Abstract
Patients or experimental animals previously exposed to lipopolysaccharide (LPS) become tolerant to further LPS challenge. We investigated the potential of the macrophage-activating lipopeptide 2 (MALP-2) to induce in vivo cross tolerance to tumor necrosis factor alpha (TNF-α) and LPS. MALP-2-induced tolerance could be of practical interest, as MALP-2 proved much less pyrogenic in rabbits than LPS. Whereas LPS signals via Toll-like receptor 4 (TLR4), MALP-2 uses TLR2 and TLR6. LPS-mediated cytokine release was studied in mice pretreated with intraperitoneal injections of MALP-2. No biologically active TNF-α could be detected in the serum of MALP-2-treated animals when challenged with LPS 24 or 72 h later, whereas suppression of LPS-dependent interleukin (IL)-6 lasted for only 24 h. Protection from lethal TNF-α shock was studied in galactosamine-treated mice. Dose dependently, MALP-2 prevented death from lethal TNF-α doses in TLR4−/− but not in TLR2−/− mice, with protection lasting from 5 to 24 h. To assay protection from LPS, mice were pretreated with MALP-2 doses of up to 10 μg. Five and 24 h later, the animals were simultaneously sensitized and challenged by intravenous coinjection of galactosamine and a lethal dose of 50 ng of LPS. There was only limited protection (four of seven mice survived) when mice were challenged 5 h after MALP-2 pretreatment, and no protection when mice were challenged at later times. The high effectiveness of MALP-2 in suppressing TNF-α, the known ways of biological inactivation, and low pyrogenicity make MALP-2 a potential candidate for clinical use.
Preexposure to small doses of lipopolysaccharide (LPS) is known to lead to unresponsiveness or low responsiveness, so-called tolerance, to higher doses of endotoxin given at later times. Various experimental models exist to study this phenomenon, ranging from pure in vitro work measuring the refractoriness of prestimulated cells to in vivo protection studies with animals (reviewed in references 16, 36, 63, and 67). In such studies, tumor necrosis factor alpha (TNF-α) has been reported to be one important mediator responsible for lethal LPS toxicity (11, 15, 57). However, interleukin (IL)-12 (5, 25), IL-18 (54), gamma interferon (IFN-γ) (4, 9, 26, 57), granulocyte macrophage colony-stimulating factor (2), or other mediators may also be involved.
Since administration of LPS for protection of patients from gram-negative septic shock is limited by the pyrogenicity and systemic toxicity of endotoxin, attempts have been made to make use of other, hopefully less harmful substances to achieve cross tolerance against LPS (24, 30, 33, 35, 50, 51, 53, 55). These substances, like LPS, are all macrophage activators, since induction of tolerance is mediated by macrophages (14). Another common feature is that these substances signal through the recently discovered family of Toll-like receptors (TLRs). The signal pathways of distinct TLRs are very similar but not identical, usually involving the participation of the cell internal adaptor molecule myeloid differentiation factor 88 (MyD88) (28, 48, 59). Since LPS-mediated induction of tolerance requires functional TLR4 (32), it was of interest to examine the possibility of inducing cross tolerance to LPS by prior stimulation through TLR2 and -6 with the 2-kDa macrophage-activating lipopeptide (MALP-2)
The in vitro studies of Sato et al. have shown that MALP-2 can induce cross tolerance to LPS, as shown by decrease of TNF-α production and reduced activation of selected genes, in MALP-2-pretreated macrophages (55, 56). MALP-2, formerly called MDHM (mycoplasma-derived high molecular material), acts on murine macrophages (7, 45, 46, 47) and human monocytes (27), leading to release of proinflammatory cytokines, chemokines, nitric oxide, and prostaglandins, and has pronounced in vivo activity (7, 38). LPS and MALP-2 use similar signal transduction pathways and lead to comparable in vitro stimulation of macrophages (28, 59). However, while LPS requires TLR4 for signaling (52), MALP-2 needs the cooperation of TLR2 and TLR6 for stimulation (44, 60). In addition, activation of NF-κB and IFN-responsive genes by LPS is possible without MyD88 through alternative pathways (29, 61), whereas there is no evidence for a MyD88-independent pathway for MALP-2 (29).
Because of this, and as TLRs are expressed differently on various tissues and potential target cells (10, 12, 40, 49), it is to be expected that the reactions of an experimental animal to systemic administration of either LPS or MALP-2 may differ appreciably. One example is the pyrogenic response in the rabbit: while about 1 ng of LPS causes a considerable rise in body temperature (13), about 1,000 times more MALP-2 is required to achieve the same effect (see Results). Also, natural killer (NK) cells, which play an important role in LPS toxicity (20), express very little or no TLR2 (12, 22) and are not expected to react to a MALP-2 stimulus. We wished, therefore, to test the capacity of the less pyrogenic and possibly less toxic MALP-2 to induce cross tolerance to LPS in an animal model.
Protection of mice against LPS challenge can be measured very conveniently in the galactosamine model in which mice are sensitized to LPS or TNF-α by several orders of magnitude (34). We used this model which indicates lethal TNF-α levels raised after LPS challenge (15) to study the effect of pretreatment with MALP-2 on the induction of cross tolerance to LPS or the lethal effect of TNF-α. Our data show that MALP-2 can provide protection from lethal TNF-α doses but that protection against LPS is incomplete.
MATERIALS AND METHODS
Macrophage activators.
