Abstract
Lipids that are found only in the cell envelope of pathogenic mycobacteria, such as those containing multiple methyl-branched fatty acids, have long been thought to play a role in pathogenesis. Among these complex lipids, sulfolipids have been the most extensively studied over the last 50 years. The numerous biological effects exhibited by purified sulfolipids on phagocytic cells led to the idea that these molecules are probably important virulence factors facilitating the intracellular survival of Mycobacterium tuberculosis. However, definitive evidence to support this concept has been lacking. The recent construction of an isogenic sulfolipid-deficient mutant of M. tuberculosis H37Rv (Sirakova et al., J. Biol. Chem. 276:16833-16839, 2001) has for the first time provided the opportunity to directly assess the contribution of these complex lipids to pathogenesis. In the present study, we show that against all expectations, sulfolipid deficiency does not significantly affect the replication, persistence, and pathogenicity of M. tuberculosis H37Rv in mice and guinea pigs or in cultured macrophages.
Tuberculosis remains a major health problem worldwide (26). Mycobacterium tuberculosis, the causative agent of this disease in humans, has the capacity to infect, replicate in, and persist inside host macrophages. The virulence factors enabling M. tuberculosis to escape the bactericidal mechanisms of these cells, notably by blocking phagosome-lysosome fusion, are essentially unknown (6). A study conducted some 55 years ago by Middlebrook and collaborators (21) recognized that the curious ability of the tubercle bacillus to grow in culture as serpentine cords was relatively restricted to virulent strains. Dubos and Middlebrook (7) also observed that the virulent cord-forming strains invariably absorbed the cationic dye neutral red, while most attenuated strains of M. tuberculosis and saprophytic mycobacteria did not.
In a search for the component of virulent mycobacteria that induced uptake of the dye, Middlebrook and coworkers (20) isolated sulfolipids. This family of molecules is composed of sulfated trehalose esters acylated with three to four acyl groups consisting of one short saturated fatty acid (palmitic acid or stearic acid) and different combinations of long-chain multiple methyl-branched fatty acids (the phthioceranic and hydroxyphthioceranic acids) (for a review, see references 9 and 11). Consistent with their suspected role in virulence, sulfolipids are found only in the human pathogen M. tuberculosis (9). Interestingly, sulfolipids are present in the virulent laboratory strain M. tuberculosis H37Rv but absent from the avirulent strain M. tuberculosis H37Ra (20).
Examination of some 60 clinical isolates of M. tuberculosis from different geographical origins suggested a highly significant statistical correlation between the production of sulfolipids in culture and the rank order of virulence for the guinea pig (8, 12, 15, 18). The most virulent strains were generally prolific producers of sulfolipids, whereas attenuated strains were notably deficient in sulfolipid production. However, the observation that some attenuated strains were proficient in sulfolipid synthesis and that a few virulent strains were devoid of these molecules led the authors to the careful conclusion that the presence of sulfolipids may not be a sufficient requirement for the expression of virulence (8, 12).
In parallel with this work carried out on M. tuberculosis field isolates, other studies conducted on purified sulfolipid molecules showed that the principal sulfolipid SL-I, although completely innocuous when injected even at high doses into mice, exhibited a synergistic potentiation of the toxicity of cord factor administered simultaneously (19). In vitro, SL-I induced swelling and disruption of mitochondrial membranes and strongly inhibited mitochondrial oxidative phosphorylation (19). Goren and collaborators (14) showed that M. tuberculosis sulfolipids are capable of preventing phagosome-lysosome fusion in cultured macrophages, suggesting a major role for sulfolipids in one of the most distinctive pathogenic functions of the tubercle bacillus. Other remarkable properties of sulfolipids drawn from in vitro studies include their ability to modulate the oxidative response and the cytokine secretion of human monocytes and neutrophils (2, 22, 27, 28). Finally, since most of the biological activities of sulfolipids were invoked from their amphiphilic and anionic properties, it was also proposed that sulfolipids may disturb the functions of phagosomal and lysosomal membranes by distorting their structure and that they may inactivate lysosomal hydrolases by interacting with the cationic sites of these enzymes (12).
Therefore, since 1947, a considerable wealth of in vivo and in vitro data that point to a role of sulfolipids in the virulence of the tubercle bacillus have been published. However, because of the lack of an isogenic strain of M. tuberculosis deficient in the production of sulfolipids, the precise contribution of these complex sulfated glycolipids to pathogenesis could not be measured and their biological activities in an infected host could not be directly tested.
Recently, Sirakova and collaborators (24) constructed a pks2 knockout strain of M. tuberculosis H37Rv that is deficient in the synthesis of phthioceranic and hydroxyphthioceranic acids and, consequently, devoid of sulfolipids. In the present study, we analyzed the virulence of this sulfolipid-deficient mutant in different cellular and animal models (mouse and guinea pig), using the same virulence evaluation criteria as those used in the early studies describing the virulence attenuation of sulfolipid-deficient clinical isolates of M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. tuberculosis H37Rv (ATCC 25618) and the M. tuberculosis H37Rv pks2 mutant (strain msl2) were grown at 37°C in Middlebrook 7H9 broth (Difco) supplemented with 10% ADC enrichment (5% bovine serum albumin fraction V, 2% dextrose, 0.003% beef catalase) and 0.05% Tween 80 or on agar Middlebrook 7H11 medium (Difco) supplemented with OADC (0.05% oleic acid, 5% bovine serum albumin fraction V, 2% dextrose, 0.004% beef catalase, 0.85% NaCl). Hygromycin B was added to the culture medium of the msl2 strain at a concentration of 50 μg/ml.
