Abstract
The variant surface antigens of Plasmodium falciparum are an important component of naturally acquired immunity and an important vaccine target. However, these proteins appear to elicit primarily variant-specific antibodies. We tested if naked DNA immunization can elicit more cross-reactive antibody responses and allow simultaneous immunization with several variant constructs. Mice immunized with plasmid DNA expressing variant cysteine-rich interdomain region 1 (CIDR1) domains of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) developed antibodies that were reactive to the corresponding PfEMP1s as measured by an enzyme-linked immunosorbent assay, flow cytometry, and agglutination of parasitized erythrocytes (PEs). We observed some cross-reactive immune responses; for example, sera from mice immunized with one domain agglutinated PEs of various lines and recognized heterologous domains expressed on the surface of Chinese hamster ovary (CHO) cells. We found no significant antigenic competition when animals were immunized with a mixture of plasmids or immunized sequentially with individual constructs. Moreover, mixed or sequential immunizations resulted in greater cross-reactive agglutination responses than immunization with a single domain. Recombinant protein (Sc y179) immunization after priming with DNA (prime-boost regimen) increased antibody titers to the homologous domain substantially but seemed to diminish the cross-reactive responses somewhat. The titer of agglutinating antibodies was previously shown to correlate with protection. Surprisingly, the agglutination titers of sera from DNA immunization were high, similar to those of pooled human hyperimmune sera. These sera also appeared to give limited low-titer variant transcending agglutination. Thus, DNA immunization appears to be a very useful tool for developing variant antigen vaccines.
The Plasmodium falciparum variant antigens play an important role in the host-parasite interaction. This family of proteins is involved in parasite adhesion and sequestration and in immune evasion by antigenic variation. These proteins, designated P. falciparum erythrocyte membrane protein 1 (PfEMP1), contribute directly to the virulence and pathogenesis of falciparum malaria (5, 6, 7, 30, 33). Among the pathogenic properties of PfEMP1 are formation of rosettes with uninfected erythrocytes, bridging of clumps of infected erythrocytes through platelets, and involvement in placental malaria and cerebral malaria by mature parasitized erythrocyte (PE) adhesion in these (and other) organs (5-7, 13, 17, 25, 35, 36, 45).
Antibodies to PfEMP1 are a major component of protective immunity, particularly during early childhood (9-12) and pregnancy (7, 19, 37, 44). This immune response correlates with protection from clinical episodes with parasites expressing previously experienced PfEMP1s but may not protect against unrecognized variants (8, 10, 24, 37, 44). These properties contribute to the establishment of chronic infection.
We recently demonstrated that immunization with the minimal CD36 binding region from the cysteine-rich interdomain region 1 (CIDR1) of MCvar1 protected Aotus monkeys from homologous challenge but not from heterologous challenge (3). A combination of several CIDR1 domains may be more effective and may lead to immune responses against heterologous PfEMP1s. Immunization with naked DNA is possibly the only efficient way to accomplish such a task. Generation of a large number of immunogens (vectors) is relatively easy, and the method permits coinjection of many members of a variant gene family at the same time (14, 15, 27, 38, 39, 43). A limited number of domains may be sufficient, as immunization with one domain appears to prime the immune response against other heterologous CIDR1 domains (3). DNA immunization is known to elicit relatively low antibody titers, and clinical protection appears to be associated with agglutinating antibodies and the titer of these antibodies. However, new approaches, such as use of a prime-boost regimen, use of CpG oligonucleotides, and electroporation, have demonstrated that it is possible to obtain higher antibody titers (16, 26, 28, 31, 32, 41-43, 48). Moreover, priming with DNA immunization may be boosted by exposure to the protein during infection, thus reducing the parasite load and eliminating the appearance of clinical symptoms.
We immunized mice in various ways with vectors expressing three variant CIDR1 domains. We found that immunization elicited good antibody responses to the PE surface and that immunization with all three constructs did not reduce antibody titers. The antibody responses to the PE surface measured by agglutination were similar to those of a pooled hyperimmune serum from humans living in an endemic area. We also found that the immunization elicited low levels of cross-reactive antibodies. The results of this study support the conclusion that there should be further development of DNA-based CIDR1 vaccines.
MATERIALS AND METHODS
Construction of plasmids for DNA immunization.
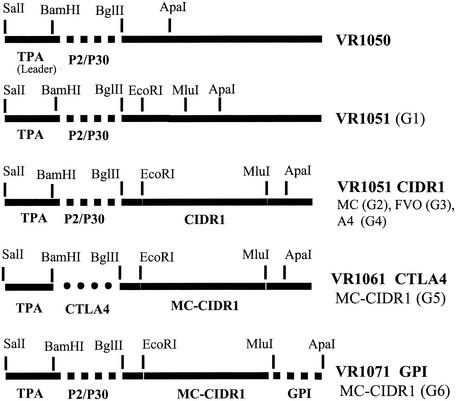
The VR1050 plasmid containing a TPA leader sequence fused to the P2P30 universal T-cell epitope was used (43). The plasmid was modified to include EcoRI and MluI sites between the original BglII and ApaI sites (VR1051) (Fig. 1). MC CIDR1 (residues 395 to 852; accession no. AAB60251), A4 CIDR1 (residues 401 to 846; accession no. L42244), and FVO CIDR1 (residues 1 to 480; accession no. AF286005) were amplified from genomic DNA by PCR and cloned into the VR1051 vector between the EcoRI and MluI sites. The MC CIDR1 was further modified to include a glycosylphosoinositol (GPI) anchor for cell surface expression cloned from the pSRα5 vector (Affymax Research Institute, Santa Clara, Calif.) between the MluI and ApaI sites (VR1071 GPI-MC CIDR1) (Fig. 1). In another modification of the VR1051 MC CIDR1 vector the P2P30 sequences were replaced by cloning the CTLA-4 binding domain (20) between the BamHI and EcoRI sites (VR1061 CTLA4-MC CIDR1). The entire insert of each of the constructs was sequenced. Plasmid DNA was produced and purified by using two cesium chloride gradients (Lofstrand Labs, Gaithersburg, Md.) and was dissolved at a concentration of 1 mg/ml in endotoxin-free phosphate-buffered saline (PBS).
FIG. 1.
Construction of plasmid VR1051 and the various DNA plasmids used in this study. G, group.