For induction of tolerance, the active and natural S-[2,3-bispalmitoyloxy-(2R)-propyl]cysteinyl-GNNDESNISFKEK enantiomer of MALP-2 was used. It was synthesized and purified by high-performance liquid chromatography (HPLC) as described previously (43, 47, 59). Active fractions were collected in glassware that was made endotoxin free by heat treatment for 4 h at 180°C. Synthetic MALP-2 was kept as a stock solution of about 1.3 mg/ml in water-2-propanol (2/1 [vol/vol]) at 4°C and was half-maximally active at 2.6 pg/ml. The exact peptide content was determined by amino acid analysis. LPS from smooth-form Salmonella enterica serovar Typhimurium was prepared by the phenol-water method (64), and LPS from Salmonella enterica serovar Abortus-equi was prepared by the PCP method of Galanos et al. (17). For in vivo induction of tolerance, MALP-2 or LPS was diluted with nonpyrogenic isotonic saline for injection. Human recombinant TNF-α (rTNF-α) was a kind gift of Knoll GmbH, Ludwigshafen, Germany.
Animal experiments.
All animal experiments were registered and complied with the appropriate German guidelines of the “Tierschutzgesetz.”
Mice.
Outbred female NMRI mice as well as C57BL/10ScSn LPS-sensitive mice were obtained from Harlan Winkelmann (Borchen, Germany). They were between 9 and 13 weeks of age when used. C3H/HeJ LPS-low-responder mice were obtained from Jackson Laboratory, Bar Harbor, Maine. C57BL/6 × 129Sv TLR2−/− mice and C57BL/10ScCr mice, the latter carrying a mutation leading to a lack of TLR4 expression (52) and an impaired IFN-γ response to bacteria (65) due to a defective IL-12 response (42), were bred in the specific pathogen-free facilities of the Max Planck Institute.
Determination of macrophage-stimulating activity by NO release assay.
The in vitro macrophage-stimulating activities of MALP-2 and LPS were determined with a nitric oxide (NO) release assay as previously described (46). Briefly, resident peritoneal exudate cells from C3H/HeJ mice or C57BL/6 × 129Sv TLR2−/− mice served as the macrophage source. A total of 105 cells were seeded in Dulbecco's modified Eagle's medium containing 5% heat-inactivated fetal calf serum, 2 mM glutamine, and 2.5 × 10−5 M 2-mercaptoethanol (culture medium) in 96-well microtiter plates and simultaneously stimulated with 25 ng of recombinant IFN-γ (rIFN-γ)/ml (a generous gift of G. R. Adolf, Ernst Boehringer Institut für Arzneimittelforschung, Vienna, Austria) and a serial dilution of macrophage-activating material. After an incubation period of 45 to 48 h, nitrate was reduced with nitrate reductase (Boehringer), and nitric oxide was determined as the sum of nitrate and nitrite by using Griess reagent.
Pyrogenicity of MALP-2 in rabbits.
The pyrogenic activity of synthetic MALP-2 was tested by Harlan Bioservice for Science GmbH (Walsrode, Germany) in White New Zealand rabbits weighing approximately 2.8 kg each. The rabbits were housed individually in an area of uniform temperature between 19 and 21°C. Before application of MALP-2, the rectal temperature of each rabbit was determined four times every 30 min. MALP-2 was injected in a volume of 2 ml of pyrogen-free saline within 15 s into the ear vein, and rectal temperature was then measured every 30 min. When preparing very dilute MALP-2 preparations for injection, it should be taken into account that MALP-2 readily adsorbs to glassware.
In vivo induction of tolerance.
Tolerance was induced by a single intraperitoneal (i.p.) injection of 2.3 μg of synthetic MALP-2 or 1 μg of Salmonella serovar Typhimurium LPS in 0.4-ml volumes of pyrogen-free isotonic saline by using groups of 18 animals for each treatment. Control mice received physiological saline. After different time intervals, six mice from each group were challenged by i.p. injection of 1 μg of serovar Typhimurium LPS in 0.4 ml of pyrogen-free isotonic saline. Mice were killed 1 h after LPS challenge by asphyxiation in CO2 and bled by cardiac puncture. Sera were kept frozen until determination of TNF-α levels by biological assay and IL-6 levels by enzyme-linked immunosorbent assay (ELISA).
Determination of IL-6 levels.
IL-6 levels were determined in a capture ELISA using the IL-6-specific monoclonal antibody MM600C (mouse immunoglobulin G1; Endogen, Cambridge, Mass.) as a capture antibody and a biotinylated monoclonal antibody from clone 6 B4 (a kind gift from J. van Snick) for determination. An authentic standard preparation of mouse recombinant IL-6 (Boehringer, Mannheim, Germany) was used for calculation of IL-6 activity in the samples. The detection limit of IL-6 was 1.5 ng/ml.
TNF-α cytotoxicity assay.
TNF-α levels were determined by a cytotoxicity assay using TNF-α-sensitive L929 cells (clone C5F6) as targets (15). Cells were plated at a density of 5 × 104 cells/well in 96-well microtiter plates and incubated for 3 h at 37°C in humidified 7.5% CO2 in air. After exposure to TNF-α for 20 h in the presence of 4 μg of actinomycin D/ml, the viability of C5F6 cells was determined by staining the surviving cells with crystal violet. The TNF-α activity was calibrated using a standard preparation of mouse rTNF-α (Boehringer). The detection limit was 5 pg/ml.
Protection against LPS and TNF-α in galactosamine-treated mice.
