Abstract
Chagas' disease, the leading cause of heart failure in Latin America, results from infection with the intracellular protozoan parasite Trypanosoma cruzi. Host cell responses elicited in the myocardium early in the infective process are thought to be critical for establishment of infection by this pathogen; however, these changes have not been well characterized. We report here that primary cardiomyocytes undergo hypertrophy as an early response to T. cruzi infection. The T. cruzi-elicited hypertrophic response is characterized by increased expression of genes encoding the contractile proteins MyHCβ and MyHCα, followed by an approximately twofold increase in cell size. Hypertrophy was observed in both parasite-containing and noninfected cell populations represented in T. cruzi-infected cultures, indicating the involvement of a soluble mediator in this process. Conditioned medium harvested from T. cruzi-infected cultures, which contained significant levels of interleukin-1β (IL-1β) but not endothelin-1 or tumor necrosis factor alpha, was sufficient to induce hypertrophy in isolated cardiomyocytes. Addition of a high-affinity receptor chimera, IL-1 trap, to cardiomyocyte cultures blocked the overall increase in cell size elicited by T. cruzi. These novel findings indicate that IL-1β, which is rapidly induced in response to T. cruzi, promotes cardiomyocyte hypertrophy early in the infective process and may contribute to maintenance of cardiomyocyte function during establishment of T. cruzi infection in the heart.
The intracellular protozoan pathogen Trypanosoma cruzi causes Chagas' disease, the leading cause of cardiomyopathy and heart failure in regions of endemicity in Latin America (19). While the acute stages of Chagas' disease are generally asymptomatic, the ability of T. cruzi to infect and persist within cells of the myocardium is a prerequisite for the development of Chagasic cardiomyopathy (25). Modulation of host cellular responses early in the T. cruzi infective process promotes parasite internalization (4) and is likely to influence intracellular survival of this pathogen. Thus, initial cardiomyocyte responses to T. cruzi are predicted to play a critical role in the establishment of long-term infection in the heart and thereby influence disease progression. Several studies have examined T. cruzi-dependent activation of host cell signaling pathways (4) and transcriptional responses (24) in a wide range of nonprofessional phagocytic cells; however, few have addressed T. cruzi-induced responses in cardiac myocytes (10, 15), the critical site of infection and pathology in the host.
Cardiomyocytes are postmitotic cells that respond to a variety of cellular stresses by initiating a hypertrophic program, which typically involves reactivation of an embryonic pattern of gene expression, increased production of myofibrils followed by an increase in cell size (20). Hypertrophy is a common endpoint that can be initiated in cardiomyocytes by a variety of external stimuli through multiple signaling pathways. Despite the potential for extensive cross talk between early signaling pathways, distinct molecular and morphological patterns of hypertrophy can arise from different stimuli (8, 18, 20). The net effect of these signaling, transcriptional, and cellular changes is increased myocardial contractility and increased cardiac output, ensuring maintenance of cardiac function in the short term. If prolonged, cardiac hypertrophy can lead to failure (12).
In Chagasic cardiomyopathy, hypertrophy has generally been considered to be a feature of chronic disease: i.e., an indirect consequence of persistent inflammation and myocardial damage (2). Recent studies have shown increased expression in rodents of a vasoactive peptide, endothelin-1 (ET-1), and a hypertrophic factor, cardiotrophin-1 (CT-1), as early as 10 to 15 days postinfection with T. cruzi (6). ET-1 has been implicated in the pathogenesis of Chagas' disease and is established as a causative agent of cardiac hypertrophy in T. cruzi-infected animals (14, 16). Because several factors with hypertrophic activity, including the proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α), are more rapidly induced in the hearts of T. cruzi-infected animals (7, 21), these observations suggest that cardiomyocyte hypertrophy is likely to be an important feature of acute as well as chronic Chagas' disease. Here, we demonstrate that within the first 48 h of infection, T. cruzi elicits hypertrophy in isolated cardiomyocytes, characterized by an increase in mean cell size and upregulation of genes encoding contractile proteins. Our data demonstrate that IL-1β, secreted by T. cruzi-infected cardiomyocytes, is a critical soluble mediator of the overall hypertrophic response for both parasite-infected and uninfected cell populations.
MATERIALS AND METHODS
Cardiomyocyte isolation.
