be associated with acrodermatitis chronicum atrophicans (3, 29, 51).
During the course of disease, Borrelia organisms are introduced by the bite of an infected Ixodes tick, disseminate throughout the host, and elicit a strong immune response. Antibodies against B. burgdorferi proteins are detected within the first few weeks of infection and can be found throughout the course of disease (12-14, 16, 22). Passive transfer of sera from infected animals (4) or monospecific antisera against OspA (17), OspC (53), or DbpA (19) can protect against B. burgdorferi infection, indicating that the humoral response confers protective immunity.
Complement plays an important role in the clearance of many bacteria. Deposition of components of the complement system on the surface of bacteria can lead to opsonization, lysis of the bacterium through development of the membrane attack complex (MAC), and local inflammatory reactions. Activation of complement can occur through the classical, alternative, or mannose-binding lectin pathways, all of which rely on the function of complement component C3. Cleavage of C3 by the C4b2a (classical and lectin pathways) or C3bBb complexes (alternative pathway) is a critical step in the complement cascade and results in release of the soluble anaphylatoxin C3a and deposition of C3b on the surface of the bacterium. C3b is a ligand for complement receptors on macrophages and other cell types; it is also a subunit of complexes involved in the cleavage of C5 to C5a and C5b, which have anaphylactic and chemotactic activity and initiate MAC formation, respectively. C3b-mediated phagocytosis and MAC formation can both result in enhanced killing of the microbe (for a review, see Tomlinson [50]).
Microbial pathogens have evolved different mechanisms to protect themselves from complement, including inactivation of C3b and binding of host complement regulatory factors (reviewed by Joiner [23]). Lyme disease Borrelia organisms have varied abilities to survive in the presence of complement in vitro. In general, B. afzelii is resistant, B. burgdorferi is partially resistant, and B. garinii is sensitive to killing by complement in normal human serum (7, 25, 49). Alitalo et al. (1) have shown that, in vitro, B. afzelii and B. burgdorferi are able to bind the host complement regulatory proteins factor H and factor H-like protein 1. Both of these proteins are cofactors for host protein factor I, which cleaves and inactivates C3b. Hellwage et al. (20) determined that the outer surface protein E (OspE), a member of the Erp family of proteins, binds specifically to both factor H and factor H-like protein 1. Studies by Stevenson et al. (48) further showed that the Erp family in general binds factor H; however, the individual Erp proteins exhibited different binding affinities to factor H from different mammalian species. The ability of Borrelia to bind these host complement inhibitory proteins may permit the bacterium to evade killing by complement and thereby promote persistent infection. Furthermore, the species-specific binding of factor H by the Erp proteins may explain the varied ability of Borrelia genospecies to infect different hosts.
The role of complement in antibody-mediated killing of Borrelia has been examined previously. Schmitz et al. (44) reported that immune sera from hamsters had differing abilities to passively protect against Borrelia infection in complement-depleted, naive hamsters. Sera obtained at 1 and 10 weeks postinfection were not able to passively protect cobra venom factor-treated hamsters from B. burgdorferi 297 infection, but sera obtained 3 weeks postinfection were able to protect against infection. The authors also reported that in vitro, 3-week serum was able to inhibit growth of B. burgdorferi in the presence of heat-inactivated serum. Bockenstedt et al. (5) compared the progression of B. burgdorferi infection in mice deficient in complement component C5 and in wild-type mice. They reported that arthritis resolution was not affected by the absence of this component of the complement cascade and that passive immunization with immune sera and monoclonal antibodies was protective in C5-deficient mice. Mice deficient in the complement components C3 or C5 have also been shown to clear infection by relapsing fever Borrelia at the same rate as wild-type mice (11, 32). These studies suggest that antibodies generated during infection are able to kill Borrelia in a complement-independent manner.
The present study was designed to further define the role of the complement system during infection of the mammalian host with Lyme disease Borrelia. Mice that are genetically deficient in C3 expression (C3−/−) were inoculated with low-passage B. burgdorferi B31, and the ability of the spirochete to disseminate, persist, and cause disease was examined. Spirochete numbers and histopathology were determined at different time points during infection to define the importance of complement activation in the pathogenesis and immune clearance of B. burgdorferi. C3−/− mice generally had higher spirochete loads than wild-type mice. However, the rate of clearance varied at different tissue locations, indicating that a complex relationship exists between B. burgdorferi infection, complement, and the host tissue environment. Furthermore, B. garinii Ip90 exhibited a lower median infectious dose (ID50) in C3−/− mice relative to wild-type mice, which may explain in part the relatively low infectivity of B. garinii strains in mouse models.
MATERIALS AND METHODS
Bacterial and mouse strains.
The experimental protocol was approved by the Laboratory Animal Care Advisory Committee at the University of Texas—Houston Health Sciences Center and satisfied all campus and National Institutes of Health rules for the humane use of laboratory animals. Mice were housed in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (Rockville, Md.), placed in groups of one to three in polycarbonate cages with beta-chip bedding, wire bar lids, and microisolator tops and provided food and water ad libitum. The Association for Assessment and Accreditation of Laboratory Animal Care is a private, nonprofit organization that promotes the humane treatment of animals in science through a voluntary accreditation program.