Protection against LPS was investigated in C57BL/10ScSn mice, and protection against human rTNF-α was investigated in C57BL/10ScCr mice and C57BL/6 × 129Sv TLR2−/− mice. Groups of mice were pretreated with an i.p. injection of different doses of MALP-2 in 0.2 ml of pyrogen-free isotonic saline. The mice were challenged 5 and 24 h later either by intravenous (i.v.) injection of a mixture of 50 ng of Salmonella serovar Abortus-equi LPS with 20 mg of d-galactosamine (Roth, Karlsruhe, Germany) or by i.p. injection of 1 μg of human rTNF-α with 20 mg of d-galactosamine, each in a volume of 0.2-ml saline. Control mice received LPS or TNF-α-d-galactosamine without MALP-2 pretreatment. Protection against TNF-α in TLR2−/− mice was investigated after pretreatment with 10 μg of MALP-2. Mice were challenged 1 day later as described above. Control mice were challenged without pretreatment. In all cases, lethality was scored up to 24 h after challenge with LPS or TNF-α.
RESULTS
Lack of cross contamination of MALP-2 and LPS.
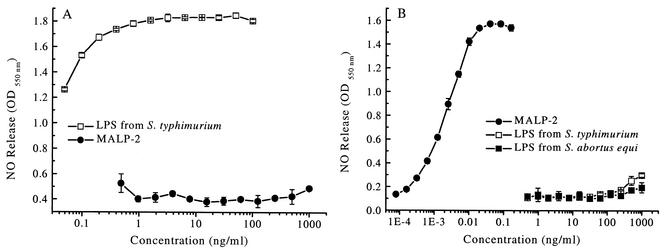
For specific stimulation of selected TLRs, it is very important to ascertain that the reagents, such as MALP-2 and LPS, are free of cross contamination. To this end, the LPS and MALP-2 preparations used in our experiments were tested in the sensitive nitric oxide release assay with peritoneal exudate cells from C3H/HeJ LPS-low-responder mice and from mice with a deficient TLR2 receptor (TLR2−/−) (62) which do not react to MALP-2. Peritoneal exudate cells from TLR2-deficient mice were not activated by MALP-2 at concentrations of up to 1 μg/ml (larger doses were not tested). In this system, LPS, acting via the TLR4 receptor, induced half-maximal NO release at <0.04 ng/ml (Fig. 1A), indicating that MALP-2 contained <0.05% LPS, if any. Peritoneal exudate cells from C3H/HeJ mice, on the other hand, showed no activity when stimulated with LPS concentrations of up to 250 ng/ml, whereas half-maximal stimulation was achieved with as little as 2 pg of MALP-2/ml (<0.001%) (Fig. 1B).
FIG. 1.
Assay of cross contamination of MALP-2 with LPS and vice versa. Nitric oxide release was determined from peritoneal exudate cells of TLR2−/− (A) and TLR4-defective (B) mice in response to LPS and MALP-2. Nitric oxide release was measured as the sum of nitrite and nitrate after 48-h stimulation with the indicated concentrations of MALP-2 and LPS from serovar Abortus-equi and serovar Typhimurium. Values are means ± standard deviation of triplicate cultures. OD550, optical density at 550 nm.
Pyrogenicity of MALP-2.
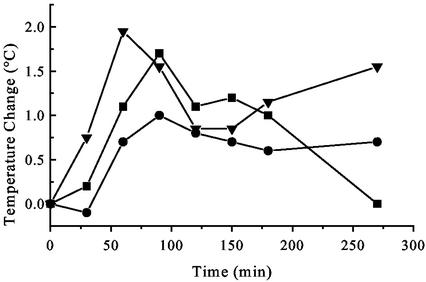
Crude preparations of mycoplasmal membranes have previously been shown to induce a rise in body temperature (45). Having identified MALP-2 as an important macrophage-activating component of Mycoplasma fermentans, we measured the pyrogenicity of the synthetic pure substance in rabbits, also with a view toward possible application in patients. As shown in Fig. 2, 5 μg of synthetic MALP-2 gave a biphasic temperature response. The minimal effective dose was about 1.5 μg/animal.
FIG. 2.
Pyrogenicity of MALP-2 in the rabbit. Rectal temperature was measured after injection of 5 μg of MALP-2 into the ear vein. The curves show the temperature increases of three individual White New Zealand rabbits.
In vivo application of MALP-2 induces cross tolerance to LPS.
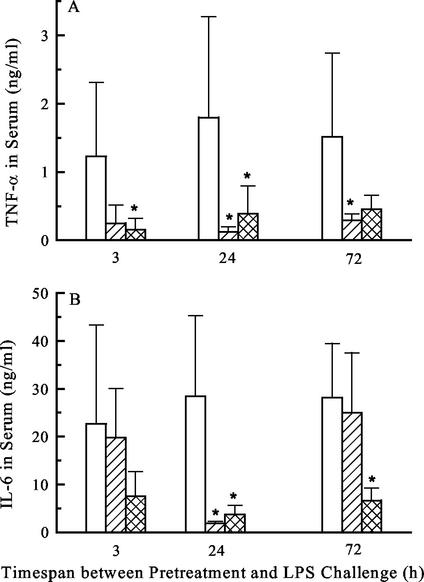
Pilot experiments have shown that i.p. injection of MALP-2 in the form of heat-killed mycoplasmas induced refractoriness of peritoneal macrophages to ex vivo stimulation with either MALP or LPS, when IL-6 release was measured (U. Deiters and P. F. Mühlradt, unpublished results). In follow-up experiments, the LPS-mediated release of the proinflammatory cytokines TNF-α and IL-6 were studied in mice pretreated with synthetic MALP-2 or control mice that received saline only. NMRI outbred mice were used to exclude atypical results through possible genetic peculiarities. At various time points after MALP-2 treatment, the animals were challenged with serovar Typhimurium LPS, and 1 h after challenge the animals were sacrificed and TNF-α and IL-6 levels in serum were determined. As shown in Fig. 3, very little biologically active TNF-α could be measured in the serum after challenge with 1 μg of LPS, when the animals were pretreated with MALP-2 24 or 72 h prior to challenge. The situation with IL-6 was somewhat different, as significant suppression of LPS-dependent serum IL-6 was only seen 24 h after MALP-2 administration. When the challenge dose of LPS was increased to 15 μg, no significant cross tolerance by pretreatment with MALP-2 was noticeable (data not shown).
