Abstract
Tuberculous pleurisy is a severe inflammatory response induced by Mycobacterium tuberculosis organisms that have escaped from lung granulomata into the pleural space during pulmonary infection. We have used the guinea pig model of tuberculous pleurisy to examine several aspects of the immune response to this antigen-specific inflammatory event. Pleurisy was induced by injection of heat-killed M. tuberculosis H37Rv directly into the pleural space of guinea pigs previously vaccinated with M. bovis BCG. Four animals were euthanized each day over a period of 9 days. Fluid in the pleural cavity was analyzed for transforming growth factor β1 (TGF-β1) and total interferon (IFN) protein levels. In addition, RNA was obtained from pleural cells and examined for TGF-β1, tumor necrosis factor alpha (TNF-α), IFN-γ, and interleukin-8 (IL-8) expression by real-time PCR. Finally, pleural cells were examined for the ability to proliferate in response to concanavalin A and purified protein derivative (PPD) in vitro. In the pleural fluid, TGF-β1 protein concentrations increased over the course of the inflammatory response while IFN protein levels were not significantly altered. Expression of TGF-β1 mRNA peaked on days 3 and 4, and IFN-γ mRNA expression peaked on day 3 and then returned to background levels. TNF-α mRNA expression was highest on days 2 to 4, and IL-8 mRNA levels remained elevated between days 2 and 5, peaking on day 3 before returning to background levels. PPD-induced proliferative responses were evident by day 3 and remained present throughout the study. Analysis of cytokine expression during tuberculous pleurisy may lead to a better understanding of the self-healing nature of this manifestation of tuberculosis.
Tuberculosis is a leading infectious disease worldwide. Approximately one-third of the world's population is infected with Mycobacterium tuberculosis (10). The predominant extrapulmonary manifestation of this disease is pleurisy, which occurs in approximately 10% of untreated individuals who test positive by the tuberculin test (15). Tuberculous pleurisy is caused by a severe delayed-type hypersensitivity reaction in response to the rupture of a subpleural focus of M. tuberculosis infection and subsequent escape of the organism, or its antigens, into the pleural space (15).
Evidence that tuberculous pleurisy is the result of a cell-mediated immune response rather than tissue destruction by active infection with M. tuberculosis has come from several experimental studies with guinea pigs and rabbits. Pleural effusions were induced upon intrapleural injection of purified protein derivative (PPD) from M. tuberculosis into animals previously sensitized to mycobacteria or their components (1, 30). Additionally, presensitization to mycobacteria was required in order for pleural effusions to develop in guinea pigs injected intrapleurally with viable M. bovis BCG (3, 28). Adoptive transfer of cells from immune guinea pigs to naive animals prior to intrapleural administration of PPD resulted in development of pleural effusions in the recipient animals, while transfer of immune serum did not (1). Finally, treatment of immunized guinea pigs with antilymphocyte serum prevented the development of pleural effusions upon delivery of PPD to the pleural space (18).
Further evidence that a specific cell-mediated immune response causes tuberculous pleurisy comes from studies of patients suffering from this manifestation of tuberculosis. Events that occur in the pleural space of patients with tuberculous pleurisy are not mirrored in peripheral blood. Elevated levels of tumor necrosis factor (TNF) and gamma interferon (IFN-γ) have been found in pleural fluid compared with those in the serum of tuberculous pleurisy patients (6). In addition, CD4+ T-helper cells of the Th1 phenotype are the predominant cell type in the pleural fluid, and these cells proliferate and produce cytokines such as IFN-γ and IL-2 (7, 8). Compared with that of individuals with nontuberculous pleurisy, pleural fluid from patients with tuberculous pleurisy contained elevated levels of transforming growth factor β (TGF-β) and IFN-γ but not TNF-α (20). The immune response to pleurisy may be the appropriate response to M. tuberculosis organisms, since this clinical manifestation resolves without therapy (9, 24).
Previously, a guinea pig model of tuberculous pleurisy was developed in our laboratory to mimic human tuberculous pleurisy. In this model, M. bovis BCG-vaccinated animals received bilateral intrapleural injections of heat-killed virulent M. tuberculosis (23). Pleural effusions were examined 7 days postinduction, and greater concentrations of lymphocytes were found in the pleural fluid than in the peripheral blood. In addition, pleural fluid cells showed significantly increased proliferation upon stimulation with PPD than did peripheral blood mononuclear cells (PBMC). In agreement with human studies, higher levels of IFN and TNF were found in the pleural fluid than in the peripheral blood, and pleural effusion cells stimulated in vitro with PPD produced greater levels of bioactive TNF and IFN than did PBMC (S. W. Phalen, M. S. Smith, and D. N. McMurray, Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995, abstr. U-105, 1995). On the basis of this information, the guinea pig appears to be a relevant animal model for the study of tuberculous pleurisy.
In this study, we analyzed several aspects of tuberculous pleurisy over the course of 9 days in order to elucidate the effect of vaccination on the development of a cytokine profile that characterizes a protective, antigen-specific inflammatory response in the guinea pig. Pleural fluid was analyzed for the presence of TGF-β1 and total IFN protein levels, expression of TGF-β1, IFN-γ, TNF-α, and IL-8 mRNAs by pleural fluid cells was quantified, and the proliferative capacity of these cells in response to a T-cell mitogen (concanavalin A [ConA]) and to the mycobacterium-specific antigen PPD was examined.