Primary cultures of ventricular cardiomyocytes from neonatal Sprague-Dawley rats (Charles River Laboratories) were prepared as described previously (3). Briefly, pups were sacrificed, and their hearts were removed and placed in Hanks' balanced salt solution (HBSS). Ventricular tissue was digested overnight in HBSS-1% trypsin (Sigma), washed, and further digested with HBSS-1% collagenase (Worthington Biochemical). To enrich for cardiomyocytes, isolated cells were preplated twice in Dulbecco's modified Eagle's medium (DMEM) onto 10-cm2 dishes, resulting in supernatants containing ∼90 to 95% cardiomyocytes. Cells were then plated onto laminin-coated dishes (BD Biosciences), allowed to adhere for 24 h, and treated with 1 mM bromodeoxyuridine. Cells were shifted to serum-free media 24 h prior to use in experiments.
Parasite infections.
T. cruzi (Y strain) was propagated in monolayers of LLC-MK2 cells in DMEM (Gibco) with 2% fetal bovine serum (FBS), and infective trypomastigotes were harvested as described previously (23). Parasites were washed in DMEM, and 2 ×107 parasites per ml were incubated with cardiomyocytes for 2 h at 37°C in 5% CO2 to allow invasion. The remaining extracellular parasites were aspirated, fresh medium was added, and cultures were incubated for a total of 8, 24, or 48 h. Where indicated, cardiomyocytes were stimulated with ET-1 (10−7 M), CT-1 (1 nM), or IL-1β (1 ng/ml) and/or incubated in the presence of the murine homologue of the receptor chimera IL-1 trap (1 μg/ml) (9), generously provided by N. Stahl (Regeneron Pharmaceuticals).
CCM.
To generate cardiomyocyte-conditioned media (CCM), cardiomyocytes were mock treated or infected with T. cruzi for 48 h as described above. Culture supernatants were collected, filtered through a 0.4-μm-pore-diameter membrane (Millipore), and incubated with serum-starved cardiomyocytes for 24 h.
Immunofluorescence.
Following infection of cardiomyocytes on coverslips, cells were rinsed with phosphate-buffered saline (PBS) and fixed with 2% paraformaldehyde-PBS. Cardiomyocytes were stained with a mouse anti-α-actinin monoclonal antibody (Sigma) (1:1,000) followed by rabbit anti-mouse Alexa 488 (1:2,000) (Molecular Probes) in Tris-buffered saline (TBS)-1% bovine serum albumin (BSA) containing 0.1% saponin. Parasites were immunostained with a rabbit anti-T. cruzi antibody (23) followed by Alexa 488 anti-rabbit (1:2,000) (Molecular Probes). DNA was stained with DAPI (4′,6′-diamidino-2-phenylindole). Slides were visualized with a Nikon TE300, and images were captured with an Orca-100 charge-coupled device camera (Hammamatsu).
FACS analysis.
Following various treatments, cardiomyocytes were trypsinized, and isolated cells fixed with 2% PFA-PBS. Immunostaining with a rabbit anti-T. cruzi antibody followed by Alexa 488 goat-anti-rabbit (1:3000) (Molecular Probes) was carried out as described above. A minimum of 10,000 cells for each condition were analyzed by fluorescence-activated cell sorter (FACS) (FACScalibur; Becton Dickinson) measuring fluorescein isothiocyanate (FITC) fluorescence and forward scatter (FSC). Data were analyzed with Cell Quest software (Becton Dickinson). The geometric mean of FSC data is used as a correlate of cell size. Fold change in population cell size was calculated as the ratio geometric mean FSC (treated)/geometric mean FSC (control).
RPA.
The RNase protection assay (RPA) was performed as follows. 32P-labeled probes specific for the genes coding for GAPDH, SERCA, myosin heavy chain α (MyHCα), and MyHCβ were generated by T7 RNA polymerase transcription (Maxiscript; Ambion) from 1 μg of gel-purified plasmid DNA (plasmids generously provided by Mark Jeong, Denver Health Medical Center). Probes were hybridized to 5 μg of total RNA extracted from cardiomyocytes at 8 and 24 h postinfection according to the manufacturers' recommendations (RPA III; Ambion). Protected probes were separated on 6% polyacrylamide gels mixed with Tris-borate-EDTA (TBE), and the relative intensity of each probe was determined by PhosphorImager analysis (Molecular Dynamics).
Northern hybridization.
