Abstract
The rat middle ear and lung clearance model has been used to show that the nontypeable Haemophilus influenzae 26-kDa outer membrane protein OMP26 is highly efficacious as a mucosal immunogen, inducing significantly enhanced clearance in immunized rats upon direct challenge of these two anatomic sites. Similarly, the chinchilla model of middle ear and nasopharyngeal clearance has been used to show that two P5 fimbrin adhesin-derived immunogens, LB1 and lipoprotein D (LPD)-LB1(f)2,1,3, are highly efficacious as parenteral immunogens. Both induced significantly augmented clearance of nontypeable H. influenzae upon challenge of these sites. Here, these three nontypeable H. influenzae immunogens in addition to six bovine serum albumin and keyhole limpet hemocyanin conjugates of the synthetic peptide LB1(f) were assayed for relative efficacy in the reciprocal rodent model system. OMP26 was assayed in the chinchilla host by a parenteral immunization route, with clearance of the middle ear and nasopharynx used as outcome measures. Both LB1 and LPD-LB1(f)2,1,3 were assayed in the rat host with a mucosal immunization route and clearance of nontypeable H. influenzae from the lungs and middle ears as outcome measures. Both of the immunogens were found to induce a high-titered and specific immune responses in the heterologous host system. Moreover, each was found to be highly efficacious in the reciprocal host system, providing strong support for the continued development and inclusion of both OMP26 and P5 fimbrin-derived peptides as candidate vaccine antigens directed at otitis media caused by nontypeable H. influenzae.
Developing an effective immunogen and selecting an appropriate adjuvant and dosing regimen for a vaccine to prevent otitis media is an important endeavor, particularly given the rapid increase in antibiotic-resistant strains of the bacteria responsible (2, 8) and the increasing costs of treatment (9, 11, 18, 28). Several outer membrane proteins of nontypeable Haemophilus influenzae, its lipooligosaccharide, and synthetic peptides and recombinant proteins derived from nontypeable H. influenzae outer membrane proteins and detoxified lipooligosaccharide conjugates have all been investigated as possible immunogens against bacterial otitis media in both the rat and chinchilla models (1, 3, 5-7, 10, 17, 19, 20, 22-24, 26, 29).
The P5 fimbrin is a protein with four surface-exposed loops, some of which appear to be quite heterogeneous (31), and has a role in adherence (4, 29). Studies in the rat and chinchilla models have found that this antigen is protective (4, 32), particularly when the vaccine is based on loop 3, which can be divided into three groups (3). Three peptides have been designed for this region (LB11, LB12, and LB13). This antigen protects against infection via an antibody-mediated mechanism, since both active immunization and passive transfer of anti-LB1 serum significantly reduced the severity and incidence of otitis media in chinchillas (3, 22). Immunization with these peptides requires conjugation to another protein or peptide carrier to provide T-cell help, and both the promiscuous T-cell epitope from the measles virus protein and the H. influenzae protein lipoprotein D have been used in this capacity as carriers (3, 4). In the chinchilla model, antibody directed against both a 40-mer synthetic chimeric peptide (LB1) and a recombinant fusion protein called lipoprotein D (LPD)-LB1(f)2,1,3 provided significant protection from the development of nontypeable H. influenzae-induced otitis media (3, 22, 23). In these studies, protection was conferred in the rat and chinchilla when either homologous or heterologous nontypeable H. influenzae strains were used to challenge animals immunized with the 26-kDa outer membrane protein OMP26, LB1, or LPD-LB1(f)2,1,3.
The nontypeable H. influenzae OMP26 protein has a 26-kDa molecular mass in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. This 174-amino-acid protein has a calculated mass of 21.73 kDa, and the preprotein has a 23-amino-acid N-terminal signal sequence (13). The N-terminal and C-terminal regions of the protein are hydrophobic and separated by a hydrophilic middle. This outer membrane protein is located in a highly conserved region of genetic organization, and homologous sequences appear to be highly conserved in gram-negative bacteria, suggesting an important function. In recent studies, we showed that OMP26 was able to significantly enhance pulmonary clearance of nontypeable H. influenzae in a rat model in which animals were immunized via intestinal Peyer's patches and then boosted intratracheally (13, 23).
Lipoprotein D is a 42-kDa membrane-associated protein (21). The N-terminal sequence contains the consensus sequence for bacterial lipoproteins, Leu-Ala-Gly-Cys, and the protein is highly conserved across nontypeable H. influenzae strains. The acylated form of the protein is more immunogenic than the nonacylated form (1). The efficacy of this protein in protection of a nontypeable H. influenzae infection in animal studies has varied. In animal studies, lipoprotein D or protein D (PD), a recombinant deacylated form of lipoprotein D (LPD), was reported not to protect rats from otitis media, and in the chinchilla model, lipoprotein D immunization did not significantly enhance clearance of nontypeable H. influenzae from the nasopharynx, although a shorter (but not significant) clearance of bacteria from the middle ear was found (27).
In the present study, we decided to test the efficacy of each in the reciprocal animal model systems (e.g., test LB1 and LPD-LB1[f]2,1,3 in rats, whereas OMP26 was to be tested in chinchillas). These two models incorporate different routes of immunization (mucosal versus parenteral), use different challenge regimens, and measure different parameters of pathogenesis and protection. Thus, being able to demonstrate efficacy of these immunogens in an additional model system would provide very strong support for their importance as candidates for a vaccine directed at otitis media caused by nontypeable H. influenzae.
MATERIALS AND METHODS
Animals.
A total of 69 DA and 27 Sprague Dawley male rats, aged between 8 and 10 weeks and weighing 160 to 202 g and 298 to 352 g, respectively, were used. Rats were bred and maintained under specific-pathogen-free conditions except for removal during immunizations and the final bacterial challenge.
A total of 44 adult chinchillas (Chinchilla lanigera) (mean weight, 575 ± 78 g) with no evidence of middle ear infection by either otoscopy or tympanometry upon enrollment in the study were used. Animals were rested for 10 days upon arrival and were then nominally bled by cardiac puncture for collection of preimmune serum. Serum samples were stored at −70°C until used individually to verify that no significant existing immunoreactivity to any OMP of nontypeable H. influenzae strain 86-028NP was evident in any of the 44 chinchillas.
Chinchilla studies were approved by the Children's Research Institute Animal Care and Use Committee, and rat studies were approved by the University of Canberra Animal Ethics Committee.
Nontypeable H. influenzae and adenovirus isolates.
