Abstract
Actinobacillus actinomycetemcomitans, an oral bacterium implicated in the etiology of periodontal diseases, produces a leukotoxin that selectively lyses primate neutrophils and monocytes, the major populations of defense cells in the periodontium. Though lysis requires expression of the receptor lymphocyte function-associated molecule 1 (LFA-1) on the cell surface, not all LFA-1-expressing leukocyte populations are equally susceptible to the toxin. In this study, the susceptibility of human leukocytes to leukotoxin-induced lysis is compared to their expression of LFA-1 and the activity of caspase 1. Cytolysis was determined by the activity of lactate dehydrogenase released from peripheral human leukocytes after 1-h exposure to leukotoxin. Monocytes were lysed at leukotoxin concentrations of ≥5 ng/ml, while the corresponding values for neutrophils and lymphocytes were approximately 10 times greater. Similar LFA-1 expression was found in all susceptible cell populations irrespective of their degree of sensitivity to the toxin. Exposure of monocytes to leukotoxin increased their caspase 1 activity about fivefold within 10 to 20 min. Presence of the caspase 1 inhibitor Ac-YVAD-CMK significantly blocked the leukotoxin-induced lysis of monocytes only. At sublytic concentrations, leukotoxin induced no apoptotic activity in monocytes, as revealed by the lack of caspase 3 activation and DNA fragmentation. Monocytes are the most lysis-sensitive leukocytes for A. actinomycetemcomitans leukotoxin. Their lysis by this toxin depends on caspase 1 activation and proceeds through a process that differs from classical apoptosis.
Actinobacillus actinomycetemcomitans, a gram-negative coccoid bacillus and a member of the oral microflora, is implicated in the etiology of certain types of periodontal diseases and also in systemic infections (42, 47). This bacterium has several virulence factors, including production of lipopolysaccharide and cell cycle inhibitors such as a cytolethal distending toxin, a chaperonin, and a leukotoxin (1, 4, 12, 17, 26, 50). Leukotoxin is assumed to enable A. actinomycetemcomitans to evade the main defense line of the periodontal pocket and to significantly contribute to the pathogenesis of periodontal disease (12, 27). Strains isolated from patients with aggressive forms of periodontitis have an enhanced production of leukotoxin compared to those isolated from healthy subjects or patients with chronic adult periodontitis (14, 53). In Africans suffering from aggressive periodontitis, a specific clone of A. actinomycetemcomitans is thought to act as an exogenous pathogen (9, 16). This clone, designated JP2, produces copious amounts of leukotoxin due to a 530-bp deletion in the promoter region of the leukotoxin gene operon (8).
The leukotoxin of A. actinomycetemcomitans shares considerable molecular homology (35 to 70%) with toxins of the RTX (repeat in toxin) family, which are produced by other gram-negative pathogens such as Escherichia coli, Actinobacillus pleuropneumoniae, Bordetella pertussis, and Mannheimia haemolytica (34, 49). Among the RTX toxins, leukotoxin exhibits unique specificity for primate leukocytes (5, 43). It lyses polymorphonuclear leukocytes (PMNL) and monocytes (44, 46), and it also induces degranulation of PMNL (10, 21) and apoptosis in T lymphocytes (35). The domain of the toxin that binds to the human target cells has been mapped (29), and a β2-integrin, the lymphocyte function-associated molecule 1 (LFA-1), was shown to be a target cell receptor involved in leukotoxin-induced cell lysis (31).
Even though expression of LFA-1 appears to be a prerequisite for the cells to be leukotoxin susceptible (31), some leukocyte populations expressing the receptor, such as lymphocytes, seem to resist lysis to a greater extent than other cells, e.g., PMNL (35, 44, 46). On the other hand, myelocytes and lymphocytes derived from a human hematopoietic tumor cell line were found to be sensitive to the leukotoxin-induced killing (41). Although there is a lack of comparative studies on the cytolysis kinetics of different leukocyte populations, the earlier observations indicate a possible involvement of additional mechanisms besides the LFA-1 expression.
Previous studies with A. actinomycetemcomitans leukotoxin have mainly been focused on the interactions of the toxin with PMNL (3, 21, 22, 24, 36, 46) and promyelocytic carcinoma cell lines (29, 31). Based on the results of this research, the mechanism behind cell lysis is currently assumed to be the formation of pores by leukotoxin in the cytoplasmic membrane of the susceptible cells (32). In this process, LFA-1 is suggested as playing a key role in the binding and orientation of the leukotoxin molecules on the cell surface, this orientation being necessary for pore formation (30). Recently, very low concentrations of leukotoxin were reported to induce abundant secretion of interleukin 1β (IL-1β) by monocytes (A. Johansson, P. Kelk, L. Hänström, G. Belibasakis, and S. Kalfas, Progr. IADR/AADR/CADR 80th Gen. Sess., abstr. 1757, 2002). In monocytes, secretion of active IL-1β demands cleavage of the precursor pro-IL-1β, which occurs through the IL-1 converting enzyme, caspase 1 (45). Caspase 1 is involved in Salmonella-induced rapid cell death, which leads to the activation and production of bioactive cytokines (37).
In this report, the A. actinomycetemcomitans leukotoxin-induced cytolysis of various human leukocyte populations is compared to their LFA-1 expression and caspase 1 activity. The results show that human monocytes are the most leukotoxin-sensitive leukocytes and that monocyte lysis involves activation of caspase 1 by leukotoxin, a mechanism not observed with other human leukocytes.
MATERIALS AND METHODS
Leukotoxin and leukocyte preparations.
Leukotoxin was purified from A. actinomycetemcomitans strain HK 1519, belonging to the highly leukotoxic clone JP2 (8). The purification procedure has previously been described in detail (21, 22). The leukotoxin preparation was essentially free of lipopolysaccharide (<0.001% of total protein).