FIG. 3.
Cross tolerance to LPS. Groups of six mice were i.p. injected with saline (controls) (white bars), 2.3 μg of MALP-2 (hatched bars), or 1 μg of serovar Typhimurium LPS (cross-hatched bars). After the indicated times, mice were challenged with 1 μg of LPS. One hour later, animals were sacrificed and sera were tested for release of TNF-α by biological assay (A) and of IL-6 by ELISA (B). Values are means of results for six animals ± standard deviation. ∗, significantly different (P < 0.05) from values for control groups pretreated with saline, as calculated by Student's t test.
Protection from lethal TNF-α shock in galactosamine-sensitized mice by MALP-2 pretreatment is TLR2 dependent.
Galactosamine-treated mice show greatly increased sensitivity to TNF-α (15). It was therefore of interest to try to protect mice from a lethal dose of TNF-α by pretreatment with MALP-2. These experiments were performed with groups of six C57BL/10ScCr mice which carry mutations leading to a lack of TLR4 expression (52) and an impaired IFN-γ response to bacteria (65) due to a defective IL-12 response (42). These mice are particularly suitable for these studies as effects of endogenously generated IFN-γ through IL-12 can be excluded. C57BL/10ScCr mice are as sensitive to TNF-α as the respective wild-type mice (18). The results are presented in Table 1 and show that pretreatment with even small doses of MALP-2 prevented death due to TNF-α given 5 and 24 h later. However, pretreatment with 10-μg doses of MALP-2 followed by TNF-α challenge 5 h later led to the deaths of 50% of the animals, probably because of a synergistic overlap at this time point of effects from an overdose of MALP-2 and TNF-α. This synergy subsided after 24 h, when full protection from TNF-α was achieved (Table 1).
TABLE 1.
Effects of a lethal dose of human rTNF-α in galactosamine-treated C57BL/10ScCr mice after MALP-2 pretreatment
| MALP-2 dose (μg/mouse) for i.p. pretreatment | Time (h) before challenge | No. dead after i.p. challenge with 1 μg of TNF-α/mousea |
|---|---|---|
| None | 5 | |
| 0.4 | 5 | 1 |
| 0.4 | 24 | 1 |
| 2 | 5 | 0 |
| 2 | 24 | 2 |
| 10 | 5 | 3 |
| 10 | 24 | 0 |
Six mice were in each group. Simultaneously with the human rTNF-α challenge, mice were given 20 mg of galactosamine. Death in unprotected mice occurred within 6 to 8 h after human rTNF-α application.
To show that the abovementioned protective effects were TLR2 dependent, these experiments were repeated with TLR2-defective mice. Three of three TLR2−/− mice given no MALP-2 pretreatment and challenged i.v. with 1 μg of TNF-α along with 20 mg of galactosamine/mouse died within 6 to 8 h after challenge, and four of four TLR2−/− mice pretreated i.p. with 10 μg of MALP-2/mouse 24 h before similar challenge also died. Thus, MALP-2 provided no protection in these mice.
Protection by MALP-2 against lethal LPS shock is only partial.
Groups of seven C57BL/10ScSn LPS-sensitive mice were i.p. injected with various doses of synthetic MALP-2. Mice were simultaneously sensitized and challenged 5 and 24 h later by i.v. coinjection of galactosamine with a lethal dose of 50 ng of LPS from serovar Abortus-equi. Even a high dose of 10 μg of MALP-2 per mouse gave only a partial protection against LPS lethality which lasted for a short time span (Table 2).
TABLE 2.
Effects of a lethal dose of LPS from serovar Abortus-equi in galactosamine-treated C57BL/10ScSn mice after MALP-2 pretreatment
| MALP-2 dose (μg/mouse) for i.p. pretreatment | Time (h) before challenge | No. dead after i.v. challenge with 50 ng of LPS/mousea |
|---|---|---|
| None | 7 | |
| 0.4 | 5 | 7 |
| 0.4 | 24 | 6 |
| 2 | 5 | 7 |
| 2 | 24 | 6 |
| 10 | 5 | 3 |
| 10 | 24 | 7 |
Seven mice were in each group. Simultaneously with the LPS challenge, mice were given 20 mg of galactosamine. Death in unprotected mice occurred within 6 to 8 h after LPS application.
DISCUSSION
The recent in vitro studies of Sato et al. have suggested that the potent macrophage activator MALP-2 can induce cross tolerance to LPS (55, 56). The present work extends this finding to in vivo protection experiments. As pointed out above, several attempts have been made in the past to induce cross tolerance with a variety of compounds, including a synthetic analogue of the murein lipoprotein which was used for in vitro studies with human monocytes (33). These studies were apparently not extended to in vivo systems, as conventional bacterial lipopeptides with three fatty acid residues show little in vivo activity (19). In contrast, MALP-2 lacks the amide-bound fatty acid and is very active in vivo, causing release of cytokines and chemokines and concurrent leukocyte infiltration (7, 38). MALP-2 could therefore be expected to be effective in experimental animals for the induction of cross tolerance to LPS. Moreover, MALP-2 proved much less pyrogenic than LPS in the rabbit when i.v. injected (Fig. 2). The rabbit was chosen for this experiment as it is customarily used for pyrogenicity studies, being similarly sensitive to LPS as humans.