Recombinant protein.
The recombinant protein Sc y179 was expressed in Saccharomyces cerevisiae VK1 cells and was purified from the supernatant by nickel-nitrilotriacetic acid-agarose chromatography as described previously (3). Recombinant protein Pp MC-179 was expressed in Pichia pastoris and was purified by using nickel-nitrilotriacetic acid followed by size exclusion chromatography and reverse-phase high-performance liquid chromatography (47). Bacterial glutathione S-transferase (GST) and GST-r179 (MC rC1-2 [1-179]) were prepared as described previously (4).
Immunization.
Ten groups, each containing five female BALB/c mice that were 6 to 8 weeks old, were used. All DNA immunizations were intradermal. Group 1 was immunized with control VR1051 DNA, and groups 2, 3, and 4 were immunized with MC CIDR1, FVO CIDR1, and A4 CIDR1, respectively. Groups 5 and 6 were immunized with CTLA4-MC CIDR1 and GPI-MC CIDR1, respectively. Each mouse was immunized four times with 50 μg of DNA in 50 μl of PBS on days 0, 21, 42, and 63. Group 7 (sequential) was immunized sequentially with MC CIDR1 followed by FVO CIDR1 and A4 CIDR1 for the first three injections and then with an equal mixture (17 μg each) of the three vectors for the fourth injection. Mice in group 8 (mixed) were immunized with equal amounts (17 μg each) of MC CIDR1, FVO CIDR1, and A4 CIDR1. Group 9 (prime-boost regimen) was immunized with MC CIDR1 DNA for the first three injections and then subcutaneously with 50 μg of recombinant Sc y179 in incomplete Freund's adjuvant. Group 10 (Sc y179) was immunized once subcutaneously with 50 μg of recombinant Sc y179 in complete Freund's adjuvant and then three times subcutaneously with 50 μg of recombinant Sc y179 in incomplete Freund's adjuvant. Serum samples were collected 10 days after each immunization.
Preadsorption of sera.
Two milligrams of recombinant protein, either GST or GST-r179, was immobilized overnight on 600 μl of glutathione agarose beads (Pierce Endogen, Rockford, Ill.). Sixteen microliters of sera was diluted 1:50 in PBS-1% bovine serum albumin-0.1% azide and adsorbed three times for 1.5 h at room temperature with 100 μl of coated beads.
Parasites and cell lines.
P. falciparum parasites were cultivated as described previously (22). The following parasite strains and isolates were used: Malayan Camp rosetting positive (MC R+), MC R−, FCR3-CD36, FCR3-ICAM1, FCR3-CSA, ItA4, Santa Lucia (SL), and RB8 R+. Parasites of the FVO line expressing the FVOvar1 PfEMP1 were tested in Aotus erythrocytes. These parasites are known to express antigenically variant PfEMP1s (22). Chinese hamster ovary (CHO) K1 cells stably expressing the CIDR1 domains of MC R+ (MCvar1395-852; accession no. AAB60251), FVO (FVOvar11-480; accession no. AF286005), ItA4 (A4var401-846; accession no. L42244), and A4tres (A4Tvar375-724; accession no. AF193424) and MC R+ CIDR2γ (MCvar11273-1714; accession no. AAB60251) were cloned and grown in culture as described previously (23).
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was performed with the pPMC-y179 recombinant protein (47). One hundred microliters of a 1-μg/ml solution of the recombinant protein in PBS was added to Immunolon 4 HBX plates and incubated overnight at 5°C. The plates were washed and then blocked for 2 h room temperature with 20 mM Tris-150 mM NaCl-0.1% Tween 20 (pH 8.0) (TBS) containing 5% skim milk (TBS-milk). Sera were diluted in TBS-milk (100 μl) and incubated for 1 h at room temperature with the antigen. The plates were washed four times with TBS and incubated for 45 min at room temperature with a 1:5,000 dilution of peroxidase-conjugated goat anti-mouse immunoglobulin G (Jackson Immunoresearch Labs, West Grove, Pa.). After washing, 100 μl of SureBlue substrate (KPL, Gaithersburg, Md.) was added, the reaction was stopped after incubation for 10 min at room temperature by addition of 100 μl of TMB stop solution (KPL), and the results were read at 450 nm.
Agglutination.
Agglutination assays were performed with mature-stage PEs at a level of parasitemia of 5 to 8% as previously described (22). Agglutination scoring was preformed in a blind fashion by using only a number assigned to each tube. Agglutination scores (22) were based primarily on the size of the agglutinate and secondary scoring for the number of agglutinates, as follows: 0, no agglutination; 1, agglutinates consisting of 10 to ≤20 PEs; 2, agglutinates consisting of 21 to 50 PEs; 3, agglutinates consisting of 51 to 100 PEs; 4, agglutinates consisting of 101 to 250 PEs; 5, agglutinates consisting of >250 PEs. After the size was scored, the number of agglutinates was counted and added to the size score, as follows: 0, <10 agglutinates; 0.25, 11 to 25 agglutinates; 0.5, 26 to 50 agglutinates; 0.75, >50 agglutinates.
Flow cytometry assays.
The binding of antibodies to the surface of transfected CHO cells was measured by flow cytometry as described previously (22). Sera for each group were pooled and tested at a 1:40 dilution. Monoclonal antibody 179 (antiepitope tag) was tested at a concentration of 10 μg/ml. Flow cytometry was performed by using a Becton-Dickinson FACSCalibur and the Flowjo 3.4 analysis software (Tri Star, San Carlos, Calif.).
RESULTS
DNA immunization induces an antibody response to the minimal CD36 binding domain (M2) of MC CIDR1.
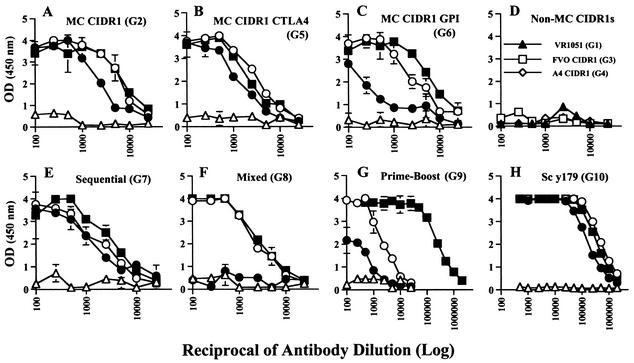
Mice (groups of five BALB/c mice) were immunized four times with 50 μg of DNA by intradermal injection. The intradermal route was reported to give higher antibody titers than intramuscular or subcutaneous injection (1). The length of the injection regimen was short (63 days), and an extended time between injections may enhance the immune response. Mice belonging to groups immunized with the Mcvar1 sequence, either with DNA (groups 2, 5, 6, 7, and 8), with DNA followed by the recombinant protein Sc y179 (group 9), or with recombinant protein Sc y179 alone (group 10), were positive as determined by ELISA (Fig. 2), with the recombinant protein Pp MC-179 (47) representing the M2 minimal binding region of MC CIDR1.