Preparation and infection of murine bone marrow macrophages.
Bone marrow cells were flushed from the femurs and tibias of 6- to 8-week-old BALB/c mice (CERJanvier, Le Genest St. Isle, France) and suspended in Dulbecco's modified Eagle's medium with 1 g of d-glucose, 3.7 g of NaHCO3, and 1.0289 g of N-acetyl-l-alanyl-l-glutamine (Biochrom, Berlin, Germany) per liter, enriched with 10% heat-inactivated fetal calf serum (Dominique Dutscher) and 10% L-cell-conditioned medium. Macrophages were seeded in 24-well plates (TPP, Geneva, Switzerland) (105 cells per well in a volume of 1 ml) and allowed to differentiate for 7 days. For experiments with activated macrophages, 100 U of gamma interferon (R&D Systems, Minneapolis, Minn.) per ml and 10 ng of tumor necrosis factor alpha (R&D Systems) per ml were added to the medium 24 h before infection and maintained throughout the infection process.
The infection assay was performed as follows. Glycerol stocks of the H37Rv wild-type and msl2 strains were washed in phosphate-buffered saline (pH 7.4) with 0.05% Tween 80, and bacterial concentrations were adjusted to 5 × 104 bacteria per ml in cell culture medium. An aliquot of the bacterial suspensions used to infect the macrophages was plated onto Middlebrook 7H11 agar to establish the exact number of bacteria in the inoculum. Cells were then infected at 37°C in a 5% CO2 atmosphere for 4 h at a multiplicity of infection of 1 bacterium to 2 macrophages. Infection was terminated by removing the overlaying medium and washing each well three times with Dulbecco's modified Eagle's medium before 1 ml of fresh culture medium was added per well. At 4 h and 3, 6, and 8 days, the infected macrophage monolayers (three wells per strain) were lysed in 200 μl of cell culture lysis reagent (Promega, Lyon, France), and the number of viable intracellular CFU was evaluated upon plating of the lysis solution onto 7H11 or 7H11 plus hygromycin.
Preparation and infection of THP-1 monocytes.
THP-1 cells were obtained from the American Type Culture Collection and grown at 37°C in a 5% CO2 atmosphere in RPMI 1640 medium (Life Technologies) containing 0.446 g of l-alanyl-l-glutamine per liter, 2 g of sodium bicarbonate per liter, 2 g of glucose per liter, 10 mM HEPES (Life Technologies), and 10% fetal bovine serum. THP-1 cell suspensions were adjusted to a concentration of 2 × 105 cells per ml in warm RPMI 1640 medium supplemented with 10 ng of phorbol 12-myristate 13-acetate (Sigma) per ml, seeded in tissue culture plates (1 ml per well), and allowed to differentiate for 24 h. The medium was then removed and replaced by 1 ml of bacterial suspension in RPMI 1640 medium containing 2 × 104 CFU per ml (multiplicity of infection, 1:10 bacterium/macrophage ratio). After 24 h at 37°C, the medium was removed and the wells were washed three times with RPMI 1640 medium to remove extracellular bacteria. At 24 h and on days 2, 5 and 7, cells (three wells per strain) were lysed with 200 μl of cell culture lysis reagent (Promega), and the number of viable intracellular CFU was counted by plating serial dilutions of the lysis solution onto Middlebrook 7H11 or 7H11 plus hygromycin agar.
M. tuberculosis growth and persistence in mice.
Female BALB/c mice (6 to 8 weeks old) purchased from CERJanvier (Le Genest St. Isle, France) were infected via the aerosol route with the M. tuberculosis H37Rv wild-type and msl2 mutant strains. M. tuberculosis aerosols were generated from bacterial suspensions consisting of 2 × 107 CFU per ml in phosphate-buffered saline (pH 7.4) with 0.05% Tween 80. Mice were exposed to the aerosols for 15 min. Five mice were used per experimental point and per strain. At various times after infection (days 1, 7, 14, 21, 42, 63, and 105), lungs and spleens were removed aseptically and homogenized in Sauton medium diluted 1:4 and supplemented with Middlebrook OADC. Viable bacteria in the organs of infected animals were enumerated by plating serial dilutions of the organ homogenates onto solid 7H11 or 7H11 plus hygromycin medium.