Human leukocytes were isolated from an enriched leukocyte fraction (buffy coat) of venous blood. The blood was taken from donors visiting the University Hospital blood bank in Umeå, Sweden. Informed consent was given by all subjects. Mononuclear leukocytes were isolated by isopycnic centrifugation in Lymphoprep (Nycomed AB, Lidingö, Sweden) as described previously (48). The mononuclear leukocyte-containing fraction was collected, and the cells were washed three times (250 × g, 5 min) with phosphate-buffered saline (PBS) to remove the platelets. The cell pellet was resuspended in culture medium RPMI 1640 containing 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, Mo.) to yield 5 × 106 cells/ml. The suspension was distributed into a 24-well culture plate (Nunc A/S, Roskilde, Denmark) at 1 ml/well and incubated at 37°C in 5% CO2 for 2 h to allow the monocytes to adhere. The nonadherent lymphocytes were removed by two rinses with 1 ml of PBS, harvested by centrifuging, and resuspended in culture medium to a concentration of 4 × 106 cells/ml. The adherent cells consisted of approximately 95% monocytes (48), in a total number of about 106 cells/well. To each well, 250 μl of culture medium was added.
PMNL were isolated from the buffy coat by dextran sedimentation and isopycnic centrifugation in Lymphoprep (Nycomed AB) as described elsewhere (22). The final suspension in culture medium contained 4 × 106 PMNL/ml.
Cells of the human carcinoma promyelocytic cell line HL-60 (ATCC CCL-240) were cultured in RPMI 1640 with 10% FBS, as described above. In some experiments, the cells had been cultured for 72 h in the presence of 5 μM phorbol 12-myristate 13-acetate (PMA) or 1% dimethyl sulfoxide (DMSO) to differentiate toward macrophage- or neutrophil-like cells, respectively (51).
Cytolysis.
Leukotoxin-induced cytolysis was determined by the release of the cytosol enzyme lactate dehydrogenase (LDH) as described earlier (23). The cells were incubated for 60 min at 37°C in the presence of up to 300 ng of leukotoxin per ml of culture medium. Thereafter, the mixture was placed on ice for 10 min and the cells were pelleted by centrifuging at 1,000 × g and 4°C for 5 min. The activity of the enzyme released from damaged cells into the supernatant was measured, and the activity was expressed as a percentage of the total LDH activity released from cells lysed by exposure to 0.1% Triton X-100 for 60 min.
Any involvement of caspase 1 in leukotoxin-induced cell lysis was further examined with the use of the caspase inhibitors Ac-YVAD-CMK (Calbiochem, La Jolla, Calif.) and N-1700 (Bachem, Bubendorf, Switzerland). Ac-YVAD-CMK inhibits both caspase 1 and caspase 4 (27a), while N-1700 is considered to be specific for caspase 4. The inhibitors were added to the leukocyte suspension at a final concentration of 100 μM, 15 min before exposure to leukotoxin. Following incubation for 1 h, the extent of cytolysis was assayed by the activity of the LDH released extracellularly.
DNA fragmentation analysis.
By detecting DNA fragmentation (52), a possible induction of apoptosis was examined in monocytes incubated for 20 h in the presence of low concentrations (0.1 to 10 ng/ml) of leukotoxin. Briefly, the cells were lysed with 0.5% sodium dodecyl sulfate at 65°C for 1 h, and the lysate was treated with 20 μg of RNase A/ml and 20 μg of proteinase K (Sigma-Aldrich)/ml for 1 h at 56°C. The lysate was extracted twice with an equal volume of chloroform, and the DNA in the water phase was precipitated with 2 volumes of 300 mM sodium acetate in 75% ethanol at −20°C. The precipitate was collected by centrifuging (13,000 × g for 10 min at 4°C) and washed twice in 70% ethanol. Following air drying and dissolving the pellet in 16 μl of H2O, the sample was run in an agarose gel electrophoresis (1.5% agarose; 135 V; 75 min), and the gel was stained with ethidium bromide and examined under UV light to detect any DNA fragmentation.
Analysis of LFA-1 expression.
Detection of the receptor LFA-1 on the leukocyte surface was assayed with the monoclonal mouse antibodies MHM 23 (Dakopats AB, Älvsjö, Sweden). These antibodies react with the αL chain (CD11a in LFA-1) of human integrins. Cell suspensions were prepared as described above. For monocyte suspensions, the adherent cells were treated with 0.2% EDTA and detached with a rubber cell scraper. All cells were fixed with 2% paraformaldehyde in PBS (pH 7.2) for 10 min at room temperature. The cells were washed once with PBS containing 1% bovine serum albumin (PBS-BSA) and harvested by centrifuging at 300 × g for 5 min. The cell pellet was suspended in PBS-BSA containing the monoclonal antibodies and incubated at 37°C for 1 h under gentle agitation. Thereafter, the cells were washed once in PBS-BSA and resuspended in PBS-BSA containing 1% fluorescein isothiocyanate-labeled monoclonal antibodies against mouse immunoglobulin G (clone CLB-1; Dakopats AB). Following incubation in the dark as described above, the leukocytes were washed once in PBS-BSA and suspended in PBS to a final concentration of 106 leukocytes/ml before being analyzed with a fluorescence-activated cell scanner (Facstarplus) equipped with the software LYSIS II (Becton Dickinson Immunocytometry Systems).
Ten thousand cells were analyzed from each sample, and the data were plotted as the distribution of leukocytes according to their degree of fluorescence. The relative number of leukocytes that exhibited fluorescence values indicative of reactivity with the antibodies was then calculated.
