Abstract
Infection of bovine ligated loops with the Salmonella enterica serotype Typhimurium wild type but not a sipA sopABDE2 mutant resulted in fluid accumulation, polymorphonuclear cell infiltration, and expression of CXC chemokines, particularly GROα. None of these sipA sopABDE2-dependent responses was observed in murine-ligated loops. The majority of GROα transcripts localized to bovine intestinal epithelium. Thus, different disease outcomes between mice (i.e., no diarrhea) and calves (i.e., diarrhea) may be due to differences in sipA sopABDE2-dependent CXC chemokine gene expression in epithelial cells.
Salmonella enterica serotype Typhimurium causes enterocolitis in humans, a localized infection characterized by diarrhea and by pathological changes that are most severe in the intestine and mesenteric lymph nodes (24, 25). In contrast, S. enterica serotype Typhi causes typhoid fever, a systemic infection characterized by fever, while diarrhea is considered to be an insignificant symptom developing in only one-third of patients (28). A striking difference between the host responses elicited during infections with serotype Typhimurium and serotype Typhi in humans is the type of inflammation observed in the intestine. Analysis of biopsy samples reveals that inflammation in the intestines of patients infected with serotype Typhimurium is characterized by an infiltrate that is composed primarily of polymorphonuclear (PMN) cells (6, 27), while inflammation in typhoid fever patients is caused predominantly by infiltration with monocytes (21, 39). Similarly, the predominant cell type (representing 95% of fecal leucocytes) in stools from typhoid fever patients is mononuclear, whereas PMN cells predominate (representing 75% of fecal leucocytes) in stool samples from enterocolitis patients (13).
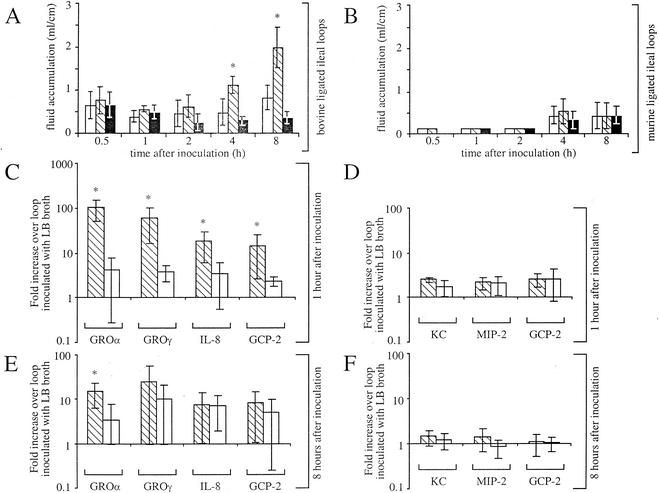
Serotype Typhimurium induces fluid accumulation and PMN cell influx in bovine, but not murine ligated ileal loops.
Experimental infections of calves or mice with serotype Typhimurium are commonly used as animal models to study the pathogenesis of typhoid fever or enterocolitis, respectively. Serotype Typhimurium causes a typhoid fever-like disease without diarrhea in mice, while infection of calves results in a localized infection characterized by diarrhea (49). We compared the host responses to infection with serotype Typhimurium in mice and calves using the ligated-ileal-loop model. Bovine ligated-ileal-loop surgeries were carried out as described previously (34, 50). In mice, anesthesia was induced and maintained with Propofol (Abbot Laboratories, Chicago, Ill.), an approximately 2-cm incision was made in the abdomen, an ileal loop of 5 to 8 cm was ligated and injected with 0.15 ml of sterile Luria-Bertani (LB) broth or 1 × 108 CFU. To prevent dehydration, mice were given two doses of 0.5 ml of sterile saline subcutaneously. Fluid accumulation and inflammatory changes elicited by the serotype Typhimurium wild type (IR715) (41) were compared to those elicited by a sipA sopABDE2 mutant (ZA21) (50) and sterile LB broth. The sipA (sspA), sopA, sopB (sigD), sopD, and sopE2 genes encode effector proteins of the invasion-associated type III secretion system (TTSS-1) that are required for eliciting PMN cell influx and fluid accumulation in bovine ligated ileal loops (11, 18, 34, 47, 50). Fluid accumulation in bovine ligated ileal loops inoculated with the serotype Typhimurium wild type (IR715) was significantly higher (P > 0.05) than that elicited by inoculation with LB broth or the sipA sopABDE2 mutant (ZA21) at 4 and 8 h postinfection (Fig. 1A). In contrast, inoculation of murine-ligated ileal loops with either the serotype Typhimurium wild type (IR715), the sipA sopABDE2 mutant (ZA21), or sterile LB broth did not result in significant differences in fluid accumulation at any of the time points collected (Fig. 1B). The serotype Typhimurium wild type was recovered at approximately 10-fold-higher numbers (P > 0.05) from bovine and murine Peyer's patches than the sipA sopABDE2 mutant at early times (1 h) but not at later times (8 h) postinfection (data not shown). Reduced recovery of the sipA sopABDE2 mutant from tissue collected at early times after infection may be explained by the requirement of SopB, SopE2, and SipA for invasion of human intestinal epithelial cells by serotype Typhimurium in vitro (17, 29, 51, 52).
FIG. 1.