Indeed, i.p. administration of MALP-2 caused a significant reduction of serum TNF-α and IL-6 levels following a secondary LPS challenge, a state of at least partial unresponsiveness that lasted up to 72 h (Fig. 3). We could then show that MALP-2 was able to protect galactosamine-treated TLR2+/+ mice from a lethal dose of TNF-α (Table 1) and that this effect was TLR2 dependent (see above). However, preexposure of animals to MALP-2 under conditions that protected them from lethal TNF-α did not result in protection from a lethal LPS dose (Table 2).
At first sight, MALP-2- as well as LPS-induced tolerance to LPS could be due to diminished TLR4 availability. It has been shown in vitro that downregulation of TLR4 may play a role in tolerance induction towards LPS (52, 55). However, Sato et al. showed that TLR4 cell surface expression is rather enhanced after MALP-2 stimulation (55). Sato et al. were also able to exclude a possible participation of IL-10, since macrophages from IL-10-deficient mice were equally well tolerized by MALP-2 (55). Likewise, modulation of expression of CD14, whose role for MALP-2 binding is still under debate, does not seem to play any role (55).
A possible mechanism of MALP-induced cross tolerance to LPS may be a dysfunction of common components of the signal transducing pathways used by LPS and MALP-2. In this context Li et al. showed low levels of IL-1 receptor-associated kinase (IRAK) protein and low IRAK kinase activity persisting 9 h after LPS removal in endotoxin-tolerant THP-1 cells (37). In addition, MyD88 and IRAK do not associate in these tolerant cells (37). Similarly, Medvedev et al. described a reduced association of MyD88 with IRAK and TLR4 in tolerant human monocytes (41). Another mechanism that has been discussed is the involvement of IRAK-M as a negative regulator of TLR signaling (31). Thus, macrophages from IRAK-M−/− mice failed to develop endotoxin tolerance (31). With regard to mechanisms further downstream, in vitro data from the laboratory of Ziegler-Heitbrock demonstrated in murine as well as human cells that restimulation with LPS leads to mobilization of an NF-κB p50p50 homodimer, a species that does not transactivate (66).
When considering possible mechanisms of tolerance induction, it is difficult to extrapolate from in vitro findings to in vivo processes. In many of the experiments referred to in the preceding paragraph, cells were exposed to LPS for many hours, LPS was “washed away,” and cells were immediately restimulated. In our studies, and those of others using lipoteichoic acid (LTA) (35), bolus injections were done for priming as well as challenge and up to 24 h lay between priming and challenge. Thus, for example, in vitro cross tolerance experiments with LTA and LPS did not result in LPS tolerance in LTA-pretreated THP-1 cells (23), but LTA was able to induce protection against LPS in mice (35). Whereas in vitro studies deal with well-defined cell populations, there are many more potentially interacting cell populations to consider in an experimental animal. The circulation and breakdown of the tolerizing substance, cytokines, or soluble receptor species also need to be taken into account. Cytokines may thus be neutralized, quickly broken down or excreted, such that most mediators may never reach concentrations which occur in cell culture. Furthermore, the composition of certain cell populations or the concentration of some circulating hormones may change in the experimental animal reacting to a systemic stimulus. Moreover, tolerizing agents that are active in cell culture may in an experimental animal bind to neutralizing serum components, may never reach the relevant target cells at effective concentrations, or, last but not least, may be too toxic to be applied in animals or humans.
It has been shown previously that tolerance in an experimental animal is at least partly due to unresponsive macrophages (14). However, other cells and factors may contribute. While IL-10 has been ruled out as being protective in IL-10-deficient mice (3), a role of IL-13, produced, for example, by mast cells, has to be considered, especially for downregulation of TNF-α (8). It has also been shown that there is an increase in immature macrophages in LPS-tolerized animals (39). A similar situation is likely to occur and to contribute to tolerance in our experiments, as i.p. administration of MALP-2 changes the composition of peritoneal exudate cells (7). Lastly, high levels of glucocorticoids which inhibit macrophage activation persist in tolerant animals (58). The processes leading to tolerance are thus multifactorial, and there may be different mechanisms responsible for tolerance effects observed at 5 or 72 h after priming.
Several reasons may explain why protection from TNF-α can be achieved by MALP pretreatment while there was poor protection, if any, from LPS. First, it must be emphasized that TNF-α is one of the more important but not the only known mediator to induce lethal shock. There are data that support the involvement of IL-12, IL-18, granulocyte macrophage colony-stimulating factor, and IFN-γ (2, 4, 5, 9, 25, 26, 54, 57). Second, while macrophages can respond to both MALP-2 and LPS, being thus theoretically sensitive to cross tolerance, other cells, such as NK cells, also play a role in LPS toxicity (20), but they are TLR2 negative (12, 22) and thus are not expected to become tolerized by MALP-2. Third, several reports have appeared showing that LPS, via TLR4, stimulates genes via a MyD88-independent pathway (1, 21, 29, 61) while MALP-2, via TLR2, does not (29). It is thus likely that activation of some of these genes may contribute to LPS toxicity and that they can be silenced by prestimulation with LPS but not with MALP-2.