Nontypeable H. influenzae strains 86-028NP, 1128, 1729 MEE, and 1715 have been described previously (29), and these isolates were maintained frozen in skim milk plus 20% (vol/vol) glycerol. Strain 772 has also been described previously (21) and was stored frozen in brain heart infusion (BHI) broth containing 25% (vol/vol) horse blood and 10% (vol/vol) glycerol. Bacteria were recovered from frozen stocks, streaked onto chocolate agar (BBL, Cockeysville, Md.), and incubated at 37°C for 18 h in a humidified atmosphere containing 5% CO2. Adenovirus serotype 1 is a pediatric isolate and has also been described previously (30).
Immunization for pulmonary challenge.
DA rats were immunized mucosally with either recombinant lipoprotein D (20 μg), LPD-LB1(f)2,1,3 (10 μg), or conjugates of keyhole limpet hemocyanin (KLH) or bovine serum albumin (BSA) to a peptide representing LB1(f) group 1 (LB11), group 2a (LB12a), or group 3 (LB13) for a total of six immunogens, as detailed in Table 1. Lipoprotein D (1), LPD-LB1(f)2,1,3 (3), and the LB1(f) peptides (3, 4, 22) have been described elsewhere; however, the amino acid sequences of the immunogens used in the present study are also shown in Table 2. Control groups consisted of (i) untreated animals, (ii) sham-treated animals, for which incomplete Freund's adjuvant (Sigma) emulsified in phosphate-buffered saline (PBS) was used in the primary inoculation and PBS alone was used in the booster inoculation, and (iii) animals immunized with KLH (Sigma) and BSA (Sigma), administered at doses equivalent to those given the active-immunization group.
TABLE 1.
Immunogens and doses used in the rat studies
| Immunogen | Strain from which immunogen was derived | Immunization dose (μg)
|
|
|---|---|---|---|
| Primary | Boost | ||
| BSA-LB11 | NTHI 1128 | 10 | 10 |
| KLH-LB11 | NTHI 1128 | 10 | 10 |
| BSA-LB12a | NTHI 1729 | 10 | 10 |
| KLH-LB12a | NTHI 1729 | 10 | 10 |
| BSA-LB13 | NTHI 1715 | 10 | 10 |
| KLH-LB13 | NTHI 1715 | 10 | 10 |
| LB1(f) combinationa | 25 | 50 | |
| KLH-LB11 | (8.3) | (16.6) | |
| BSA-LB12a | (8.3) | (16.6) | |
| KLH-LB13 | (8.3) | (16.6) | |
| LPD-LB1(f)2,1,3a | 10 | 10 | |
| LPD | NTHI 772 | 20 | 20 |
The LB1(f) combination was a mix of three of the protein-LB1 conjugates, as indicated with the doses given in parentheses, and belonging to the different strains listed in the table.
TABLE 2.
Amino acid sequences of LB1 immunogens used in the rat and chichilla models of infection of the airway by nontypeable H. influenzaea
Underlined, lowercase sequences represent linker peptides inserted into the sequence of the recombinant polypeptide immunogen LPD-LB1(f)2,1,3. Red, consensus sequence of majority group 1 isolates; blue, consensus sequence of minority group 2a isolates; green, consensus sequence of minority group 3 isolates (3). All are given as incorporated into recombinant polypeptide immunogen LPD-LB1(f)2,1,3.
Immunization for middle ear challenge.
Rats were immunized with either lipoprotein D (20 μg), LPD-LB1(f)2,1,3 (10 μg), or a mix comprising KLH-LB1(f) (group 1), BSA-LB1(f) (group 2a), and KLH-LB1(f) (group 3) (Table 1). Control groups were as described above.
As previously described, LB1, used for chinchillas, is a 40-mer chimeric synthetic peptide that combines a B-cell epitope of P5 fimbrin [LB1(f)] with a promiscuous T-cell epitope of measles virus fusion protein. Recombinant OMP26 (rOMP26) with the 23-amino-acid leader peptide has been described previously (13).
Active immunization.
Immunization was performed essentially as described previously (13, 23, 25). Briefly, rats were sedated with sodium pentobarbital given intraperitoneally, and the small intestine was exposed to enable inoculation of intestinal Peyer's patches with a 1:1 mixture of the immunogen emulsified with incomplete Freund's adjuvant so that each rat received the doses described in Table 1 (total volume delivered was 50 μl). Fourteen days postimmunization, rats received a boost, delivered intratracheally to the lungs, of immunogen in 50 μl of PBS at the dose indicated in Table 1. A live nontypeable H. influenzae challenge was delivered 21 days postimmunization either intratracheally (pulmonary challenge) or intrabullarly (middle ear challenge). Pulmonary challenge has been described previously (21).
Each rat received approximately 5 × 108 CFU (viability count was confirmed by concurrent overnight culture). Intrabullar challenge was achieved with a 26-gauge needle to pierce the bulla that was exposed through an incision in the neck and injection of a 20-μl volume of live nontypeable H. influenzae at a concentration of 5 × 108 CFU/ml. The skin wound was sutured, and the animal was allowed to recover. Four hours after challenge, rats were sacrificed, and middle ear lavage fluid or bronchoalveolar lavage fluid and lung tissue were collected (where applicable).
Four cohorts of 11 chinchillas each were established. All immunizations were delivered subcutaneously, and boosting doses were received 30 days after the primary immunization. The primary doses were as follows: 10 μg of LB1 (the 40-mer chimeric peptide) in 500 μl of S5; 10 μg of LB1 in 200 μl of complete Freund's adjuvant; 10 μg of rOMP26 in 500 μl of S5; and 500 μl of S5 only. S5 is an adjuvant formulation that includes aluminum salts, monophosphoryl lipid A, and QS21. The boosting doses were identical; however, complete Freund's adjuvant was replaced with an equal volume of incomplete Freund's adjuvant for delivery of LB1 in the boost.
The cohort receiving LB1 in complete or incomplete Freund's adjuvant served as the positive control based on significant efficacy demonstrated previously (3, 4, 22). The adjuvant-only cohort served as a negative control. Ten days after the boost, all animals were bled by cardiac puncture for collection of immune serum. Serum samples were pooled by cohort and stored at −70°C until use. At this time, chinchillas also received 6 × 106 50% tissue culture infectious doses of adenovirus intranasally. Seven days later, chinchillas were challenged both intranasally and transbullarly with approximately 108 and 2,500 CFU of nontypeable H. influenzae 86-028NP, respectively. Actual challenge doses received were confirmed by plate count. Animal procedures have been described in detail before (3, 4).
Assessment of serum titer and specificity by ELISA and Western blot.