Low-passage B. burgdorferi B31 clone 5A4 and B. garinii Ip90 were used in this study. Clone 5A4 is highly infectious in mice, and plasmid analysis revealed that it contains all 19 plasmids examined (38). B. garinii Ip90, graciously provided by Alan Barbour, University of California at Irvine School of Medicine, was initially isolated from ticks collected in eastern Russia (26) and had been passed through C3H/HeN mice to ensure infectivity. Strains were passaged in vitro fewer than five times following mouse infection. As described by Circolo et al. (8), C57BL/6 mice deficient in C3 were generated by replacing the 5′-flanking region of the C3 gene with a neomycin resistance marker. These C3-deficient (C3−/−) mice produce no detectable C3 protein and no complement activity. Wild-type C57BL/6 littermates were used for C3 positive controls. Bacteria were grown from frozen stocks in BSK-II medium to late log phase and diluted to 106 organisms per ml, and groups of 10 mice per time point were infected with 105 organisms by intradermal injection at the base of the tail (34). Mice were sacrificed at 2, 4, 8, and 12 weeks postinoculation, and tissues were harvested. Uninfected control animals were included at each time point. In experiments examining the infectivity of B. garinii Ip90, groups of C3−/− mice and C57BL/6 mice were inoculated intradermally with 10-fold-decreasing doses of bacteria, and ID50 values were determined according to the method of Reed and Muench (40). For quantitation of bacterial numbers, one tibiotarsal joint, half of the heart (bisected lengthwise), and one ear from each mouse were frozen and stored at −80°C. One joint was submerged in 10% buffered formalin for histology and one ear, half of the heart, and the bladder were cultured in BSK-II medium to determine the presence of spirochetes and to confirm infection.
DNA preparation for PCR quantitation.
DNA preparation was performed as described previously (31) with minor modifications. Briefly, individual tissues were incubated in 0.1% collagenase A at 37°C overnight followed by addition of an equal volume of a 0.2-mg/ml proteinase K (Sigma Chemical Co., St. Louis, Mo.) solution. After an overnight incubation at 55°C, DNA was recovered by phenol-chloroform extraction and ethanol precipitation. After digestion with DNase-free RNase (Sigma Chemical Co.) at 1 mg/ml, samples were again extracted and DNA was recovered by precipitation. This precipitate was resuspended in 1.5 ml of water, and the DNA content was determined by measuring the absorbance at 260 nm.
Quantitation of Borrelia.
Quantitative DNA analyses were performed using the LightCycler PCR system (Roche Diagnostics, Indianapolis, Ind.). Amplification was performed with 200 ng of sample DNA in a 10-μl final volume containing 50 mM Tris (pH 8.3), 3 mM MgCl2, 4.5 μg of bovine serum albumin, a 200 μM concentration of deoxynucleoside triphosphates, a 1:30,000 dilution of SYBR Green I (Molecular Probes Inc., Eugene, Oreg.), a 5 μM concentration of each primer, and 0.5 U of Platinum Taq DNA polymerase (Invitrogen Corp., Carlsbad, Calif.), which contains a Taq-specific antibody. Amplification was performed for 40 cycles, with each cycle consisting of heating at 20°C per s to 95°C with a 1-s hold, cooling at 20°C per s to 60°C with a 1-s hold, and heating at 1°C per s to 82°C. To minimize the inclusion of nonspecific products, the fluorescent signal was collected at 82°C at the end of each cycle. Melting curves were used to confirm the specificity of the assessed PCR products. The relative starting copy number present in each sample was determined by cycle threshold detection using the LightCycler analysis software. Copy numbers for the single-copy mouse nidogen gene and B. burgdorferi recA were calculated by using the LightCycler relative quantification software (Roche) and known external standards. The reported recA values were corrected by normalization to the nidogen gene copy number. The oligonucleotide primers used to detect murine nidogen were nidoF (5′-CCA GCC ACA GAA TCA CAT CC-3′) and nidoR (5′-GGA CAT ACT CTG CTG CCA TC-3′). The oligonucleotide primers used to detect B. burgdorferi recA were nTM17.F (5′-GTG GAT CTA TTG TAT TAG ATG AGG CTC TCG-3′) and nTM17.R (5′-GCC AAA GTT CTG CAA CAT TAA CAC CTA AAG-3′) (31).
Assessment of lesions in tibiotarsal joints.
Histopathologic evaluation was performed on multiple longitudinal sections of each tibiotarsal joint from mice sacrificed at 2, 4, 8, and 12 weeks postinfection and on control mice (46). Joints were fixed in 10% neutral-buffered formalin, decalcified, embedded in paraffin, sectioned at 5-μm thickness, and stained with hematoxylin and eosin. Sections were evaluated in a blinded fashion and assessed for lesions as described previously (52), including acute and chronic inflammation, tendon sheath thickness, and reactive or reparative responses. Each characteristic was assigned a score ranging from 0 to 5, with 5 representing the most severe lesion and 0 indicating normal tissue appearance. Scores for individual characteristics of lesions were incorporated into the overall lesion score reported in this study.
Antibody concentrations.
Serum samples were taken at each sacrifice time point and assayed by enzyme-linked immunosorbent assay to determine total immunoglobulin (Ig) and B. burgdorferi-specific Ig content. Microtiter plates were coated at 5 μg/ml with either sonicated B. burgdorferi B31 clone 5A4 or goat antibody to mouse IgG, IgM, and IgA (Southern Biotechnology Associates, Birmingham, Ala.). Serum dilutions were added to plates for 90 min at 37°C before washing to remove unbound material. Bound murine Ig was detected by addition of horseradish peroxidase-conjugated antibodies to murine IgG or IgM (Southern Biotechnology). Ig content was quantified by comparing with standard curves generated on the same plates using purified mouse IgG or IgM (Southern Biotechnology).