As discussed in a recent review (6), protection from an overdose of LPS or resulting high levels of TNF-α does not reflect the situation of clinical sepsis but rather that of intoxication. It remains to be seen from further experiments, and in different experimental models, whether or not MALP-2 could be useful in clinical trials, e.g., for a preventive treatment of sepsis patients, as under these conditions the onset of exposure to LPS and other microbial stimulants is gradual. The high effectiveness of MALP-2 in suppressing TNF-α, the known ways of biological inactivation (44), and the low pyrogenicity as opposed to the highly pyrogenic LPS which persists in biologically active form in liver cells for 3 days (13) make MALP-2 a potential candidate for clinical use.
Acknowledgments
This work was supported by grants Mu 672/2-3 and Mu 672/2-4 from the Deutsche Forschungsgemeinschaft. U. Deiters is grateful for an award from the Förderverein der GBF.
We thank T. Hirsch for excellent technical assistance.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Arbibe, L., J.-P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533-540. [DOI] [PubMed] [Google Scholar]
- 2.Basu, S., A. R. Dunn, M. W. Marino, H. Savoia, G. Hodgson, G. J. Lieschke, and J. Cebon. 1997. Increased tolerance to endotoxin by granulocyte-macrophage colony-stimulating factor-deficient mice. J. Immunol. 159:1412-1417. [PubMed] [Google Scholar]
- 3.Berg, D. J., R. Kühn, K. Rajewsky, W. Müller, S. Menon, N. Davidson, G. Grünig, and D. Rennick. 1995. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Schartzman reaction but not to endotoxin tolerance. J. Clin. Investig. 96:2339-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Car, B. D., V. M. Eng, B. Schnyder, L. Ozmen, S. Huang, P. Gallay, D. Heumann, M. Aguet, and B. Ryffel. 1994. Interferon-γ receptor deficient mice are resistant to endotoxic shock. J. Exp. Med. 179:1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson, W. E., H. Yu, J. Dierksheide, K. Pfeffer, P. Bouchard, R. Clark, J. Durbin, A. S. Baldwin, J. Peschon, P. R. Johnson, G. Ku, H. Baumann, and M. A. Caligiuri. 1999. A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. J. Immunol. 162:4943-4951. [PubMed] [Google Scholar]
- 6.Deitch, E. A. 1998. Animal models of sepsis and shock: a review and lessons learned. Shock 9:1-11. [DOI] [PubMed] [Google Scholar]
- 7.Deiters, U., and P. F. Mühlradt. 1999. Mycoplasmal lipopeptide MALP-2 induces the chemoattractant proteins MIP-1α, MCP-1, and MIP-2 and promotes leukocyte infiltration in mice. Infect. Immun. 67:3390-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Santo, E., C. Meazza, M. Sironi, P. Fruscella, A. Mantovani, J. D. Sipe, and P. Ghezzi. 1997. IL-13 inhibits TNF production but potentiates that of IL-6 in vivo and ex vivo in mice. J. Immunol. 159:379-382. [PubMed] [Google Scholar]
- 9.Doherty, G. M., J. R. Lange, H. N. Langstein, H. R. Alexander, C. M. Buresh, and J. A. Norton. 1992. Evidence for IFN-γ as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J. Immunol. 149:1666-1670. [PubMed] [Google Scholar]
- 10.Faure, E., O. Equils, P. A. Sieling, L. Thomas, F. X. Zhang, C. J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-κB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058-11063. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler, V. B., I. Loof, E. Sander, V. Voehringer, C. Galanos, and M. A. Fournel. 1992. Monoclonal antibody to tumor necrosis factor-alpha prevents lethal endotoxin sepsis in adult rhesus monkeys. J. Lab. Clin. Med. 120:574-588. [PubMed] [Google Scholar]
- 12.Flo, T. H., O. Halaas, S. Torp, L. Ryan, E. Lien, B. Dybdahl, A. Sundan, and T. Espevik. 2001. Differential expression of Toll-like receptor 2 in human cells. J. Leukoc. Biol. 69:474-481. [PubMed] [Google Scholar]
- 13.Freudenberg, M. A., and C. Galanos. 1985. Alterations in rats in vivo of the chemical structure of lipopolysaccharide from Salmonella abortus equi. Eur. J. Biochem. 152:353-359. [DOI] [PubMed] [Google Scholar]
- 14.Freudenberg, M. A., and C. Galanos. 1988. Induction of tolerance to lipopolysaccharide (LPS)-d-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect. Immun. 56:1352-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freudenberg, M. A., and C. Galanos. 1991. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect. Immun. 59:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freudenberg, M. A., R. Salomao, A. Sing, I. Mitov, and C. Galanos. 1998. Reconciling the concepts of endotoxin sensitization and tolerance. Prog. Clin. Biol. Res. 397:261-268. [PubMed] [Google Scholar]
- 17.Galanos, C., O. Lüderitz, and O. Westphal. 1979. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-Pyrexal). Zentbl. Bakteriol. Infektkrankh. Hyg. Abt. 1 Orig. Reihe A 243:226-244. [PubMed] [Google Scholar]