Kinetics of fluid accumulation and CXC chemokine expression in bovine and murine ligated ileal loops. (A and B) Fluid accumulation elicited in bovine (A) and murine ligated (B) ileal loops is shown. Each data point represents the average (±standard deviations) from three independent experiments. Asterisks indicate that the amount of fluid elicited by serotype Typhimurium wild type (IR715; hatched bars) was significantly higher (P < 0.05) than that elicited by the sipA sopABDE2 mutant (ZA21; open bars) and sterile LB broth (solid bars). (C to F) The change in induction (n-fold) of CXC chemokine gene expression in bovine (C and E) or murine (D and F) ileal Peyer's patches infected with wild-type (hatched bars) or sipA sopABDE2 mutant (open bars) strains compared to expression in Peyer's patches inoculated with LB broth at 1 h (C and D) or 8 h (E and F) after inoculation is shown below. Asterisks indicate a significant increase in chemokine mRNA level in loops infected with the wild type (IR715) compared to those inoculated with the sipA sopABDE2 mutant (ZA21).
Hematoxylin-and-eosin-stained sections of bovine and murine ileal Peyer's patches were examined by light microscopy. The degree of inflammatory cell infiltration and the severity of intestinal epithelial detachment were scored by two pathologists as described previously (34). A higher degree of monocyte infiltration was observed in sections collected from both bovine and murine ileal Peyer's patches infected with the serotype Typhimurium wild type (IR715) than in those collected from loops infected with the sipA sopABDE2 mutant (ZA21) or sterile LB broth (Table 1). The host response observed in ligated ileal loops of mice differed from that in calves mainly by the degree of PMN cell infiltration. Infection with the serotype Typhimurium wild type (IR715) resulted in perivascular PMN cell infiltration in the bovine lamina propria by 1 h postinfection, and this inflammatory response progressed to severe diffuse PMN cell infiltration in the bovine ileal mucosa and submucosa by 8 h postinfection (Table 1). The sipA sopABDE2 mutant (ZA21) caused only a mild PMN cell infiltration in the bovine ileal mucosa with minimal lesions. In contrast, the serotype Typhimurium wild type and the sipA sopABDE2 mutant (ZA21) elicited only a mild PMN cell infiltration in the murine ileal mucosa.
TABLE 1.
Development of inflammatory infiltrates and tissue injury in the bovine and murine ileal mucosa following inoculation with the serotype Typhimurium wild type, the sipA sopABDE2 mutant, or sterile LB broth
| Loop source | Pathological change | Loop inoculated with | Avga qualitative score of pathological changesb in tissue collected at the following times (h) after inoculation of loops
|
||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | |||
| Calves | Tissue-associated PMN infiltration | Wild type | 1.7 | 2.7 | 3.3 | 2.7 | 4.0 |
| sipA sopABDE2 mutant | 1.7 | 1.3 | 2.7 | 1.7 | 2.3 | ||
| LB broth | 2.0 | 2.0 | 2.0 | 1.3 | 1.0 | ||
| Intravascular PMN infiltration | Wild type | 0.7 | 1.7 | 2.3 | 1.3 | 2.3 | |
| sipA sopABDE2 mutant | 1.0 | 0.7 | 2.3 | 2.0 | 1.7 | ||
| LB broth | 1.0 | 0.5 | 1.3 | 1.3 | 1.0 | ||
| Monocyte infiltration | Wild type | 2.0 | 1.7 | 1.7 | 2.3 | 3.0 | |
| sipA sopABDE2 mutant | 1.9 | 1.3 | 2.3 | 1.7 | 2.0 | ||
| LB broth | 1.7 | 2.0 | 1.7 | 1.7 | 1.3 | ||
| Detachment of surface epithelia | Wild type | 0.0 | 1.0 | 1.4 | 1.7 | 3.3 | |
| sipA sopABDE2 mutant | 0.7 | 0.3 | 2.0 | 1.0 | 1.3 | ||
| LB broth | 0.0 | 1.4 | 1.0 | 0.3 | 0.7 | ||
| Mice | Tissue-associated PMN infiltration | Wild type | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 |
| sipA sopABDE2 mutant | 0.0 | 0.3 | 0.7 | 0.7 | 1.3 | ||
| LB broth | 1.0 | 0.0 | 0.0 | 0.0 | 0.3 | ||
| Intravascular PMN infiltration | Wild type | 0.5 | 0.7 | 1.0 | 1.0 | 1.0 | |
| sipA sopABDE2 mutant | 0.3 | 1.0 | 2.3 | 3.0 | 2.7 | ||
| LB broth | 1.0 | 1.0 | 0.7 | 0.0 | 1.3 | ||
| Monocyte infiltration | Wild type | 1.5 | 3.0 | 3.3 | 3.0 | 3.5 | |
| sipA sopABDE2 mutant | 2.0 | 2.3 | 1.3 | 2.3 | 2.3 | ||
| LB broth | 1.7 | 1.0 | 1.7 | 0.7 | 2.3 | ||
| Detachment of surface epithelia | Wild type | 0.0 | 0.7 | 2.0 | 2.0 | 4.8 | |
| sipA sopABDE2 mutant | 0.3 | 0.3 | 0.0 | 0.0 | 1.0 | ||
| LB broth | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | ||
Each data point represents the average score from three independent experiments.
The histopathological changes of murine and bovine Peyer's patches were assessed in a blinded manner by two veterinary pathologists and independently scored on a scale of 0 to 5, with 5 being the highest level of histologic changes.
The exposure of the ileal mucosa to a large bacterial inoculum for the duration of the experiment represents a physiological limitation of the ligated-ileal-loop assay. This limitation may be responsible for the severe detachment of surface epithelia observed within only 8 h after inoculation of murine ligated ileal loops with the serotype Typhimurium wild type (Table 1), while little or no inflammatory changes are observed in the intestinal mucosa at 1 day after oral infection of mice (20, 36). In calves, on the other hand, lesions developed with kinetics similar to those observed during an oral infection (34, 43). Intravascular PMN cells were seen in the microvasculature of the murine ileal serosa in all treatment groups (data not shown), suggesting that the response in the mouse was due to surgical manipulations rather than to infection.