M. tuberculosis growth and persistence in guinea pigs.
Forty female Hartley guinea pigs were obtained from Charles River Laboratories (Willmington, Mass.). For aerosol infections, thawed aliquots of M. tuberculosis H37Rv and sulfolipid-deficient strain msl2 were diluted in double-distilled sterile water to the desired inoculum concentrations. The 40 guinea pigs were randomly separated into two groups of 20. Each group was then infected via the aerosol route with a low dose of either the wild-type or the mutant bacteria. Briefly, the nebulizer compartment of a Middlebrook air-borne infection apparatus (Glas-Col, Terre Haute, Ind.) was filled with 5 ml of distilled water containing a suspension of bacteria known to deliver approximately 20 to 50 bacteria into the lungs. All animals were weighed once a week. Means and standard errors of the mean values for each group were calculated and compared over time. Five guinea pigs from each group were sacrificed 11, 31, 65, and 100 days after aerosol infection. No animal died unexpectedly.
At necropsy, all lung lobes were removed from the thorax individually to enable separate manipulations with each lobe. In addition, the tracheobronchial lymph nodes, the left quadrate sublobe of the liver, and the spleen were removed. The number of viable bacteria in the lungs was monitored over time by plating serial 10-fold dilutions of right cranial lung lobe homogenates onto nutrient Middlebrook 7H11 agar. Bacterial load was assessed in the same way in half of each lymph node, liver, and spleen sample. Bacterial colony formations were counted after 21 days of incubation at 37°C in a 5% CO2 incubator. The data were expressed as the log10 value of the mean number of bacteria recovered. Means and standard errors of the mean values for each group were calculated and compared over time.
Assessment of intradermal reaction to M. tuberculosis purified protein derivative in guinea pigs.
At 29, 63, and 98 days postinfection, five guinea pigs from each group were shaved along the dorsum and received an intradermal injection of 1 μg of M. tuberculosis purified protein derivative suspended in 100 μl of sterile saline. At the same time, 100 μl of sterile saline was injected at a caudal site to act as a negative control. At 48 h after injection, the diameter of the erythema associated with the injections was measured independently by two people. Means and standard errors of the means of the values recorded by the two people were calculated and compared.
Histopathological assessment of guinea pig tissues.
The right middle lung lobe was first slowly infused through the major vessels at the hilus with 10% neutral buffered formalin to prevent alveolar collapse and assist in histopathological interpretation. The lobe was then submerged in the formalin. Half of each of the lymph node, liver, and spleen samples was also submerged in formalin. After a minimum of 48 h, the tissue was prepared and sectioned for light microscopy with lobe orientation designed to allow maximum surface area to be seen. Consecutive sections were stained with hematoxylin and eosin. Sections were examined by a veterinary pathologist without prior knowledge of the experimental groups and evaluated at least twice to verify the reproducibility of the observations.
Immunohistochemistry.
Mouse anti-guinea pig monoclonal antibodies specific for guinea pig CD4 (clone CT7), and CD8 (clone CT6) were purchased from Serotec (Oxford, England). In each case, the Serotec mouse immunoglobulin G1-negative control (clone W3/25) was used as the isotype control. F(ab′)2 rabbit anti-mouse immunoglobulin-biotin, also from Serotec, was used as the secondary antibody. At necropsy, the left cranial lung lobe was first slowly infused with 20% optimal cutting temperature compound (OCT; Tissue-Tek, Inc. Torrance, Calif.) in phosphate-buffered saline solution through the major vessels at the hilus. Lung lobes were then placed in a tissue mold, completely surrounded by OCT, frozen in a bath of liquid nitrogen, and stored at −70°C until used. Serial sections from each lung, 5 μm thick, were cut in a cryotome (CM 1850; Leica, Bannockburn, Ill.), employing the Instrumedics Inc. (Hackensack, N.J.) Tape Transfer System, fixed in cold acetone for 5 to 10 min, and then air dried. Next, the sections were washed in APK buffer solution (Vetana Medical Systems, Tucson, Ariz.) for 15 to 20 min and incubated in a 1:50 (CD4) or 1:100 (CD8) solution of Protein Block-goat serum (Biogenex, San Ramon, Calif.) and primary antibody for 30 min. The sections were then placed on a Nexus automated immunostainer (Ventana Medical Systems, Tucson, Ariz.). The labeled avidin-alkaline phosphatase and Fast Red-naphthol detection kit was employed. The secondary antibody diluted 1:100 in APK buffer was incubated for 30 min at room temperature. Sections were counterstained with Meyer's hematoxylin. Sections of spleen were also examined to act as positive controls.
Photomicroscopy and morphometry.
Photomicroscopy was performed with an Olympus AH-2 microscope linked to a Sony SKC-DK5 digital camera and Adobe Photoshop 6.0 software.
Statistics.
Statistical analysis was performed with Student's t test (Satterthwaite, unequal variances method) on the SAS system. Differences were significant considered a P of <0.05.
Biochemical analysis of sulfolipid content in wild-type and msl2 mutant of M. tuberculosis.
Radiolabeling with [1-14C]propionate was used to check the sulfolipid content of the M. tuberculosis H37Rv and msl2 strains grown under axenic conditions or recovered from the lungs of guinea pigs 65 days postinfection. For this purpose, sodium [1-14C]propionate (0.5 μCi per ml; specific activity, 50 mCi/mmol; ICN) was added to 5 ml of 7H9 cultures of M. tuberculosis H37Rv and msl2 (optical density at 600 nm of 1), and incubation was continued at 37°C under agitation for a further 24 h. Lipids were then extracted from bacterial pellets and analyzed by thin-layer chromatography and autoradiography as described previously (24).