Enzyme-linked immunosorbent assay (ELISAs) were performed on dilutions of individual serum samples and bronchoalveolar lavage fluid or pooled middle ear lavage fluid from rats (within groups). Each was assayed against lipoprotein D and the 40-mer LB1 chimeric peptide. Polysorb microtiter wells (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) containing either lipoprotein D (1 μg/ml) or LB1 (5 μg/ml), and the assay was performed as previously described (13). Horseradish peroxidase-conjugated immunoglobulins used were goat anti-rat immunoglobulin G (IgG) (1:2,000) and IgA (1:1,000) (Fc specific; Nordic Immunological Laboratories), which were developed with the substrate tetramethylbenzidine (Fluka, Buchs, Switzerland) in phosphate citrate buffer, pH 5, containing 0.05% (vol/vol) H2O2. The reaction was stopped with 100 μl of 0.5 M H2SO4. Plates were read at 450 nm on a plate reader (Bio-Rad Laboratories, Hercules, Calif.). Plate background was determined by rows coated with coating buffer alone and treated the same as test wells, and between-plate variation was assessed by comparison of control samples repeated on each plate. Quantitation of anti-lipoprotein D or LB1 IgG and IgA was achieved by inclusion of standards for rat IgG ranging from 31 to 500 ng/ml and rat IgA ranging from 7.8 to 125 ng/ml (Serotec, Oxford, United Kingdom). Western blots were performed as previously described (13) with pooled serum samples (within group).
ELISA assays were performed with dilutions of pooled chinchilla serum samples from each cohort. Serum samples were assayed against nontypeable H. influenzae whole OMP preparation (from strain 86-028NP) (0.5 μg/well), rOMP26, and the LB1 40-mer chimeric peptide (0.2 μg/well) in 96-well microtiter plates (Dynatech, Horsham, Pa.) as previously described (3, 4, 29). The titer of each serum pool was defined as the reciprocal of the dilution consistently yielding an optical density at 490 nm value that showed a twofold increase over that of wells containing all components but immune serum. Western blotting was also performed as described previously (29), with pooled immune serum diluted 1:100 as the primary antibody and horseradish peroxidase-protein A (Zymed) diluted 1:200 as the secondary antibody. Color was developed with 4-chloro-1-naphthol (Sigma).
Assessment of bacterial clearance.
Four hours after nontypeable H. influenzae challenge, rats were killed by overdose of sodium pentobarbital (intraperitoneally). Blood was collected by cardiac puncture for serum. The trachea was exposed, and the lungs were lavaged with five 2-ml volumes of PBS, and this bronchoalveolar lavage fluid was pooled. The lungs were removed following lavage, freed of other connected tissues, and homogenized in 10 ml of PBS. Bronchoalveolar lavage fluid and lung homogenates were assessed for bacterial counts by plating 10-fold serial dilutions of the washes onto chocolate agar and incubated for CFU determination. At 4 h post-middle ear challenge, the rats were euthanized, and the middle ear was lavaged with three 50-μl volumes of PBS injected and recovered through the tympanic membrane. The pooled lavage fluid (middle ear lavage fluid) was assessed for bacterial counts as described. After assessment for bacterial counts, both middle ear lavage and bronchoalveolar lavage fluids were centrifuged at 1,000 rpm at 4°C for 10 min, and the supernatants were removed for storage at −20°C until required for antibody assays.
Chinchillas were blindly evaluated by otoscopy and tympanometry daily or every 2 days from the time of adenovirus inoculation until 35 days after nontypeable H. influenzae challenge. Signs of tympanic membrane inflammation were rated on a 0 to 4+ scale, and tympanometry was used to monitor changes in middle ear pressure, tympanic width, and tympanic membrane compliance as previously described (3, 22). Signs of respiratory tract infection, including ruffling of fur, conjunctivitis, altered character of nasal/ocular secretions, wheezing, labyrinthitis, and cornering behavior, were also recorded daily.
A nasopharyngeal lavage was performed on all chinchillas on days 1, 4, 7, 10, 14, 18, 21, 28, and 35 after nontypeable H. influenzae challenge by passive inhalation of 500 μl of pyrogen-free sterile saline. Epitympanic taps were attempted on the same schedule as nasopharyngeal lavage fluids when an effusion was considered to be of sufficient volume to be retrieved (any ears scored as 2.0 or greater for inflammation). Nasopharyngeal lavage and epitympanic tap fluids were maintained on ice until serially diluted. Dilutions of nasopharyngeal lavage fluids were then cultured on chocolate agar plates containing bacitracin (BBL) to limit growth of other normal nasopharyngeal flora. Dilutions of epitympanic tap fluids were cultured on chocolate agar plates (BBL). All plates were incubated at 37°C for 48 h to semiquantitate CFU of nontypeable H. influenzae/ml. Animals were also tabulated as having a colonized or cleared status based on culture results as previously described (3, 22). Finally, blood was obtained for serum samples from all chinchillas via cardiac puncture, prior to sacrifice, on day 35 postchallenge.
Statistical methods.
For the rat model data, the bacterial clearance and antibody data were assessed for statistical significance by an independent t test on log10-transformed data (Macintosh Systat). For the chinchilla model data, a log rank test was used to compare cohorts for relative time to bacterial clearance of the nasopharynx, as determined by culture-negative status, and illustrated with Kaplan-Meier survival analysis curves. Cox proportional hazard regression analyses were additionally performed to further elaborate the differences between the cohorts. An alpha level of 0.05 was accepted as significant and was adjusted for multiple comparisons with the method of Bonferroni.
RESULTS
Characterization of antisera.
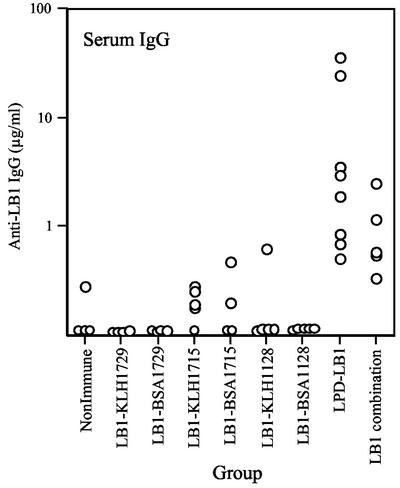
Antisera generated by rats against LB1(f) peptide conjugates and lipoprotein D were quantitated by ELISA by measurement of the reciprocal dilution that gave an optical density within the range of the rat immunoglobulin standards assayed on the same plate. LPD-LB1(f)2,1,3 was also assessed but the results were not different from those generated by the LB1(f) immunogens or lipoprotein D alone. Levels of IgG specific to the 40-mer LB1 in the serum samples were very low for all animals in the groups immunized with either the BSA or KLH conjugate. Significant titers (P < 0.05) were detected following immunization with either LPD-LB1(f)2,1,3 or the combination of KLH-LB11, BSA-LB12a and KLH-LB13 (Fig. 1). The only detectable LB1-specific antibody in the lavage were in two rats in the LPD-LB1(f)2,1,3 group for IgG, and no rats had detectable levels of IgA (data not shown). The lipoprotein D- and LPD-LB1(f)2,1,3-immunized groups had high levels of lipoprotein D-specific antibody in both serum samples and lavage (Table 3). There were low levels of anti-lipoprotein D IgG in both the serum samples and the lavage of some nonimmune rats; however, there was a >1,000-fold increase in the levels measured in the immunized groups. There was no significant difference in the levels of IgG or IgA to lipoprotein D following immunization with either lipoprotein D alone or the LPD-LB1(f)2,1,3 group construct.