Statistical analysis.
Data were analyzed using SigmaPlot (SPSS Science, Chicago, Ill.) and Microsoft Excel (Redmond, Wash.) software. The two-tailed Student t test was used to analyze quantitative PCR results. Values of P < 0.05 were considered to be significant.
RESULTS
Numbers of spirochetes in tissue samples.
To determine if the complement system is important in controlling the dissemination and spirochetal load of B. burgdorferi B31 in tissues of the mouse model, C3−/− C57BL/6 mice and wild-type C57BL/6 mice were inoculated intradermally with 105 organisms. Ten mice from each strain were sacrificed at 2, 4, 8, and 12 weeks postinfection. Two uninfected mice from each strain were also sacrificed at each time point to serve as negative controls. The spirochete numbers in the tibiotarsal joint, ear, and half of the heart were determined by quantitative real-time PCR as described in Materials and Methods and are displayed in Fig. 1. All values are normalized to the number of mouse genome equivalents present in the sample, using the mouse nidogen gene as the target for amplification and parallel quantification.
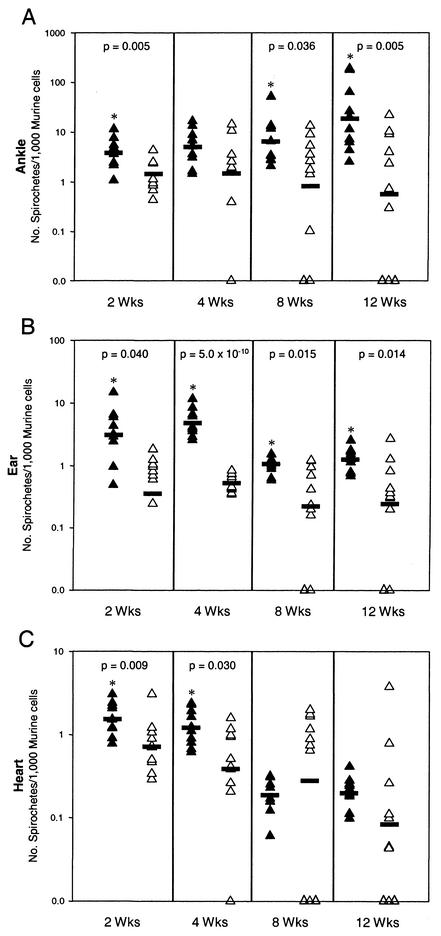
FIG. 1.
Spirochete numbers in tissues from C57BL/6 C3−/− and C57BL/6 wild-type mice. ▴, C3−/− mice; ▵, C57BL/6 mice; _, average. *, P < 0.05.
By 2 weeks postinfection, spirochetes had disseminated and infected all tissues in both strains of mice (Fig. 1). The average spirochetal loads of all three tissues were significantly higher in the C3−/− background (P = 0.005, 0.040, and 0.009 for the ankle, ear, and heart tissues, respectively). Joint, ear, and heart tissues from C3−/− mice had averages of 3.93, 3.12, and 1.55 spirochetes per 1,000 murine cells, respectively, compared with an average of 1.44, 0.36, and 0.73 spirochetes per 1,000 murine cells in wild-type littermates. By 4 weeks, the average number of spirochetes in each of the tissues was still consistently higher in C3−/− mice than in the wild-type background, with significantly higher values in the ear (P = 0.004) and heart (P = 0.030). At 8 weeks postinfection, the average number of spirochetes in the ear and heart tissues from C3−/− mice dropped dramatically from 4.81 to 1.06 copies per 1,000 mouse cells in the ear and 1.21 to 0.19 copies in the heart. A similar decrease in bacterial numbers was not observed in the ankle tissues. Spirochete numbers remained greater in the ankle (P = 0.036) and ear (P = 5.0 × 10−10) tissues of the C3−/− mice, but higher numbers were observed in the hearts of the wild-type mice. At 12 weeks after infection, a higher number of organisms was again observed in the joints (P = 0.005) and ears (P = 0.015) of infected C3−/− mice; however, the average number of spirochetes was not significantly different in the heart tissue of the two groups of mice. Interestingly, a much higher degree of variation in the numbers of spirochetes in the tibiotarsal joints of individual C3−/− mice was detected at the 12-week time point than observed at other time points or in other tissues.
The differences in spirochete numbers in the two mouse backgrounds implicate the involvement of the complement system in controlling the number of spirochetes, especially during the early stages of infection. Furthermore, the highly variable course of B. burgdorferi infection in the three tissues of the C3−/− mice demonstrates the complexity of the interactions of the immune system with B. burgdorferi during the course of infection.
All tissues were consistently culture positive in these studies; however, we were not able to detect spirochete DNA by quantitative PCR in some instances (zero values in Fig. 1). Negative PCR results were obtained only from wild-type C57BL/6 mice and not from the complement-deficient mice. Eight C57BL/6 mice had at least one tissue that had no detectable Borrelia DNA; four of these mice yielded negative PCR results in all three tissues tested, and one mouse had negative results in two of the tissues tested. These tissues were isolates at later time points, with the first one isolated at week 4 and the remaining six at weeks 8 and 12 (three mice at each time point). The fact that these tissues were culture positive yet did not contain detectable levels of B. burgdorferi DNA indicates that extremely small numbers of organisms were present; these results are consistent with more-effective immune clearance in the wild-type (C3+/+) mice.