MATERIALS AND METHODS
Reagents.
RPMI 1640 medium supplemented with HEPES buffer, 10% Hartley guinea pig serum (Harlan Laboratories, Indianapolis, Ind.), 2-mercaptoethanol (10 μM), l-glutamine (2 μM), penicillin (100 U/ml), and streptomycin (100 μg/ml) was used in all experiments. ConA (Sigma, St. Louis, Mo.) and PPD (Statens Seruminstitut, Copenhagen, Denmark) were used to stimulate lymphoproliferation.
Animals and vaccination.
Outbred Hartley strain guinea pigs from Charles River Breeding Laboratories, Inc., were used in this study. Guinea pigs were vaccinated by intradermal injection of 0.1 ml (103 CFU) of M. bovis BCG (Danish 1331 strain; Statens Seruminstitut). All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee.
Mycobacterium preparation and pleurisy induction.
M. tuberculosis H37Rv (ATCC 27294; American Type Culture Collection, Manassas, Va.) was removed from storage at −80°C and rapidly thawed. The organisms were vortexed and sonicated with an Ultrasonics sonicator (Heat Systems-Ultrasonics, Inc., Plainview, N.Y.) for 45 to 60 s at an output setting of 8.0 to disrupt bacterial clumps. The bacteria were then heat killed by incubation in an 80°C water bath for 2 h and diluted in sterile, endotoxin-free 0.9% sodium chloride solution (Sigma) to a final concentration of 5.5 × 107 CFU/ml. Five weeks after vaccination, guinea pigs were anesthetized in accordance with the previous protocol (23) and pleurisy was induced by injection of 1 ml of heat-killed M. tuberculosis H37Rv bilaterally into the pleural cavity (day 0).
Necropsy and cell isolation.
At daily intervals following pleuritis induction, four guinea pigs were euthanized each day over a period of 9 days by intraperitoneal injection of an overdose (100 mg/kg) of sodium pentobarbital (Sleepaway; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). Upon euthanasia, fluid in the pleural cavity was collected and the area was washed two times with ice-cold phosphate-buffered saline to remove accumulated inflammatory cells. Pleural exudate was centrifuged at 200 × g for 15 min. The fluid was then transferred to a new conical tube and centrifuged at 1,400 × g for 20 min to remove all traces of cells and stored at −80°C until needed. Pleural cells were treated with ACK lysing buffer (0.15 M NH4Cl, 0.01 M KHCO3, 0.1 mM Na2EDTA) to remove erythrocytes and then washed twice with phosphate-buffered saline. The cells were then resuspended in 1 ml of RPMI medium, and viable cells were counted on a hemacytometer by the trypan blue exclusion method.
Differential cell counts.
Cells obtained from the pleural cavity were examined in order to determine the cell types present. Cells were centrifuged onto silanated glass slides (CSA-100; PGC Scientifics, Gaithersburg, Md.) at 140 × g for 5 min (Cytospin 2; Shandon Southern Instrument, Inc., Sewickley, Pa.). Cells were stained with Diff-Quik (Dade Behring Inc., Newark, Del.) and viewed under a light microscope in order to determine the relative immune cell composition by cell morphology.
Measurement of cytokines in pleural fluid.
In order to determine TGF-β1 protein concentrations, pleural fluid was analyzed by enzyme-linked immunosorbent assay (ELISA; Human TGF-β1 DuoSet ELISA Development System; R&D Systems; Minneapolis, Minn.). Prior to assay, pleural fluid samples were activated in accordance with the manufacturer's protocol for activation of serum samples.
The guinea pig fibroblast cell line 104C1 (ATCC CRL-1405; American Type Culture Collection) and the challenge virus encephalomyocarditis virus (ATCC VR-129B) were used to determine concentrations of bioactive IFN in the pleural fluid as previously described (31). Briefly, 104C1 cells were seeded onto 96-well plates at a concentration of 3 × 104/well and incubated at 37°C in 5% CO2 for 24 h. Human IFN-α (400 U/ml) and pleural fluid samples were twofold serially diluted and added (50%, vol/vol) to the cells, and the plate was incubated for 20 to 24 h. Encephalomyocarditis virus was then added to the plate at a concentration of 900 PFU/well, and the plate was incubated for 24 to 30 h. WST-1/1-methoxy PMS (Dojindo, Kumamoto, Japan) was added to the cells, and the plate was incubated for 2 to 4 h at 37°C. Color development was stopped with 1 N H2SO4. The optical density at 450 nm was measured with a microtiter plate reader with a reference filter at 630 nm.
Total-RNA isolation and real-time PCR.