TTSS-1 effector genes are required for induction of CXC chemokine gene expression in bovine—but not murine—Peyer's patches.
The major difference detected between the inflammatory infiltrates in calves and mice was the presence or absence of a large amount of PMN cells in the ileal mucosa (Table 1). Since trafficking of leukocytes is largely controlled by chemokines (23), we investigated whether the different compositions of inflammatory infiltrates observed in mice and calves in response to serovar Typhimurium infection may be reflected by the expression in infected tissue of different subsets of these chemoattractants. According to the number and arrangement of N-terminal cysteine residues, chemokines are divided into four subfamilies, including CX3C (three amino acid residues between the first two cysteine residues), CXC (one amino acid between the first two cysteine residues), CC (the first two cysteine residues being adjacent), and XC (lacking the first cysteine residue) (53). Different subsets of chemokines direct the migration of specific subsets of leukocytes (23). For instance, monocyte chemotactic protein 1 (MCP-1), MCP-2, macrophage inflammatory protein 1α (MIP-1α), and RANTES (i.e., regulated upon activation, normal T-cell expressed and secreted) act mainly on monocytes and belong to a subgroup of human CC chemokines, which are encoded by genes clustered on human chromosome 17q11.2 (53). In contrast, interleukin 8 (IL-8), growth-related oncogene α (GROα), GROγ, and granulocyte chemotactic protein 2 (GCP-2) are encoded by genes clustered on human chromosome 4q12-q13 and belong to a subgroup of human CXC chemokines controlling migration of PMN cells (53). While counterparts of the human genes encoding MCP-1, MCP-2, MIP-1α, RANTES, IL-8, GCP-2, GROα, and GROγ are also present in the bovine host (1, 30-32, 44, 45), mice exhibit a number of genetic differences. That is, mice do not possess the CC chemokine MCP-1 but instead express a functional analogue, the monocyte chemoattractant JE (3). Furthermore, mice do not possess IL-8, GROα, or GROγ, but instead produce the CXC chemokines keratinocyte-derived chemokine (KC) and macrophage inflammatory protein 2 (MIP-2) (4, 33, 42, 46). Murine KC and MIP-2 share sequence homology with human and bovine GRO proteins (31) and are involved in controlling PMN cell trafficking (38). Unlike other cytokines, CXC and CC chemokines are generally not stored within cells; rather, their production is induced at the transcriptional level upon appropriate stimulation (2, 10). We therefore investigated the expression of chemokines in bovine and murine tissues by detecting transcripts at 0.5, 1, 2, 4, and 8 h postinfection by using semiquantitative reverse transcriptase PCR (RT-PCR) as described previously (35) and by using primers specific to bovine MCP-1, MCP-2, MIP-1α, RANTES, IL-8, GCP-2, GROα, and GROγ as well as primers specific to murine JE, MCP-2, MIP-1α, RANTES, KC, GCP-2, and MIP-2 (Table 2).
TABLE 2.
Primers used in this study for RT-PCR and real-time PCR
| PCR type | Target | Primer sequencea | Annealing temp (°C) | No. of cycles | Product or amplicon size (bp) |
|---|---|---|---|---|---|
| RT | |||||
| b-IL-8 | TGCCTAAACCCCAAGGAAAAGTG AACCCTACACCAGACCCACACAGAAC | 53.5 | 25 | 205 | |
| b-GROα | GATTCACCTCAAGAACATCCAGAGC AGAACTGCCAAACACATTCACACC | 55.0 | 25 | 396 | |
| b-GROγ | CAAAGAGGGAAAAGAGGAATCACC AAGGGCTGGCATAATGTGGG | 52.0 | 25 | 335 | |
| b-GCP-2 | TTCGCCACTATGAGACTGCTATCC TCCAGACAGACTTCCCTTCCATTC | 60.0 | 25 | 284 | |
| b-MCP-1 | AAACCAAACTCCAAAGCCTTGAG TTCTTGCGAGGACACTTCCACC | 52.5 | 25 | 335 | |
| b-MCP-2 | ATTCTGTGTCTGCTGCTCGTGG TTCAAGGCTTCGGTGTTC | 55.3 | 25 | 283 | |
| b-MIP-1α | TCTGCCCTTGCCTGTTGTTC TCGGTGATGTATTCCTGGACCC | 55.2 | 25 | 252 | |
| b-RANTES | CCAGGAGTATTTCTACACCAGC AGCACTGAGGGTCTTTCACAGC | 56.5 | 25 | 298 | |
| m-KC | TGGGATTCACCTCAAGAAC AGTGTTGTCAGAAGCCAGCG | 53.9 | 25 | 355 | |
| m-MIP-2 | CCCAGACAGAAGTCATAGCCAC AATAAGTGAACTCTCAGACAGCG | 55.8 | 25 | 366 | |
| m-GCP-2 | GGCATTTCTGTTGCTGTTCACG CTTTCTTCTCTTCACTGGGGTCAG | 56.3 | 25 | 343 | |
| m-JE | GGAAAAATGGATCCACACCTTGC TCTCTTCCTCCACCACCATGCAG | 58.3 | 25 | 581 | |
| m-MCP-2 | TGCTTCTTTGCCTGCTGCTC TGCTTGTAACATCTCTCTGCCTGG | 56.8 | 25 | 358 | |
| m-MIP-1α | TGACCTGGAACTGAATGC TGTGACCAACTGGAGGGATG | 54.4 | 25 | 242 | |
| m-RANTES | CATCCTCACTGCAGCCGCC CCAAGCTGGCTAGGACTAGAG | 56.2 | 25 | 319 | |
| Real time | |||||
| b-IL-8 | AAGTGGGTGCAGAAGGTTGTG GGAGCATGGGTTTTTCCTTTC | 79 | |||
| b-GCP-2 | CCAGTGTCCCCAGGAAGCT GTCCAGGAGCCTTATGGAAGTCT | 103 | |||
| b-GROα | TTACTTTTTGTAGAGAAGATTGTCAGTTGTT CCAAGGGATATTTAGATCATTGTCATT | 121 | |||
| b-GROγ | TTGGATGGCTGTTCCAGAAGTA GCCTTAGGAGGTGGTGATTCCT | 78 | |||
| b-GAPDH | TTCTGGCAAAGTGGACATCGT GCCTTGACTGTGCCGTTGA | 92 | |||
| m-KC | ACCCAAACCGAAGTCATAGCC TTCAGGGTCAAGGCAAGCC | 60 | |||
| m-MIP-2 | TGAGTGTGACGCCCCCA TTTTTGACCGCCCTTGAGAG | 71 | |||
| m-GCP-2 | ACGCTGCGCAGCATCA GCTCCGTTGCGGCTATG | 59 |
Sequences for bovine and murine chemokine genes were obtained from GenBank. Top row, primer 1; bottom row, primer 2.