- 18.Galanos, C., M. A. Freudenberg, T. Katschinski, R. Salomao, H. Mossmann, and Y. Kumazawa. 1992. Tumor necrosis factor and host response to endotoxin, p. 75-104. In J. L. Ryan, and D. C. Morrison (ed.), Bacterial endotoxic lipopolysaccharides, vol. III. Immunopharmacology and pathophysiology. CRC Press, Boca Raton, Fla. [Google Scholar]
- 19.Hauschildt, S., H. U. Beuscher, G. Jung, W. Bessler, and A. Ulmer. 1994. Intraperitoneal injection of synthetic bacterial lipopeptides does not cause a rise in circulating inflammatory cytokines. FEMS Immunol. Med. Microbiol. 8:77-82. [DOI] [PubMed] [Google Scholar]
- 20.Heremans, H., C. Dillen, J. van Damme, and A. Billiau. 1994. Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. Eur. J. Immunol. 24:1155-1160. [DOI] [PubMed] [Google Scholar]
- 21.Horng, T., G. M. Barton, and R. Medzhitov. 2001. TIRAP: an adaptor molecule in the toll signaling pathway. Nature Immunol. 2:835-841. [DOI] [PubMed] [Google Scholar]
- 22.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdörfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 23.Jacinto, R., T. Hartung, C. McCall, and L. Li. 2002. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J. Immunol. 168:6136-6141. [DOI] [PubMed] [Google Scholar]
- 24.Jin, L., D. P. Raymond, T. D. Crabtree, S. J. Pelletier, C. K. Rudy, T. L. Pruett, and R. G. Sawyer. 2002. Preexposure of murine macrophages to CpG-containing oligonucleotides results in nuclear factor κB p50 homodimer-associated hyporesponsiveness. Surgery 132:245-251. [DOI] [PubMed] [Google Scholar]
- 25.Karp, C. L., M. Wysocka, X. Ma, M. Marovich, R. E. Factor, T. Nutman, M. Armant, L. Wahl, P. Cuomo, and G. Trinchieri. 1998. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur. J. Immunol. 28:3128-3136. [DOI] [PubMed] [Google Scholar]
- 26.Katschinski, T., C. Galanos, A. Coumbos, and M. A. Freudenberg. 1992. Gamma interferon mediates Propionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect. Immun. 60:1994-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann, A., E. Rischkowsky, P. F. Mühlradt, D. Gemsa, and H. Sprenger. 1999. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans-derived lipoprotein MALP-2. Infect. Immun. 67:6303-6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 29.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Mühlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 30.Kiani, A., A. Tschiersch, E. Gaboriau, F. Otto, A. Seiz, H. P. Knopf, P. Stutz, L. Faber, U. Haus, C. Galanos, R. Mertelsmann, and R. Engelhadt. 1997. Downregulation of the proinflammatory cytokine response to endotoxin by pretreatment with the nontoxic lipid A analog SDZ MRL 953 in cancer patients. Blood 90:1673-1683. [PubMed] [Google Scholar]
- 31.Kobayashi, K., L. D. Hernandez, J. E. Galán, C. A. Janeway, Jr., R. Medzhitov, and R. A. Flavell. 2002. IRAK-M is a negative regulator of toll-like receptor signaling. Cell 110:191-202. [DOI] [PubMed] [Google Scholar]
- 32.Kraatz, J., L. Clair, J. L. Rodriguez, and M. A. West. 1999. Macrophage TNF secretion in endotoxin tolerance: role of SAPK, p38, and MAPK. J. Surg. Res. 83:158-164. [DOI] [PubMed] [Google Scholar]
- 33.Kreutz, M., U. Ackermann, S. Hauschildt, S. W. Krause, D. Riedel, W. Bessler, and R. Andreesen. 1997. A comparative analysis of cytokine production and tolerance induction by bacterial lipopeptides, lipopolysaccharides and Staphylococcus aureus in human monocytes. Immunology 92:396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann, V., M. A. Freudenberg, and C. Galanos. 1987. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J. Exp. Med. 165:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 36.Lehner, M. D., and T. Hartung. 2002. Endotoxin tolerance mechanisms and beneficial effects in bacterial infections. Rev. Physiol. Biochem. Pharmacol. 144:95-141. [DOI] [PubMed] [Google Scholar]
- 37.Li, L., S. Cousart, J. Hu, and C. E. McCall. 2000. Characterization of interleukin-1 receptor associated kinase in normal and endotoxin-tolerant cells. J. Biol. Chem. 275:23340-23345. [DOI] [PubMed] [Google Scholar]
- 38.Lührmann, A., U. Deiters, J. Skokowa, M. Hanke, J. E. Gessner, P. F. Mühlradt, R. Papst, and T. Tschernig. 2002. In vivo effects of a synthetic 2-kilodalton macrophage-activating lipopeptide of Mycoplasma fermentans after pulmonary application. Infect. Immun. 70:3785-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madonna, G. S., and S. N. Vogel. 1985. Early endotoxin tolerance is associated with alterations in bone marrow-derived macrophage precursor pools. J. Immunol. 135:3763-3771. [PubMed] [Google Scholar]
- 40.Matsugushi, T., K. Takagi, T. Musikacharoen, and Y. Yoshikai. 2000. Gene expression of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood 95:1378-1385. [PubMed] [Google Scholar]
- 41.Medvedev, A. E., A. Lentschat, L. M. Wahl, D. T. Golenbock, and S. N. Vogel. 2002. Dysregulation of LPS-induced toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 169:5209-5216. [DOI] [PubMed] [Google Scholar]