Total RNA was isolated from cells with RNeasy columns, and contaminating DNA was removed by on-column treatment with RNase-free DNase (QIAGEN, Valencia, Calif.). Reverse transcription, performed with TaqMan Reverse Transcription Reagents, and real-time PCR, performed with SYBR Green I double-stranded DNA binding dye (Applied Biosystems, Foster City, Calif.), were carried out in accordance with our previously published protocol (19). Real-time primers for guinea pig TGF-β1, IFN-γ, TNF-α, IL-8, and 18S were designed with Primer Express software (Applied Biosystems) and are described in Table 1. The only available sequence data for these guinea pig genes is cDNA; thus, it is not known whether the primers span introns. However, reverse transcriptase-negative controls were used to ensure that amplification was not due to contaminating genomic DNA. In order to ensure that only the correct gene was amplified, dissociation curves for the PCR samples were created by an additional denaturation step at 95°C for 15 s, annealing at 60°C for 20 s, and a slow increase in temperature back to 95°C with a ramp time of 19 min, 59 s. Fold induction of mRNA was determined from the threshold cycle values normalized for 18S expression and then normalized to the value derived from healthy, pleuritis-free animals.
TABLE 1.
Primer sequences used for quantitative PCRa
| mRNA | Primerb |
|---|---|
| TGF-β1 | F, 5′ CATCGATATGGAGCTGGTGAAG 3′ |
| R, 5′ GCCGTAATTTGGACAGGATCTG 3′ | |
| TNF-α | F, 5′ CCTACCTGCTTCTCACCCATACC 3′ |
| R, 5′ TTGATGGCAGAGAGAAGGTTGA 3′ | |
| IFN-γ | F, 5′ ATTTCGGTCAATGACGAGCAT 3′ |
| R, 5′ GTTTCCTCTGGTTCGGTGACA 3′ | |
| IL-8 | F, 5′ GGCAGCCTTCCTGCTCTCT 3′ |
| R, 5′ CAGCTCCGAGACCAACTTTGT 3′ | |
| 18S | F, 5′ TGCATGGCCGTTCTTAGTTG 3′ |
| R, 5′ AGTTAGCATGCCAGAGTCTCGTT 3′ |
Primers were designed with Primer Express software as described in Materials and Methods.
F, forward; R, reverse.
Lymphoproliferation.
Cells from the pleural effusion were analyzed for the ability to proliferate in response to ConA (10 μg/ml) and PPD (12.5 and 25 μg/ml) in vitro. Pleural cells were seeded onto 96-well plates (2 × 105/well) in medium alone or in the presence of ConA or PPD and cultured for 96 h in accordance with our standard procedure (11). [3H]thymidine was added at a concentration of 1 μCi/well for the final 6 h of culture. Cells were harvested onto glass fiber filters, and proliferative activity was quantified by counts per minute on a liquid scintillation counter (LS8000; Beckman Instruments, Inc., Fullerton, Calif.). Stimulation indices were calculated by division of the counts per minute of stimulated cells by the counts per minute of unstimulated cells from the same animal.
Statistical analysis.
Analysis of variance was used to test the effect of the treatment variable (day postinduction) on the dependent variables. Mean differences between groups were tested for statistical significance at the 95% confidence interval by Duncan's post hoc analysis. For statistical analysis of proliferation data, the general linear model was used to test the effects of the independent variables (day postinduction and stimulation with ConA or with PPD at 12.5 or 25 μg/ml) on the dependent variables. The type I experiment-wise error rate of 0.05 was used to control for multiple comparisons. These statistical tests were run on SAS software (release 8.01; SAS Institute, Inc., Cary, N.C.).
RESULTS
Cell and fluid accumulation and gross pathology in guinea pigs with experimental tuberculous pleurisy.
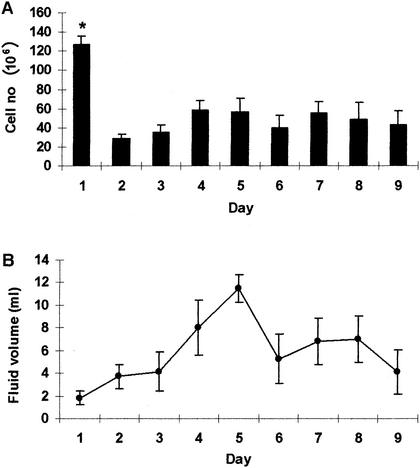
Pleurisy was induced in guinea pigs via injection of heat-killed M. tuberculosis H37Rv bilaterally into the pleural space on day 0. Guinea pigs were euthanized on days 1 to 9, and pleural fluid and cells were removed as described in Materials and Methods. Figure 1 shows cellular influx and fluid accumulation in the pleural space throughout the experiment. On day 1 postinduction, considerable numbers of cells were present in the pleural fluid (127.25 × 106 ± 8.56 × 106) of three of the four guinea pigs studied (Fig. 1A). By day 2, the cell numbers decreased significantly (P = 0.001) and remained between 28.44 × 106 (± 4.94 × 106) and 58.69 × 106 (± 9.69 × 106) during the course of the experiment. There was no concurrent influx of fluid into the pleural space with the cell migration that occurred on day 1 (Fig. 1B); rather, fluid levels in the pleural space peaked on day 5. Examination of the cellular concentration per milliliter of pleural fluid revealed that cells were most concentrated on day 1 postinduction, averaging >50 × 106 cells/ml of pleural fluid (P < 0.0001). The concentration of pleural cells fell to <10 × 106 cells/ml on day 2 and remained at that level (data not shown).
FIG. 1.