Caspase 1 and 3 analysis.
The total amount of caspase 1 in leukocytes was analyzed by an enzyme-linked immunosorbent assay (ELISA; R&D Systems Inc., Minneapolis, Minn.). Briefly, leukocytes isolated from buffy coat as described above were suspended in RPMI (106 cells/ml) containing 10% FBS and 0.1% Triton X-100, and the suspension was incubated for 1 h at room temperature to lyse the cells. The supernatant cell lysates obtained after centrifugation (1,000 × g; 4°C, 5 min) were analyzed for amounts of cell-bound caspase 1.
The activity of caspase 1 was determined in a colorimetric assay with a substrate (WEHD-pNA) specific for this enzyme (R&D Systems, Inc.). Monocytes were exposed to leukotoxin (10 ng/ml) for up to 1 h. At the end of the incubation, the cells were pelleted by centrifuging (1,000 × g; 4°C, 5 min), the supernatant was aspirated, and the cells were lysed in accordance with the manufacturer's instructions. Cell lysate and the initial supernatant were analyzed for the activity of cell-bound and released caspase 1, respectively.
The intracellular caspase 3 activity was measured by a fluorometric immunosorbent enzyme assay using a commercial kit (caspase 3 activity assay; Roche Molecular Biochemicals, Mannheim, Germany). Monocytes were exposed to different concentrations of leukotoxin (0 to 10 ng/ml) for 6 h before determining the activity of caspase 3. Cells were lysed, and the cleavage of a fluorescent substrate (Ac-DEVD-AFC) was quantified in accordance with the manufacturer's protocol. Furthermore, the activity of caspase 3 was determined in a colorimetric assay with a specific substrate (DEVD-pNA) for this enzyme (R&D Systems, Inc.). Monocytes were exposed to leukotoxin (10 ng/ml) for up to 1 h. At the end of the incubation, the cells were pelleted by centrifuging (1,000 × g; 4°C, 5 min), the supernatant was aspirated, and the cells were lysed in accordance with the manufacturer's protocol. Cell lysate and the initial supernatant were analyzed, and the total activity of caspase 3 was determined in each sample.
ELISA for IL-1β.
The effect of leukotoxin on the amounts of IL-1β secreted into the culture medium of the monocytes was analyzed by ELISA using a commercially available kit (OptEIA; BD Pharmingen, San Diego, Calif.). Monocytes were exposed to leukotoxin (10 ng/ml) for up to 1 h. At the end of the incubation, the culture supernatant was aspirated, and the amount of IL-1β was quantified in accordance with the manufacturer's protocol.
Statistics.
The significance of differences was assessed with Student's t test. P values of ≤0.05 were considered indicative of a statistically significant difference.
RESULTS
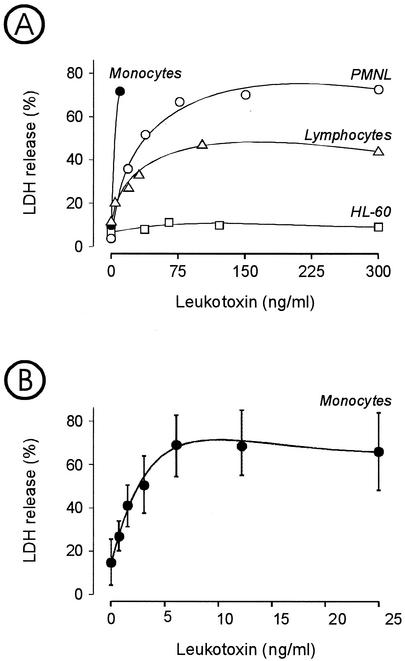
The leukotoxin concentration required for cell lysis varied for the different leukocyte populations (Fig. 1). Monocytes were the most sensitive cells, followed by PMNL and lymphocytes, while HL-60 cells appeared to be resistant. Maximal lysis of PMNL occurred at an approximately 10 times greater leukotoxin concentration than that for monocytes (Fig. 1A). Under the same conditions, only half of the lymphocyte population was susceptible to leukotoxin-induced lysis, since the maximum LDH release caused by the toxin was approximately 50% of the total LDH extracted by treating with Triton X-100. Monocyte lysis was induced at leukotoxin concentrations of >1 ng/ml (Fig. 1B).
FIG. 1.
Extracellular release of LDH, indicative of cell lysis, from different cell populations incubated for 1 h with various concentrations of leukotoxin. (A) Comparative kinetics for different human peripheral blood leukocytes and the cancer cell line HL-60. (B) Kinetics of monocyte lysis at low leukotoxin concentrations. Mean values ± standard deviations of four experiments are shown.