FIG. 2.
Antibody reactivity with the yeast recombinant protein Pp MC-179 as determined by ELISA. Titers were determined after the first (▵), second (•), third (○), and fourth (▪) injections. Immunization was performed with DNA (A to F), with DNA followed by protein (Sc y179) boosting (G), or with recombinant protein alone (H). The ELISA data for non-MC CIDR1 immunization (D) are the data for the fourth injection. The results are means ± standard deviations for duplicate wells. G, group; OD (450 nm), optical density at 450 nm.
The three groups immunized with DNA constructs of MC CIDR1, including MC CIDR1 (group 2) CTLA4-MC CIDR1 (group 5), and GPI-MC CIDR1 (group 6), had similar antibody titers (Fig. 2). The enhancement of antibody titers reported previously for constructs expressing the CTLA-4 binding domain (20) was not observed (Fig. 2B). We also did not observe a significant change if the expressed proteins were secreted (group 2) or were expressed on the surface of the cells via a GPI anchor (group 6). However, the small size of each group may affect the significance of such differences. In most groups antibody titers were almost maximal after the second injection, but some groups (groups 6, 8, and 9) exhibited the maximal response only after three injections. These differences were attributed mainly to the small sample size that intensified differences between individual animals or to the smaller amount (one-third) of specific MC CIDR1 plasmid DNA used for group 8. Mice immunized with the control VR1051 vector or with constructs expressing FVO CIDR1 (group 3) or A4 CIDR1 (group 4) were negative for reactivity with Pp MC-179 as determined by ELISA (Fig. 2).
We also tested a prime-boost regimen, in which mice were given three injections of MC CIDR1 DNA and were boosted once with 50 μg of Sc y179 recombinant protein. This procedure was reported previously to significantly enhance the antibody response (26, 28). Indeed, mice immunized with the prime-boost regimen (group 9) or with recombinant protein alone (group 10) had significantly higher antibody titers than mice immunized with DNA alone (Fig. 2). After the protein boost the antibody titers of the prime-boost group (group 9) were similar to those of the recombinant protein immunization group (group 10). No reactivity was found with an unrelated merozoite surface protein 1 (yMSP1-19) recombinant protein produced in S.cerevisiae, indicating that the elevated response was directed against the Sc y179 recombinant protein and not contaminating yeast proteins (data not shown).
To overcome the restricted antibody response to PfEMP1, we may need to immunize simultaneously with several var gene sequences. Therefore, we examined the effect of injecting all three constructs at the same time (group 8, mixed) on antibody titers. In this case, the total amount of DNA was kept at 50 μg by using only 17 μg of DNA for each construct. Injection of all three constructs initially slowed the antibody response to the M2 region of MC CIDR1 (Pp MC-179), probably due to the smaller specific amounts of MC CIDR1, although initial antigenic competition cannot be ruled out. However, after the third injection the antibody responses were similar to the responses after immunization with a single construct (Fig. 2).
Another possible way to overcome the variant-specific response to PfEMP1 is by sequential injection of variant CIDR1s in the hope that such exposure will enhance the response to cross-reactive epitopes. Therefore, we immunized mice sequentially with MC CIDR1 followed by the FVO CIDR1 and A4 CIDR1. The last (fourth) injection was injection of all three constructs (17 μg each) to boost the antibody response. Interestingly, the last injection did not have a significant effect on antibody titers to Pp MC-179 (Fig. 2E), and the antibody titers resulting from the single initial (first) immunization with MC CIDR1 were only somewhat less than the antibody titers observed for the other MC CIDR1 DNA immunization groups (Fig. 2). It is likely that the longer elapsed time from the initial injection was the main reason for the similar titers obtained for groups 7 (sequential) and 2 (MC CIDR1 DNA) and not the effect of exposure to heterologous CIDR1 DNAs. The heterologous sequences did not elicit a response to Pp MC-179 on their own. Thus, it is possible that given sufficient time, one or two immunizations is sufficient to elicit the maximal antibody titers.
Cross-reactive antibody responses after CIDR1 DNA immunization.
We further investigated antibody responses to full-length CIDR1 domains and tested if this type of vaccination can elicit less variant-specific responses. Since we did not have correctly folded full-length CIDR1 recombinant protein from these genes, we tested sera from the last bleed by flow cytometry by using various CIDR1s expressed on the surface of CHO cells (Table 1). Sera from all immunizations in which MC CIDR1 was used were reactive with CHO cells expressing this domain (Table 1), in agreement with the ELISA results (Fig. 2). At the dilution tested (1:40) sera from groups that were not immunized or boosted with recombinant protein showed comparable median fluorescence intensities (435 to 498 U). Sera from group 6 (GPI-MC CIDR1) reacted with all clones tested, probably because test clones had the same epitope tag and GPI anchor as the immunogen, and thus were excluded from the assay. Interestingly, the prime-boost group (group 9) had a higher median fluorescence intensity than group 10 immunized only with recombinant protein (Table 1). Sera from groups 3 (FVO CIDR1) and 4 (A4 CIDR1) were reactive with CHO cells expressing the corresponding domains. Sera from either the mixed group (group 8) or the sequential group (group 7) were reactive with all three constructs (Table 1).
TABLE 1.