Ten micrograms of total RNA was prepared from cardiomyocytes by using RNAeasy (Qiagen), separated on 1% agarose gels containing 0.2 M formaldehyde, and blotted onto Hybond N+ nylon membranes (Amersham). Membranes were hybridized sequentially with 32P-labeled probes (Rediprime II; Amersham-Pharmacia) for atrial natriuretic factor (ANF) (kindly provided M. Kuroski de Bold, Ottawa Heart Institute) or rat GAPDH (Clontech) in ExpressHYB (Clontech) and exposed to Hyperfilm (Amersham-Pharmacia), and densitometric analysis was performed.
ELISA.
Five hundred microliters of culture supernatant collected from control, ET-1-stimulated, or T. cruzi-infected monolayers at 24 and 48 h posttreatment was clarified by centrifugation at 14,000 × g for 10 min. Secreted IL-1β, TNF-α, and ET-1 were measured in supernatants using antibody-specific ELISA kits as directed by the manufacturer (R&D for TNF-α and IL-1β; Peninsula Labs for ET-1) and analyzed with a Spectra Max 190 enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices).
Statistical analysis.
Student's t test was used for comparison between control and experimental samples. Data represented as fold change over control were analyzed with a one-sample t test. Values of P < 0.05 were considered significant.
RESULTS
Cardiomyocyte hypertrophy is an early host response to T. cruzi infection.
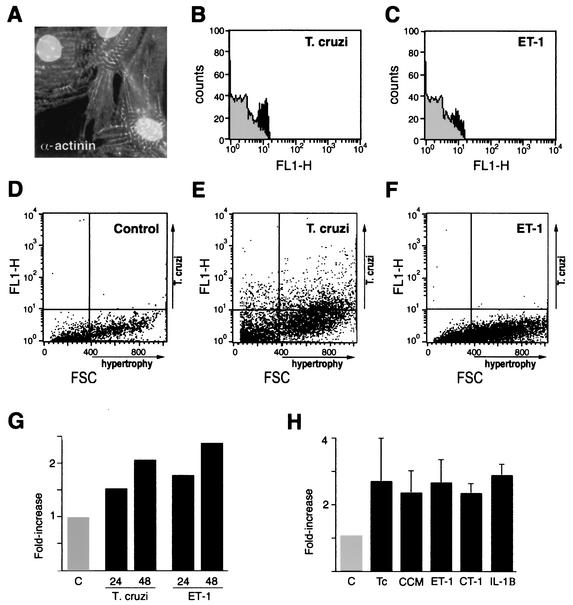
T. cruzi infection of myocardial cells is a decisive step in the pathogenesis of Chagas' disease; however, little is known regarding early responses of cardiomyocytes to this pathogen. To determine whether T. cruzi infection induces adaptive hypertrophy early in the infective process, FACS analysis of cultured, infected cardiomyocytes was carried out to examine changes in cell size. As compared to mock-infected controls, a marked increase in the population cell size was observed in cardiomyocytes analyzed from T. cruzi-infected cultures, as indicated by increased sarcomeric α-actinin staining (Fig. 1A and B) and FCS analysis (Fig. 1D to G), in which significant increases in cell size were observed by 48 h postinfection. Similar results were obtained following treatment of cardiomyocytes with ET-1, a well-characterized hypertrophic stimulant (Fig. 1C, F, and G). Since the efficiency of T. cruzi infection of cardiomyocytes varied between 25 and 60% of the total cells in culture, cardiomyocyte populations analyzed by flow cytometry contained both parasite-infected and noninfected cells. To distinguish the parasite-containing cell population from the noninfected cells, intracellular staining for T. cruzi was carried out prior to FACS analysis (Fig. 1D to F). As demonstrated (Fig. 1E), hypertrophic cells were detected in both parasite-infected and noninfected cardiomyocyte populations, suggesting a role for soluble mediators in this process.
FIG. 1.
T. cruzi induces hypertrophy in isolated cardiomyocytes. (A) Immunofluorescence staining of sarcomeric α-actinin in fixed cardiomyocytes. (B and C) FACS analysis of relative α-actinin expression in cardiomyocytes infected with T. cruzi (B; black) or stimulated with ET-1 (C; black) for 48 h compared to mock-treated controls (gray). Shown are representative plots depicting relative cell size by FSC of mock-treated (D), T. cruzi-infected (E), and ET-1-stimulated (F) cardiomyocytes stained with anti-T. cruzi antibodies to detect intracellular parasites (y axis). (G) T. cruzi infection of cardiomyocytes causes an increase in population cell size. FSC analysis of fixed cardiomyocytes infected with T. cruzi or treated with ET-1 for 24 or 48 h is shown. The data are represented as the ratio of the geometric mean of the treated cell population to that of the mock-treated control. (H) Cardiomyocyte hypertrophy induced following T. cruzi infection (Tc) or stimulation of cells with conditioned medium from T. cruzi-infected cells (CCM) and represented as an average from three independent experiments ± standard deviation.