Infection with the serotype Typhimurium wild type (IR715) caused a significant (P < 0.05) elevation in the expression of two bovine CC chemokines (MCP-1 and MCP-2) and two bovine CXC chemokines (GROα and GROγ) in tissue from bovine Peyer's patches compared to infection with the sipA sopABDE2 mutant (ZA21) (Table 3). Induction of GROα gene expression was most pronounced, being significantly (P < 0.05) elevated at all time points and reaching a peak at 1 h postinfection of bovine loops. In contrast, only the expression of two murine CC chemokines (i.e., JE and RANTES) was significantly (P < 0.05) higher in murine Peyer's patches infected with the serotype Typhimurium wild type (IR715) than in those infected with the sipA sopABDE2 mutant (ZA21) (Table 3). These data showed that the TTSS-1 effector genes sipA, sopA, sopB, sopD, and sopE2 were only required in the calf for eliciting elevated expression of PMN cell chemoattractants (i.e., GROα and GROγ).
TABLE 3.
Expression profile of chemokine genes in bovine and murine ileal Peyer's patches determined by RT-PCR
| Chemokine | Loop inoculated with | Avg.a intensity of RT-PCR productsb from tissue collected at the following times (h) after inoculation of loops
|
||||
|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | ||
| Bovine GROα | Wild type | 21,659 | 43,862 | 39,525 | 41,398 | 44,374 |
| sipA sopABDE2 mutant | 1,681 | 7,308 | 8,595 | 22,765 | 10,401 | |
| LB broth | 2,366 | 3,256 | 924 | 3,019 | 3,658 | |
| Bovine GROγ | Wild type | 12,736 | 43,331 | 44,574 | 35,691 | 43,014 |
| sipA sopABDE2 mutant | 6,159 | 12,780 | 30,285 | 37,517 | 23,707 | |
| LB broth | 3,202 | 5,622 | 3,213 | 4,187 | 9,227 | |
| Bovine IL-8 | Wild type | 25,839 | 38,272 | 37,428 | 37,213 | 36,497 |
| sipA sopABDE2 mutant | 19,988 | 28,175 | 25,751 | 38,657 | 31,395 | |
| LB broth | 6,430 | 12,314 | 11,371 | 17,239 | 11,525 | |
| Bovine GCP-2 | Wild type | 20,906 | 30,238 | 28,735 | 38,998 | 34,338 |
| sipA sopABDE2 mutant | 22,666 | 23,162 | 32,724 | 31,325 | 36,786 | |
| LB broth | 15,025 | 14,587 | 11,331 | 10,291 | 18,894 | |
| Bovine MCP-1 | Wild type | 19,865 | 34,807 | 29,242 | 23,868 | 27,201 |
| sipA sopABDE2 mutant | 5,566 | 7,482 | 16,178 | 16,824 | 11,345 | |
| LB broth | 3,616 | 3,846 | 2,506 | 1,153 | 772 | |
| Bovine MCP-2 | Wild type | 38,738 | 49,545 | 44,096 | 44,129 | 52,014 |
| sipA sopABDE2 mutant | 21,107 | 30,192 | 38,427 | 49,680 | 43,826 | |
| LB broth | 20,511 | 22,795 | 22,415 | 20,622 | 19,531 | |
| Bovine MIP-1α | Wild type | 3,150 | 4,711 | 6,019 | 5,824 | 5,353 |
| sipA sopABDE2 mutant | 7,216 | 4,817 | 3,699 | 6,524 | 5,791 | |
| LB broth | 2,067 | 4,096 | 4,977 | 2,330 | 2,483 | |
| Bovine RANTES | Wild type | 41,907 | 42,042 | 42,018 | 34,219 | 39,325 |
| sipA sopABDE2 mutant | 46,135 | 43,184 | 43,064 | 39,054 | 38,471 | |
| LB broth | 32,058 | 41,615 | 39,265 | 30,620 | 29,846 | |
| Murine KC | Wild type | 47,636 | 52,148 | 50,812 | 52,744 | 53,246 |
| sipA sopABDE2 mutant | 40,688 | 50,866 | 50,594 | 51,208 | 49,622 | |
| LB broth | 18,581 | 42,701 | 39,421 | 50,579 | 40,688 | |
| Murine GCP-2 | Wild type | 8,616 | 16,655 | 47,264 | 54,468 | 56,123 |
| sipA sopABDE2 mutant | 6,878 | 6,359 | 41,984 | 52,237 | 48,917 | |
| LB broth | 4,738 | 5,689 | 15,315 | 45,895 | 39,984 | |
| Murine MIP-2 | Wild type | 37,232 | 52,471 | 51,698 | 51,403 | 56,766 |
| sipA sopABDE2 mutant | 26,215 | 52,049 | 55,777 | 56,673 | 44,451 | |
| LB broth | 15,546 | 33,140 | 40,279 | 56,931 | 44,342 | |
| Murine JE | Wild type | 28,370 | 27,814 | 28,592 | 30,706 | 33,599 |
| sipA sopABDE2 mutant | 7,686 | 14,969 | 13,464 | 6,401 | 6,417 | |
| LB broth | 12,960 | 15,756 | 12,344 | 15,927 | 17,240 | |
| Murine MCP-2 | Wild type | 12,243 | 38,362 | 45,164 | 43,510 | 49,735 |
| sipA sopABDE2 mutant | 1,528 | 25,614 | 43,678 | 48,953 | 35,019 | |