FIG. 1.
LB1-specific levels of IgG in the serum of rats immunized with KLH or BSA conjugates of the different LB1(f) peptides or LPD-LB1(f)2,1,3.
TABLE 3.
Comparison of LPD-specific antibody
| Immunization group | Mean anti-LPD (μg/ml) as determined by ELISAa ± SEM (n = 5)
|
||
|---|---|---|---|
| Serum IgG | BAL
|
||
| IgG | IgA | ||
| Nonimmune | 5.4 ± 3.4 | 0.92 ± 0.60 | 0 |
| LPD | 14,200 ± 1,142b | 935 ± 195b | 119 ± 29b |
| LPD-LB1(f)2,1,3 | 11,472 ± 907b | 523 ± 43b | 92 ± 23b |
Antibody in serum and bronchalveolar lavage fluid (BAL) of rats post-mucosal immunization with either LPD or LPD-LB1(f)2,1,3 and at the time of bacterial challenge.
Titer significantly different from the nonimmune group (P < 0.005). There were no significant differences between the LPD and LPD-LB1(f)2,1,3 groups.
Antisera generated against LB1 or rOMP26 by chinchillas were characterized by ELISA and Western blotting for titer and specificity. Reciprocal serum antibody titers were determined in triplicate for each serum pool (Table 4) and showed that a high-titered and specific immune response had been elicited against each immunogen preparation. Animals receiving adjuvant only did not demonstrate a reciprocal immune titer greater than 103 against either a whole OMP preparation, LB1, or rOMP26. Please note that in the screening of preimmune serum samples of each animal enrolled in the present study by both ELISA and Western blot, there was no significant response noted to either OMP within the outer membrane of nontypeable H. influenzae strain 86-028NP, LB1 or rOMP26.
TABLE 4.
Reciprocal titers in chinchilla serum
| Cohort | Median reciprocal titera against:
|
||
|---|---|---|---|
| LB1 | rOMP26 | 86-028NP OMPb | |
| S5 | 1 × 103 | 100 | 1 × 103 |
| rOMP26/S5 | 550 | 5 × 104 | 1 × 103 |
| LB1/CFA | 5 × 104 | 1 × 103 | 1 × 103 |
| LB1/S5 | 5 × 104 | 100 | 1 × 103 |
Titers of ≥104 are shown in bold type.
OMP, whole outer membrane protein preparation.
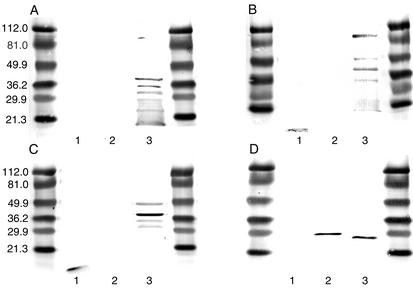
As confirmation, by Western blotting (Fig. 2), no reactivity was shown by the anti-S5 serum pool (panel A) against either LB1 or rOMP26. Chinchillas immunized with this strong adjuvant did recognize several outer membrane proteins within the nontypeable H. influenzae whole OMP preparation (Fig. 2, lane 3), as we have previously described for other strong adjuvants (22). However, anti-LB1/complete Freund's adjuvant (Fig. 2B) and anti-LB1/S5 (Fig. 2C) serum pools both recognized LB1 (Fig. 2, lane 1), as well as the fully and partially denatured species of P5 fimbrin in the whole OMP preparation from the nontypeable H. influenzae challenge strain 86-028NP (lane 3) as reported previously (3). Anti-rOMP26/S5 (Fig. 2D) recognized rOMP26 (lane 2) and a band of approximately 28 kDa (lane 3) in the 86-028NP OMP preparation which is presumed to be native OMP26. Note that the banding pattern typical of lane 3 in Fig. 2A, B, and C was also present in blots obtained with hyperimmune serum from chinchillas that had received rOMP26 plus the adjuvant S5; however, due to the speed with which the band recognizing rOMP26 in lane 2 and native OMP 26 in lane 3 developed, these are not apparent in lane 3 of Fig. 2D. Anti-rOMP26/S5 did not recognize LB1 in lane 1 of Fig. 2D, as expected.
FIG. 2.
Western blot composite showing reactivity of chinchilla samples with (A) anti-S5, (B) anti-LB1/complete Freund's adjuvant, (C) anti-LB1/S5, and (D) anti-rOMP26 against LB1 (lanes 1), rOMP26 (lanes 2), and a whole OMP preparation from nontypeable H. influenzae strain 86-028NP (lanes 3).
Clearance of rat lung.
Bacterial clearance was measured in the rats at 4 h postchallenge as has been described previously for this infection model (13, 14). There were differences in the ability of the different LB1(f) conjugates to enhance clearance of their homologous nontypeable H. influenzae strains (note: immunization with either BSA or KLH alone does not enhance clearance of nontypeable H. influenzae; data not shown). LB11 was most protective when conjugated to KLH, whereas LB12a only significantly cleared the nontypeable H. influenzae when conjugated to BSA (Table 5). LB13 was the least protective of the peptides and only significantly enhanced clearance of the bacteria from the lung tissue when conjugated to KLH. The degree of clearance from the lung tissue was comparable to that observed for the other two protective LB1(f) conjugates. KLH-LB11 and BSA-LB12a enhanced clearance more effectively from the bronchoalveolar area than from the lung tissue, as demonstrated by the clearance in the bronchoalveolar lavage fluid. There was an 84.5% and a 91.5% reduction in the bacterial load in the bronchoalveolar lavage fluid for the KLH-LB11- and BSA-LB12a-immunized groups, respectively, compared with 74.9% and 72.4% enhanced clearance in the lung tissue during the first 4 h of infection.
TABLE 5.