Reactivities of sera from immunized mice with CHO K1 cells expressing various CIDR1 domains as determined by flow cytometry
| Group | Fluorescence intensitya
|
|||||
|---|---|---|---|---|---|---|
| CHO K1 control | MC CIDR1 | FVO CIDR1 | A4 CIDR1 | A4tres CIDR1 | MC CIDR2 | |
| VR1051 control (group 1) | 6 | 4 | 5 | 3 | 3 | 3 |
| MC CIDR1 (group 2) | 7 | 435 | 45 | 39 | 4 | 4 |
| FVO CIDR1 (group 3) | 10 | 6 | 274 | 4 | 4 | 4 |
| A4 CIDR1 (group 4) | 8 | 10 | 45 | 157 | 4 | 5 |
| CTLA4-MC CIDR1 (group 5) | 8 | 450 | 26 | 11 | 3 | 4 |
| Sequential (group 7) | 9 | 498 | 63 | 102 | 3 | 4 |
| Mixed (group 8) | 6 | 491 | 249 | 158 | 3 | 3 |
| Prime-boost (group 9) | 10 | 797 | 9 | 5 | 4 | 4 |
| Sc y179 (group 10) | 9 | 691 | 30 | 5 | 4 | 7 |
| Monoclonal antibody 179b | 3 | 423 | 257 | 202 | 164 | 323 |
Reactivity is expressed as the median fluorescence intensity (in arbitrary units).
Monoclonal antibody 179 recognizes an epitope tag incorporated into the carboxyl terminus of each clone. GPI-MC CIDR1 (group 6) sera reacted with the epitope tag recognized by monoclonal antibody 179 and present on the surface of all the clones tested.
In addition to their reactivity with MC CIDR1, sera from group 2 (MC CIDR1) also recognized FVO CIDR1 and A4 CIDR1, but the reactivity was only 10% of the MC CIDR1 reactivity (Table 1). The group 2 sera, like all the other sera tested, failed to recognize the A4tres CIDR1 or the MC CIDR2γ, indicating that the cross-reactive response was limited (Table 1). Sera from group 5 (CTLA4-MC CIDR1) exhibited a similar pattern, although the reactivities with FVO CIDR1 and particularly with A4 CIDR1 were lower than the reactivity of the group 2 sera. Some reactivity with FVO CIDR1 but not with A4 CIDR1 was detected with sera from recombinant Sc y179 immunization. However, sera from the prime-boost group (group 9) had no reactivity with either CIDR1 domain. Thus, exposure to recombinant protein appears to elevate the response to the homologous construct but reduce or eliminate responses to heterologous CIDR1 domains.
Not all DNA constructs elicited antibodies that cross-reacted with heterologous domains expressed on CHO cells (Table 1). The FVO CIDR1 construct (group 3) did not elicit any cross-reactive response, while the A4 constructs (group 4) elicited antibodies to FVO CIDR1 but did not elicit antibodies (or only minimal antibody response) to MC CIDR1. It is interesting that the cross-reactive responses were not reciprocal. Thus, MC CIDR1 elicited antibody responses to both FVO CIDR1 and A4 CIDR1, but these constructs failed to elicit antibody response to MC CIDR1. The reason for such nonreciprocal responses is not clear at this point.
Simultaneous immunization with all three constructs mixed together in equal amounts (group 8) elicited antibodies to all three CIDR1s (Table 1). We found no decrease in the antibody reactivity of group 8 sera regardless the reduced amount of DNA used (50 μg of total DNA, 17 μg of each construct) compared to the reactivities of sera from mice immunized individually with each construct. Thus, we obtained no significant evidence that there is antigenic competition when three CIDR1 constructs are used together. Nevertheless, no response was observed with A4tres CIDR1, suggesting that a larger number of domains may be required to extend coverage.
The results obtained by flow cytometry showed that there was comparable or even greater reactivity in the sera from the sequential group (group 7) immunized with MC CIDR1 than in sera from mice immunized with MC CIDR1 DNA alone (group 2) (Table 1). However, we noted a 35% reduction in the reactivity with A4 CIDR1 and a 77% reduction in the reactivity with FVO CIDR1. This may be attributed to the number of immunizations and the shorter elapsed time from immunization, particularly for A4 CIDR1 immunization, which was the last immunization in the sequence and thus had less time to produce a maximal antibody response. We often found that the expression of FVO CIDR1 in transient transfections was significantly lower than the expression of other CIDR1 domains, so it was possible that more time and immunization were required for low-expressing constructs. A less likely alternative explanation is that exposure to FVO CIDR1 and A4 CIDR1 boosted the response to MC CIDR1 but the initial immunization with MC CIDR1 had no effect on the response to FVO CIDR1 and A4 CIDR1.
The majority of the antibody response is directed to the M2 region in MC CIDR1.
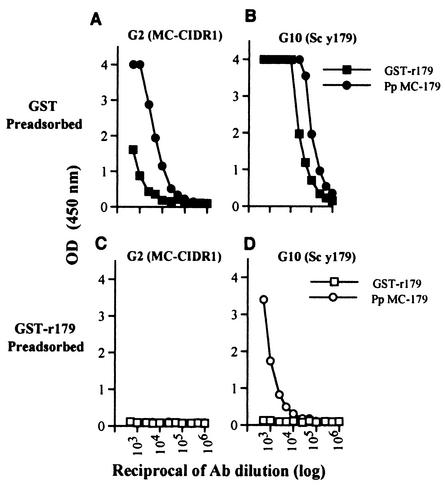
The CIDR1 domain can be divided to three parts, the M1, M2, and M3 regions, where M2 represents the minimal CD36 binding domain (23). We examined whether DNA immunization with the full-length CIDR1 directed most of the antibody response to the M2 region or to the M1 and M3 regions. We preadsorbed sera from group 2 (MC CIDR1 DNA) and from group 10 (Sc y179 recombinant protein) on GST or recombinant GST-r179 protein representing the M2 minimal CD36 binding epitope of Mcvar1 (Fig. 3 and Table 2). We then examined the preadsorbed sera by ELISA with GST-r179 and Pp MC-179 (Fig. 3) and by flow cytometry with CHO cells expressing MC CIDR1 and Pp MC-179 (Table 2). After preadsorption, sera from group 2 lost all their activity with both GST-r179 and Pp MC-179 (Fig. 3C) and with CHO MC-179 (Table 2), demonstrating that the adsorption conditions were adequate. However, the adsorbed serum exhibited about 28% reactivity with the full-length MC CIDR1 (Table 2). Sera from group 10 (Sc y179) lost all of their reactivity with CHO MC CIDR1, with CHO MC-179 (Table 2), or with GST-r179 (Fig. 3D). Thus, the major antibody response following DNA immunization with CIDR1 was the response to the minimal CD36 binding region.