| LB broth | 1,732 | 17,727 | 27,713 | 30,071 | 26,441 | |
| Murine MIP-1α | Wild type | 14,196 | 26,057 | 35,651 | 39,379 | 38,554 |
| sipA sopABDE2 mutant | 18,240 | 13,436 | 27,968 | 29,018 | 18,001 | |
| LB broth | 8,580 | 19,396 | 10,920 | 16,760 | 16,433 | |
| Murine RANTES | Wild type | 29,994 | 40,021 | 35,289 | 31,557 | 40,429 |
| sipA sopABDE2 mutant | 35,293 | 26,349 | 26,708 | 32,241 | 42,878 | |
| LB broth | 32,054 | 19,464 | 32,946 | 40,776 | 38,599 | |
Each data point represents the average of three independent experiments.
Intensity of bands was determined using the NIH image software.
To quantify the differences in CXC chemokine gene expression observed between the treatment groups, real-time PCR analyses were performed with RNA samples collected at 1 and 8 h postinoculation. Real-time PCR was performed by using the SYBR Green method according to instructions provided by the manufacturer of the PCR kit (Applied Biosystems, Foster City, Calif.). Primers for murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased (Biosource International, Camarillo, Calif.), and the remaining primers are listed in Table 2. Reverse transcription of total RNA (2 μg) in a mixture containing 100 μl of 5.5 mM MgCl2, 500 μM dNTP, 2.5 μM random hexamers, and 1.25 U of MultiScribe reverse transcriptase per μl was performed at 48°C for 30 min. Real-time PCR was performed for each cDNA sample (4 μl/reaction) in duplicate by using gene-specific primers (300 nM) and an ABI Prism 7700 thermocycler (95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min). This experiment was performed twice for each total RNA sample. The threshold cycle (CT) value was determined for each sample, and the mRNA concentration for each target gene was quantified by using a comparative CT method (Applied Biosystems). Real-time PCR amplification of GAPDH transcripts was used to normalize the cDNA concentrations of different samples (which was carried out with the assumption that expression of GAPDH does not change during infection). The normalized amount of transcripts relative to the amount of transcripts present in samples from an uninfected control loop was given as 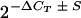 , where S is the standard deviation.
, where S is the standard deviation.
Both the serotype Typhimurium wild type (IR715) and the sipA sopABDE2 mutant (ZA21) elicited similar increases (4.8-fold or less) of murine CXC chemokine (KC, MIP-2, and GCP-2) gene expression compared to transcript levels in loops inoculated with sterile LB broth at both 1 and 8 h postinfection (Fig. 1D and F). In sharp contrast, the serotype Typhimurium wild type (IR715) elicited substantially higher CXC chemokine gene activation in the calf than the sipA sopABDE2 mutant (ZA21). These differences were most pronounced for expression of the bovine GROα and GROγ genes at 1 h after infection (Fig. 1C). In addition, expression of IL-8 and GCP-2 was consistently elevated at 1 h postinfection of bovine ligated ileal loops with the serotype Typhimurium wild type (IR715) compared to that due to infection with the sipA sopABDE2 mutant (ZA21). The fact that differences between the wild type and the sipA sopABDE2 mutant in their ability to induce expression of IL-8 and GCP-2 at 1 h postinfection were detected by real-time PCR but not by RT-PCR analysis is likely due to the higher sensitivity and accuracy of the former method. Collectively, these data further supported the idea that the TTSS-1 effector genes sipA, sopA, sopB, sopD, and sopE2 were required for elevated expression of CXC chemokine genes in bovine but not murine intestinal tissue.
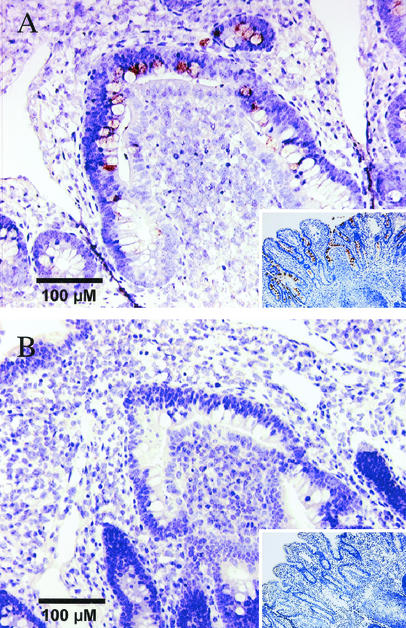
Localization of GROα transcripts in bovine ileal Peyer's patches by in situ hybridization.