Bacterial clearance in rats following immunization with LPD and LB1(f) peptides conjugated to either BSA, KLH, or LPD and pulmonary challenge
| Immunization group | No. of rats in group | Challenge strain | Bacterial clearanceb (%)
|
|
|---|---|---|---|---|
| BAL | Lung homogenate | |||
| Nonimmune | 5 | NTHI 1128 | 0 ± 17.8 | 0 ± 13 |
| BSA-LB11 | 5 | 45 ± 18 | 22.4 ± 14 | |
| KLH-LB11 | 5 | 84.5 ± 13.8* | 74.9 ± 17.8* | |
| Nonimmune | 5 | NTHI 1729 | 0 ± 14 | 0 ± 12.9 |
| BSA-LB12a | 4 | 91.5 ± 20.4* | 72.4 ± 14.8* | |
| KLH-LB12a | 5 | 48.7 ± 17.3 | 25.8 ± 13.8 | |
| Nonimmune | 5 | NTHI 1715 | 0 ± 14.8 | 0 ± 13.1 |
| BSA-LB13 | 5 | 36.9 ± 15.4 | −2.3 ± 13.5 | |
| KLH-LB13 | 5 | 33.9 ± 17 | 71.2 ± 15.5* | |
| Nonimmune | 5 | NTHI 772 | 0 ± 13.8 | 0 ± 10.9 |
| LB1 combinationb | 5 | 85.9 ± 17.4* | 87.1 ± 13.2* | |
| Nonimmune | NTHI 772 | 0 ± 12.5 | 0 ± 12 | |
| LPD | 96.7 ± 19** | 93.1 ± 12.3** | ||
| LB1-LPD | 97.6 ± 13.5** | 94 ± 0.11** | ||
Percent clearance in the immune groups was calculated as 100 minus the mean CFU recovered from the immunized group divided by the mean of the nonimmune group. The mean for the nonimmune group was assigned as 0%. The error represents the standard error of the mean, expressed as a percentage. Lungs were collected after lavage, homogenized in PBS, and aliquoted for the presence of bacteria. BAL, bronchoalveolar lavage fluid. Significance: *, P < 0.05 compared to the nonimmune group on log10 CFU transformed data; **, P < 0.001 compared to the nonimmune group on log10 CFU transformed data.
LB1 combination comprised immunization with KLH-LB11, BSA-LB12a, and KLH-LB13 and as described in Table 1.
The three conjugates of LB1(f), KLH-LB11, BSA-LB12a and KLH-LB13, were combined for immunization, and the rats were challenged with the unrelated nontypeable H. influenzae strain 772. This combination significantly enhanced clearance from both the bronchoalveolar lavage fluid and lung tissue (P = 0.018 and P = 0.001, respectively) and represented reductions of 85.9% and 87.1%, respectively, in bacterial load over 4 h compared to the nonimmune group.
Immunization with lipoprotein D or LPD-LB1(f)2,1,3 resulted in significant clearance (P < 0.001) in both the bronchoalveolar lavage fluid and lung tissue (Table 5). The bacterial recovery corresponds to an enhanced reduction in bacterial load of 96.7% for lipoprotein D and 97.6% for LPD-LB1(f)2,1,3 in the bronchoalveolar lavage fluid and 93.1% and 94% in the lung tissue, respectively, compared to the nonimmune rats during the first 4 h of infection. This level of clearance was greater than that observed for LB1(f) peptides in this model.
Clearance of rat middle ear.
Unlike in the pulmonary model, there was no significant clearance of a middle ear infection in lipoprotein D- or LPD-LB1(f)2,1,3-immunized rat (Table 6), but there was for the LB1 combination-immunized group (P < 0.05), with an 82% reduction in bacterial load. Lipoprotein D immunization enhanced clearance of the infection in three of the six rats, indicating a tendency towards a protective response. However, immunization with LPD-LB1(f)2,1,3 did not result in clearance in any of the rats in the group. LPD-LB1(f)2,1,3 rats were immunized with two 10-μg doses, whereas lipoprotein D was administered in two 20-μg doses. The results would suggest that perhaps higher immunization doses or additional immunization boosts may result in a protective immune response in the middle ear in the rat model. It should be noted, however, that lipoprotein D has a more dominating antibody response than LB1 in this model. Clearance was significant following the LB1 combination experiments, and the anti-LB1 titers obtained were equivalent to anti-LB1 induced by the LPD-LB1 immunization. Increasing the dose of lipoprotein D from 10 to 20 μg appears to only slightly increase the anti-lipoprotein D response, so it is likely that increasing the dose may result in a similar differential clearance of infection response between animals within a group immunized with 20 μg of LPD-LB1 as was observed for 2-μg doses of lipoprotein D alone.
TABLE 6.
Bacterial clearance following middle ear challenge of rats with strain 772
Clearance of chinchilla nasopharynx.
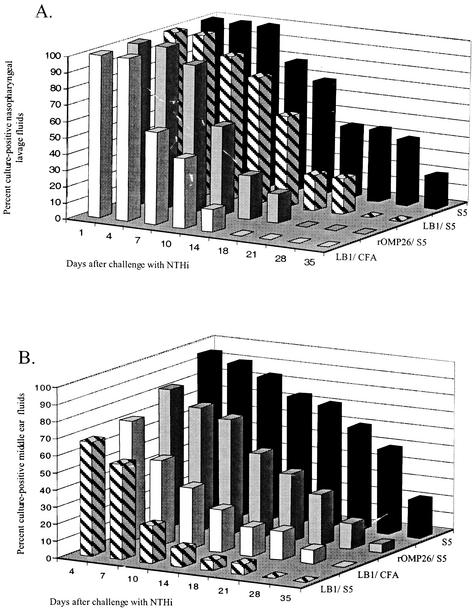
Intranasal challenge of chinchillas with nontypeable H. influenzae strain 86-028NP (actual dose, 9.4 × 107 CFU/animal) resulted in initial colonization of the nasopharynx within all cohorts, as verified by positive plate counts obtained from 95% of the nasal lavage fluids collected 1 day after challenge. Thereafter, the effect of immunization became evident as enhanced clearance in selected cohorts. The LB1/complete Freund's adjuvant cohort cleared nontypeable H. influenzae from the nasopharynx at least 17 days before the control cohort that received only S5 (Fig. 3A). The rOMP26/S5 cohort also rapidly cleared nontypeable H. influenzae from the nasopharynx. Nontypeable H. influenzae were not detected in nasopharyngeal lavage fluids of any animal in the rOMP26/S5 cohort by 21 days after challenge, 2 weeks earlier than the adjuvant-only cohort. The LB1/S5 cohort completely cleared nontypeable H. influenzae 28 days after challenge. The S5 cohort remained colonized 35 days after challenge, with 20% (2 of 10) of these animals remaining culture positive at this time point.
FIG. 3.