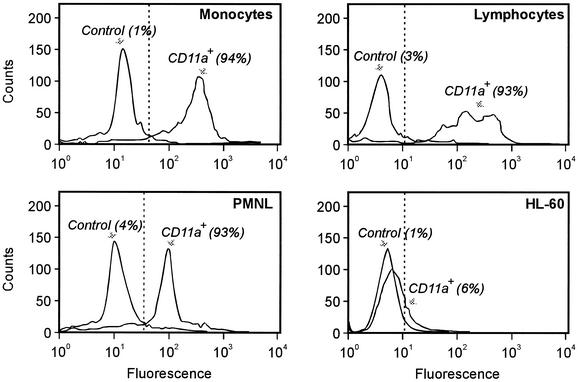
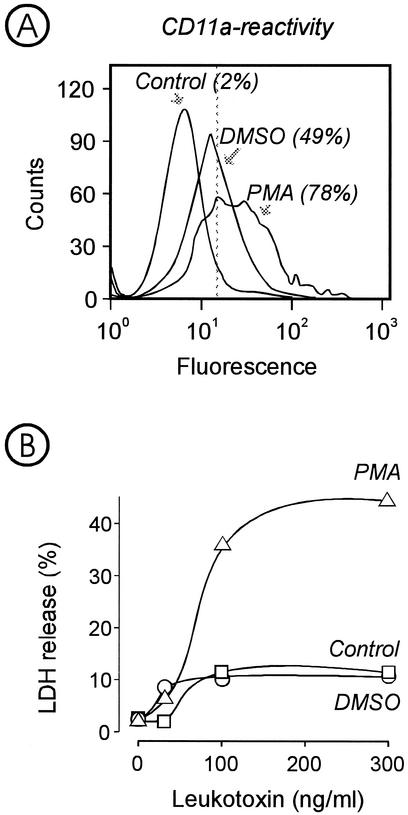
Expression of LFA-1, measured as reactivity with anti-CD11a monoclonal antibodies, was found in all leukocytes isolated from peripheral blood (Fig. 2). More than 90% of the cells in each population expressed the receptor. In contrast, the leukotoxin-resistant HL-60 cells showed negligible binding of the antibodies, the binding being limited to about 6% of the cell population. When these cells were grown in the presence of the differentiation agents PMA and DMSO, they showed increased reactivity with the antibodies (Fig. 3A). PMA induced higher receptor expression than DMSO. At the same time, only the PMA-exposed cells became leukotoxin susceptible, while those treated with DMSO were not lysed by the toxin (Fig. 3B).
FIG. 2.
Expression of LFA-1 receptors on the cell surface of different human peripheral blood leukocytes and the cancer cell line HL-60. The expression of LFA-1 was determined by the reactivity of the cells with monoclonal antibodies against CD11a. The proportion of cells reacting with the antibody (given within brackets) is the mean value of three experiments with each cell type. Representative results of cell distributions obtained by a fluorescence-assisted cell scanner are shown.
FIG. 3.
(A) Expression of LFA-1 on HL-60 cells grown in the presence of DMSO or PMA for 72 h. The expression of LFA-1 was determined by the reactivity of the cells with monoclonal antibodies against CD11a. The proportion of cells reacting with the antibody (given within brackets) is the mean value of three experiments. Representative results of cell distributions obtained by a fluorescence-assisted cell scanner are shown. (B) Extracellular release of LDH, indicative of cell lysis, from HL-60 cells grown in the presence of DMSO or PMA and incubated for 1 h with various concentrations of leukotoxin. Mean values of three experiments are shown.
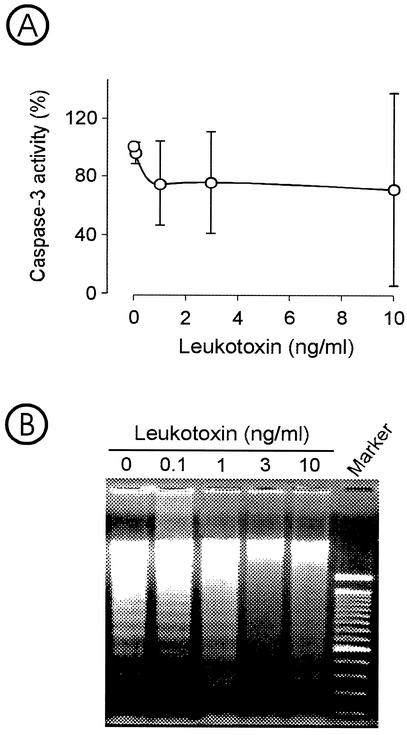
Sublytic concentrations (<1 ng/ml) of leukotoxin induced no apoptotic events in monocytes (Fig. 4). The intracellular activity of caspase 3 was not significantly affected by the leukotoxin exposure for 6 h (Fig. 4A), nor was an increased DNA fragmentation observed after a 20-h incubation of the cells compared to control cultures grown in the absence of leukotoxin (Fig. 4B). Increasing the toxin concentration to ≥3 ng/ml appeared to inhibit the activity of DNA fragmentation observed in the negative control and the test cultures with ≤1 ng of leukotoxin per ml. At the same time, the increased toxin concentration (≥3 ng/ml) resulted in increased monocyte lysis (≥50% of the cells) as mentioned above (Fig. 1B).
FIG. 4.
Determination of apoptosis in monocytes exposed to different concentrations of leukotoxin. (A) Caspase 3 activity in lysate from monocytes exposed for 6 h. The intracellular caspase 3 activity was measured by a fluorometric immunosorbent enzyme assay and expressed as the percentage of control values for cultures incubated without leukotoxin. Values are means ± standard deviations of four experiments. (B) DNA fragmentation in monocytes exposed to leukotoxin for 20 h. The DNA was separated by agarose electrophoresis and stained with ethidium bromide. The experiment was performed in triplicate. Representative results are shown.