Cell and fluid accumulation in the pleural space of guinea pigs with tuberculous pleurisy. Cells (A) and pleural fluid (B) in the pleural space of pleuritic guinea pigs were quantified between days 1 and 9 of the inflammatory response. Cells were stained with trypan blue, and four squares of a hemocytometer were counted and averaged to obtain cell numbers, which are expressed in millions. Total milliliters of pleural fluid were assessed after the fluid was centrifuged and removed from cells. Data and error bars represent average cell number or fluid accumulation for three or four animals ± the standard error of the mean. An asterisk indicates a significant difference in the cell number compared with that on any other day.
The cellular composition in the pleural exudate was examined throughout the course of the inflammatory response. Despite some technical difficulties with the stained cell samples, it was determined that on day 1, 56.8% ± 4.4% of the cells were neutrophils, followed by 24.1% ± 4.6% monocytes and 10.6% ± 3.6% lymphocytes. By day 5, while 46.2% ± 9% of the cells were neutrophils, the lymphocyte population increased a considerable amount (31.9% ± 8%) and the monocyte population fell to 14.9% ± 3.7% of the total cells. Finally, at the end of the experiment (day 9), 62.1% ± 4.3% of the cells were neutrophils, lymphocytes were reduced to 10.3% ± 1.4%, as seen at the beginning of the inflammatory response, and monocytes remained the same (16.1% ± 5.3%).
Upon gross examination of the pleural space, the diaphragm was thickened on day 1 postinduction compared with that of uninfected animals. Around day 5, granulomata began to appear and the pleura became thick and fibrous. This condition continued to worsen throughout the remainder of the experiment (data not shown).
Detection of cytokine proteins in tuberculous pleural fluid.
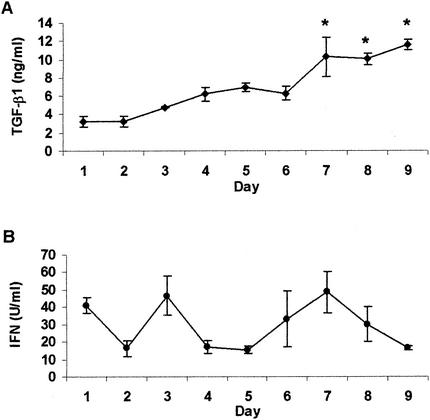
TGF-β1 and total bioactive IFN protein levels were examined in the pleural fluid of pleuritic guinea pigs euthanized between days 1 and 9. TGF-β1 levels were determined by analysis with a human TGF-β1 ELISA. Because of the nature of TGF-β1 and the sensitivity of the ELISA, acid activation of the samples was required, resulting in readings for total in vivo TGF-β1 levels only. Bioactive guinea pig TGF-β1 and human TGF-β1 have 98% sequence homology, and we have previously used human TGF-β1 to exacerbate pulmonary tuberculosis disease in guinea pigs (13). Therefore, we are confident that the human ELISA is able to accurately detect levels of guinea pig TGF-β1 in the pleural fluid. Figure 2 shows that the total levels of TGF-β1 continually increased throughout the development of the antigen-specific inflammatory response. TGF-β1 protein levels were relatively low during the first 2 days postinduction and gradually increased throughout the experiment (Fig. 2A). Concentrations of TGF-β1 from day 4 through day 9 reached significantly higher levels than those present on day 1. Concentrations peaked on days 7 to 9, when levels were significantly higher than those observed on days 1 to 4 and on day 6 (P < 0.0001). Bioactive IFN protein levels, on the other hand, followed no consistent pattern, peaking on days 1, 3, and 7 (Fig. 2B). No statistically significant differences were observed in IFN protein concentrations throughout the inflammatory response.
FIG. 2.
Cytokine protein concentrations in pleural fluid. TGF-β1 (A) and total bioactive IFN (B) protein levels in pleural fluid of guinea pigs during 9 days of tuberculous pleurisy were measured. TGF-β1 protein levels were analyzed by ELISA and are expressed in nanograms per milliliter. Total IFN protein levels were analyzed by bioassay as described in Materials and Methods and are expressed in units per milliliter. Data and error bars represent the mean ± the standard error of the mean for three or four animals per day. An asterisk indicates a significant increase in TGF-β1 levels above that on all days except day 5.
mRNA expression in cells collected from tuberculous pleural fluid.