RESULTS
Growth characteristics of a sulfolipid-deficient mutant in murine and human macrophages.
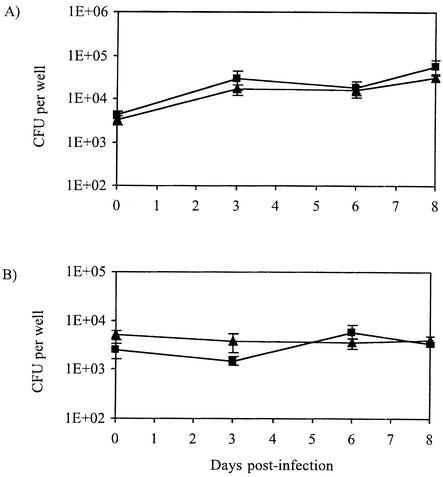
The numerous demonstrated biological effects of purified sulfolipid molecules on the activation and phagocytic functions of cultured murine and human macrophages suggested that the production of sulfolipids supports the intracellular growth of M. tuberculosis. In order to test this hypothesis, activated and nonactivated bone marrow macrophages from BALB/c mice and human THP-1 monocyte-derived macrophages were infected with the M. tuberculosis sulfolipid-deficient strain (msl2) or with the wild-type parental strain M. tuberculosis H37Rv, and the survival of intracellular mycobacteria was assessed by enumeration of CFU over time. In nonactivated mouse bone marrow macrophages, the mutant and wild-type strains exhibited no significant difference in growth over a period of 8 days (Fig. 1A). Over the same period of time, the two strains did not show any difference in persistence in activated mouse bone marrow macrophages (Fig. 1B). Likewise, in human THP-1 monocyte-derived macrophages, the sulfolipid-deficient strain and the wild-type H37Rv replicated similarly over a period of 7 days (data not shown). Therefore, sulfolipids are unlikely to be involved in the survival of M. tuberculosis inside phagocytic cells or in its resistance to the respiratory burst that accompanies macrophage activation.
FIG. 1.
Growth of M. tuberculosis H37Rv (▴) and sulfolipid-deficient mutant msl2 (▪) in nonactivated (A) and activated (B) murine (BALB/c) bone marrow macrophages. The multiplicity of infection used was 1 bacterium per 2 macrophages. The reported values represent the means and standard deviations (error bars) of data obtained from three independent wells.
Multiplication and persistence of sulfolipid-deficient mutant in mice.
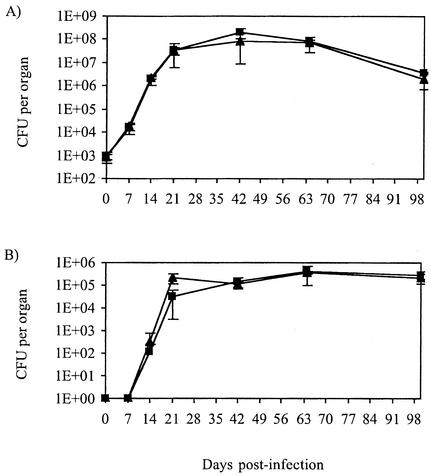
After performing virulence assays on cultured macrophages, the in vivo mouse model of infection was used to analyze the effect of sulfolipid deficiency on virulence. The ability of the wild-type and mutant strains to replicate and survive in the lungs and spleen of BALB/c mice was studied by CFU analysis after aerosol exposure. Figure 2 shows that after air-borne infection with bacterial inocula that implanted 1,000 bacilli in the lungs (as assessed by CFU counts the day after infection), the bacterial organ burdens in the mice infected with the sulfolipid-deficient mutant and the parental strain H37Rv were identical throughout a 105-day period. The two strains proliferated in lungs and spleen for 6 weeks and then persisted in both tissues. Therefore, sulfolipid deficiency does not compromise the growth of M. tuberculosis H37Rv in the lungs or spleens of BALB/c mice.
FIG. 2.
Multiplication and persistence of wild-type H37Rv (▴) and sulfolipid-deficient mutant msl2 (▪) M. tuberculosis strains in the lungs (A) and spleen (B) of BALB/c mice infected via the aerosol route. Results are expressed as means ± standard deviations (error bars) of CFU counts for five infected mice.
Multiplication and persistence of sulfolipid-deficient mutant in guinea pigs.
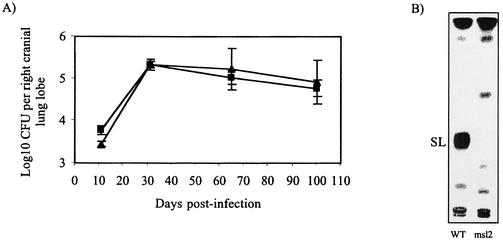
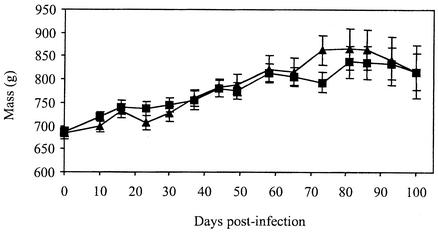
We then assessed the in vivo growth characteristics of the M. tuberculosis H37Rv wild-type and sulfolipid-deficient strains in guinea pigs. As in BALB/c mice, the mutant and wild-type strains replicated equally well in guinea pigs, giving rise to comparable bacterial burdens in the lungs (Fig. 3), thoracic lymph node, liver, and spleen (data not shown) at all time points of the infection. Consistent with these observations, the mass changes of msl2-infected guinea pigs followed the same progression as that of H37Rv-infected guinea pigs over the 100 days of the infection (Fig. 4) with no statistically significant differences between the two groups.