Three-dimensional bar graphs showing (A) percentage of animals per cohort that were culture positive for nontypeable H. influenzae in nasopharyngeal lavage fluids over time and (B) percentage of ears per cohort that were culture positive for nontypeable H. influenzae in middle ear fluids over time.
Cox regression analysis was used to compare the time to clearance of nontypeable H. influenzae from the nasopharynx among the cohorts. When each cohort was compared to the sham (S5) cohort, the LB1/complete Freund's adjuvant and rOMP26/S5 cohorts were significantly more likely to clear nontypeable H. influenzae from this site (P ≤ 0.01). There was no statistically significant difference noted for the LB1/S5 cohort (P ≤ 0.07); however, this cohort yielded clearance kinetics that were likely biologically relevant, as nontypeable H. influenzae was eradicated from the nasopharynx at least 7 days earlier than in the S5 cohort. Moreover, by day 18 after challenge, only 22% of animals were culture positive in the LB1/S5 cohort, versus 44% of the controls.
Clearance of chinchilla middle ear.
Chinchillas were also challenged transbullarly with an actual dose of 1.8 × 103 CFU/ear. Figure 3B illustrates the percentage of culture-positive middle ear fluids retrieved by epitympanic taps. The LB1/S5 cohort completely cleared nontypeable H. influenzae from the middle ears on day 28; however, by day 10 after challenge, 78% of all ears in this cohort (14 of 18) were already culture negative for nontypeable H. influenzae. The middle ear fluids in the LB1/complete Freund's adjuvant cohort were culture negative for nontypeable H. influenzae for all animals in the cohort only upon the final day of the study; however, on day 28 this cohort had only 8% of ears still culture positive for nontypeable H. influenzae versus 50% of the controls. The rOMP26/S5 and S5 cohorts remained culture positive on day 35; however, only 5% of ears in the rOMP26/S5 cohort remained culture positive compared to 22% in the S5 cohort at this time point. By day 18 after challenge, 60% of the rOMP26/S5 ears were already culture negative for nontypeable H. influenzae, versus 28% of the S5 cohort animal's ears.
Cox regression analysis was used to compare the time to clearance of nontypeable H. influenzae from middle ear fluids among the cohorts. When each cohort was compared to the sham (S5) cohort, the LB1/S5 and LB1/complete Freund's adjuvant cohorts were found to be significantly more likely to clear nontypeable H. influenzae from this site (P ≤ 0.001 and 0.005, respectively). The rOMP26/S5 cohort data yielded a P value of 0.02 that was determined to be not statistically significant due to the adjustment for multiple comparisons; however, the clearance kinetics generated in this cohort would suggest biological relevance.
DISCUSSION
Many factors contribute to both the pathogenesis of bacterial otitis media and the host's ability to resolve these infections and return the tympanum to homeostatic conditions. It has become apparent, through the efforts of many laboratories, that the greatest likelihood for the successful development of a nontypeable H. influenzae-directed vaccine relies upon the careful selection of conserved and protective immunogens (16, 27). Ideally, the vaccine must induce a balanced and broadly protective immune response in very young children.
Toward this goal, our laboratories have focused recently on two nontypeable H. influenzae outer membrane proteins that have the shared qualities of at least partial surface exposure, relative conservancy, and the ability to induce an immune response that results in significantly augmented clearance of bacteria upon challenge. OMP26 is a well-conserved surface protein of approximately 26 kDa with homology to other Skp transporter proteins and to OmpH (13, 23). Rats immunized with OMP26 via a mucosal route respond in a dose-dependent fashion (21). Following homologous or heterologous challenge, OMP26-immunized animals demonstrate significantly enhanced pulmonary clearance 4 h after challenge. Recombinant forms of OMP26 (one possessing and the other lacking the 23-amino-acid leader peptide) induce significant IgG and IgA titers in bronchoalveolar lavage fluid and serum; however, the larger preprotein is more efficacious at enhancing pulmonary clearance and inducing a greater cell-mediated immune response (13). Moreover, the larger 28-kDa rOMP26 induces higher overall levels of systemic and mucosal antibodies equivalent to those induced by the isolated native protein.
Likewise, immunogens derived from an area of limited diversity (4, 12, 31) within the OMP P5-homologous adhesin P5 fimbrin induce the production of antibodies, upon parenteral immunization, that significantly augment clearance of a homologous challenge isolate from the middle ears and nasopharynges of chinchillas (3, 4). Moreover, passive transfer of P5 fimbrin-directed antibodies significantly prevents the development of otitis media in animals receiving either anti-LB1 or anti-LPD-LB1(f)2,1,3. This protection is conferred against both homologous and heterologous challenge (3, 4, 22, 27).
While both the rat and the chinchilla model systems measure reduction in bacterial counts from recovered lavage fluids as one outcome for judging efficacy, each model is also unique in multiple ways. The advantages of the chinchilla model of otitis media include the sustainability of nontypeable H. influenzae in the nasopharynx and middle ear over several weeks. In contrast, the rat model investigates both lung and middle ear infections, focusing on the capacity of the immune system to invoke an early response to the presence of nontypeable H. influenzae. The rat model is one of mucosal immunization, with the primary dose delivered directly to the Peyer's patches, followed by a boost via the trachea 14 days later (23, 25). Direct challenge of the lungs or middle ears occurs 7 days after the boost. Bacteria recovered in bronchoalveolar lavage fluid, middle ear lavage fluid, and lung homogenates can be measured 4 or 6 h after challenge of the lung and middle ear, respectively. Also, absolute and differential counts of white blood cells recruited to these sites can be made, the induced immunoglobulins can be isotyped, and specific cytokines produced during infection can be measured (14, 15).
Conversely, the chinchilla model is one of either active parenteral immunization, as described here, or can be one of passive transfer. Animals are then directly challenged in the middle ears and nasopharynx. or, after passive transfer, juvenile animals are challenged exclusively intranasally, allowing the bacteria to ascend the virus-compromised eustachian tube to induce otitis media (3, 4, 22). Bacterial counts from middle ear and nasopharyngeal lavage fluids are determined over a 5-week period of observation, and total immunoglobulin titer (predominantly IgG) is assayed. Tympanic membrane inflammation and changes in middle ear pressure are recorded for the entire disease course. Histopathological assessment of the tympanic membrane and inferior bulla mucosa are also typically evaluated (29). Therefore, to further strengthen the argument for development of each of these immunogens, we wanted to evaluate each in the reciprocal host system. Our hypothesis was that demonstrated efficacy in two rodent models, each with a different immunization regimen, adjuvant, and schedule of dosing, boosting, challenging, and assessing, would be extremely supportive of data gathered previously in a single host model.