FIG. 3.
Antibody reactivities of group 2 and 10 sera after preadsorption. Sera obtained after the fourth injection of MC CIDR1 DNA (group 2) (A and C) or Sc y179 recombinant protein (group 10) (B and D) were preadsorbed with GST (A and B) or with GST-r179 (C and D) and examined by ELISA with GST-r179 (▪ and □) or with the yeast recombinant protein Pp MC-179 (• and ○). The results are means ± standard deviations for duplicate wells. G, group; OD (450 nm), optical density at 450 nm; Ab, antibody.
TABLE 2.
Reactivities of preadsorbed sera as determined by flow cytometry
| Group | Preadsorption | Fluorescence intensitya
|
|
|---|---|---|---|
| CHO MC CIDR1 | CHO MC-179 | ||
| MC CIDR1 (group 2) | GST | 139 | 153 |
| GST-r179 | 39 | 3 | |
| Sc y179 (group 10) | GST | 495 | 511 |
| GST-r179 | 3 | 2 | |
Reactivity is expressed as the median fluorescence intensity (in arbitrary units).
We also noted that group 2 serum was much less reactive with the bacterial GST-r179 recombinant than with the yeast Pp MC-179 recombinant (Fig. 3A). The results were less pronounced with GST-preadsorbed group 10 sera (Fig. 3B). One possible explanation for this is that the yeast recombinant Pp MC-179 contained a larger amount of correctly folded (functional) proteins than GST-r179, allowing more antibodies to bind.
Interestingly, group 10 sera preadsorbed with GST-r179 had no reactivity with GST-r179, but significant activity with Pp MC-179 still remained (Fig. 3D). The lack of reactivity with GST-179 (Fig. 3D) and the lack of reactivity with CHO cells expressing MC CIDR1 or MC-179 (Table 2) support the idea that the results were not due to inadequate preadsorption. The recombinant protein Pp MC-179 used for the ELISA was highly purified and was not likely to have any significant amount of contaminating yeast proteins. We believe that the unadsorbed reactivity represented epitopes that appeared in both yeast proteins (Sc y179 and Pp MC-179) but were not present in the bacterial product and the proteins expressed by CHO cells.
Agglutination with MC R+ PEs.
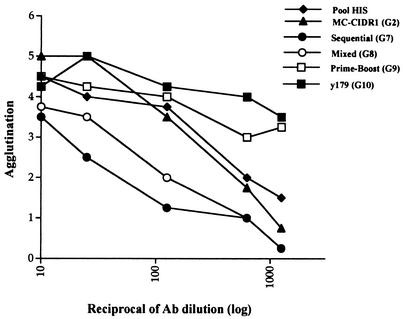
We recently demonstrated that in Aotus monkeys antibody-mediated agglutination is highly correlated with protection (3). Other studies also have shown that there is a correlation between clinical immunity and agglutination. Therefore, we tested sera from immunized mice for agglutination with MC R+ PEs at various dilutions (Fig. 4). Altogether, we found high agglutinating antibody titers with MC R+ PEs. Group 2 serum (MC CIDR1) had an agglutination profile similar to that of a pooled human hyperimmune serum. The agglutination properties of the sera from mice immunized with the recombinant protein (group 10) or primed and boosted (group 9) were even higher (Fig. 4), in agreement with their higher ELISA titers and flow cytometry reactivities (Fig. 2 and Table 1).
FIG. 4.
Agglutination titers of various sera after the fourth injection with MC R+ PEs. Sera obtained from group 2, 7, 8, 9, and 10 mice after the fourth injection and a pooled human hyperimmune serum (Pool HIS) were tested for agglutination with MC R+ PEs at various dilutions. Ab, antibody.
Both the mixed (group 8) and sequential (group 7) immunization sera had lower agglutination titers despite the fact that they (particularly the G8 group) had ELISA titers and flow cytometry reactivities comparable to those of group 2 (MC CIDR1 DNA). This may indicate that there are differences between the epitopes or the availability of epitopes expressed by PfEMP1 on the PE surface and the epitopes or the availability of epitopes expressed by recombinant CIDR1 proteins. It is also possible that exposure to heterologous CIDR1 domains altered the qualitative and quantitative antibody responses to various epitopes of the MC PfEMP1.
DNA immunization can induce low-titer variant-transcending agglutinating antibodies.
One of the major objectives of this work was to determine if immunization with naked DNA could elicit variant-transcending responses or cover various parasite lines by immunization with multiple constructs. The most relevant antibodies are those that recognize the surface of the PE. We tested the various sera for agglutination with PEs known to express antigenically variant PfEMP1 (Table 3). Sera were tested mostly at a dilution of 1:5, and some sera were tested at a dilution of 1:10 to allow detection of low levels of cross-reactive agglutination. Surprisingly, most sera showed cross-reactive agglutination with several strains, although the reactivity was significantly reduced or eliminated at dilutions higher than 1:25 (Baruch, unpublished data). None of the sera reacted with the non-CD36-binding clone FCR3-CSA, as this parasite is believed to express PfEMP1 that is structurally different than CD36-binding PfEMP1s (21). The MC CIDR1 DNA immunizations (groups 2, 5, and 6) elicited agglutinating antibodies to all strains tested (except FCR3-CSA), although the reactivity with SL PEs was minimal. The highest cross-reactivity was with A4 and FCR3-ICAM-1 PEs (Table 3). FVO CIDR1 (group 3) DNA immunization elicited cross-reactive agglutination that was greatest with A4 PEs and minimal for FCR3-ICAM and SL PEs. A4 CIDR1 (group 4) sera did not recognize or had minimal activity with FCR3-CD36, FCR3-ICAM, and SL PEs and displayed good recognition of MC R+ and MC R− PEs. The sera from the prime-boost group (group 9) and the recombinant protein Sc y179 group (group 10) gave somewhat lower levels of cross-reactive agglutination than sera from DNA immunization, in agreement with the flow cytometry results. The mixed group (group 8) and particularly the sequential group (group 7) sera had the most prevalent cross-reactive responses and failed to agglutinate only the FCR3-CSA PEs. Thus, although DNA immunization results in lower antibody titers, it appears to elicit more cross-reactive antibody responses to the P. falciparum variant antigen, PfEMP1.
TABLE 3.