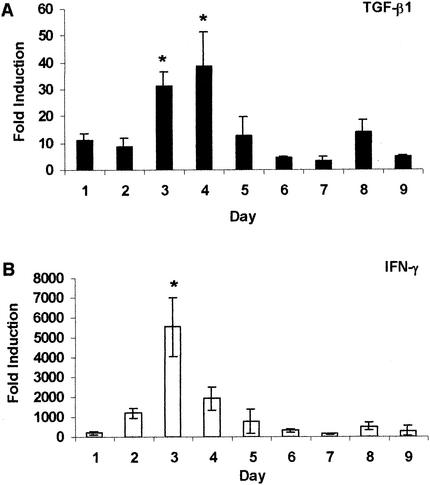
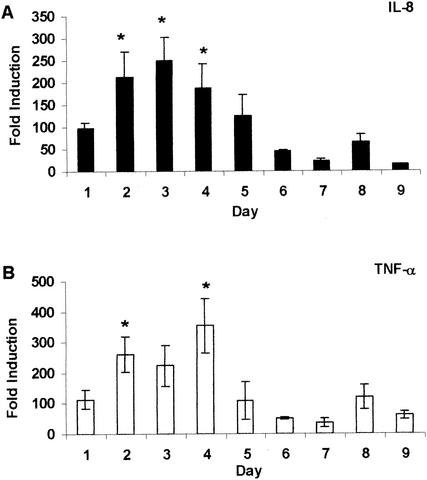
Expression of TGF-β1, IFN-γ, IL-8, and TNF-α mRNAs was examined by real-time quantitative reverse transcription PCR at daily intervals during the course of the antigen-specific inflammatory response, and the results are illustrated in Fig. 3 and 4. Expression of TGF-β1 mRNA (Fig. 3A) was modest during the first 2 days postinduction (approximately 10 times that of negative controls) and increased significantly on days 3 and 4 (P < 0.002). By day 5, TGF-β1 mRNA expression decreased and remained low through the rest of the study. IFN-γ mRNA expression reached levels much higher than that of TGF-β1 mRNA expression, starting at approximately 200-fold higher than that of negative controls on day 1 following pleuritis induction (Fig. 3B). Expression of IFN-γ mRNA increased significantly and peaked on day 3 with a fold induction >5,000 times greater than that of negative controls (P < 0.0001). On day 4, IFN-γ mRNA expression decreased drastically, and by day 6, it had returned to levels equivalent to those seen on day 1. IL-8 mRNA expression, like IFN-γ mRNA expression, rose from day 1, peaked on day 3, and fell until day 6 (Fig. 4A). However, the changes were much more subtle. On days 2 to 4, IL-8 mRNA expression was significantly higher than on days 6, 7, and 9 (P = 0.0008), with the highest expression approximately 250 times that of negative controls. Figure 4B shows that the TNF-α mRNA level was relatively low on day 1 (approximately 100 times that of negative controls) and increased between days 2 and 4, reaching levels significantly higher than those on days 6, 7, and 9 (P = 0.002). By day 5, expression returned to the levels seen on day 1 and remained low throughout the period of study.
FIG. 3.
Expression of TGF-β1 and IFN-γ mRNAs during tuberculous pleurisy. Expression of TGF-β1 (A) and IFN-γ (B) mRNAs was quantified in pleural fluid cells obtained from guinea pigs with tuberculous pleurisy between days 1 and 9. Fold induction was determined from the threshold cycle values normalized for 18S expression and then normalized to the values derived from healthy, pleuritis-free animals. Data and error bars represent the mean of three or four animals ± the standard error of the mean. An asterisk indicates a significant increase in mRNA expression compared to that on all other days.
FIG. 4.
Expression of IL-8 and TNF-α mRNAs during tuberculous pleurisy. Expression of IL-8 (A) and TNF-α (B) mRNAs was quantified in pleural fluid cells obtained from guinea pigs with tuberculous pleurisy euthanized on days 1 to 9. Fold induction was calculated as described in the legend to Fig. 3. Data and error bars represent the mean of three or four animals ± the standard error of the mean. An asterisk indicates a significant increase in mRNA expression compared with that on days 6, 7, and 9.
Proliferation of pleural lymphocytes in response to ConA and PPD.
Cells were removed daily from the pleural space and examined for the ability to proliferate in response to ConA, a T-cell mitogen, and PPD, a mycobacterium-specific antigen. Throughout the development of the local inflammatory response, spontaneous proliferation was extremely high, ranging from 1,452 to 12,520 cpm, and averaged 5,158 ± 479 cpm. Comparatively, spontaneous proliferation for healthy, nonpleuritic guinea pigs ranged from 500 to 1,000 cpm.
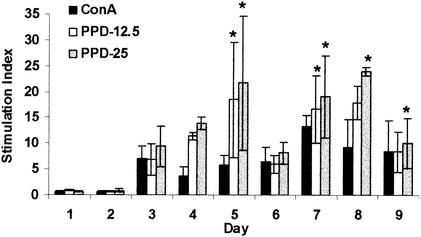
As Fig. 5 shows, on days 1 and 2, cells did not respond at levels greater than spontaneous proliferation levels. However, by day 3, and throughout the remainder of the experiment, proliferation in response to ConA and both concentrations of PPD was evident. Proliferative responses were significantly greater than that seen on day 1 postinduction upon stimulation with PPD at 12.5 μg/ml on days 5 and 7 (P < 0.05) and on days 5 and 7 to 9 in response to PPD at 25 μg/ml (P < 0.02). On the other hand, ConA-induced proliferation did not reach levels significantly higher than those of day 1 during the study period.
FIG. 5.
Proliferation of pleural exudate cells in tuberculous pleurisy. Pleuritic guinea pigs were euthanized on days 1 to 9, and cells were removed from the pleural cavity. Cells were cultured for 96 h in the presence of ConA (10 μg/ml) or PPD (12.5 or 25 μg/ml). Proliferative responses are reported as stimulation indices (counts per minute of stimulated cells divided by counts per minute of unstimulated cells). Data and error bars represent the mean of three or four animals ± the standard error of the mean. An asterisk indicates a significant increase in proliferation compared to similarly stimulated cells on day 1.