Arthritis development in C3−/− mice.
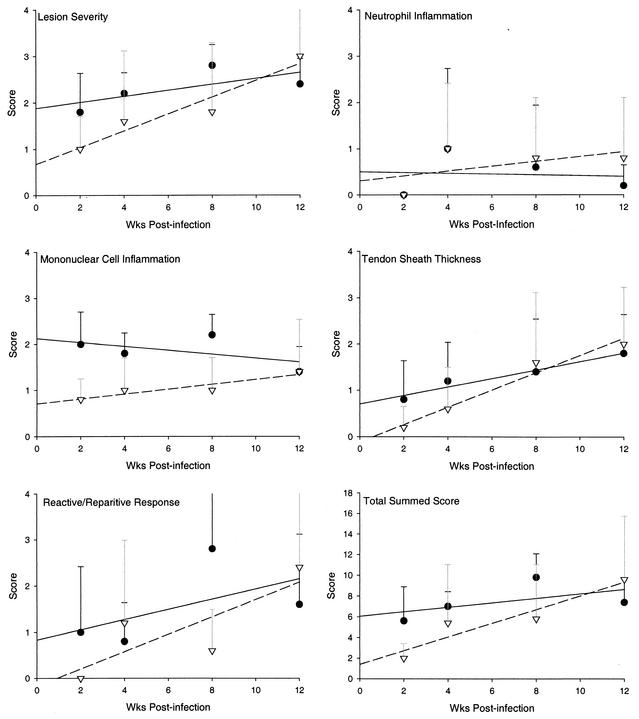
Infection with B. burgdorferi can lead to the development of arthritis in the large joints of Lyme borreliosis patients and experimentally infected mice. As mentioned previously, the complement components C3a and C5a are powerful anaphylatoxins that have important functions in the recruitment of inflammatory cells, degranulation of mast cells, and increasing vascular permeability, but they would not be produced in response to infection in the C3−/− mice. To determine if the degree of inflammation and severity of arthritis was affected by a deficiency in complement activation, the tibiotarsal joints of C3−/− and wild-type mice infected with B. burgdorferi B31 were examined to determine the extent of histopathology in these tissues. Sections were scored in a blinded fashion, and the mean scores and trend lines for each lesion characteristic are depicted in Fig. 2. The severity of arthritis tended to increase over time in both C3−/− and C57BL/6 mice, and the character and distribution of lesions were similar in both groups. Lesions in C3−/− mice tended to be more severe than in C57BL/6 mice at 2, 4, and 8 weeks postinfection, although there were no significant statistical differences between the two groups. Neutrophilic inflammatory responses were not a significant component of the lesions; however, mononuclear inflammation was notably higher in C3−/− mice at 2, 4, and 8 weeks postinfection (Fig. 2 and 3). In general, no substantial differences in the character and distribution of tendon sheath thickness and reactive and reparative responses were observed between C3−/− and C57BL/6 mice except at 8 weeks postinfection, when new bone formation and periosteal remodeling were more intensive in C3−/− mice than in wild-type mice (Fig. 2 and 3). Uninoculated control mice (n = 9) all had a total histopathology score of 0, except for one mouse with a score of 6. These results show that the lack of C3a and C5a production does not result in a decrease in the development of arthritis in the mouse model; in fact, lesions tended to be more severe in C3−/− mice than in wild-type mice at three of the four time points.
FIG. 2.
Mean lesion scores and trend lines from C57BL/6 mice (▵) and C3−/− (•) mice infected with B. burgdorferi 5A4. In general, lesion characteristics were more severe in C3−/− mice at earlier time points postinfection. By 12 weeks postinfection, the severity of lesions was similar in both groups.
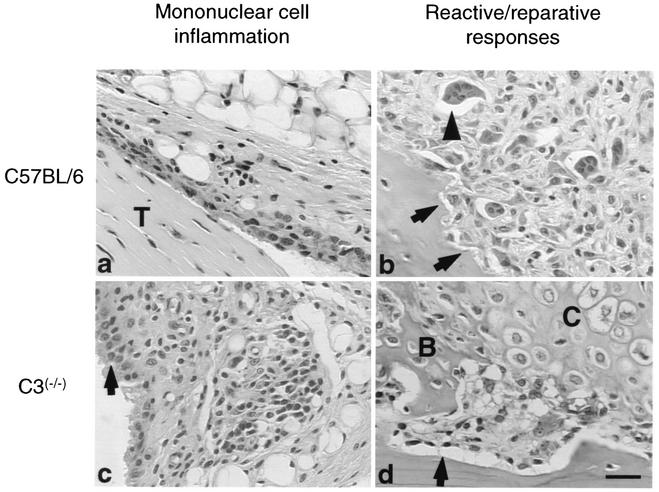
FIG. 3.
Histopathology in tibiotarsal joints of C57BL/6 and C3−/− mice induced by infection with B. burgdorferi 5A4. (a and c) Less mononuclear cell inflammation was observed in C57BL/6 mice than in C3−/− mice. The arrow is pointing to the reactive lining cells of a tendon sheath. T, tendon. (b and d) Mild periosteal remodeling and reactive changes (arrows) seen in C57BL/6 mice compared to C3−/− mice, as well as new bone (B) and cartilage (C) formation in C3−/− mice. The arrowhead is pointing to an osteoclast. Hematoxylin and eosin stain was used. Bar = 50 μm.