Agglutination of PEs from various P. falciparum clones by sera from immunized mice
| Group | PE agglutination of various P. falciparum parasite clonesa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MC R+ | MC R− | A4 | FCR3-CD36 | FCR3-ICAM | FCR3-CSA | SL | RB8 R+ | FVO | |
| VR1050 control (group 1) | 0-0.25 (3)b | 0, 0 (2) | 0, 0.25(2) | 0.25 (1) | 0.25 (1) | 0, 0 (2) | 0 (1) | 0.25 (1) | NDd |
| MC CIDR1 (group 2) | 4-5 (3)c | 1.2 (2)c | 2.3 (2)c | 1, 1.75 (2)c | 2, 3.25 (2)c | 0 (1) | 0.75 (1) | 1 (1) | ND |
| FVO CIDR1 (group 3) | 15, 1.5 (2) | 1, 1 (2) | 1, 3.25 (2)c | 1.25 (1) | 0.5 (1) | 0 (1) | 0.5 (1) | 2 (1) | 2.5 (1)c |
| A4 CIDR1 (group 4) | 1, 5.2 (2) | 2 (1) | 2, 3.75 (2)c | 0.25 (1) | 0.25 (1) | 0 (1) | 0.5 (1) | 1.5 (1) | ND |
| CTLA4-MC CIDR1 (group 5) | 5, 5 (2) | 1 (1) | 3 (1) | 1.5 (1) | 3.25 (1) | ND | 0.5 (1) | 1 (1) | ND |
| GPI-MC CIDR1 (group 6) | 4, 5 (2) | 1 (1) | 1.5, 2 (2) | 1.5 (1) | 1.25 (1) | ND | 0 (1) | 0.5 (1) | ND |
| Sequential (group 7) | 2-3.75 (3)c | 1.5, 2 (2)c | 3.5, 4 (2)c | 2.5, 3 (2)c | 1.25, 2 (2)c | 0 (1) | 2 (1) | 2 (1) | ND |
| Mixed (group 8) | 3.5-4.75 (3)c | 1.5, 2.25 (2)c | 1.5, 4 (2)c | 1.5, 3 (2)c | 1.2 (2)c | 0 (1) | 1.25 (1) | 0.25 (1) | ND |
| Prime-boost (group 9) | 4.5, 4.75 (2) | 1, 1 (2) | 1, 2.5 (2) | 0.25 (1) | 0.75 (1) | 0, 0.25 (2) | 0.25 (1) | 2.5 (1) | ND |
| Sc y179 (group 10) | 5, 5 (2) | 1.75, 2 (2) | 2, 2.25 (2) | 0.25, 0.5 (2) | 2, 2 (2) | 0 (1) | 1 (1) | 2 (1) | 0 (1) |
Unless indicated otherwise, all PE agglutination was measured at a dilution of 1:5. The values are agglutination scores from one or two independent assays or are ranges of agglutination scores from three independent assays.
The numbers in parentheses are the numbers of independent assays.
At least one assay was performed at a 1:10 serum dilution.
ND, not determined.
DISCUSSION
Anti-PfEMP1 antibodies are a major component of clinical immunity developed by residents of areas where P. falciparum is endemic (2, 8, 11, 12). However, antibodies to the PE surface are primarily variant specific, and protection develops slowly in response to many infections with parasites expressing various PfEMP1s (2, 9-12, 33, 34). Immunization of Aotus monkeys with the M2 region of CIDR1 (Sc y179) elicited primarily variant-specific antibodies but primed or directed the immune response to the corresponding region of PfEMP1 in a heterologous infection (3). Thus, it may be possible to facilitate development of clinical immunity by exposure to a limited number of these domains. The results of this DNA immunization trial support this idea. We found no adverse effect on antibody titers resulting from immunization with three CIDR1s compared to the antibody titers after immunization with each of the domains alone (Table 1). Jones et al. (27) also reported a lack of antigenic competition in Aotus monkeys immunized with a mixture of plasmids expressing three blood-stage antigens. Moreover, immunization with each of the domains sequentially also had relatively little effect on antibodies titers. One of the most significant and encouraging findings was that the immunizations elicited antibodies to the PE surface and that the reactivity had a cross-reactive antibody component detected by both agglutination and flow cytometry. However, the titers of the cross-reactive antibodies appeared to be relatively low (Tables 1 and 3). Most of the antibody response was against the M2 minimal binding region of CIDR1 (Table 2). We also observed epitope differences between DNA immunization and immunization with yeast recombinant protein. Similar results were obtained by Wang et al. (46) when they immunized mice with DNA or recombinant protein of merozoite surface protein 4. The qualitative differences may account for the apparently greater cross-reactive responses after immunization with DNA and the significant reduction in recognition of CHO cells expressing heterologous CIDR1s after exposure to the Sc y179 recombinant protein (Table 1).
Combining a large number of variant CIDR1s domains with sequential immunization may eventually break the primarily variant-specific antibody responses and divert the responses to the more cross-reactive epitopes. Such epitopes were recently found on the CIDR1 domain (22). However, there have been several examples in which the responses of humans to DNA vaccination have been significantly different than the responses observed in mice (14, 15, 26).
Targeting only a limited number of variant genes involved in pathogenic infections, such as placental malaria and noncerebral severe malaria, may be sufficient to protect against these severe forms (10, 19, 24). Most PfEMP1s expressed in isolates collected from placentas can be categorized into several (two for now) highly conserved var gene families (18, 40). In addition, chondroitin sulfate-binding PEs are recognized in a panreactive manner, and thus protection may be elicited by immunization with a single domain or a limited number of domains of PfEMP1 (19, 29). It has also been reported that one or two exposures to severe noncerebral malaria protect individuals from this form of malaria (24). Although the var gene families associated with this form have not been identified yet, it is plausible that immunization with a limited number of domains will facilitate immunity against severe malaria.