Proliferation in response to ConA and PPD was highly variable between animals euthanized on the same days, resulting in the large error bars seen in Fig. 5. This variability may explain why proliferation in response to ConA did not reach levels significantly greater than those of day 1 throughout the experiment. Cells responded equally to ConA and PPD, except on day 5, when proliferation was significantly stronger upon stimulation with PPD at 25 μg/ml than upon stimulation with ConA (P = 0.02). Taken together, these data suggest that by day 3, sufficient numbers of mycobacterial antigen-specific T lymphocytes were present in the pleural space and that they remained there throughout the infection period.
DISCUSSION
Successful pleural effusions were induced in almost all of the guinea pigs in this study, and all of the animals survived the inflammatory response throughout the 9 days of study. Fluid accumulation was highly variable between animals euthanized on the same day, as has been demonstrated in previous studies of pleurisy in guinea pigs (1, 28). This variability may be responsible for the inability to achieve statistical significance on days when fluid accumulation was clearly greater than that on day 1 (Fig. 1B). The immediate influx of cells into the pleural space illustrates the rapidity and intensity of the delayed-type hypersensitivity response that occurs upon exposure of immune animals to mycobacterial antigens in a confined anatomical location. By day 2 postinduction, cell numbers in the pleural space dropped drastically from the levels observed on day 1 (Fig. 1A). They rose slightly on day 4 and then stayed relatively constant throughout the remainder of the experiment. Yamamoto and colleagues (30) used a slightly different model to induce pleurisy and only carried the study out to 48 h; however, their findings were similar to ours. Maximum cell numbers were observed between 18 and 24 h postinduction and averaged approximately 100 × 106 cells at that time, followed by a decrease to 33 × 106 cells at the end of their study.
During the first 2 days postinduction, no proliferative responses greater than spontaneous proliferation levels were observed upon stimulation with either ConA or PPD (Fig. 5). On day 3, however, proliferative responses to both ConA and PPD rose above background levels, indicating active proliferation in response to the stimuli, and remained strong through the rest of the study. It is important to note that between days 3 and 9, with the exception of days 5 and 7, one guinea pig per group did not respond at all to ConA or PPD. Of the 22 guinea pigs whose pleural cells proliferated in response to a stimulus, 15 (68%) had greater responses to PPD than to ConA. In a similar fashion, Widstrom and Nilsson (29) generally observed less proliferation of pleural lymphocytes in response to phytohemagglutinin than in response to PPD, although in our model, responses to both the mitogen and the antigen were much higher than those they observed. In addition, we observed a high level of spontaneous proliferation by pleural exudate cells. Previous studies have demonstrated spontaneous proliferation of pleural fluid cells ranging from less than 500 to nearly 2,000 cpm in humans with tuberculous pleuritis (9, 22). Similarly, in BCG-induced pleurisy in guinea pigs, Widstrom and Nilsson (28) reported spontaneous proliferation in the range of 300 to 900 cpm. The pleural cells used in the studies described here were lymphocyte populations purified to various degrees. Therefore, it is possible that our enhanced spontaneous proliferation was due to elevated numbers of antigen-presenting cells in the unseparated population. The cells recovered from the pleural space are engaged in an intense inflammatory response; thus, it is no surprise that they proliferate in the absence of any additional stimulus.
The primary purpose of this study was to examine levels of cytokine production throughout the course of experimental tuberculous pleurisy. TGF-β1 was of primary interest because its production is greater in monocytes and at sites of disease in individuals with active pulmonary tuberculosis than in healthy tuberculin converters (27). In addition, in patients with active tuberculosis, and in our guinea pig model, TGF-β1 neutralization improves T-lymphocyte function (12, 26). In our experiment, TGF-β1 protein concentrations in pleural fluid (Fig. 2A) and mRNA levels in cells obtained from the pleural cavity (Fig. 3A) were measured throughout the 9-day study. In the beginning of the experiment, TGF-β1 protein levels were relatively low and continually rose to a peak on days 7 to 9. By day 4, TGF-β1 protein reached levels significantly greater than those of days 1 and 2, significantly increased again on day 7, and then remained elevated through day 9. TGF-β1 protein levels were not measured in healthy, pleuritis-free guinea pigs because of a lack of pleural fluid in these animals. TGF-β1 mRNA expression was, however, analyzed in resident pleural cells of healthy animals and used as the calibrator against which the fold induction of TGF-β1 mRNA was measured throughout the experiment. TGF-β1 mRNA expression in pleural cells significantly increased above the day 1 level on days 3 and 4 and then dropped and remained low. TGF-β1 undergoes extensive posttranslational regulation in the cell and, once released into the extracellular milieu, must be activated in order to exert its biological effects. As a result, there is often no obvious relationship between mRNA expression and protein production (4, 17). It is possible that the increased mRNA expression that occurred on days 3 and 4 is responsible for the increased protein levels observed later in the experiment, specifically on days 7 through 9.
IFN-γ is an important cytokine in tuberculous pleurisy, and its level is elevated in the pleural fluid of patients with this manifestation of tuberculosis (6, 20). As previously discussed, IFN-γ mRNA expression was measured in cells of healthy, pleuritis-free guinea pigs and used as the control; however, the same could not be done for total IFN protein levels because of a lack of pleural fluid in healthy animals. In our guinea pig model, IFN-γ mRNA expression peaked on day 3. We examined total IFN protein levels by the use of a bioassay previously designed in our laboratory (31) and found various concentrations of bioactive IFN protein present throughout the experiment. As a result of our inability to detect significant differences in bioactive IFN protein concentrations and the fact that the bioassay detects all three species of interferon, we cannot say whether increased IFN-γ mRNA expression (Fig. 3B) led to increased protein production.