Antibody responses towards B. burgdorferi in C3−/− mice.
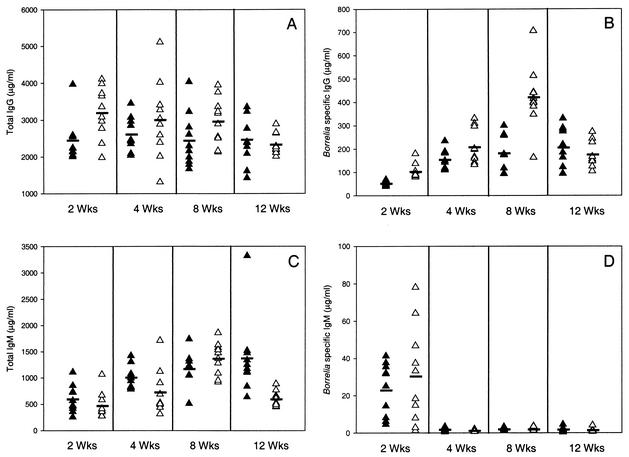
To determine whether the above findings were due to direct effects of C3 deficiency on B. burgdorferi infection and not from an inability of the C3−/− mice to mount an efficient antibody response, the antibody titers of the C3−/− mice were determined and compared to those of wild-type mice. Sera from mice infected with B. burgdorferi B31 were analyzed at 2, 4, 8, and 12 weeks postinfection, and the serum concentrations of IgM and IgG antibodies and B. burgdorferi-specific IgM and IgG for each strain of mouse were determined by enzyme-linked immunosorbent assay. The serum concentrations of total IgM and IgG were comparable between the C3−/− and wild-type mice, with an increase in total IgG seen in infected mice of both backgrounds over the uninfected controls (Fig. 4). Anti-B. burgdorferi IgM antibodies were present 2 weeks after infection but declined by 4 weeks in both strains, whereas B. burgdorferi-specific IgG antibodies appeared by 2 weeks after infection and were maintained throughout the course of the study. The presence of B. burgdorferi-specific antibodies was not detected in uninfected controls. These results indicate that a deficiency in complement activation did not result in an inability of the C3−/− mice to mount an antibody response against B. burgdorferi in this study.
FIG. 4.
Antibody concentrations in C3−/− mice and C57BL/6 wild-type mice infected with B. burgdorferi. ▴, C3−/− mice; ▵, C57BL/6 mice; _, average.
Infection by complement-sensitive B. garinii.
While B. burgdorferi B31 exhibits resistance to in vitro lysis by complement from several mammalian species, B. garinii has been shown to be sensitive to complement from rodents (including BALB/c mice, Clethrionomys glareolus, Sciurus carolinensis, and Apodemus sylvaticus) (6, 27, 28). B. garinii is also less infectious in the mouse model than B. burgdorferi B31 (A. G. Barbour, personal communication). To elucidate if the increased sensitivity of B. garinii to complement may account for its decreased ability to infect the mouse model, C3−/− and wild-type mice were infected with 106 to 102 B. garinii Ip90 cells to determine the ID50 in each mouse strain. Two weeks postinfection, mice were sacrificed and tissues were cultured in BSK-II medium to detect the presence of spirochetes. In mice that were positive for B. garinii infection, organisms were only isolated from the urinary bladders (Table 1). The ID50 for wild-type mice was 1.7 × 104 spirochetes, while the ID50 in the C3−/− mice was 5.5-fold lower at 3.2 × 103 organisms. This difference in ID50 indicates that the complement system influences the infectivity of Borrelia species to some extent.
TABLE 1.
ID50 of B. garinii Ip90 in C3−/− and C57BL/6 mice
| Mouse background | Infectious dose | No. infected/no. exposed
|
Total no. of mice infected/no. exposed | % Infected | ID50 | ||
|---|---|---|---|---|---|---|---|
| Heart | Bladder | Ear | |||||
| C3−/− | 106 | 0/3 | 3/3 | 0/3 | 3/3 | 100 | |
| 105 | 0/3 | 3/3 | 0/3 | 3/3 | 100 | ||
| 104 | 0/3 | 3/3 | 0/3 | 3/3 | 100 | 3.2 × 103 | |
| 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0 | ||
| 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0 | ||
| C57BL/6 | 106 | 0/3 | 3/3 | 0/3 | 3/3 | 100 | |
| 105 | 0/3 | 3/3 | 0/3 | 3/3 | 100 | ||
| 104 | 0/3 | 1/3 | 0/3 | 1/3 | 33.33 | 1.8 × 104 | |
| 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0 | ||
| 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0 | ||
DISCUSSION
Borrelia species incubated in vitro with serum have been shown to have divergent sensitivities to lysis by complement (7, 25, 49). We have further elucidated the role of complement in the host response to Borrelia infection by comparing the course of infection by B. burgdorferi B31 in mice genetically deficient in complement component C3 to that in mice with an intact complement system. We consistently found higher numbers of organisms present in the tissues of C3−/− mice, with more dramatic differences present in tissues harvested at the earlier time points of infection. In the ear and heart, the number of organisms was significantly higher in C3−/− mice at weeks 2 and 4, followed by a prominent decrease in numbers at weeks 8 and 12. By 12 weeks, the number of organisms in the hearts of C57BL/6 littermates also decreased; however, a decrease in the average number of organisms in the ears of wild-type mice was not apparent. A slightly different trend in spirochete number was observed in the tibiotarsal joints of the C3−/− mice. A significantly higher number of spirochetes was observed in the joints of C3−/− mice at 2 weeks, revealing the importance of the complement system early in Borrelia infection and perhaps a role in slowing dissemination of Borrelia to these joints; however, the average number of spirochetes increased in the joint tissues as the infection progressed. The occurrence of outliers with high concentrations of B. burgdorferi DNA in these tissues contributed greatly to the increased average. C57BL/6 mice maintained a very consistent number of organisms in the joints throughout the course of the study, similar to the trend observed for the other two tissues. The higher variability of spirochete numbers in joint tissue of C3−/− mice may imply that the control of the numbers of Borrelia by the adaptive immune response is more dependent on the complement system in the tibiotarsal joints than in other tissues examined.