In the study of OMP26 in the chinchilla model, we have shown that rOMP26 induced a high-titered and specific immune response that was highly efficacious in the chinchilla model. Delivery with the adjuvant S5 resulted in significantly earlier clearance of nontypeable H. influenzae from the nasopharynx of all animals in the cohort. While clearance of nontypeable H. influenzae from the directly challenged middle ear of rOMP26-immunized chinchillas was not shown to be significantly enhanced (a P value of 0.0167 was required when the adjustment was made for multiple comparisons), a P value of 0.02 was obtained and is highly likely to be biologically significant nonetheless. Due to the observed significantly enhanced clearance of nontypeable H. influenzae from the nasopharynx following immunization with rOMP26, future testing of this immunogen for efficacy would be more relevant if conducted in a model in which bacteria are first allowed to colonize the nasopharynx prior to ascending the eustachian tube. The ability of this immunogen to rapidly clear nontypeable H. influenzae from the nasopharyngeal colonization site would likely greatly diminish the incidence of otitis media that developed in the latter model.
Likewise, P5 fimbrin-derived immunogens proved efficacious in the rat lung and middle ear clearance studies following a mucosal immunization regimen. Demonstrated ability to enhance pulmonary clearance is a prerequisite in our laboratory for further assessment of any immunogen in the more rigorous and tedious rat middle ear challenge model. Here both LB1(f) conjugates and LPD-LB1(f)2,1,3 were shown to induce significantly augmented clearance of nontypeable H. influenzae from both bronchoalveolar lavage fluid fluids and lung homogenates following mucosal immunization. No significant difference was noted between the two immunogens and clearance compared with that observed following lipoprotein D immunization.
The 40-mer chimeric peptide immunogen LB1 has been characterized previously (3, 4, 20) and shown to clear nontypeable H. influenzae from the middle ear and nasopharynx of chinchillas. In LB1, a 19-mer putative B-cell epitope from the nontypeable H. influenzae isolate 1128 [called LB1(f) and incorporated into the group 1 peptide conjugates used here to immunize rats] has been fused to a “promiscuous” T-cell epitope from the measles virus protein to create a 40-mer peptide. This construct was efficacious in clearing the nontypeable H. influenzae infection in chinchillas. However, this group 1 peptide-containing immunogen potentially, if no cross-protection access, only protects against approximately 76% of strains according to the distribution in 99 clinical isolates that were sequenced (3). In that study the LPD-LB1(f)2,1,3 protein was constructed to confer broader coverage by the anti-LB1(f) response. In the current study, we have constructed BSA and KLH conjugates of each LB1(f) peptide from the three groupings and investigated their efficacy against homologous nontypeable H. influenzae pulmonary challenge following mucosal immunization in the rat model. The most protective conjugates from each group were then combined as immunogens for heterologous nontypeable H. influenzae pulmonary and middle ear challenges. These results were compared with that achieved by the immunogens LPD-LB1(f)2,1,3 and LPD.
The results showed that the KLH conjugates were better for the first and third peptide groups, whereas BSA was the preferred conjugate for the second group peptide. While each of these peptides enhanced clearance of the homologous nontypeable H. influenzae strain, there were no significant titers of IgG or IgA detected to either the 40-mer peptide LB1 (Fig. 1) or LPD-LB1(f)2,1,3 (data not shown). When KLH-LB11, BSA-LB12a, and KLH-LB13 were combined, there was significant clearance of nontypeable H. influenzae following both pulmonary and middle ear challenge with a heterologous strain and measurable titers of IgG to LB1 in the serum. The titers of anti-LB1 induced by the combination immunizations were not different from the anti-LB1 induced by the LPD-LB1(f)2,1,3 protein. These results support the concept of inclusion of peptides from the three groups in a vaccine. Mucosal immunization with either lipoprotein D or LPD-LB1(f)2,1,3 enhanced clearance of a pulmonary nontypeable H. influenzae challenge; however, neither achieved significant clearance of the middle ear challenge, although lipoprotein D did show a trend towards clearance. This may be a dose effect for efficacy in the middle ear because lipoprotein D was administered in 20-μg doses and LPD-LB1(f)2,1,3 in 10-μg doses, these being the doses that enhanced the pulmonary nontypeable H. influenzae challenge. Increasing the doses of these immunogens may be required to enhance clearance in the middle ear of rats.
In summary, nontypeable H. influenzae OMP26 has previously been shown to be a highly efficacious mucosal immunogen, inducing significantly augmented clearance of bacteria from the rat lung. Here we showed that when used as a parenteral immunogen, rOMP26 is also highly effective at inducing an immune response that leads to significantly enhanced clearance of the chinchilla nasopharynx. Similarly, lipoprotein D and the P5 fimbrin-based immunogens LPD-LB1(f)2,1,3 and several BSA or KLH conjugates of LB1(f) delivered singly as well as in combination proved to be protective in a rat model of pulmonary and middle ear clearance following mucosal immunization. This study has thereby demonstrated that both protein and peptide constructs can confer protection against nontypeable H. influenzae infection, that these immunogens were highly efficacious in reciprocal animal systems and that the immunogens can confer protection when delivered either mucosally or parenterally.
Acknowledgments
This study was supported by grants from Glaxo SmithKline Biologicals and grant R01 DC02830-04 from NIDCD/NIH. We thank Glaxo SmithKline Biologicals, Belgium, for supplying some of the LB1 and lipoprotein D constructs.
We thank Joseph Jurcisek, Bobbie-Jo Kennedy, Jolene DeFiore-Hyrmer, Marilyn Kennedy, Claire Batum, Nancy Fisher, and Mojca Keglovic for technical assistance. We are also grateful to Jim Rauscher for chinchillas, Gillian Nolan for rats, Lynn Mitchell for biostatistical analyses, Joe Jurcisek for generation of figures, and Jennifer Neelans for preparation of the manuscript.