FIG. 3.
Multiplication and persistence of wild-type H37Rv and sulfolipid-deficient mutant msl2 of M. tuberculosis in the lungs of guinea pigs infected via the aerosol route. (A) Growth kinetics of M. tuberculosis H37Rv (▴) and sulfolipid-deficient mutant msl2 (▪) in the lungs of guinea pigs. Values represent the means and standard errors of the means of log10 CFU for four or five infected animals. (B) Analysis of the sulfolipid content of M. tuberculosis H37Rv (WT) and the sulfolipid-deficient mutant (msl2) recovered from the lungs of guinea pigs 65 days postinfection. Equal counts per minute of [1-14C]propionate-labeled total lipids from the two strains were applied to thin-layer chromatography Silica Gel G plates, developed with chloroform-methanol-water (90:10:1, vol/vol), and autoradiographed. SL, sulfolipids.
FIG. 4.
Change in mean total body mass of guinea pigs after aerosol infection with M. tuberculosis H37Rv (▴) or msl2 (▪). All infected guinea pigs were weighed once a week. Values represent the means and standard errors of the means for 20 (days 0 and 10), 15 (days 16 to 30), 10 (days 37 to 65), and 5 (days 73 to 100) animals from each group.
In order to check whether the msl2 mutant had reverted to the wild-type phenotype in the course of infection, the sulfolipid content of the H37Rv and msl2 strains recovered from the lungs of guinea pigs 65 days postinfection were analyzed by thin-layer chromatography. For this purpose, H37Rv and msl2 colonies recovered from the lungs of guinea pigs were grown in 7H9 medium and radiolabeled with [1-14C]propionate prior to lipid extraction and analysis. No sulfolipids were detected in the msl2 strain (Fig. 3), indicating that no reversion of the phenotype had occurred and that sulfolipid production in the pks2 knockout strain was catalyzed by no other M. tuberculosis polyketide synthase.
Histopathologic analysis.
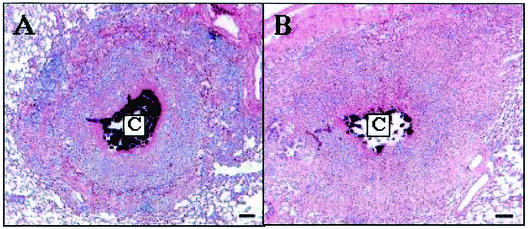
There were no significant differences in the lung, lymph node, liver, and spleen pathologies of H37Rv- and msl2-infected guinea pigs over time. In the lung, a similar progression through the previously described (25) histopathological stages of pulmonary tuberculosis in the guinea pig was observed in each group of animals. Figure 5 demonstrates a typical stage 4 lesion from each group observed in the chronic stage of the disease characterized by necrotizing granulomatous pneumonia with central mineralization. The spectra of lesions in the other organs were also similar between groups, with characteristic granulomatous inflammation and tissue destruction which increased with increasing duration of the disease (data not shown).
FIG. 5.
Typical pulmonary granulomas at 100 days postinfection from guinea pigs infected with M. tuberculosis H37Rv (A) or msl2 (B). Note mineralization of the core (C) and the similarity in size and overall architecture. Sections were stained with hematoxylin and eosin. Bar, 100 μm.
Cellular immune responses in infected guinea pigs.
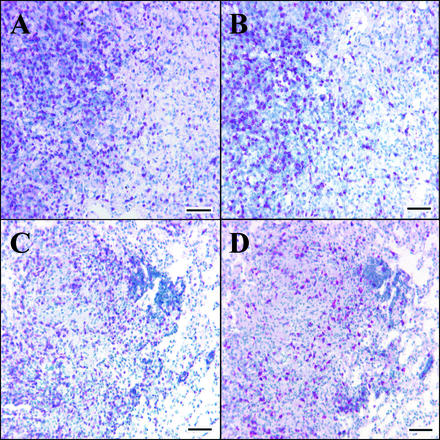
Both the distributions and the numbers of CD4 and CD8 T cells were similar in lung lesions at all time points for both groups of guinea pigs (Fig. 6). Typically, the lymphocytes were scattered evenly around the central area of each granuloma.
FIG. 6.
Representative photomicrographs of immunohistochemical staining for CD4+ (A and C) and CD8+ (B and D) lymphocytes in pulmonary granulomas of guinea pigs 100 days post-aerosol infection with M. tuberculosis H37Rv (A and B) or msl2 (C and D). Positive cells are indicated by red staining of the plasma membrane. Images are 5-μm serial sections. Note the similar numbers and arrangements of positive cells in panels A and B and in panels C and D. Bar, 10 μm.