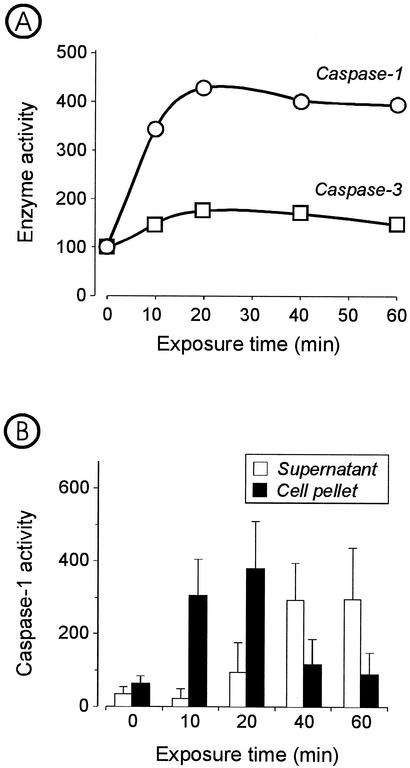
The amount of caspase 1 varied in the different leukocyte populations. Monocytes contained approximately a threefold greater amount of caspase 1 compared to PMNL and lymphocytes. The values (mean ± standard deviation) found for monocytes, PMNL, and lymphocytes were 3.0 ± 1.2, 1.0 ± 0.4, and 0.9 ± 0.4 ng/106 cells, respectively. The activity of caspase 1 in monocytes showed a fourfold increase after 20 min of exposure to leukotoxin (10 ng/ml), while the leukotoxin-mediated increase of caspase 3 activity was less than twofold (Fig. 5A). Maximal activity of caspase 1 was reached within 20 min, with most of the activity being found in the cell pellets (Fig. 5B). Upon longer incubation, the enzyme was released extracellularly and the activity, mainly recovered in the supernatant, slowly declined during the following observation period (Fig. 5B).
FIG. 5.
Effect of leukotoxin (10 ng/ml) on the activities of caspase 1 and caspase 3 in cultures of monocytes. The relative activity in cultures not exposed to leukotoxin was set at the index of 100. (A) Kinetics of relative activity of caspase 1 and caspase 3 (supernatant plus pellet). Means of pooled samples from three experiments were analyzed in triplicate. (B) Activity of caspase 1 in each supernatant and cell lysate. Data are means ± standard deviations of three experiments.
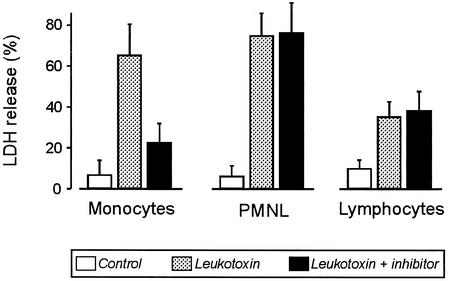
The presence of the caspase 1 inhibitor Ac-YVAD-CMK almost eliminated the leukotoxin-induced lysis of monocytes, as indicated by the extracellular LDH activity (Fig. 6). Under the same conditions, lysis of PMNL and lymphocytes proceeded unaffected, reaching LDH release values similar to those in the absence of the inhibitor (Fig. 6). The caspase 4 inhibitor N-1700 had no effect on the cytolysis of the three leukocyte populations (data not shown).
FIG. 6.
Leukotoxin-induced release of LDH, indicative of cell lysis, from human leukocytes in the presence or absence of the caspase 1 inhibitor Ac-YVAD-CMK. The leukotoxin concentration was 10 ng/ml in the monocyte cultures and 300 ng/ml in the PMNL and lymphocyte suspensions. Data are means ± standard deviations of five experiments.
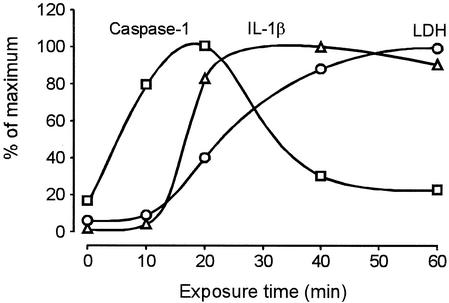
The kinetics analyses of caspase 1 activation in monocytes showed a time-dependent increase during the first 20 min after addition of leukotoxin (10 ng/ml). The secretion of IL-1β followed the kinetics of caspase 1 activity, but with a delay of about 10 min. Finally, the leukotoxin-exposed monocytes lysed, which was indicated by the increased release of LDH (Fig. 7).
FIG. 7.
Kinetic analyses of caspase 1 activation, IL-1β secretion, and LDH release in cultures of monocytes exposed to leukotoxin (10 ng/ml). The value for each parameter is expressed as the percentage of maximum. Means of pooled samples from three experiments were analyzed in triplicate.
DISCUSSION
The intensity of the inflammatory reactions in periodontitis seems to be crucial to the extent of tissue damage caused during the course of the disease. Aggressive periodontitis differs from chronic adult periodontitis in terms of the rapid bone destruction that is observed in the former disease type. In young subjects, aggressive periodontitis is strongly associated with a rich presence of A. actinomycetemcomitans (39, 42) and especially with the presence of highly leukotoxic strains (9, 14, 16). Most of the studies on the interactions of the A. actinomycetemcomitans leukotoxin with leukocytes have been focused on PMNL. These cells comprise the major leukocyte population in the periodontal pocket. Positioned between the bacterial plaque on the tooth surface and the epithelial lining, PMNL are thought to protect the soft tissues against massive bacterial invasion and the rapid spread of the infection. Nevertheless, their inability to eliminate the entire plaque by phagocytosis leads to the inflammation becoming chronic and to its apical proliferation, with concomitant destruction of the alveolar bone. Besides being lytic, leukotoxin was also shown to induce a rapid degranulation of PMNL (10, 21). This active process was suggested to contribute to the local tissue destruction through increased proteolysis caused by the released lysosomal enzymes.
It is arguable whether leukotoxin can further intensify the inflammatory response by interacting with leukocytes in the soft periodontal tissues behind the PMNL layer. The vulnerability of the toxin to proteolytic enzymes (20, 21), the lack of a bacterial secretory mechanism similar to those found for other RTX toxins (25), and the relatively high concentration of the toxin required for cytolytic activity support the hypothesis that the pathogenic action of leukotoxin is exerted in the vicinity of the PMNL layer.