- 42.Merlin, T., A. Sing, P. J. Nielsen, C. Galanos, and M. A. Freudenberg. 2001. Inherited IL-12 unresponsiveness contributes to the high LPS resistance of the Lpsd C57BL/10ScCr mouse. J. Immunol. 166:566-573. [DOI] [PubMed] [Google Scholar]
- 43.Metzger, J. W., K.-H. Wiesmüller, and G. Jung. 1991. Synthesis of N-alpha-Fmoc protected derivatives of S-(2,3-dihydroxypropyl)-cysteine and their application in peptide synthesis. Int. J. Pept. Protein Res. 38:545-554. [DOI] [PubMed] [Google Scholar]
- 44.Morr, M., O. Takeuchi, S. Akira, M. M. Simon, and P. F. Mühlradt. 2002. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur. J. Immunol. 32:3337-3347. [DOI] [PubMed] [Google Scholar]
- 45.Mühlradt, P. F., and U. Schade. 1991. MDHM, a macrophage-stimulatory product of Mycoplasma fermentans, leads to in vitro interleukin-1 (IL-1), IL-6, tumor necrosis factor, and prostaglandin production and is pyrogenic in rabbits. Infect. Immun. 59:3969-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mühlradt, P. F., and M. Frisch. 1994. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophages to release nitric oxide, TNF, and IL-6. Infect. Immun. 62:3801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mühlradt, P. F., M. Kieβ, H. Meyer, R. Süβmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muzio, M., J. Ni, P. Feng, and V. M. Dixit. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278:1612-1615. [DOI] [PubMed] [Google Scholar]
- 49.Muzio, M., D. Bosisio, N. Polentarutti, G. D'Amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 50.Nose, M., A. Uzawa, M. Nomura, Y. Ikarashi, Y. Nakata, M. Akashi, and G. Suzuki. 1998. Control of endotoxin shock by the dried preparation of low virulent Streptococcus pyogenes OK-432. Cell. Immunol. 188:97-104. [DOI] [PubMed] [Google Scholar]
- 51.Passlick, B., M. O. Labeta, J. R. Izbicki, P. Ostertag, T. Loffler, M. Siebeck, U. Pichlmeier, L. Schweiberer, and H. W. Ziegler-Heitbrock. 1995. Prevention of experimental endotoxin shock by a monocyte activator. Antimicrob. Agents Chemother. 39:2535-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. A. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 53.Roth, J., T. Aslan, B. Störr, and E. Zeisberger. 1997. Lack of cross tolerance between LPS and muramyl dipeptide in induction of circulating TNF-α and IL-6 in guinea pigs. Am. J. Physiol. 273:R1529-R1533. [DOI] [PubMed] [Google Scholar]
- 54.Sakao, Y., K. Takeda, H. Tsutsui, T. Kaisho, F. Nomura, H. Okamura, K. Nakanishi, and S. Akira. 1999. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int. Immunol. 11:471-480. [DOI] [PubMed] [Google Scholar]
- 55.Sato, S., F. Nomura, T. Kawai, O. Takeuchi, P. F. Mühlradt, K. Takeda, and S. Akira. 2000. Synergy and cross-tolerance between toll-like receptor (TLR)2- and TLR4-mediated signaling pathways. J. Immunol. 165:7096-7101. [DOI] [PubMed] [Google Scholar]
- 56.Sato, S., O. Takeuchi, T. Fujita, H. Tomizawa, K. Takeda, and S. Akira. 2002. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int. Immunol. 14:783-791. [DOI] [PubMed] [Google Scholar]
- 57.Senaldi, G., C. L. Shaklee, J. Guo, L. Martin, T. Boone, T. W. Mak, and T. R. Ulich. 1999. Protection against the mortality associated with disease models mediated by TNF and IFN-γ in mice lacking IFN regulatory factor-1. J. Immunol. 163:6820-6826. [PubMed] [Google Scholar]
- 58.Szabó, C., C. Thiemermann, C. C. Wu, M. Perretti, and J. R. Vane. 1994. Attenuation of the induction of nitric oxide synthase by endogenous glucocorticoids accounts for endotoxin tolerance in vivo. Proc. Natl. Acad. Sci. USA 91:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Mühlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi, O., T. Kawai, P. F. Mühlradt, M. Morr, J. D. Radolf, A. Zychlinsky, Y. Takeda, and S. Akira. 2001. Discrimination of microbial lipoproteins by Toll-like receptor (TLR) 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 61.Toshchakov, V., B. W. Jones, P.-Y. Perera, K. Thomas, M. J. Cody, S. Zhang, B. R. G. Williams, J. Major, T. A. Hamilton, M. J. Fenton, and S. N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 3:392-398. [DOI] [PubMed] [Google Scholar]
- 62.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 63.West, M. A., and W. Heagy. 2002. Endotoxin tolerance: a review. Crit. Care Med. 30:S64--S73. [PubMed]
- 64.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 65.Yaegashi, Y., P. Nielsen, A. Sing, C. Galanos, and M. A. Freudenberg. 1995. Interferon beta, a cofactor in the interferon gamma production induced by gram-negative bacteria in mice. J. Exp. Med. 181:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziegler-Heitbrock, H. W. L., A. Wedel, W. Schraut, M. Ströbel, P. Wendelgass, T. Sternsdorf, P. Bäuerle, J. G. Haas, and G. Riethmüller. 1994. Tolerance to lipopolysaccharide involves mobilization of nuclear factor κB with predominance of p50 homodimers. J. Biol. Chem. 269:17001-17004. [PubMed] [Google Scholar]
- 67.Ziegler-Heitbrock, H. W. L. 1995. Molecular mechanism in tolerance to lipopolysaccharide. J. Inflamm. 45:13-26. [PubMed] [Google Scholar]