Delayed-type hypersensitivity induced by sulfolipid-deficient mutant in infected guinea pigs.
In order to assess the immunogenicity of the H37Rv and msl2 strains, the cutaneous delayed-type hypersensitivity response to purified protein derivative in five guinea pigs from each group was evaluated at 29, 63, and 98 days postinfection. There were no statistically significant differences in the mean induration diameters between the two groups at any of the time points examined (Table 1), suggesting that msl2 is as immunogenic as its parental strain.
TABLE 1.
Intradermal reaction of H37Rv- and msl2-infected guinea pigs to M. tuberculosis purified protein derivativea
| Day postinfection | Mean diam of erythema (mm) ± SEM
|
|
|---|---|---|
| H37Rv infected | msl2 infected | |
| 29 | 14.7 ± 0.9 | 15.1 ± 0.9 |
| 63 | 13.3 ± 3 | 18.4 ± 1.4 |
| 98 | 14.7 ± 0.7 | 19.8 ± 4 |
At 29, 63, and 98 days postinfection, the cutaneous delayed-type hypersensitivity response to purified protein derivative was measured in five H37Rv- and five msl2-infected guinea pigs. The diameter of the erythema associated with the injection was measured independently by two people. The means and standard errors of the means of the values recorded by the two people are indicated.
DISCUSSION
Lipids that are found only in the cell envelope of pathogenic mycobacteria such as those containing multiple methyl-branched fatty acids have long been thought to play a role in pathogenesis (11). With the recent availability of genetic tools for performing insertional mutagenesis in M. tuberculosis (1, 23), mutant libraries were constructed and screened for virulence attenuation in mice by signature-tagged transposon mutagenesis. With this approach, phthiocerol dimycocerosate were the first methyl-branched fatty acid-containing lipids to be positively recognized as important for the multiplication of the tubercle bacillus in the lungs of mice (3, 4). Although the envelope of M. tuberculosis contains other similar complex lipids, such as diacylated trehaloses, triacylated trehaloses, polyacylated trehaloses, and sulfolipids, the lack of isogenic mutants deficient in their synthesis prevented studies on their involvement in pathogenesis.
Among these complex lipids, sulfolipids have most frequently been postulated to play a role in the virulence of M. tuberculosis. Certainly the most remarkable, and also controversial, biological activity associated with purified sulfolipids is their ability to prevent phagosome-lysosome fusion in murine peritoneal macrophages (5, 14, 16). More recently, the finding that the polyketide synthase gene pks2, involved in the synthesis of the methyl-branched fatty acid constituents of sulfolipids (24), is strongly upregulated upon phagocytosis of M. tuberculosis by cultured human primary macrophages (17) further strengthened the idea that sulfolipids are produced to lessen the effectiveness of activated phagocytic cells.
The construction of a sulfolipid-deficient strain of M. tuberculosis H37Rv by Sirakova and collaborators (24) enabled direct tests for the role of sulfolipids in pathogenesis to be conducted for the first time. To assess the residual virulence of this particular sulfolipid-deficient mutant of M. tuberculosis H37Rv, we used different models of infection and different virulence evaluation criteria. The occurrence of sulfolipid molecules in the human pathogen M. tuberculosis but not in any other mycobacteria from the tuberculosis complex suggested that the toxicity of sulfolipids might be directed only toward specific hosts. Therefore, the virulence of the sulfolipid-deficient mutant was analyzed not only in the mouse model but also in the guinea pig model in which the initial observations on the virulence attenuation of sulfolipid-deficient strains were made (8, 12). Similarly, the intracellular growth of the sulfolipid-deficient mutant was monitored in both murine and human macrophages. All animals were infected via the aerosol route, and the ability of the wild-type and sulfolipid-deficient strains to replicate in the lungs and to disseminate to other organs was monitored over a 100-day period by CFU count. Because organ CFU analysis is not an absolute measure of virulence, weight loss, histopathology, and cellular immune responses induced by each bacterial strain were also assessed in the guinea pig model over the same time period.
In striking contrast to the published data that suggested a major role for sulfolipids in the in vivo replication of M. tuberculosis from the very first weeks of infection (8, 12, 15, 18), our data indicate that sulfolipid deficiency does not affect the multiplication and persistence of M. tuberculosis H37Rv in either mice or guinea pigs over a 100-day period. The sulfolipid-deficient mutant was as proficient as wild-type H37Rv at replicating in infected animals. In guinea pigs, the two strains did not differ in their abilities to induce the formation of granulomas and the recruitment of CD4 and CD8 T lymphocytes at the site of infection. In addition, at all times examined, the sulfolipid-deficient mutant induced a delayed-type hypersensitivity response to purified protein derivative that was equal in intensity to that induced by the wild-type strain, indicating that it retained its immunogenicity.