Other important cytokines in tuberculous pleurisy include TNF-α and the chemokine IL-8. Barnes and colleagues (6) demonstrated that TNF-α production was greater in the pleural fluid of patients with tuberculous pleurisy than in the serum of those patients, and they demonstrated TNF-α mRNA expression in pleural lesions of these patients. Upon analysis of TNF-α mRNA expression in cells collected from the pleural space (Fig. 4B), we found the highest levels between days 2 and 4. Monocytes-macrophages are the predominant producers of TNF-α, and thus, its decreased expression after day 4 may be due to the decrease in these cells in the pleural exudate. We attempted to assess total TNF protein levels in the pleural fluid of guinea pigs with tuberculous pleurisy. However, because of the viscous nature of pleural fluid, we were not able to successfully detect the presence of TNF with a bioassay. IL-8 is increased in tuberculous pleural effusions compared with parapneumonic effusions (14). IL-8 levels have also been demonstrated to be higher in tuberculous pleural effusions than in fluid obtained from patients with congestive heart failure (21). Pleural fluid from tuberculous pleurisy patients was chemotactic for T lymphocytes, and addition of IL-8 monoclonal antibody to the fluid completely suppressed T-lymphocyte chemotaxis. IL-8 is also known to be chemotactic for neutrophils (5). In a recent study, IL-8 was produced by pleural mesothelial cells in response to stimulation with IL-1β, TNF-α, and, to a lesser degree, lipopolysaccharide (2). Additionally, mesothelial cells were chemotactic for neutrophils, and incubation of these cells with IL-8 antiserum significantly inhibited neutrophil chemotaxis. Attempts to measure IL-8 protein in the pleural fluid of guinea pigs with tuberculous pleurisy with a human IL-8 ELISA were unsuccessful. However, IL-8 mRNA expression on day 1 was approximately 100-fold greater than the levels in healthy, nonpleuritic guinea pigs, suggesting that IL-8 expression occurred early in response to the pleuritis (Fig. 4A). This increase in IL-8 mRNA expression may have been responsible for the influx of cells, namely neutrophils, into the pleural cavity on day 1. IL-8 mRNA expression increased on days 2 to 4 and, during this time, was significantly higher than levels seen later in the experiment. This increase may have been partially responsible for the influx of lymphocytes into the pleural space seen on day 5. By day 6, levels of the mRNAs of all of the cytokines measured were down-regulated and remained low until day 9. On the basis of TGF-β1 protein levels in the pleural fluid, it is possible that TGF-β1 was involved in the suppression of these cytokines at the end of the experiment. This hypothesis is supported by the findings that TGF-β1 was able to inhibit production of IFN-γ and TNF-α by nonadherent or adherent PBMC, respectively (16), and IL-8 production by endothelial cells was inhibited by TGF-β1 (25).
Tuberculous pleurisy is an intense immune response to mycobacteria that results in clearance of organisms from the pleural space. Examination of this response may shed light on the factors involved in a protective response to mycobacteria. These data provide baseline observations that will allow future experiments to be designed to test the effects of recombinant guinea pig cytokines and chemokines, or their neutralizing antibodies, injected directly into the pleural space, on the accumulation and function of immune cells drawn into a mycobacterial antigen-specific inflammatory exudate.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 AI 15495 to D.N.M.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Allen, J. C., and M. A. Apicella. 1968. Experimental pleural effusion as a manifestation of delayed hypersensitivity to tuberculin PPD. J. Immunol. 101:481-487. [PubMed]
- 2.Antony, V. B., J. W. Hott, S. L. Kunkel, S. W. Godbey, M. D. Burdick, and R. M. Strieter. 1995. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am. J. Respir. Cell Mol. Biol. 12:581-588. [DOI] [PubMed] [Google Scholar]
- 3.Antony, V. B., S. A. Sahn, A. C. Antony, and J. E. Repine. 1985. Bacillus Calmette-Guérin-stimulated neutrophils release chemotaxins for monocytes in rabbit pleural spaces and in vitro. J. Clin. Investig. 76:1514-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assoian, R. K., B. E. Fleurdelys, H. C. Stevenson, P. J. Miller, D. K. Madtes, E. W. Raines, R. Ross, and M. B. Sporn. 1987. Expression and secretion of type β transforming growth factor by activated human macrophages. Proc. Natl. Acad. Sci. USA 84:6020-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggiolini, M., A. Walz, and S. L. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes, P. F., S. J. Fong, P. J. Brennan, P. E. Twomey, A. Mazumder, and R. L. Modlin. 1990. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J. Immunol. 145:149-154. [PubMed] [Google Scholar]
- 7.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes, P. F., V. Mehra, G. R. Hirschfield, S. J. Fong, C. Abou-Zeid, G. A. Rook, S. W. Hunter, P. J. Brennan, and R. L. Modlin. 1989. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J. Immunol. 143:2656-2662. [PubMed] [Google Scholar]
- 9.Barnes, P. F., S. D. Mistry, C. L. Cooper, C. Pirmez, T. H. Rea, and R. L. Modlin. 1989. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J. Immunol. 142:1114-1119. [PubMed] [Google Scholar]
- 10.Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, M. K., R. A. Bartow, C. L. Mintzer, and D. N. McMurray. 1987. Effects of diet and genetics on Mycobacterium bovis BCG vaccine efficacy in inbred guinea pigs. Infect. Immun. 55:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai, G. 1997. Detrimental effects of dietary protein malnutrition on host acquired antituberculosis immunity and underlying mechanisms. Ph.D. thesis. Texas A&M University, College Station.