In this study, we inoculated mice with a high dose of B. burgdorferi (105/animal) to ensure a uniform infection rate. Inoculation with a lower number of organisms may yield more dramatic differences in C3−/− mice that could implicate a more significant role for complement in controlling early dissemination of B. burgdorferi. In addition, infection of C3H/HeN C3−/− mice may yield different results, in that B. burgdorferi has been reported to induce a more severe arthritis in this genetic background. However, one might expect that differences caused by the C3−/− mutation would actually be more dramatic in the relatively resistant C57BL/6 background.
Several investigators have demonstrated that antibodies against Borrelia are bactericidal in vitro in the absence of complement (2, 9, 10, 15, 24, 30, 42, 43, 45). Extending these studies to the in vivo setting, Schmitz et al. (44) reported that passive immunization with anti-Borrelia serum in hamsters was affected by the depletion of complement; however, these effects were dependent on the time that the immune serum was obtained postinfection. Serum taken from mice 3 weeks after infection was able to protect complement-depleted animals from B. burgdorferi infection, while sera from two other time points were not protective. Bockenstedt et al. (5) demonstrated the ability of immune sera to passively protect mice deficient in complement component C5 from infection by B. burgdorferi, suggesting that borrelicidal antibodies arising during infection do not require complement to kill the bacterium. This hypothesis is supported by more recent studies with relapsing fever spirochetes that have shown that an intact complement system is not necessary for proper clearance of these spirochetes from the mouse model (11, 32). Additional studies by Rathinavelu et al. (39) have also shown that OspA hyperimmune serum from C3-deficient mice was able to kill B. burgdorferi in feeding ticks and block transmission of the spirochetes to the mammalian host. The decrease in numbers of spirochetes in the ears and hearts of C3−/− mice that we have reported here is consistent with the hypothesis that complement-independent killing can occur in vivo; however, the differences in progression of disease observed in the tibiotarsal joints of C3−/− mice also indicate that there is a complex interaction between the immune system and B. burgdorferi and that several components of the immune response are necessary to properly control the course of infection.
The severity of arthritis and tissue destruction in the tibiotarsal joints of C3−/− mice developed earlier in infection than in C57BL/6 mice. Earlier development of arthritis in C3−/− mice correlates with a significantly higher average number of organisms in these tissues at the 2-week time point; however, spirochete load did not directly correlate with severity of arthritis, i.e., the C3−/− mouse with the highest numbers of Borrelia present in its tibiotarsal joints did not have the most severe arthritis. The rapid development of B. burgdorferi-induced arthritis in C3−/− mice was strikingly different than the development of rheumatoid arthritis in a similar C3-deficient mouse model. Hietala et al. (21) recently reported that collagen-induced arthritis, an animal model for rheumatoid arthritis, does not develop in C3−/− mice after immunization with bovine type II collagen. Only after a second inoculation with bovine type II collagen do the C3-deficient mice develop arthritis, but to a much lesser degree than in wild-type controls. This dissimilarity with the development of murine Lyme arthritis suggests that C3 may play a greater role in early host defense against B. burgdorferi than in subsequent arthritis development.
Arthritis in C3−/− mice continued to be more severe at the 4- and 8-week time points than in C57BL/6 littermates; however, by week 12, histopathology scores were higher in C57BL/6 mice. No correlation between spirochete number and arthritis severity was observed. The variability in spirochete numbers and arthritis development in both inbred mouse strains used in this study indicates the complexity of factors contributing to immune clearance and inflammatory responses to B. burgdorferi, which varied markedly even within each experimental group.
Host specificity of Borrelia species has been documented, and it is speculated that the three Borrelia genospecies, B. burgdorferi, B. garinii, and B. afzelii, have adapted to different reservoir hosts. Accentuating this adaptation to diverse hosts is the differing abilities of these genospecies to survive in the presence of serum from different mammalian, avian, and reptilian hosts (27). It has been hypothesized that the resistance of B. afzelii and (to a lesser extent) B. burgdorferi to lysis by complement is due to the OspE/F/Erp protein families and their ability to bind to host complement regulatory proteins (1, 20, 48). Furthermore, Stevenson et al. (48) have shown that different Erp proteins, encoded on the cp32 plasmids, have specificities for factor H proteins from different mammalian species, providing B. burgdorferi with a repertoire of factor H-specific proteins that could potentially protect the spirochete from complement of several mammalian hosts. B. garinii, believed to be primarily a bird pathogen (18, 35-37), has been shown to be sensitive to murine serum in vitro (6, 27, 28) and has a high ID50 in the mouse model (Table 1). To determine if complement was a barrier to infection of the murine model by B. garinii, we compared the ID50 of B. garinii in C3−/− mice and C57BL/6 mice. The ID50 calculated for infection in C3−/− mice was 5.5-fold lower than that for C3+/+ mice. This increased infectious dose in C57BL/6 mice indicates that B. garinii's susceptibility to murine complement is a barrier to infection; however, the ID50 of B. garinii in C3−/− mice is still much higher than the ID50 reported for B. burgdorferi in immunocompetent mice (33). These differences in infectivity imply that the evolutionary divergence of B. burgdorferi and B. garinii involve factors other than complement susceptibility.