There are a number of reasons that might explain the previous results that were not expected from previously published observations. Most of the previous studies, although providing indisputable evidence of the powerful biological activities of sulfolipids, were performed with purified sulfolipid molecules in cellular models. Important factors to be taken into consideration when interpreting the role of sulfolipids in pathogenesis from the in vitro studies are the conditions under which the biological activities of purified sulfolipids were tested. The stimulating effects of these glycolipids on the activation of phagocytic cells were found to be dose dependent (2, 22, 28). Hence, the intracellular concentration of sulfolipids appears to be critical to their biological activities. From the purification data provided by Goren (10), 1 μg of sulfolipid can be extracted from 1 to 1.3 mg of wet H37Rv bacilli (approximately 108 bacilli). The equivalent multiplicity of infection required to expose cells to concentrations of sulfolipids similar to that used in the in vitro studies (1 to 100 μg/ml) would therefore be on the order of 100 to 1,000 bacteria per cell. Such values are unlikely to be reached during the natural process of infection. In addition to the intracellular concentration, the localization of sulfolipids inside cells might affect their biological activities. The study by Goren and collaborators (14) showed that mouse macrophages exposed to sulfolipids take up these molecules by endocytosis and sequester them in secondary lysosomes. Since M. tuberculosis does not normally reside in this compartment, conclusions derived from this biological system may not all be relevant to M. tuberculosis infection in vivo.
Regarding the guinea pig experiments, an important difference between the studies published between 1948 (7) and 1982 (15) and our study is that our work was performed with an isogenic mutant strain of M. tuberculosis. Differences in the cell envelope compositions of the clinical isolates used by Goren and collaborators (12) other than those affecting sulfolipids might have been responsible for virulence attenuation. Another potential attenuation indicator was actually recognized by Goren and colleagues (13) when they analyzed more thoroughly the lipid composition of the 40 clinical isolates used in the sulfolipid study (12). Twenty-two of the 25 strains of reduced virulence were characterized by the presence of an attenuation indicator lipid that was not seen in any of the more virulent strains. This observation that the distribution of the attenuation indicator lipid (a methoxyphenolphthiocerol dimycocerosate) is restricted to attenuated strains was later extended by Goren and colleagues (15) to a larger number of M. tuberculosis clinical isolates. In the light of our results, it now seems that the presence of the attenuation indicator lipid was a more potent indicator of virulence attenuation than the absence of sulfolipids.
Finally, sulfolipids are complex molecules, and one cannot exclude the possibility that some biosynthetic precursors produced by the msl2 mutant in the absence of phthioceranic and hydroxyphthioceranic acids, such as trehalose-2′-sulfate, retain most of the toxicity of the entire sulfolipid molecules. The pathogenicity of M. tuberculosis H37Rv mutants deficient in other steps of sulfolipid synthesis (e.g., sulfation of trehalose) will be required to address this issue. Virulence is a complex phenotypic trait, and it is also possible that other factors in the M. tuberculosis H37Rv sulfolipid-deficient mutant compensate for the lack of sulfolipids. For instance, it was shown that the methylmalonyl-coenzyme A that is not used for the synthesis of phthioceranic and hydroxyphthioceranic acids in the mutant was channeled into mycocerosic and mycolipanoic acids found in phthiocerol dimycocerosates and acyl trehaloses, respectively (24). Since most of the biological activities of sulfolipids were invoked from their amphiphilic and anionic properties, it is plausible that other envelope lipids and glycolipids with similar physical properties can exert the same biological activities as sulfolipids. In support of this assumption, it is known that other glycolipids share with sulfolipids the ability to promote the toxicity of cord factor when injected simultaneously into mice (19).
In conclusion, our study sheds light on an issue raised some 55 years ago. In spite of the powerful biological activities of sulfolipids in vitro and of a remarkable correlation between the virulence of M. tuberculosis field isolates and their ability to elaborate sulfolipids, these molecules on their own do not contribute significantly to the virulence of M. tuberculosis H37Rv in the mouse and guinea pig models of infection used in this study. In view of the restricted distribution of sulfolipids to the human pathogen M. tuberculosis, one cannot exclude the possibility that these complex lipids play an important role in human pathogenesis. Moreover, it would be interesting to study whether these complex lipids play a more significant role in the virulence of other strains of M. tuberculosis with different cell envelope compositions and sulfolipid contents.
Acknowledgments
We thank G. Marchal and Eddy Maranghi for help with mouse infection and A. Murray for critical reading of the manuscript.
This work was supported by the Ministère de la Recherche et de la Technologie grant 1412146.00, the Institut Pasteur, and NIH grants AI-40488, AI-46582, and AI-35272. C.R. is a recipient of a Ministère de la Recherche fellowship.