It is widely accepted that immunization with naked DNA elicits low antibody titers (16, 26, 28, 31, 32, 41-43). Although immunization or boosting with recombinant protein gave antibody titers of 106 as determined by ELISA, we were able to detect reactivity at antibody dilutions around 10,000 with DNA immunization. What was surprising was the agglutination titers of sera from DNA immunization that were not much lower than the titers after protein immunization and similar to the titers obtained for a pooled human hyperimmune serum with MC R+ parasites. Agglutination titers were positively correlated with the degree of protection in Aotus monkeys vaccinated with Sc y179 (3). The appearance of antibodies that recognized variant strains by agglutination is also very encouraging. The prime-boost regimen suggests that even if the initial antibody titers are low, exposure to the protein or the parasite expressing PfEMP1 rapidly boosts the response. Significant boosting of antibody titers was detected in Sc y179-immunized monkeys after exposure to the homologous parasite. This was particularly impressive with animals immunized with MF59 as an adjuvant, which had low initial antibody titers (3). Thus, natural exposure to P. falciparum parasites may supply the boost part in a prime-boost immunization scheme (26) and result in rapid development of protective antibodies. New methods designed to elevate expression or immune responses to DNA immunization, such as changing the codon usage, the use of CpG oligonucleotides, electroporation, or coimmunization with granulocyte-macrophage colony-stimulating factor or cytokines, may improve antibody titers considerably (16, 26, 28, 31, 32, 39, 41-43, 48).
We believe that naked DNA immunization with domains of PfEMP1 may prime the host immune system to rapidly respond to these domains during the initial phase of an infection. We hope that sequential immunization with different combinations of var gene domains will prime for more variant-transcending immunity and that such immunity may be sufficient to protect individuals from severe disease. It is clear that such immunization will not provide sterile immunity, but it may facilitate development of clinical immunity and thus significantly reduce morbidity and mortality among people routinely exposed to P. falciparum infection.
Acknowledgments
We thank Richard Hedstrom, Naval Medical Research Institute, for providing the VR1050 plasmid for DNA immunization.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aguiar, J. C., R. C. Hedstrom, W. O. Rogers, Y. Charoenvit, J. B. Sacci, Jr., D. E. Lanar, V. F. Majam, R. R. Stout, and S. L. Hoffman. 2001. Enhancement of the immune response in rabbits to a malaria DNA vaccine by immunization with a needle-free jet device. Vaccine 20:275-280. [DOI] [PubMed] [Google Scholar]
- 2.Barragan, A., P. G. Kremsner, W. Weiss, M. Wahlgren, and J. Carlson. 1998. Age-related buildup of humoral immunity against epitopes for rosette formation and agglutination in African areas of malaria endemicity. Infect. Immun. 66:4783-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruch, D. I., B. Gamain, J. W. Barnwell, J. S. Sullivan, A. Stowers, G. G. Galland, L. H. Miller, and W. E. Collins. 2002. Immunization of Aotus monkeys with a functional domain of the Plasmodium falciparum variant antigen induces protection against a lethal parasite line. Proc. Natl. Acad. Sci. USA 99:3860-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baruch, D. I., X. C. Ma, H. B. Singh, X. Bi, B. L. Pasloske, and R. J. Howard. 1997. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood 90:3766-3775. [PubMed] [Google Scholar]
- 5.Baruch, D. I., S. J. Rogerson, and B. M. Cooke. 2002. Asexual blood stages of malaria antigens: cytoadherence. Chem. Immunol. 80:144-162. [DOI] [PubMed] [Google Scholar]
- 6.Beeson, J. G., and G. V. Brown. 2002. Pathogenesis of Plasmodium falciparum malaria: the roles of parasite adhesion and antigenic variation. Cell Mol. Life Sci. 59:258-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeson, J. G., J. C. Reeder, S. J. Rogerson, and G. V. Brown. 2001. Parasite adhesion and immune evasion in placental malaria. Trends Parasitol. 17:331-337. [DOI] [PubMed] [Google Scholar]
- 8.Bull, P. C., M. Kortok, O. Kai, F. Ndungu, A. Ross, B. S. Lowe, C. I. Newbold, and K. Marsh. 2000. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182:252-259. [DOI] [PubMed] [Google Scholar]
- 9.Bull, P. C., B. S. Lowe, N. Kaleli, F. Njuga, M. Kortok, A. Ross, F. Ndungu, R. W. Snow, and K. Marsh. 2002. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J. Infect. Dis. 185:1688-1691. [DOI] [PubMed] [Google Scholar]
- 10.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bull, P. C., and K. Marsh. 2002. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 10:55-58. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Q., M. Schlichtherle, and M. Wahlgren. 2000. Molecular aspects of severe malaria. Clin. Microbiol. Rev. 13:439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doolan, D. L., and S. L. Hoffman. 2001. DNA-based vaccines against malaria: status and promise of the multi-stage malaria DNA vaccine operation. Int. J. Parasitol. 31:753-762. [DOI] [PubMed] [Google Scholar]
- 15.Doolan, D. L., and S. L. Hoffman. 2002. Nucleic acid vaccines against malaria. Chem. Immunol. 80:308-321. [DOI] [PubMed] [Google Scholar]
- 16.Fang, Z., Y. W. Liu, Y. K. Shi, X. B. Yu, W. Q. Huang, and X. Ji. 2002. The humoral immune responses elicited in mice by inoculations with a recombinant protein or DNA based on the circumsporozoite-protein gene of Plasmodium falciparum. Ann. Trop. Med. Parasitol. 96:463-468. [DOI] [PubMed] [Google Scholar]
- 17.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 18.Fried, M., and P. E. Duffy. 2002. Two DBLgamma subtypes are commonly expressed by placental isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 122:201-210. [DOI] [PubMed] [Google Scholar]
- 19.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 20.Fukumoto, T., N. Torigoe, S. Kawabata, M. Murakami, T. Uede, T. Nishi, Y. Ito, and K. Sugimura. 1998. Peptide mimics of the CTLA4-binding domain stimulate T-cell proliferation. Nat. Biotechnol. 16:267-270. [DOI] [PubMed] [Google Scholar]