In conclusion, the presence of complement component C3 has a measurable, albeit modest, influence on the course of Lyme borreliosis in experimentally infected C57BL/6 mice. We have shown that a deficiency in complement component C3, and thus later components in the complement cascade, leads to a significantly higher number of spirochetes in tissues at early time points and an earlier development of arthritis. In addition, we have shown that the sensitivity of B. garinii to complement may in part explain the higher ID50 of this organism in mice relative to B. burgdorferi strains; however, other mechanisms are also likely to be involved in host-pathogen associations. These results differ from those obtained with relapsing fever Borrelia, in which C3 or C5 deficiencies had no measurable effect on the antibody-dependent clearance of organisms in the mouse model (11, 32). As predominantly blood-borne organisms, it is likely that the relapsing fever Borrelia spp. have developed even more substantial defenses against the effects of complement.
Acknowledgments
We thank Michelle Meadows and Robin Aller for technical assistance.
This work was supported by National Institutes of Health grants AI37277 (S.J.N.), AI32223 (J.J.W. and J.F.Z.), AI10223 (S.M.D.), and AI25011 (R.A.W.) and start-up funds from the Medical College of Ohio (R.M.W.).
Editor: D. L. Burns
REFERENCES
- 1.Alitalo, A., T. Meri, L. Ramo, T. S. Jokiranta, T. Heikkila, I. J. Seppala, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backenson, P. B., J. L. Coleman, and J. L. Benach. 1995. Borrelia burgdorferi shows specificity of binding to glycosphingolipids. Infect. Immun. 63:2811-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balmelli, T., and J. C. Piffaretti. 1995. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res. Microbiol. 146:329-340. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W., and L. K. Bockenstedt. 1993. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect. Immun. 61:4696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bockenstedt, L. K., S. Barthold, K. Deponte, N. Marcantonio, and F. S. Kantor. 1993. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect. Immun. 61:2104-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brade, V., I. Kleber, and G. Acker. 1992. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology 185:453-465. [DOI] [PubMed] [Google Scholar]
- 7.Breitner-Ruddock, S., R. Wurzner, J. Schulze, and V. Brade. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. 185:253-260. [DOI] [PubMed] [Google Scholar]
- 8.Circolo, A., G. Garnier, W. Fukuda, X. Wang, T. Hidvegi, A. J. Szalai, D. E. Briles, J. E. Volanakis, R. A. Wetsel, and H. R. Colten. 1999. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology 42:135-149. [DOI] [PubMed] [Google Scholar]
- 9.Coleman, J. L., R. C. Goger, and J. L. Benach. 1992. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect. Immun. 60:3098-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman, J. L., R. C. Rogers, P. A. Rosa, and J. L. Benach. 1994. Variations in the ospB gene of Borrelia burgdorferi result in differences in monoclonal antibody reactivity and in production of escape variants. Infect. Immun. 62:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly, S. E., and J. L. Benach. 2001. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J. Immunol. 167:3029-3032. [DOI] [PubMed] [Google Scholar]
- 12.Craft, J. E., R. L. Grodzicki, M. Shrestha, D. K. Fischer, M. Garcia-Blanco, and A. C. Steere. 1984. The antibody response in Lyme disease. Yale J. Biol. Med. 57:561-565. [PMC free article] [PubMed] [Google Scholar]
- 13.Craft, J. E., R. L. Grodzicki, and A. C. Steere. 1984. Antibody response in Lyme disease: evaluation of diagnostic tests. J. Infect. Dis. 149:789-795. [DOI] [PubMed] [Google Scholar]
- 14.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 15.Escudero, R., M. L. Halluska, P. B. Backenson, J. L. Coleman, and J. L. Benach. 1997. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect. Immun. 65:1908-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feder, H. M., Jr., M. A. Gerber, S. W. Luger, and R. W. Ryan. 1992. Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin. Infect. Dis. 15:788-793. [DOI] [PubMed] [Google Scholar]
- 17.Fikrig, E., L. K. Bockenstedt, S. W. Barthold, M. Chen, H. Tao, P. Ali-Salaam, S. R. Telford, and R. A. Flavell. 1994. Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J. Infect. Dis. 169:568-574. [DOI] [PubMed] [Google Scholar]
- 18.Gylfe, Å., B. Olsen, D. Strasevicius, N. Marti Ras, P. Weihe, L. Noppa, Y. Ostberg, G. Baranton, and S. Bergstrom. 1999. Isolation of Lyme disease Borrelia from puffins (Fratercula arctica) and seabird ticks (Ixodes uriae) on the Faeroe Islands. J. Clin. Microbiol. 37:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson, M. S., D. R. Cassatt, B. P. Guo, N. K. Patel, M. P. McCarthy, D. W. Dorward, and M. Hook. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 66:2143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 21.Hietala, M. A., I. M. Jonsson, A. Tarkowski, S. Kleinau, and M. Pekna. 2002. Complement deficiency ameliorates collagen-induced arthritis in mice. J. Immunol. 169:454-459. [DOI] [PubMed] [Google Scholar]