Editor: B. B. Finlay
REFERENCES
- 1.Akkoyunlu, M., A. Melhus, C. Capiau, O. van Opstal, and A. Forsgren. 1997. The acylated form of protein D of Haemophilus influenzae is more immunogenic than the nonacylated form and elicits an adjuvant effect when it is used as a carrier conjugated to polyribosyl ribitol phosphate. Infect. Immun. 65:5010-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsarraf, R., C. J. Jung, J. Perkins, C. Crowley, N. W. Alsarraf, and G. A. Gates. 1999. Measuring the indirect and direct costs of acute otitis media. Arch. Otolaryngol. Head Neck Surg. 125:12-18. [DOI] [PubMed] [Google Scholar]
- 3.Bakaletz, L. O., B. J. Kennedy, L. A. Novotny, G. Dequesne, J. Cohen, and Y. Lobet. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 67:2746-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakaletz, L. O., E. R. Leake, J. M. Billy, and P. T. Kaumaya. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15:955-961. [DOI] [PubMed] [Google Scholar]
- 5.Barenkamp, S. J. 1996. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect. Immun. 64:1246-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barenkamp, S. J. 1986. Protection by serum antibodies in experimental nontypable Haemophilus influenzae otitis media. Infect. Immun. 52:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barenkamp, S. J., and J. W. St. Geme 3rd. 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol. Microbiol. 19:1215-1223. [DOI] [PubMed] [Google Scholar]
- 8.Bondy, J., S. Berman, J. Glazner, and D. Lezotte. 2000. Direct expenditures related to otitis media diagnoses: extrapolations from a pediatric medicaid cohort. Pediatrics 105:E72.. [DOI] [PubMed] [Google Scholar]
- 9.Brook, I., and A. E. Gober. 1999. Resistance to antimicrobials used for therapy of otitis media and sinusitis: effect of previous antimicrobial therapy and smoking. Ann. Otol. Rhinol. Laryngol. 108:645-647. [DOI] [PubMed] [Google Scholar]
- 10.DeMaria, T. F., D. M. Murwin, and E. R. Leake. 1996. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect. Immun. 64:5187-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derecola, A., D. L. Butler, R. L. Kaplan, L. A. Miller, and J. A. Poupard. 1999. A 5-year surveillance study of 44,691 isolates of Haemophilus influenzae project Beta-Alert 1993-1997. Antimicrob. Agents Chemother. 43:185-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duim, B., L. D. Bowler, P. P. Eijk, H. M. Jansen, J. Dankert, and L. van Alphen. 1997. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect. Immun. 65:1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Adhami, W., J. M. Kyd, D. A. Bastin, and A. W. Cripps. 1999. Characterization of the gene encoding a 26-kilodalton protein (OMP26) from nontypeable Haemophilus influenzae and immune responses to the recombinant protein. Infect. Immun. 67:1935-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foxwell, A. R., J. M. Kyd, and A. W. Cripps. 1998. Characteristics of the immunological response in the clearance of non-typeable Haemophilus influenzae from the lung. Immunol. Cell Biol. 76:323-331. [DOI] [PubMed] [Google Scholar]
- 15.Foxwell, A. R., J. M. Kyd, and A. W. Cripps. 1998. Kinetics of inflammatory cytokines in the clearance of non-typeable Haemophilus influenzae from the lung. Immunol. Cell Biol. 76:556-559. [DOI] [PubMed] [Google Scholar]
- 16.Foxwell, A. R., J. M. Kyd, and A. W. Cripps. 1998. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol. Mol. Biol. Rev. 62:294-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green, B. A., M. E. Vazquez, G. W. Zlotnick, G. Quigley-Reape, J. D. Swarts, I. Green, J. L. Cowell, C. D. Bluestone, and W. J. Doyle. 1993. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect. Immun. 61:1950-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, M., and E. R. Wald. 1996. Emerging resistance to antibiotics: impact on respiratory infections in the outpatient setting. Ann. Allergy Asthma Immunol. 77:167-173. [DOI] [PubMed] [Google Scholar]
- 19.Gu, X. X., J. Sun, S. Jin, S. Barenkamp, D. Lim, J. Robbins, and J. Battey. 1997. Detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins confers protection against otitis media in chinchillas. Infect. Immun. 65:4488-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase, E. M., A. A. Campagnari, J. Sarwar, M. Shero, M. Wirth, C. U. Cumming, and T. F. Murphy. 1991. Strain-specific and immunodominant surface epitopes of the P2 porin protein of nontypeable Haemophilus influenzae. Infect. Immun. 59:1278-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janson, H., L. O. Heden, A. Grubb, M. R. Ruan, and A. Forsgren. 1991. Protein D, an immunoglobulin D-binding protein of Haemophilus influenzae: cloning, nucleotide sequence, and expression in Escherichia coli. Infect. Immun. 59:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy, B. J., L. A. Novotny, J. A. Jurcisek, Y. Lobet, and L. O. Bakaletz. 2000. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect. Immun. 68:2756-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyd, J. M., and A. W. Cripps. 1998. Potential of a novel protein, OMP26, from nontypeable Haemophilus influenzae to enhance pulmonary clearance in a rat model. Infect. Immun. 66:2272-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyd, J. M., A. W. Cripps, and T. F. Murphy. 1998. Outer-membrane antigen expression by Moraxella (Branhamella) catarrhalis influences pulmonary clearance. J. Med. Microbiol. 47:159-168. [DOI] [PubMed] [Google Scholar]
- 25.Kyd, J. M., M. L. Dunkley, and A. W. Cripps. 1995. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect. Immun. 63:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loosmore, S. M., Y. P. Yang, R. Oomen, J. M. Shortreed, D. C. Coleman, and M. H. Klein. 1998. The Haemophilus influenzae HtrA protein is a protective antigen. Infect. Immun. 66:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poolman, J. T., L. Bakaletz, A. Cripps, P. A. Denoel, A. Forsgren, J. Kyd, and Y. Lobet. 2000. Developing a nontypeable Haemophilus influenzae (nontypeable H. influenzae) vaccine. Vaccine 19(Suppl. 1):S108-S115. [DOI] [PubMed] [Google Scholar]
- 28.Richter, S. S., P. L. Winokur, A. B. Brueggemann, H. K. Huynh, P. R. Rhomberg, E. M. Wingert, and G. V. Doern. 2000. Molecular characterization of the beta-lactamases from clinical isolates of Moraxella (Branhamella) catarrhalis obtained from 24 U. S. medical centers during 1994-1995 and 1997-1998. Antimicrob. Agents Chemother. 44:444-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sirakova, T., P. E. Kolattukudy, D. Murwin, J. Billy, E. Leake, D. Lim, T. DeMaria, and L. Bakaletz. 1994. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect. Immun. 62:2002-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki, K., and L. O. Bakaletz. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect. Immun. 62:1710-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb, D. C., and A. W. Cripps. 1998. Secondary structure and molecular analysis of interstrain variability in the P5 outer-membrane protein of non-typeable Haemophilus influenzae isolated from diverse anatomical sites. J. Med. Microbiol. 47:1059-1067. [DOI] [PubMed] [Google Scholar]
- 32.Webb, D. C., and A. W. Cripps. 2000. A P5 peptide that is homologous to peptide 10 of OprF from Pseudomonas aeruginosa enhances clearance of nontypeable Haemophilus influenzae from acutely infected rat lung in the absence of detectable peptide-specific antibody. Infect. Immun. 68:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]