- 13.Dai, G., and D. N. McMurray. 1999. Effects of modulating TGF-β1 on immune responses to mycobacterial infection in guinea pigs. Tuber. Lung Dis. 79:207-214. [DOI] [PubMed] [Google Scholar]
- 14.Dlugovitzky, D., L. Rateni, A. Torres-Morales, J. Ruiz-Silva, R. Pinesky, B. Canosa, O. Molteni, and O. Bottasso. 1997. Levels of interleukin-8 in tuberculous pleurisy and the profile of immunocompetent cells in pleural and peripheral compartments. Immunol. Lett. 55:35-39. [DOI] [PubMed] [Google Scholar]
- 15.Ellner, J. J., P. F. Barnes, R. S. Wallis, and R. L. Modlin. 1988. The immunology of tuberculous pleurisy. Semin. Respir. Infect. 3:335-342. [PubMed] [Google Scholar]
- 16.Espevik, T., I. S. Figari, M. R. Shalaby, G. A. Lackides, G. D. Lewis, H. M. Shepard, and M. A. Palladino, Jr. 1987. Inhibition of cytokine production by cyclosporin A and transforming growth factor β. J. Exp. Med. 166:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehrl, J. H., L. M. Wakefield, A. B. Roberts, S. Jakowlew, M. Alvarez-Mon, R. Derynck, M. B. Sporn, and A. S. Fauci. 1986. Production of transforming growth factor β by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163:1037-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibowitz, S., L. Kennedy, and M. H. Lessof. 1973. The tuberculin reaction in the pleural cavity and its suppression by antilymphocyte serum. Br. J. Exp. Pathol. 54:152-162. [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2002. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 70:5471-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda, J., N. Ueki, T. Ohkawa, N. Iwahashi, T. Nakano, T. Hada, and K. Higashino. 1993. Local production and localization of transforming growth factor-β in tuberculous pleurisy. Clin. Exp. Immunol. 92:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace, E., M. Gjomarkaj, M. Melis, M. Profita, M. Spatafora, A. M. Vignola, G. Bonsignore, and C. H. Mody. 1999. Interleukin-8 induces lymphocyte chemotaxis into the pleural space: role of pleural macrophages. Am. J. Respir. Crit. Care Med. 159:1592-1599. [DOI] [PubMed] [Google Scholar]
- 22.Pettersson, T., M. Klockars, and H. Riska. 1981. PHA and PPD reactivity of lymphocytes in pleural effusions. Chest 80:44-47. [DOI] [PubMed] [Google Scholar]
- 23.Phalen, S. W., and D. N. McMurray. 1993. T-lymphocyte response in a guinea pig model of tuberculous pleuritis. Infect. Immun. 61:142-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roper, W. H., and J. J. Waring. 1955. Primary serofibrinous pleural effusion in military personnel. Am. Rev. Tuberc. Pulm. Dis. 71:616-634. [DOI] [PubMed] [Google Scholar]
- 25.Smith, W. B., L. Noack, Y. Khew-Goodall, S. Isenmann, M. A. Vadas, and J. R. Gamble. 1996. Transforming growth factor-β1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J. Immunol. 157:360-368. [PubMed] [Google Scholar]
- 26.Toossi, Z., and J. J. Ellner. 1998. The role of TGF-β in the pathogenesis of human tuberculosis. Clin. Immunol. Immunopathol. 87:107-114. [DOI] [PubMed] [Google Scholar]
- 27.Toossi, Z., P. Gogate, H. Shiratsuchi, T. Young, and J. J. Ellner. 1995. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J. Immunol. 154:465-473. [PubMed] [Google Scholar]
- 28.Widstrom, O., and B. S. Nilsson. 1982. Low in vitro response to PPD and PHA in lymphocytes from BCG-induced pleurisy in guinea pigs. Eur. J. Respir. Dis. 63:435-441. [PubMed] [Google Scholar]
- 29.Widstrom, O., and B. S. Nilsson. 1982. Pleurisy induced by intrapleural BCG in immunized guinea pigs. Eur. J. Respir. Dis. 63:425-434. [PubMed] [Google Scholar]
- 30.Yamamoto, S., C. J. Dunn, F. Capasso, D. A. Deporter, and D. A. Willoughby. 1975. Quantitative studies on cell-mediated immunity in the pleural cavity of guinea pigs. J. Pathol. 117:65-73. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto, T., A. Jeevan, K. Ohishi, Y. Nojima, K. Umemori, S. Yamamoto, and D. N. McMurray. 2002. A new assay system for guinea pig interferon biological activity. J. Interferon Cytokine Res. 22:793-797. [DOI] [PubMed] [Google Scholar]