- 22.Hilton, E., A. Tramontano, J. DeVoti, and S. K. Sood. 1997. Temporal study of immunoglobin M seroreactivity to Borrelia burgdorferi in patients treated for Lyme borreliosis. J. Clin. Microbiol. 35:774-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joiner, K. A. 1988. Complement evasion by bacteria and parasites. Annu. Rev. Microbiol. 42:201-230. [DOI] [PubMed] [Google Scholar]
- 24.Katona, L. I., S. Ayalew, J. L. Coleman, and J. L. Benach. 2000. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J. Immunol. 164:1425-1431. [DOI] [PubMed] [Google Scholar]
- 25.Kraiczy, P., K. P. Hunfeld, S. Breitner-Ruddock, R. Wurzner, G. Acker, and V. Brade. 2000. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology 201:406-419. [DOI] [PubMed] [Google Scholar]
- 26.Kriuchechnikov, V. N., E. I. Korenberg, S. V. Shcherbakov, V. Kovalevskii Iu, and M. L. Levin. 1988. Identification of borrelia isolated in the USSR from Ixodes persulcatus Schulze ticks. Zh. Mikrobiol. Epidemiol. Immunobiol. 12:41-44. [PubMed] [Google Scholar]
- 27.Kurtenbach, K., S. De Michelis, S. Etti, S. M. Schafer, H. S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 28.Kurtenbach, K., H. S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunemann, J. D., S. Zarmas, S. Priem, J. Franz, R. Zschenderlein, E. Aberer, R. Klein, L. Schouls, G. R. Burmester, and A. Krause. 2001. Rapid typing of Borrelia burgdorferi sensu lato species in specimens from patients with different manifestations of Lyme borreliosis. J. Clin. Microbiol. 39:1130-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, J., C. Gingrich-Baker, P. M. Franchi, P. Bulger, and R. T. Coughlin. 1995. Molecular analysis of neutralizing epitopes on outer surface proteins A and B of Borrelia burgdorferi. Infect. Immun. 63:2221-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman, K., Jr., and R. C. Johnson. 1981. In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect. Immun. 31:465-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris, S. J., C. J. Carter, J. K. Howell, and A. G. Barbour. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 60:4662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norris, S. J., J. K. Howell, S. A. Garza, M. S. Ferdows, and A. G. Barbour. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen, B., D. C. Duffy, T. G. Jaenson, A. Gylfe, J. Bonnedahl, and S. Bergstrom. 1995. Transhemispheric exchange of Lyme disease spirochetes by seabirds. J. Clin. Microbiol. 33:3270-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen, B., T. G. Jaenson, and S. Bergstrom. 1995. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 61:3082-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen, B., T. G. Jaenson, L. Noppa, J. Bunikis, and S. Bergstrom. 1993. A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature 362:340-342. [DOI] [PubMed] [Google Scholar]
- 38.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathinavelu, S., A. Broadwater, and A. M. De Silva. 2003. Does host complement kill Borrelia burgdorferi within ticks? Infect. Immun. 71:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 41.Rosa, P. A. 1997. Microbiology of Borrelia burgdorferi. Semin. Neurol. 17:5-10. [DOI] [PubMed] [Google Scholar]
- 42.Sadziene, A., A. G. Barbour, P. A. Rosa, and D. D. Thomas. 1993. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect. Immun. 61:3590-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadziene, A., M. Jonsson, S. Bergstrom, R. K. Bright, R. C. Kennedy, and A. G. Barbour. 1994. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect. Immun. 62:2037-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitz, J. L., S. D. Lovrich, S. M. Callister, and R. F. Schell. 1991. Depletion of complement and effects on passive transfer of resistance to infection with Borrelia burgdorferi. Infect. Immun. 59:3815-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scriba, M., J. S. Ebrahim, T. Schlott, and H. Eiffert. 1993. The 39-kilodalton protein of Borrelia burgdorferi: a target for bactericidal human monoclonal antibodies. Infect. Immun. 61:4523-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seiler, K. P., Z. Vavrin, E. Eichwald, J. B. Hibbs, Jr., and J. J. Weis. 1995. Nitric oxide production during murine Lyme disease: lack of involvement in host resistance or pathology. Infect. Immun. 63:3886-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suhonen, J., K. Hartiala, H. Tuominen-Gustafsson, and M. K. Viljanen. 2000. Borrelia burgdorferi—induced oxidative burst, calcium mobilization, and phagocytosis of human neutrophils are complement dependent. J. Infect. Dis. 181:195-202. [DOI] [PubMed] [Google Scholar]
- 50.Tomlinson, S. 1993. Complement defense mechanisms. Curr. Opin. Immunol. 5:83-89. [DOI] [PubMed] [Google Scholar]
- 51.van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 52.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162:948-956. [PubMed] [Google Scholar]
- 53.Zhong, W., L. Gern, T. Stehle, C. Museteanu, M. Kramer, R. Wallich, and M. M. Simon. 1999. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive, but not active immunization using recombinant OspC. Eur. J. Immunol. 29:946-957. [DOI] [PubMed] [Google Scholar]