In the tissues surrounding the inflamed periodontal pocket, monocytes and macrophages are the predominant leukocytes. These cells accumulate in large numbers and play a decisive role in the regulation of the inflammatory reactions and the tissue breakdown and remodeling (15). The present study demonstrates that human monocytes are the most sensitive leukocytes for leukotoxin-induced lysis. The critical toxin concentration is approximately 10 times lower than the one found for other cells. This concentration could reasonably be reached in the periodontal tissues provided that the toxin is released from the bacterial surface and that it is not immediately destroyed by proteases. The first condition was recently tested (24a), and the findings indicated that a protein-rich environment, like the one of the periodontal pocket, promotes the complete release of active leukotoxin from the surface of A. actinomycetemcomitans. Serum can also protect the toxin against proteolytic degradation, either competitively due to its protein content or mainly by its protease inhibitors (20). Thus, it seems possible that interactions of leukotoxin with tissue macrophages can occur in the periodontium.
Expression of the β2-integrin LFA-1 on the leukocyte plasma membrane has been shown crucial for sensitivity to leukotoxin-induced cytolysis (31). High levels of expression of LFA-1 were found with all human leukocytes used in this study. The nonsensitive human promyelocytic carcinoma HL-60 cells showed no LFA-1 expression. Differentiation of HL-60 cells towards monocytes that were induced by PMA activated LFA-1 expression and increased the leukotoxin sensitivity. Nevertheless, differentiation by DMSO towards PMNL left the cells resistant to the toxin despite the increased LFA-1 expression. It seems that LFA-1 is a surface molecule necessarily involved in the cytolytic interaction of leukotoxin with human leukocytes, as previously suggested (31). However, expression of this integrin may not be the only condition required, since the type and possibly the level of differentiation appear to affect the sensitivity of HL-60 cells to the A. actinomycetemcomitans leukotoxin.
All populations of human leukocytes expressed LFA-1 at a high level (>90%). Remarkably, the expression did not correlate with the sensitivity of the target cells to the toxin. This finding also supports the hypothesis that other molecules may be involved in the cytolytic interaction. In a recent study (11), a correlation was reported between cytolysis induced by another RTX toxin, the leukotoxin of M. haemolytica, and the expression of CD18, the common β-subunit of the β2-integrins. CD18 was suggested to mediate leukotoxin-induced lysis of bovine leukocytes (11), and β2-integrins have been identified as the receptors for M. haemolytica leukotoxin on these leukocytes (2, 33). It is tempting to suggest a similar interaction of human leukocyte CD18 with A. actinomycetemcomitans leukotoxin as the possible mechanism behind the present lack of correlation, since there are differences in the expression of β2-integrins among the various leukocyte populations (13).
The existence of different cytolytic mechanisms induced by the leukotoxin in the various leukocytes could constitute another possible explanation. The cellular pathway for leukotoxin-induced cell death is at the moment not fully understood. A low concentration of leukotoxin activates apoptotic events in HL-60 cells but, with increasing concentrations, the plasma membrane integrity totally collapses and results in an uncontrolled leakage of cytosol components (28). It has been suggested that leukotoxin at low concentrations causes small pores in the plasma membrane that allow influx of Ca2+, which activates apoptotic pathways, while the presence of high concentrations leads to toxin oligomerization in the plasma membrane and to the formation of large pores, the cause of the rapid cytolysis (30). Human monocytes differ in this aspect from HL-60 cells, since they do not undergo apoptosis in the presence of sublytic concentrations of leukotoxin as found presently. Furthermore, the lytic concentration for monocytes is much lower than the corresponding one for HL-60 cells, the concentration being probably too low to allow oligomerization.
Recently, a new cytotoxic mechanism was found in macrophages induced by Salmonella enterica serovar Typhimurium (7, 37). The Salmonella invasive protein B activates caspase 1 (18) when released intracellularly in the infected leukocytes and leads to a rapid cell necrosis through the so-called inflammatory death pathway (6). A similar phenomenon has been reported for Shigella species (19). Initially, the cytotoxic effect was characterized as apoptosis, but a more extensive analysis of the mechanisms involved led to the suggestion that the phenomenon is a necrotic rather than an apoptotic event (7). Although apoptosis and necrosis are thought to be distinct forms of cell death, there is increasing evidence that the morphological and biochemical characteristics of these events represent only the extreme ends in a wide range of possible cell deaths (38). The S. enterica serovar Typhimurium-induced cytolysis involves no activation of caspase 3 and is characterized by rapid changes in the plasma membrane integrity. This caspase 1-dependent cell death involves both apoptotic and necrotic pathways, which indicates a mechanism for cytotoxicity at the interface between apoptosis and necrosis.
This paper is the first report on an RTX toxin-induced inflammatory death pathway similar to the one described for the invasins of Salmonella and Shigella species. Monocyte lysis by the leukotoxin of A. actinomycetemcomitans is caspase 1 dependent, since the caspase 1 inhibitor Ac-YVAD-CMK can block it. On the other hand, the inhibitor protected neither PMNL nor lymphocytes, indicating the involvement of different pathways in the cytolytic interactions of these leukocytes with the toxin.
The interaction of leukotoxin with monocytes results in a rapid increase in caspase 1 activity. Caspase 1 activates proinflammatory cytokines, and this activation leads to acute inflammation that is responsible for severe tissue destruction (40, 54). In caspase 1 knockout mice, Shigella infection induces no macrophage death and the animals develop a chronic inflammation without clearing the bacterial infection (40). Possibly, the rapid tissue destruction observed in aggressive A. actinomycetemcomitans-associated periodontitis is the result of the interaction of leukotoxin with the macrophages accumulated in the periodontal tissues.
Acknowledgments
This study was supported in part by the Swedish Medical Research Council (project K98-24X-12684-01A), the Research Fund of Västerbotten County, the Swedish Dental Society, and the Swedish Patent Revenue Fund.