- 21.Gamain, B., S. Gratepanche, L. H. Miller, and D. I. Baruch. 2002. Molecular basis for the dichotomy in Plasmodium falciparum adhesion to CD36 and chondroitin sulfate A. Proc. Natl. Acad. Sci. USA 99:10020-10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamain, B., L. H. Miller, and D. I. Baruch. 2001. The surface variant antigens of Plasmodium falciparum contain cross-reactive epitopes. Proc. Natl. Acad. Sci. USA 98:2664-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamain, B., J. D. Smith, L. H. Miller, and D. I. Baruch. 2001. Modifications in the CD36 binding domain of the Plasmodium falciparum variant antigen are responsible for the inability of chondroitin sulfate A adherent parasites to bind CD36. Blood 97:3268-3274. [DOI] [PubMed] [Google Scholar]
- 24.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 25.Heddini, A., F. Peterson, O. Kai, J. Shafi, J. Obiero, Q. Chen, A. Barragan, M. Wahlgren, and K. Marsh. 2001. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect. Immun. 69:5849-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman, S. L., and D. L. Doolan. 2000. Can malaria DNA vaccines on their own be as immunogenic and protective as prime-boost approaches to immunization? Dev. Biol. 104:121-132. [PubMed] [Google Scholar]
- 27.Jones, T. R., R. A. Gramzinski, J. C. Aguiar, B. K. Sim, D. L. Narum, S. R. Fuhrmann, S. Kumar, N. Obaldia, and S. L. Hoffman. 2002. Absence of antigenic competition in Aotus monkeys immunized withPlasmodium falciparum DNA vaccines delivered as a mixture. Vaccine 20:1675-1680. [DOI] [PubMed] [Google Scholar]
- 28.Jones, T. R., D. L. Narum, A. S. Gozalo, J. Aguiar, S. R. Fuhrmann, H. Liang, J. D. Haynes, J. K. Moch, C. Lucas, T. Luu, A. J. Magill, S. L. Hoffman, and B. K. Sim. 2001. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. J. Infect. Dis. 183:303-312. [DOI] [PubMed] [Google Scholar]
- 29.Lekana Douki, J. B., B. Traore, F. T. Costa, T. Fusai, B. Pouvelle, Y. Sterkers, A. Scherf, and J. Gysin. 2002. Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood 100:1478-1483. [DOI] [PubMed] [Google Scholar]
- 30.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenic basis of malaria. Nature 415:673-679. [DOI] [PubMed] [Google Scholar]
- 31.Nagata, T., M. Uchijima, A. Yoshida, M. Kawashima, and Y. Koide. 1999. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem. Biophys. Res. Commun. 261:445-451. [DOI] [PubMed] [Google Scholar]
- 32.Narum, D. L., S. Kumar, W. O. Rogers, S. R. Fuhrmann, H. Liang, M. Oakley, A. Taye, B. K. Sim, and S. L. Hoffman. 2001. Codon optimization of gene fragments encoding Plasmodium falciparum merozoite proteins enhances DNA vaccine protein expression and immunogenicity in mice. Infect. Immun. 69:7250-7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newbold, C. I. 1999. Antigenic variation in Plasmodium falciparum: mechanisms and consequences. Curr. Opin. Microbiol. 2:420-425. [DOI] [PubMed] [Google Scholar]
- 34.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75:281-292. [DOI] [PubMed] [Google Scholar]
- 35.Ockenhouse, C. F., M. Ho, N. N. Tandon, G. A. Van Seventer, S. Shaw, N. J. White, G. A. Jamieson, J. D. Chulay, and H. K. Webster. 1991. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J. Infect. Dis. 164:163-169. [DOI] [PubMed] [Google Scholar]
- 36.Pain, A., D. J. Ferguson, O. Kai, B. C. Urban, B. Lowe, K. Marsh, and D. J. Roberts. 2001. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc. Natl. Acad. Sci. USA 98:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 38.Rogers, W. O., J. K. Baird, A. Kumar, J. A. Tine, W. Weiss, J. C. Aguiar, K. Gowda, R. Gwadz, S. Kumar, M. Gold, and S. L. Hoffman. 2001. Multistage multiantigen heterologous prime boost vaccine for Plasmodium knowlesi malaria provides partial protection in rhesus macaques. Infect. Immun. 69:5565-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers, W. O., W. R. Weiss, A. Kumar, J. C. Aguiar, J. A. Tine, R. Gwadz, J. G. Harre, K. Gowda, D. Rathore, S. Kumar, and S. L. Hoffman. 2002. Protection of rhesus macaques against lethal Plasmodium knowlesi malaria by a heterologous DNA priming and poxvirus boosting immunization regimen. Infect. Immun. 70:4329-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe, J. A., S. A. Kyes, S. J. Rogerson, H. A. Babiker, and A. Raza. 2002. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J. Infect. Dis. 185:1207-1211. [DOI] [PubMed] [Google Scholar]
- 41.Sedegah, M., G. T. Brice, W. O. Rogers, D. L. Doolan, Y. Charoenvit, T. R. Jones, V. F. Majam, A. Belmonte, M. Lu, M. Belmonte, D. J. Carucci, and S. L. Hoffman. 2002. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8+-T-cell populations. Infect. Immun. 70:3493-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedegah, M., W. Weiss, J. B. Sacci, Jr., Y. Charoenvit, R. Hedstrom, K. Gowda, V. F. Majam, J. Tine, S. Kumar, P. Hobart, and S. L. Hoffman. 2000. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J. Immunol. 164:5905-5912. [DOI] [PubMed] [Google Scholar]
- 43.Sim, B. K., D. L. Narum, H. Liang, S. R. Fuhrmann, N. Obaldia 3rd, R. Gramzinski, J. Aguiar, J. D. Haynes, J. K. Moch, and S. L. Hoffman. 2001. Induction of biologically active antibodies in mice, rabbits, and monkeys by Plasmodium falciparum EBA-175 region II DNA vaccine. Mol. Med. 7:247-254. [PMC free article] [PubMed] [Google Scholar]
- 44.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 45.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 46.Wang, L., J. G. Menting, C. G. Black, A. Stowers, D. C. Kaslow, S. L. Hoffman, and R. L. Coppel. 2000. Differences in epitope recognition, isotype and titer of antisera to Plasmodium falciparum merozoite surface protein 4 raised by different modes of DNA or protein immunization. Vaccine 19:816-824. [DOI] [PubMed] [Google Scholar]
- 47.Yipp, B. G., D. I. Baruch, C. Brady, A. G. Murray, S. Looareesuwan, P. Kubes, and M. Ho. 2002. Recombinant PfEMP1 peptide inhibits and reverses cytoadherence of clinical Plasmodium falciparum isolates in vivo. Blood 101:331-337. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, L., E. Nolan, S. Kreitschitz, and D. P. Rabussay. 2002. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim. Biophys. Acta 1572:1-9. [DOI] [PubMed]