The most dramatic changes in the host response observed during this study were a TTSS-1-dependent induction of CXC chemokine gene expression, particularly GROα, in the bovine ileal mucosa (Fig. 1). Previous in vitro studies have shown that infection of human epithelial cell lines with Salmonella serotypes can elicit the production of various CXC chemokines (8, 19, 26, 48), thereby suggesting that epithelial cells may be a source of these PMN cell chemoattractants in vivo. However, expression of CXC chemokines can be induced in vitro upon appropriate stimulation in nearly every type of cell that has been examined (2, 10), thereby illustrating the need to experimentally test the assumption that epithelial cells are a significant source of GROα production in vivo. To this end, we localized bovine GROα transcripts in bovine tissue by using in situ hybridization in tissue collected 1 h after inoculation. The bovine GROα gene was PCR amplified from a bovine cDNA library by using the primers listed in Table 2 (b-GROα) and labeled with psoralen-biotin according to instructions provided by the manufacturer (Ambion). This probe was used for in situ hybridization by using 5-μm sections of formalin-fixed and paraffin-embedded tissue samples. Deparaffinized sections were treated with a target retrieval solution at 95°C for 40 min (Dako) and with proteinase K (DAKO) at room temperature for 5 min. Endogenous peroxidase activity was quenched by incubating the sections in a solution of 0.3% hydrogen peroxide in methanol for 20 min. After denaturation of RNA for 5 min at 65°C, hybridization was carried out at 37°C for 1 h in a humidified chamber. Specificity of the labeling was confirmed in two control experiments in which the GROα probe was either omitted or replaced by biotinylated bacterial plasmid DNA. Hybridization of probes was detected by subsequent incubation with a primary streptavidin-peroxidase concentrate followed by one cycle of signal amplification with biotinyl tyramide solution and a secondary streptavidin-peroxidase concentrate. Sections were incubated with a diaminobenzidine solution, counterstained with Meyer's hematoxylin, dehydrated, and mounted.
In samples from loops infected with the serotype Typhimurium wild type (IR715), bovine GROα transcripts were detected primarily in enterocytes lining the intestinal crypts, in the base of absorptive villi, and in the follicle-associated epithelium of lymphoid nodules in Peyer's patches (Fig. 2A). Positive signals were observed less frequently in epithelial cells located at the tips of absorptive villi and occasionally in mononuclear leukocytes in the lamina propria. However, PMN cells were negative for the production of GROα transcripts. The amount of GROα transcripts detected in sections collected from loops inoculated with the sipA sopABDE2 mutant (ZA21) or LB broth was strongly reduced (Fig. 2B) compared to that in sections from wild-type-infected loops. These data demonstrate that intestinal epithelial cells represent the principal cell type producing GROα in the initial (1 h postinoculation) response to serotype Typhimurium infection.
FIG. 2.
Localization of GROα transcripts in bovine ileal Peyer's patches by in situ hybridization. The brown signal is produced by hybridization with GROα-specific mRNA, and slides were counterstained with hematoxylin to visualize cells (blue signal). (A) Section of bovine ileal Peyer's patches 1 h after infection with the serotype Typhimurium wild type (IR715). The insert shows a section at a lower magnification. (B) Section of bovine ileal Peyer's patches 1 h after inoculation with sterile LB broth. The insert shows a section at a lower magnification.
The precise mechanism for the sipA sopABDE-dependent induction of GROα gene expression in bovine epithelial cells is presently unclear. Serotype Typhimurium induces the expression of IL-1β mRNA in bovine intestinal tissue (35), but it is not known whether this cytokine is proteolytically activated and subsequently contributes to the induction of CXC chemokine gene expression in epithelial cells. The production of GROα by human intestinal epithelial cell lines in response to S. enterica serotype Dublin infection suggests a direct interaction between bacteria and enterocytes as an alternate mechanism for the sipA sopABDE-dependent production of this CXC chemokine (48). Whether the TTSS-1 is required for inducing GROα production in human epithelial cells has not previously been studied. However, the serotype Typhimurium TTSS-1 is required for eliciting the production of human IL-8 in cultured epithelial cells (14) and was essential for inducing bovine IL-8 production in vivo (Fig. 1). It is presently a matter of debate whether TTSS-1-mediated invasion is required for IL-8 production (7, 12). TTSS-1-mediated invasion of epithelial cells may facilitate recognition by Nods or Toll-like receptors (16, 37), thereby triggering proinflammatory signaling events leading to CXC chemokine gene expression (15). An alternative mechanism by which TTSS-1 effector proteins may elicit CXC chemokine gene expression in epithelial cells is by directly engaging components of proinflammatory intracellular signaling cascades (5, 9, 22, 40). Regardless of whether CXC chemokine expression is induced through Toll-like receptors or by direct interaction of TTSS-1 effector proteins with intracellular targets, a correlate of the hypothesis that CXC chemokine expression results from a direct interaction between bacteria and enterocytes is that the relevant proinflammatory signaling cascades must differ between murine and bovine intestinal epithelium, since the wild type and the sipA sopABDE mutant elicited similar CXC chemokine profiles in the mouse (Fig. 1D and F).
In summary, our data provide convincing evidence that induction of CXC chemokine expression accompanied by PMN cell infiltration is a key event that distinguishes the host response in calves from that elicited in mice during serotype Typhimurium infection. Furthermore, our study suggests that future studies should focus on GROα as the main CXC chemokine, the expression of which is induced by the TTSS-1 of serotype Typhimurium during enterocolitis in calves.