Editor: J. D. Clements
REFERENCES
- 1.Akifusa, S., S. Poole, J. Lewthwaite, B. Henderson, and S. P. Nair. 2001. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect. Immun. 69:5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambagala, T. C., A. P. Ambagala, and S. Srikumaran. 1999. The leukotoxin of Pasteurella haemolytica binds to β2 integrins on bovine leukocytes. FEMS Microbiol. Lett. 179:161-167. [DOI] [PubMed] [Google Scholar]
- 3.Baehni, P., C. C. Tsai, W. P. McArthur, B. F. Hammond, and N. S. Taichman. 1979. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect. Immun. 24:233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belibasakis, G., A. Johansson, Y. Wang, R. Claesson, C. Chen, S. Asikainen, and S. Kalfas. 2002. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. Eur. J. Oral Sci. 110:336-373. [DOI] [PubMed] [Google Scholar]
- 5.Berthold, P., D. Forti, I. R. Kieba, J. Rosenbloom, N. S. Taichman, and E. T. Lally. 1992. Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiol. Immunol. 7:24-27. [DOI] [PubMed] [Google Scholar]
- 6.Boise, L. H., and C. M. Collins. 2001. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 9:64-67. [DOI] [PubMed] [Google Scholar]
- 7.Brennan, M. A., and B. T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31-40. [DOI] [PubMed] [Google Scholar]
- 8.Brogan, J. M., E. T. Lally, K. Poulsen, M. Kilian, and D. R. Demuth. 1994. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 62:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueno, L. C., M. P. Mayer, and J. M. DiRienzo. 1998. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J. Periodontol. 69:998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claesson, R., A. Johansson, G. Belibasakis, L. Hanstrom, and S. Kalfas. 2002. Release and activation of matrix metalloproteinase 8 from human neutrophils triggered by the leukotoxin of Actinobacillus actinomycetemcomitans. J. Periodontal Res. 37:353-359. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande, M. S., T. C. Ambagala, A. P. Ambagala, M. E. Kehrli, Jr., and S. Srikumaran. 2002. Bovine CD18 is necessary and sufficient to mediate Mannheimia (Pasteurella) haemolytica leukotoxin-induced cytolysis. Infect. Immun. 70:5058-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fives-Taylor, P. M., D. H. Meyer, K. P. Mintz, and C. Brissette. 1999. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology 2000 20:136-167. [DOI] [PubMed] [Google Scholar]
- 13.Gahmberg, C. G., L. Valmu, S. Fagerholm, P. Kotovuori, E. Ihanus, L. Tian, and T. Pessa-Morikawa. 1998. Leukocyte integrins and inflammation. Cell Mol. Life Sci. 54:549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraszthy, V. I., G. Hariharan, E. M. Tinoco, J. R. Cortelli, E. T. Lally, E. Davis, and J. J. Zambon. 2000. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J. Periodontol. 71:912-922. [DOI] [PubMed] [Google Scholar]
- 15.Hassell, T. M. 1993. Tissues and cells of the periodontium. Periodontology 2000 3:9-38. [DOI] [PubMed] [Google Scholar]
- 16.Haubek, D., J. M. Dirienzo, E. M. Tinoco, J. Westergaard, N. J. Lopez, C. P. Chung, K. Poulsen, and M. Kilian. 1997. Racial tropism of a highly toxic clone of Actinobacillus actinomycetemcomitans associated with juvenile periodontitis. J. Clin. Microbiol. 35:3037-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson, B., M. Wilson, L. Sharp, and J. M. Ward. 2002. Actinobacillus actinomycetemcomitans. J. Med. Microbiol. 51:1013-1020. [DOI] [PubMed] [Google Scholar]
- 18.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 20.Johansson, A., R. Claesson, G. Belibasakis, E. Makoveichuk, L. Hanstrom, G. Olivecrona, G. Sandstrom, and S. Kalfas. 2001. Protease inhibitors, the responsible components for the serum-dependent enhancement of Actinobacillus actinomycetemcomitans leukotoxicity. Eur. J. Oral Sci. 109:335-341. [DOI] [PubMed] [Google Scholar]
- 21.Johansson, A., R. Claesson, L. Hanstrom, G. Sandstrom, and S. Kalfas. 2000. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal Res. 35:85-92. [DOI] [PubMed] [Google Scholar]
- 22.Johansson, A., L. Hanstrom, and S. Kalfas. 2000. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacteria from the subgingival flora. Oral Microbiol. Immunol. 15:218-225. [DOI] [PubMed] [Google Scholar]
- 23.Johansson, A., and S. Kalfas. 1998. Characterization of the proteinase-dependent cytotoxicity of Porphyromonas gingivalis. Eur. J. Oral Sci. 106:863-871. [DOI] [PubMed] [Google Scholar]
- 24.Johansson, A., G. Sandstrom, R. Claesson, L. Hanstrom, and S. Kalfas. 2000. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur. J. Oral Sci. 108:136-146. [DOI] [PubMed] [Google Scholar]
- 24a.Johansson, A., R. Claesson, L. Hänstrom, and S. Kalfas. 2003. Serum-mediated release of leukotoxin from the cell surface of the periodontal pathogen Actinobacillus actinomycetemcomitans. Eur. J. Oral Sci. 111:209-215. [DOI] [PubMed] [Google Scholar]
- 25.Kachlany, S. C., D. H. Fine, and D. H. Figurski. 2000. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect. Immun. 68:6094-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamin, S., W. Harvey, M. Wilson, and A. Scutt. 1986. Inhibition of fibroblast proliferation and collagen synthesis by capsular material from Actinobacillus actinomycetemcomitans. J. Med. Microbiol. 22:245-249. [DOI] [PubMed] [Google Scholar]
- 27.Kinder-Haake, S., and G. Huang. 2002. Molecular biology of the host-microbe interaction in periodontal diseases: selected topics, p. 153-156. In H. Takei, M. Newman, and F. Caranza (ed.), Carranza's clinical periodontology, 9th ed. W. B. Saunders Company, Philadelphia, Pa.