To determine whether soluble factors present in T. cruzi-infected cardiomyocyte cultures participate in the hypertrophic response, medium harvested from mock-treated or T. cruzi-infected cultures was used to stimulate uninfected cardiomyocytes. CCM from T. cruzi-infected cells alone was capable of inducing a significant increase in population cell size compared to medium from mock-treated controls (Fig. 1H). The effect of CCM mimicked that of T. cruzi infection or stimulation with the hypertrophic agents ET-1, CT-1, and IL-1β (Fig. 1H). Combined, the data indicate that T. cruzi induces a rapid hypertrophic response in cardiomyocytes that is mediated, at least in part, by a diffusible host cell or parasite factor(s) released into the medium from infected cardiomyocytes.
Atypical modulation of cardiomyocyte hypertrophic response genes by T. cruzi.
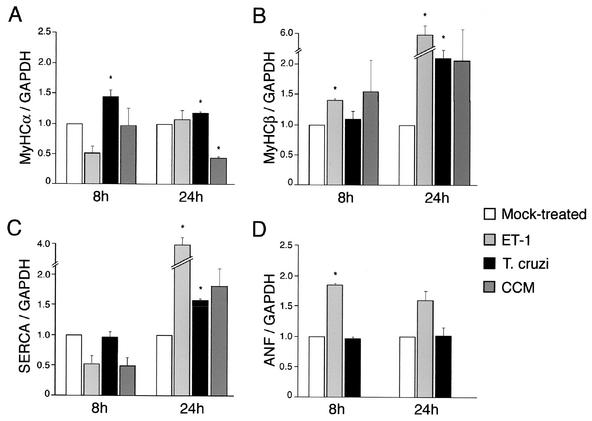
Reversion to the fetal program of cardiomyocyte gene expression is central to many hypertrophic response pathways and commonly characterized by reactivation of ANF and MyHCβ gene expression and downregulation of the genes coding for MyHCα and SERCA (18). To assess whether T. cruzi infection affects expression of these hypertrophic response genes, their expression relative to that of the gene coding for GAPDH was determined at 8 and 24 h postinfection in at least three independent experiments (Fig. 2). Because ET-1 has been implicated as an important soluble mediator of cardiac hypertrophy in Chagasic cardiomyopathy (14), it was used as a positive hypertrophic stimulant in these experiments. Overall, the expression data represented in Fig. 2 demonstrate that significant and reproducible increases in the expression of the MyHCα, MyHCβ, and SERCA genes occur following infection of cardiomyocytes with T. cruzi. Notable differences in the profile of gene expression were seen when comparing the cardiomyocyte responses to T. cruzi, ET-1, and CCM. Most striking was the observation that ANF transcript levels were consistently unaltered by T. cruzi, while a significant increase in ANF expression was observed in response to ET-1 (Fig. 2D). In addition, increased MyHCα transcript abundance was observed at 8 and 24 h following T. cruzi infection; however, ET-1 failed to modulate expression of this marker, and stimulation of cells with CCM from T. cruzi-infected cardiomyocytes resulted in a twofold decrease in MyHCα mRNA levels by 24 h (Fig. 2A). A slight increase in SERCA mRNA levels was observed at 24 h postinfection or post-CCM treatment (Fig. 2C). Collectively, these data indicate that while T. cruzi, CCM, and ET-1 can promote marked increases in cell size (Fig. 1), distinct patterns of cardiomyocyte gene expression are observed in response to these various stimuli. However, a common feature of the response elicited by T. cruzi, CCM, or ET-1 was the consistent increase in expression of MyHCβ observed at 24 h (Fig. 2B). The observation that the T. cruzi-induced response was not faithfully reproduced by the addition of CCM likely reflects a more complex transcriptional response elicited in cells harboring intracellular parasites than that in cells stimulated with soluble factors alone.
FIG. 2.