Acknowledgments
We thank Josely Figueiredo for technical assistance in bovine ligated-ileal-loop surgeries, Ellen Kasari for advice on murine ligated- ileal-loop surgeries, John Roths for technical assistance with photomicrography, and Alan Patranella and the staff members of Laboratory Animal Resources and Research Facilities, Texas A&M University, for providing animal care.
This project was supported by the Texas Agricultural Experiment Station project 8409 and Public Health Service grant AI44170. Work in A.J.B.'s and L.G.A.'s laboratories was further supported by Public Health Service grant AI40124 and USDA/NRICGP grant numbers 2002-35204-11624 and 2002-02140. J.N. is supported by CAPES, Brasília, Brazil.
Editor: F. C. Fang
REFERENCES
- 1.Aust, G., E. Brylla, I. Lehmann, S. Kiessling, and K. Spanel-Borowski. 1999. Cloning of bovine RANTES mRNA and its expression and regulation in ovaries in the periovulatory period. FEBS Lett. 463:160-164. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 3.Boring, L., J. Gosling, F. S. Monteclaro, A. J. Lusis, C. L. Tsou, and I. F. Charo. 1996. Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein 1α receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J. Biol. Chem. 271:7551-7558. [DOI] [PubMed] [Google Scholar]
- 4.Bozic, C. R., L. F. Kolakowski, Jr., N. P. Gerard, C. Garcia-Rodriguez, C. von Uexkull-Guldenband, M. J. Conklyn, R. Breslow, H. J. Showell, and C. Gerard. 1995. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 154:6048-6057. [PubMed] [Google Scholar]
- 5.Criss, A. K., M. Silva, J. E. Casanova, and B. A. McCormick. 2001. Regulation of Salmonella-induced neutrophil transmigration by epithelial ADP-ribosylation factor 6. J. Biol. Chem. 276:48431-48439. [DOI] [PubMed] [Google Scholar]
- 6.Day, D. W., B. K. Mandal, and B. C. Morson. 1978. The rectal biopsy appearances in Salmonella colitis. Histopathology 2:117-131. [DOI] [PubMed] [Google Scholar]
- 7.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin 8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckmann, L., J. R. Smith, M. P. Housley, M. B. Dwinell, and M. F. Kagnoff. 2000. Analysis by high-density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J. Biol. Chem. 275:14084-14094. [DOI] [PubMed] [Google Scholar]
- 9.Friebel, A., H. Ilchmann, M. Aepfelbacher, K. Ehrbar, W. Machleidt, and W. D. Hardt. 2001. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 276:34035-34040. [DOI] [PubMed] [Google Scholar]
- 10.Furie, M. B., and G. J. Randolph. 1995. Chemokines and tissue injury. Am. J. Pathol. 146:1287-1301. [PMC free article] [PubMed] [Google Scholar]
- 11.Galyov, E. E., M. W. Wood, R. Rosqvist, P. B. Mullan, P. R. Watson, S. Hedges, and T. S. Wallis. 1997. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 25:903-912. [DOI] [PubMed] [Google Scholar]
- 12.Gewirtz, A. T., A. M. Siber, J. M. Madara, and B. A. McCormick. 1999. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect. Immun. 67:608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris, J. C., H. L. Dupont, and R. B. Hornick. 1972. Fecal leukocytes in diarrheal illness. Ann. Intern. Med. 76:697-703. [DOI] [PubMed] [Google Scholar]
- 14.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 15.Hobert, M. E., K. A. Sands, R. J. Mrsny, and J. L. Madara. 2002. Cdc42 and Rac1 regulate late events in Salmonella typhimurium-induced interleukin 8 secretion from polarized epithelial cells. J. Biol. Chem. 277:51025-51032. [DOI] [PubMed] [Google Scholar]
- 16.Inohara, N., Y. Ogura, and G. Nunez. 2002. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr. Opin. Microbiol. 5:76-80. [DOI] [PubMed] [Google Scholar]
- 17.Jepson, M. A., B. Kenny, and A. D. Leard. 2001. Role of sipA in the early stages of Salmonella typhimurium entry into epithelial cells. Cell. Microbiol. 3:417-426. [DOI] [PubMed] [Google Scholar]
- 18.Jones, M. A., M. W. Wood, P. B. Mullan, P. R. Watson, T. S. Wallis, and E. E. Galyov. 1998. Secreted effector proteins of Salmonella dublin act in concert to induce enteritis. Infect. Immun. 66:5799-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingsley, R. A., R. L. Santos, A. M. Keestra, L. G. Adams, and A. J. Bäumler. 2002. Salmonella enterica serotype Typhimurium ShdA is an outer membrane fibronectin-binding protein that is expressed in the intestine. Mol. Microbiol. 43:895-905. [DOI] [PubMed] [Google Scholar]
- 21.Kraus, M. D., B. Amatya, and Y. Kimula. 1999. Histopathology of typhoid enteritis: morphologic and immunophenotypic findings. Mod. Pathol. 12:949-955. [PubMed] [Google Scholar]
- 22.Lee, C. A., M. Silva, A. M. Siber, A. J. Kelly, E. Galyov, and B. A. McCormick. 2000. A secreted salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. USA 97:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 24.Mandal, B. K., and J. Brennand. 1988. Bacteraemia in salmonellosis: a 15-year retrospective study from a regional infectious diseases unit. BMJ 297:1242-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal, B. K., and V. Mani. 1976. Colonic involvement in salmonellosis. Lancet i:887-888. [DOI] [PubMed] [Google Scholar]
- 26.McCormick, B., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGovern, V. J., and L. J. Slavutin. 1979. Pathology of salmonella colitis. Am. J. Surg. Pathol. 3:483-490. [DOI] [PubMed] [Google Scholar]
- 28.Miller, S. I., E. L. Hohmann, and D. A. Pegues. 1995. Salmonella (including Salmonella typhi), p. 2013-2033. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed., vol. 2. Churchill Livingstone, New York, N.Y.