- 27a.Kondo, S., B. P. Barna, T. Morimura, J. Takeuchi, J. Yuan, A. Akbasak, and G. H. Barnett. 1995. Interleukin-1 beta-converting enzyme mediates cisplatin-induced apoptosis in malignant glioma cells. Cancer Res. 55:6166-6171. [PubMed] [Google Scholar]
- 28.Korostoff, J., J. F. Wang, I. Kieba, M. Miller, B. J. Shenker, and E. T. Lally. 1998. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect. Immun. 66:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lally, E. T., E. E. Golub, and I. R. Kieba. 1994. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J. Biol. Chem. 269:31289-31295. [PubMed] [Google Scholar]
- 30.Lally, E. T., R. B. Hill, I. R. Kieba, and J. Korostoff. 1999. The interaction between RTX toxins and target cells. Trends Microbiol. 7:356-361. [DOI] [PubMed] [Google Scholar]
- 31.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. J. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a β2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463-30469. [DOI] [PubMed] [Google Scholar]
- 32.Lear, J. D., U. G. Furblur, E. T. Lally, and J. C. Tanaka. 1995. Actinobacillus actinomycetemcomitans leukotoxin forms large conductance, voltage-gated ion channels when incorporated into planar lipid bilayers. Biochim. Biophys. Acta 1238:34-41. [DOI] [PubMed] [Google Scholar]
- 33.Li, J., and K. D. Clinkenbeard. 1999. Lipopolysaccharide complexes with Pasteurella haemolytica leukotoxin. Infect. Immun. 67:2920-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig, A. 1996. Cytolytic toxins from gram-negative bacteria. Microbiologia 12:281-296. [PubMed] [Google Scholar]
- 35.Mangan, D. F., N. S. Taichman, E. T. Lally, and S. M. Wahl. 1991. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect. Immun. 59:3267-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McArthur, W. P., C. C. Tsai, P. C. Baehni, R. J. Genco, and N. S. Taichman. 1981. Leukotoxic effects of Actinobacillus actinomycetemcomitans: modulation by serum components. J. Periodontal Res. 16:159-170. [DOI] [PubMed] [Google Scholar]
- 37.Monack, D. M., W. W. Navarre, and S. Falkow. 2001. Salmonella-induced macrophage death: the role of caspase-1 in death and inflammation. Microbes Infect. 3:1201-1212. [DOI] [PubMed] [Google Scholar]
- 38.Nicotera, P., M. Leist, and E. Ferrando-May. 1999. Apoptosis and necrosis: different execution of the same death. Biochem. Soc. Symp. 66:69-73. [DOI] [PubMed] [Google Scholar]
- 39.Novak, M. J., and K. F. Novak. 1996. Early-onset periodontitis. Curr. Opin. Periodontol. 3:45-58. [PubMed] [Google Scholar]
- 40.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 41.Simpson, D. L., P. Berthold, and N. S. Taichman. 1988. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 56:1162-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slots, J., and M. Ting. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontology 2000 20:82-121. [DOI] [PubMed] [Google Scholar]
- 43.Stanley, P., V. Koronakis, and C. Hughes. 1998. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol. Mol. Biol. Rev. 62:309-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taichman, N. S., R. T. Dean, and C. J. Sanderson. 1980. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect. Immun. 28:258-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornberry, N. A., H. G. Bull, J. R. Calaycay, K. T. Chapman, A. D. Howard, M. J. Kostura, D. K. Miller, S. M. Molineaux, J. R. Weidner, J. Aunins, et al. 1992. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768-774. [DOI] [PubMed] [Google Scholar]
- 46.Tsai, C. C., W. P. McArthur, P. C. Baehni, B. F. Hammond, and N. S. Taichman. 1979. Extraction and partial characterization of a leukotoxin from a plaque-derived gram-negative microorganism. Infect. Immun. 25:427-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Winkelhoff, A. J., and J. Slots. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontology 2000 20:122-135. [DOI] [PubMed] [Google Scholar]
- 48.Walsh, L. J., F. Stritzel, K. Yamazaki, P. S. Bird, E. Gemmell, and G. J. Seymour. 1989. Interleukin-1 and interleukin-1 inhibitor production by human adherent cells stimulated with periodontopathic bacteria. Arch. Oral Biol. 34:679-683. [DOI] [PubMed] [Google Scholar]
- 49.Welch, R. A. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85-111. [DOI] [PubMed] [Google Scholar]
- 50.White, P. A., M. Wilson, S. P. Nair, A. C. Kirby, K. Reddi, and B. Henderson. 1995. Characterization of an antiproliferative surface-associated protein from Actinobacillus actinomycetemcomitans which can be neutralized by sera from a proportion of patients with localized juvenile periodontitis. Infect. Immun. 63:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson, P. D., D. Hreniuk, and J. Lenard. 1989. Reduced cytotoxicity of the lysosomotropic detergent N-dodecylimidazole after differentiation of HL60 promyelocytes. Cancer Res. 49:507-510. [PubMed] [Google Scholar]
- 52.Wyllie, A. H. 1980. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555-556. [DOI] [PubMed] [Google Scholar]
- 53.Zambon, J. J., J. Slots, and R. J. Genco. 1983. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 41:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]