Expression of hypertrophic markers is modulated by T. cruzi infection. Shown is the relative abundance of MyHCα (A), MyHCβ (B), and SERCA (C) mRNA transcripts normalized to GAPDH mRNA determined by densitometric analysis of RPA gels or Northern blots (D) of total cardiomyocyte RNA hybridized sequentially with probes for ANF and GAPDH. Error bars indicate the standard error of the mean. An asterisk denotes statistical significance (P < 0.05).
IL-1β is a soluble mediator of T. cruzi-induced cardiomyocyte hypertrophy.
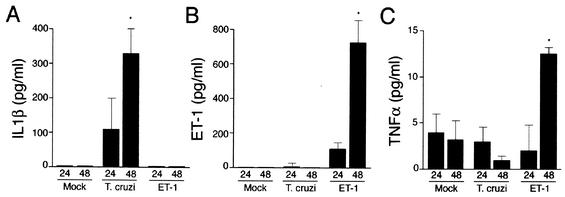
To identify putative hypertrophic factors in conditioned medium from parasite-infected cultures, ELISAs were carried out to measure the levels of IL-1β, TNF-α, and ET-1, which were predicted to be secreted by cardiomyocytes in response to T. cruzi (14, 15). Significant production of IL-1β was measured in T. cruzi-infected cultures at 24 and 48 h postinfection, with steady-state levels reaching 100 to 330 pg/ml at these time points (Fig. 3A). In contrast, ET-1 was below the level of detection (<40 pg/ml) in both mock- and T. cruzi-infected culture supernatants and could only be detected in cultures that had been stimulated with ET-1 (Fig. 3B). Low levels of TNF-α (∼5 pg/ml) were measured in culture supernatants of T. cruzi-infected cells (Fig. 3C), which was unexpected, given that TNF-α transcripts were previously shown to be induced following infection of isolated cardiomyocytes with T. cruzi (15). Thus, ET-1 and TNF-α were unlikely to function as soluble mediators of T. cruzi-induced cardiomyocyte hypertrophy in this system, whereas IL-1β was produced in sufficient quantity to cause an increase in MyHCβ transcript levels and promote an approximately twofold increase in cell size (data not shown).
FIG. 3.
T. cruzi-infected cardiomyocytes secrete IL-1β. Shown are the results of ELISA analysis of IL-1β (A), ET-1 (B), and TNF-α (C) in conditioned medium harvested from mock-treated, T. cruzi-infected, or ET-1-stimulated cardiomyocytes at 24 and 48 h posttreatment in duplicate. The data are representative of three independent experiments. Error bars indicate the standard deviation from the mean. An asterisk denotes statistical significance (P < 0.05).
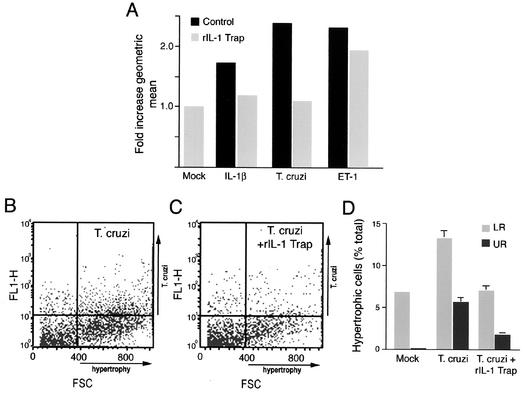
To test whether IL-1β secreted by T. cruzi-infected cardiomyocytes could mediate the observed hypertrophic response, infections were carried out in the presence and absence of recombinant IL-1 trap (rIL-1 trap), a multicomponent IL-1 receptor chimera that exhibits high affinity for IL-1α and IL-1β (9). While rIL-1 trap alone caused control cells to undergo a slight increase in size (data not shown), incubation of T. cruzi-infected cardiomyocytes with rIL-1 trap for 48 h abrogated the overall increase in population cell size induced by this pathogen (Fig. 4A). Specificity of the effect was demonstrated in the ability of rIL-1 trap to inhibit IL-1β-mediated hypertrophy but not the ET-1-stimulated response (Fig. 4A). Moreover, the presence of rIL-1 trap resulted in a decrease in the overall number of hypertrophic cardiomyocytes found in both the infected (Fig. 4D, upper right quadrant) and noninfected (Fig. 4D, lower right quadrant) populations present in T. cruzi-infected cardiomyocyte cultures. Independent analysis of T. cruzi-infected cardiomyocytes by immunofluorescence microscopy indicated that parasite infectivity, intracellular replication, and host cell viability were not adversely affected by the addition of rIL-1 trap (data not shown). In contrast, attempts to block the CCM-mediated hypertrophic response with rIL-1 trap consistently resulted in a loss of cell viability, suggesting that IL-1β may provide a protective function under these conditions. Overall, the dramatic reduction in overall size of cardiomyocyte populations by rIL-1 trap provides compelling evidence that IL-1β secreted by T. cruzi-infected cells promotes hypertrophy in isolated cardiomyocytes infected with T. cruzi.