The first two authors contributed equally to this work.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bardarov, S., J. Kriakov, C. Carriere, S. Yu, C. Vaamonde, R. A. M. Adam, B. R. Bloom, G. F. Hatfull, and W. R. Jacobs, Jr. 1997. Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10961-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brozna, J. P., M. Horan, J. M. Rademacher, K. M. Pabst, and M. J. Pabst. 1991. Monocyte responses to sulfolipid from Mycobacterium tuberculosis: inhibition of priming for enhanced release of superoxide, associated with increased secretion of interleukin-1 and tumor necrosis factor alpha and altered protein phosphorylation. Infect. Immun. 59:2542-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camacho, L. R., D. Ensergueix, E. Perez, B. Gicquel, and C. Guilhot. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34:257-267. [DOI] [PubMed] [Google Scholar]
- 4.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79-83. [DOI] [PubMed] [Google Scholar]
- 5.D'Arcy Hart, P., and M. R. Young. 1988. Polyanionic agents inhibit phagosome-lysosome fusion in cultured macrophages: a reply to the suggestion of Goren, Vatter, and Fiscus to the contrary. J. Leukoc. Biol. 43:179-182. [DOI] [PubMed] [Google Scholar]
- 6.Deretic, V., and R. A. Fratti. 1999. Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31:1603-1609. [DOI] [PubMed] [Google Scholar]
- 7.Dubos, R. J., and G. Middlebrook. 1948. Cytochemical reaction of virulent tubercle bacilli. Am. Rev. Tuberc. 58:698-699. [DOI] [PubMed] [Google Scholar]
- 8.Gangadharam, P. R. J., M. L. Cohn, and G. Middlebrook. 1963. Infectivity, pathogenicity and sulpholipid fraction of some Indian and British strains of tubercle bacilli. Tubercle 44:452-455. [DOI] [PubMed] [Google Scholar]
- 9.Goren, M. B. 1990. Mycobacterial fatty acid esters of sugars and sulfosugars, p. 363-461. In M. Kates (ed.), Handbook of lipid research: glycolipids, phosphoglycolipids and sulfoglycolipids, vol. 6. Plenum Press, New York, N.Y.
- 10.Goren, M. B. 1970. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. Purification and properties. Biochim. Biophys. Acta 210:116-126. [DOI] [PubMed] [Google Scholar]
- 11.Goren, M. B., and P. J. Brennan. 1979. Mycobacterial lipids: chemistry and biologic activities, p. 63-193. In G. P. Youmans (ed.), Tuberculosis. W. B. Saunders Company, Philadelphia, Pa.
- 12.Goren, M. B., O. Brokl, and W. B. Schaefer. 1974. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infect. Immun. 9:142-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goren, M. B., O. Brokl, and W. B. Schaefer. 1974. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: phthiocerol dimycocerosate and the attenuation indicator lipid. Infect. Immun. 9:150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goren, M. B., P. D'Arcy Hart, M. R. Young, and J. A. Armstrong. 1976. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 73:2510-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goren, M. B., J. M. Grange, V. R. Aber, B. W. Allen, and D. A. Mitchinson. 1982. Role of lipid content and hydrogen peroxyde susceptibility in determining the guinea-pig virulence of Mycobacterium tuberculosis. Br. J. Exp. Pathol. 63:693-700. [PMC free article] [PubMed] [Google Scholar]
- 16.Goren, M. B., A. E. Vatter, and J. Fiscus. 1987. Polyanionic agents do not inhibit phagosome-lysosome fusion in cultured macrophages. J. Leukoc. Biol. 41:122-129. [DOI] [PubMed] [Google Scholar]
- 17.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grange, J. M., V. R. Aber, B. W. Allen, D. A. Mitchinson, and M. B. Goren. 1978. The correlation of bacteriophage types of Mycobacterium tuberculosis with guinea-pig virulence and in vitro-indicators of virulence. J. Gen. Microbiol. 108:1-7. [DOI] [PubMed] [Google Scholar]
- 19.Kato, M., and M. B. Goren. 1974. Synergistic action of cord factor and mycobacterial sulfatides on mitochondria. Infect. Immun. 10:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middlebrook, G., C. M. Coleman, and W. B. Schaefer. 1959. Sulfolipid from virulent tubercle bacilli. Proc. Natl. Acad. Sci. USA 45:1801-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middlebrook, G., R. J. Dubos, and C. Pierce. 1947. Virulence and morphological characteristics of mammalian tubercle bacilli. J. Exp. Med. 86:175-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pabst, M. J., J. M. Gross, J. P. Brozna, and M. B. Goren. 1988. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J. Immunol. 140:634-640. [PubMed] [Google Scholar]
- 23.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirakova, T. D., A. K. Thirumala, V. S. Dubey, H. Sprecher, and P. E. Kolattukudy. 2001. The Mycobacterium tuberculosis pks2 gene encodes the synthase for the hepta- and octamethyl branched fatty acids required for sulfolipid synthesis. J. Biol. Chem. 276:16833-16839. [DOI] [PubMed] [Google Scholar]
- 25.Turner, O. C., R. J. Basaraba, and I. M. Orme. 2003. Immunopathogenesis of pulmonary granulomas in guinea pig following infection with Mycobacterium tuberculosis. Infect. Immun. 71:864-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. 2002. Tuberculosis fact sheet no. 104. http://www.who.int/mediacentre/factsheets/who104/en/index.html. (Online.)
- 27.Zhang, L., D. English, and B. R. Andersen. 1991. Activation of human neutrophils by Mycobacterium tuberculosis-derived sulfolipid I. J. Immunol. 146:2730-2736. [PubMed] [Google Scholar]
- 28.Zhang, L., M. B. Goren, T. J. Holzer, and B. R. Andersen. 1988. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect. Immun. 56:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]