- 29.Mirold, S., K. Ehrbar, A. Weissmuller, R. Prager, H. Tschape, H. Russmann, and W. D. Hardt. 2001. Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1 (SPI1), SPI5, and sopE2. J. Bacteriol. 183:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modi, W. S., M. R. Amarante, M. Hanson, J. E. Womack, and A. Chidambaram. 1998. Assignment of the mouse and cow CXC chemokine genes. Cytogenet. Cell. Genet. 81:213-216. [DOI] [PubMed] [Google Scholar]
- 31.Modi, W. S., and T. Yoshimura. 1999. Isolation of novel GRO genes and a phylogenetic analysis of the CXC chemokine subfamily in mammals. Mol. Biol. Evol. 16:180-193. [DOI] [PubMed] [Google Scholar]
- 32.Morsey, M. A., Y. Popowych, J. Kowalski, G. Gerlach, D. Godson, M. Campos, and L. A. Babiuk. 1996. Molecular cloning and expression of bovine interleukin-8. Microb. Pathog. 20:203-212. [DOI] [PubMed] [Google Scholar]
- 33.Oquendo, P., J. Alberta, D. Z. Wen, J. L. Graycar, R. Derynck, and C. D. Stiles. 1989. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet α-granule proteins. J. Biol. Chem. 264:4133-4137. [PubMed] [Google Scholar]
- 34.Santos, R. L., R. M. Tsolis, S. Zhang, T. A. Ficht, A. J. Baumler, and L. G. Adams. 2001. Salmonella-induced cell death is not required for enteritis in calves. Infect. Immun. 69:4610-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos, R. L., S. Zhang, R. M. Tsolis, A. J. Bäumler, and L. G. Adams. 2002. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 39:200-215. [DOI] [PubMed] [Google Scholar]
- 36.Santos, R. L., S. Zhang, R. M. Tsolis, R. A. Kingsley, L. G. Adams, and A. J. Bäumler. 2001. Animal models of Salmonella infections: enteritis vs. typhoid fever. Mircrob. Infect. 3:237-247. [DOI] [PubMed] [Google Scholar]
- 37.Sieling, P. A., and R. L. Modlin. 2002. Toll-like receptors: mammalian “taste receptors” for a smorgasbord of microbial invaders. Curr. Opin. Microbiol. 5:70-75. [DOI] [PubMed] [Google Scholar]
- 38.Song, F., K. Ito, T. L. Denning, D. Kuninger, J. Papaconstantinou, W. Gourley, G. Klimpel, E. Balish, J. Hokanson, and P. B. Ernst. 1999. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J. Immunol. 162:2275-2280. [PubMed] [Google Scholar]
- 39.Sprinz, H., E. J. Gangarosa, M. Williams, R. B. Hornick, and T. E. Woodward. 1966. Histopathology of the upper small intestines in typhoid fever. Biopsy study of experimental disease in man. Am. J. Dig. Dis. 11:615-624. [DOI] [PubMed] [Google Scholar]
- 40.Steele-Mortimer, O., L. A. Knodler, S. L. Marcus, M. P. Scheid, B. Goh, C. G. Pfeifer, V. Duronio, and B. B. Finlay. 2000. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J. Biol. Chem. 275:37718-37724. [DOI] [PubMed] [Google Scholar]
- 41.Stojiljkovic, I., A. J. Bäumler, and F. Heffron. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 177:1357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tekamp-Olson, P., C. Gallegos, D. Bauer, J. McClain, B. Sherry, M. Fabre, S. van Deventer, and A. Cerami. 1990. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J. Exp. Med. 172:911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Baumler. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wempe, F., J. Hanes, and K. H. Scheit. 1994. Cloning of the gene for bovine monocyte chemoattractant protein-2. DNA Cell Biol. 13:1-8. [DOI] [PubMed] [Google Scholar]
- 45.Wempe, F., A. Henschen, and K. H. Scheit. 1991. Gene expression and cDNA cloning identified a major basic protein constituent of bovine seminal plasma as bovine monocyte-chemoattractant protein-1 (MCP-1). DNA Cell Biol. 10:671-679. [DOI] [PubMed] [Google Scholar]
- 46.Wolpe, S. D., and A. Cerami. 1989. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 3:2565-2573. [DOI] [PubMed] [Google Scholar]
- 47.Wood, M. W., M. A. Jones, P. R. Watson, A. M. Siber, B. A. McCormick, S. Hedges, R. Rosqvist, T. S. Wallis, and E. E. Galyov. 2000. The secreted effector protein of Salmonella dublin, SopA, is translocated into eukaryotic cells and influences the induction of enteritis. Cell. Microbiol. 2:293-303. [DOI] [PubMed] [Google Scholar]
- 48.Yang, S. K., L. Eckmann, A. Panja, and M. F. Kagnoff. 1997. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology 113:1214-1223. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, S., R. A. Kingsley, R. L. Santos, H. Andrews-Polymenis, M. Raffatellu, J. Figueiredo, J. Nunes, R. M. Tsolis, L. G. Adams, and A. J. Bäumler. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, D., L. M. Chen, L. Hernandez, S. B. Shears, and J. E. Galan. 2001. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 39:248-259. [DOI] [PubMed] [Google Scholar]
- 52.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]
- 53.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]