FIG. 4.
rIL-1 trap blocks T. cruzi-induced cardiomyocyte hypertrophy. (A) Relative population cell size of cardiomyocytes treated with medium alone (mock), IL-1β (1 ng/μl), or ET-1 (10−7 M) or infected with T. cruzi for 48 h in the presence (black) or absence (gray) of 1 μg of rIL-1 trap per ml. Fixed cells were analyzed by FACS, and FSC data are represented as the ratio of the geometric mean of the treated population to that of the mock-treated population. (B and C) Representative FACS plots of T. cruzi-infected cells at 48 h stained for intracellular T. cruzi (y axis) and incubated in the absence (B) or presence (C) of 1 μg of rIL-1 trap per ml. (D) rIL-1 trap inhibits hypertrophy of both T. cruzi-infected (upper right quadrant [UR]) and noninfected (lower right quadrant [LR]) cells in the total cell population. The data are represented as the average of three experiments ± standard deviation.
DISCUSSION
The complex array of host cellular responses elicited by intracellular pathogens is likely to reflect both host defense mechanisms and pathways utilized for pathogen survival. Establishment of cardiomyocyte infection by the intracellular parasite T. cruzi is critical to the development of Chagasic cardiomyopathy (2). In this study, we have exploited an isolated cardiomyocyte culture system to begin to characterize early host responses induced in this clinically relevant cell type following T. cruzi infection. Potentially confounding effects of secondary inflammation and/or host factors produced by other cell types were avoided in this simplified in vitro system, thereby permitting detection of cardiomyocyte-specific responses. Our studies reveal that as an early response to T. cruzi infection, cardiomyocytes undergo a rapid hypertrophic response that is mediated by the secreted proinflammatory cytokine IL-1β. Parasite-induced cardiomyocyte hypertrophy, which occurs in both T. cruzi-infected and uninfected myocytes in culture, was inhibited by the addition of a soluble multicomponent IL-1 receptor (IL-1 trap). These novel findings indicate that in addition to its primary immune regulatory function, IL-1β may play an important role as a mediator of cardiomyocyte hypertrophy as part of the acute response to T. cruzi infection in the heart.
ET-1 has been implicated in the pathogenesis of Chagas' disease (16). A recent study that utilized a targeted cardiac-specific deletion of ET-1 further demonstrated that cardiac myocytes are an important source of ET-1 production in animal models of T. cruzi infection, where reduced ET-1 expression correlated with reduced hypertrophy (14). Interestingly, the results presented in the present study clearly demonstrate that ET-1 is not produced by isolated cardiomyocytes as an early response to T. cruzi infection. Furthermore, we show that ET-1 does not play a significant role in the induction of parasite-induced cardiomyocyte hypertrophy in a simplified in vitro culture system. These data suggest that induction of ET-1 production from cardiomyocytes is not a primary response to T. cruzi infection and likely requires additional autocrine and/or paracrine factors produced by other cell types (e.g., myocardial and/or infiltrating inflammatory cells) absent from our in vitro cardiomyocyte culture system. Consistent with this idea, increases in ET-1 expression in the hearts of T. cruzi-infected rodents were not detectable until 10 to 15 days postinfection (17). In contrast, IL-1β is rapidly upregulated during the initiation of cardiac infection by T. cruzi (3, 15, 21). Since IL-1β is a known hypertrophic mediator (13), it is plausible that cardiomyocyte hypertrophy, mediated by this proinflammatory cytokine, may be a critical, unrecognized feature of acute Chagas' disease. As infection progresses beyond the initiation period (3 to 5 days), it is predicted that a complex array of host factors released from infiltrating cells as well as other known hypertrophic mediators produced during acute infection, including ET-1 (17), CT-1 (6), and TNF-α (3, 15, 21), would modulate an initial hypertrophic response mediated by IL-1β.
While T. cruzi factors responsible for induction of IL-1β expression in cardiomyocytes have yet to be determined, it is well established that a family of T. cruzi glycosylphosphatidyl inositol-linked surface mucin-like molecules (1) trigger proinflammatory cytokine expression in macrophages (5) through the activation of Toll-like receptor 2 (TLR2) (5). Since cardiomyocytes express TLR2, which has been shown to be an antiapoptotic signal under oxidative stress, (11), it is tempting to speculate that this innate signaling pathway may regulate the induction of IL-1β expression in cardiomyocytes and the concomitant hypertrophic response triggered by T. cruzi.
Overall, the results from the present study begin to challenge the concept that cardiomyocyte hypertrophy in Chagas' disease is restricted to a late stage of T. cruzi infection, following chronic inflammation and myocardial damage. Instead, we provide evidence that IL-1β-mediated cardiomyocyte hypertrophy occurs in isolated cardiomyocytes in response to T. cruzi and may play an important role in the maintenance of cardiomyocyte function during the initial phases of infection. IL-1β is produced by other myocardial cells, including vascular endothelial cells, following T. cruzi infection (22), indicating that local secretion of IL-1β could promote cardiomyocyte hypertrophy regardless of the site of initial site of parasite invasion. Our novel findings provide the basis for further investigation of the role of IL-1β-mediated cardiomyocyte hypertrophy in acute Chagas' disease.
Acknowledgments
We thank K. Shibata and G. Koren, Brigham and Women's Hospital, for advice on neonatal rat cardiomyocyte isolation. We are extremely grateful to P. Thomas and O. Atochin for assistance with FACS analysis. We acknowledge M. Fisher for technical assistance and G. Reed, A. Woolsey, M. Unnikrishnan, and J. Daily for helpful discussions.
This research has been supported in part by the Investigators in Pathogenesis of Infectious Diseases Award from the Burroughs Wellcome Fund and National Institutes of Health grants R01 AI47960 to B.A.B. and K08 AI50803 to C.A.P.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Acosta-Serrano, A., I. C. Almeida, L. H. Freitas-Junior, N. Yoshida, and S. Schenkman. 2001. The mucin-like glycoprotein super-family of Trypanosoma cruzi: structure and biological roles. Mol. Biochem. Parasitol. 114:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Arnaiz, M. R., L. E. Fichera, and M. Postan. 2002. Cardiac myocyte hypertrophy and proliferating cell nuclear antigen expression in Wistar rats infected with Trypanosoma cruzi. J. Parasitol. 88:919-925. [DOI] [PubMed] [Google Scholar]
- 3.Baliga, R. R., D. R. Pimental, Y. Y. Zhao, W. W. Simmons, M. A. Marchionni, D. B. Sawyer, and R. A. Kelly. 1999. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am. J. Physiol. 277:H2026-H2037. [DOI] [PubMed] [Google Scholar]
- 4.Burleigh, B. A., and A. M. Woolsey. 2002. Cell signalling and Trypanosoma cruzi invasion. Cell Microbiol. 4:701-711. [DOI] [PubMed] [Google Scholar]
- 5.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar, B., P. C. Melby, D. Pennica, and G. L. Freeman. 1998. Overexpression of cardiotrophin-1 and gp130 during experimental acute Chagasic cardiomyopathy. Immunol. Lett. 61:89-95. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekar, B., P. C. Melby, D. A. Troyer, J. T. Colston, and G. L. Freeman. 1998. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am. J. Pathol. 152:925-934. [PMC free article] [PubMed] [Google Scholar]
- 8.Chien, K. R. 1993. Molecular advances in cardiovascular biology. Science 260:916-917. [DOI] [PubMed] [Google Scholar]
- 9.Economides, A. N., L. R. Carpenter, J. S. Rudge, V. Wong, E. M. Koehler-Stec, C. Hartnett, E. A. Pyles, X. Xu, T. J. Daly, M. R. Young, J. P. Fandl, F. Lee, S. Carver, J. McNay, K. Bailey, S. Ramakanth, R. Hutabarat, T. T. Huang, C. Radziejewski, G. D. Yancopoulos, and N. Stahl. 2003. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat. Med. 9:47-52. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira, L. R., E. F. Abrantes, C. V. Rodrigues, B. Caetano, G. C. Cerqueira, A. C. Salim, L. F. Reis, and R. T. Gazzinelli. 2002. Identification and characterization of a novel mouse gene encoding a Ras-associated guanine nucleotide exchange factor: expression in macrophages and myocarditis elicited by Trypanosoma cruzi parasites. J. Leukoc. Biol. 72:1215-1227. [PubMed] [Google Scholar]
- 11.Frantz, S., R. A. Kelly, and T. Bourcier. 2001. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J. Biol. Chem. 276:5197-5203. [DOI] [PubMed] [Google Scholar]
- 12.Frey, N., and E. N. Olson. 2003. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 65:45-79. [DOI] [PubMed] [Google Scholar]
- 13.Harada, E., O. Nakagawa, M. Yoshimura, M. Harada, M. Nakagawa, Y. Mizuno, Y. Shimasaki, M. Nakayama, H. Yasue, K. Kuwahara, Y. Saito, and K. Nakao. 1999. Effect of interleukin-1 beta on cardiac hypertrophy and production of natriuretic peptides in rat cardiocyte culture. J. Mol. Cell. Cardiol. 31:1997-2006. [DOI] [PubMed] [Google Scholar]
- 14.Huang, H., M. Yanagisawa, Y. Y. Kisanuki, L. A. Jelicks, M. Chandra, S. M. Factor, M. Wittner, L. M. Weiss, R. G. Pestell, V. Shtutin, J. Shirani, and H. B. Tanowitz. 2002. Role of cardiac myocyte-derived endothelin-1 in chagasic cardiomyopathy: molecular genetic evidence. Clin. Sci. (London) 103(Suppl. 1):263S-266S. [DOI] [PubMed]
- 15.Machado, F. S., G. A. Martins, J. C. Aliberti, F. L. Mestriner, F. Q. Cunha, and J. S. Silva. 2000. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation 102:3003-3008. [DOI] [PubMed] [Google Scholar]
- 16.Petkova, S. B., H. Huang, S. M. Factor, R. G. Pestell, B. Bouzahzah, L. A. Jelicks, L. M. Weiss, S. A. Douglas, M. Wittner, and H. B. Tanowitz. 2001. The role of endothelin in the pathogenesis of Chagas' disease. Int. J. Parasitol. 31:499-511. [DOI] [PubMed] [Google Scholar]
- 17.Petkova, S. B., H. B. Tanowitz, H. I. Magazine, S. M. Factor, J. Chan, R. G. Pestell, B. Bouzahzah, S. A. Douglas, V. Shtutin, S. A. Morris, E. Tsang, L. M. Weiss, G. J. Christ, M. Wittner, and H. Huang. 2000. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc. Pathol. 9:257-265. [DOI] [PubMed] [Google Scholar]
- 18.Schaub, M. C., M. A. Hefti, B. A. Harder, and H. M. Eppenberger. 1997. Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes. J. Mol. Med. 75:901-920. [DOI] [PubMed] [Google Scholar]
- 19.Schmunis, G. 1994. American trypanosomiasis as a public health problem. Pan American Health Organization, Washington, D.C.
- 20.Steinberg, S. F. 2000. Many pathways to cardiac hypertrophy. J. Mol. Cell. Cardiol. 32:1381-1384. [DOI] [PubMed] [Google Scholar]
- 21.Talvani, A., C. S. Ribeiro, J. C. Aliberti, V. Michailowsky, P. V. Santos, S. M. Murta, A. J. Romanha, I. C. Almeida, J. Farber, J. Lannes-Vieira, J. S. Silva, and R. T. Gazzinelli. 2000. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2:851-866. [DOI] [PubMed] [Google Scholar]
- 22.Tanowitz, H. B., M. Wittner, S. A. Morris, W. Zhao, L. M. Weiss, V. B. Hatcher, V. L. Braunstein, H. Huang, S. A. Douglas, M. Valcic, M. Spektor, and G. J. Christ. 1999. The putative mechanistic basis for the modulatory role of endothelin-1 in the altered vascular tone induced by Trypanosoma cruzi. Endothelium 6:217-230. [DOI] [PubMed] [Google Scholar]
- 23.Tardieux, I., P. Webster, J. Ravesloot, W. Boron, J. A. Lunn, J. E. Heuser, and N. W. Andrews. 1992. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell 71:1117-1130. [DOI] [PubMed] [Google Scholar]
- 24.Vaena De Avalos, S. G., I. J. Blader, M. L. Fisher, J. C. Boothroyd, and B. A. Burleigh. 2001. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J. Biol. Chem. 277:639-644. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, L., and R. L. Tarleton. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J Infect. Dis. 180:480-486. [DOI] [PubMed] [Google Scholar]