Abstract
The UspA1 and Hag proteins have previously been shown to be involved in the ability of the Moraxella catarrhalis wild-type strain O35E to bind to human Chang and A549 cells, respectively. In an effort to identify novel adhesins, we generated a plasmid library of M. catarrhalis DNA fragments, which was introduced into a nonadherent Escherichia coli strain. Recombinant E. coli bacteria were subsequently enriched for clones that gained the ability to bind to Chang and A549 cells, yielding the plasmid pELFOS190. Transposon mutagenesis of this plasmid identified the potential adhesin gene mcaP (M. catarrhalis adherence protein). Sequence analysis revealed that McaP is related to autotransporter proteins and has substantial similarity with the GDSL family of lipolytic enzymes, particularly the Moraxella bovis phospholipase B. Expression of the mcaP gene product by E. coli increased adherence to Chang, A549, and 16HBE14o− polarized human bronchial cells 50- to 100-fold. Spectrophotometric assays with p-nitrophenol derivatives also demonstrated that McaP is an esterase. Furthermore, thin-layer chromatography revealed that McaP cleaves both phosphatidylcholine and lysophosphatidylcholine. McaP releases fatty acids and glycerophosphorylcholine upon cleavage of phosphatidylcholine, thus exhibiting phospholipase B activity. The construction and characterization of isogenic M. catarrhalis O35E mutants demonstrated that the lack of McaP expression abolishes esterase activity and considerably decreases adherence to several human cell lines.
The unencapsulated, gram-negative bacterium Moraxella catarrhalis is one of the leading causes of otitis media in young children and of lower respiratory tract infections in adults with chronic obstructive pulmonary disease (COPD). In developed countries, more than 80% of children under the age of 3 years will be diagnosed at least once with otitis media, and M. catarrhalis is responsible for 15 to 25% of all of these cases (6, 7, 10, 12, 26-29, 41, 51, 60). Lower respiratory tract infections (exacerbations) have been shown to contribute to the progression of COPD, and of the approximately 20 million cases of exacerbations documented each year in the United States, up to 35% result from M. catarrhalis infections (3, 41, 43, 54, 55).
A vaccine that provides protection against M. catarrhalis is highly desirable due to this substantial morbidity as well as the growing concern over antibiotic resistance observed in clinical isolates (30). Toward this end, current research is focused on the identification of potential antigens with emphasis on the outer membrane proteins (OMPs) (26, 34, 35). While M. catarrhalis OMPs with a wide variety of functions have been identified, adhesins are particularly attractive vaccine candidates because they are surface-exposed antigens. Furthermore, adhesins generally play a crucial role in colonization and pathogenesis. For many bacteria, adherence to epithelial surfaces contributes to the evasion of host clearance mechanisms (4, 25, 59). Previous studies with M. catarrhalis have identified UspA1 (32, 36), UspA2 (2, 8, 36), Hag (14, 15, 17, 19, 24, 45, 47; M. M. Holm and E. R. Lafontaine, unpublished data), lipooligosaccharide (24), OMPCD (49), and UspA2H (32) as potential adhesins. Of these, only UspA1 and UspA2H have been directly shown to mediate adherence to human cells (32, 36). However, while UspA1 is expressed by virtually all strains tested to date, only ∼20% of clinical isolates contain an uspA2H gene (32, 37).
The present study describes the identification of the novel M. catarrhalis OMP McaP. While this protein was identified based on its ability to function as an adhesin, we report that it also displays the enzymatic activity of an esterase and a phospholipase B (PLB).
MATERIALS AND METHODS
Strains, plasmids, tissue culture (TC) cell lines, and growth conditions.
The bacterial strains and plasmids described in this study are listed in Table 1. M. catarrhalis was routinely cultured at 37°C on Todd-Hewitt medium (Difco). When cultured on agar plates, M. catarrhalis was incubated in an atmosphere of 92.5% air-7.5% CO2. Antimicrobial supplementation for M. catarrhalis involved the use of 20 μg of kanamycin (KAN)/ml, 5 μg of Zeocin (Invitrogen)/ml, 15 μg of spectinomycin/ml, or 1 μg of chloramphenicol/ml. Escherichia coli recombinant clones were cultured in Luria-Bertani (LB) medium (Difco) supplemented with the following antibiotic concentrations, where indicated: 50 μg of KAN/ml, 15 μg of chloramphenicol/ml, or 100 μg of ampicillin/ml. To detect lipolytic activity of E. coli recombinant clones, LB agar plates were supplemented with 1% (vol/vol) Tween 80 (Sigma) as previously reported (63).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| M. catarrhalis | ||
| O35E | Wild-type isolate | 2 |
| O35E.M | mcaP isogenic mutant of strain O35E | This study |
| O35E.ZCS | Isogenic mutant of strain O35E; but expresses McaP | 47 |
| O35E.ZCSM | mcaP isogenic mutant of strain O35E.ZCS; lacks expression of Hag, UspA1, UspA2, and McaP | This study |
| E. coli | ||
| EPI300 | Cloning strain | Epicentre |
| TOP10 | Cloning strain | Invitrogen |
| Plasmids | ||
| pCC1 | Cloning vector | Epicentre |
| pBR322 | Cloning vector | New England Biolabs |
| pUC18K1 | Source of the Kanr cassette | 38 |
| pELFOS190 | Adherent recombinant clone containing an ≈30-kb M. catarrhalis O35E.ZCS DNA insert and expressing mcaP | This study |
| pELFOS190TN6 | Adherence-negative mutant of pELFOS190 containing a transposon insertion in mcaP | This study |
| pELCCF1 | pELFOS190 digested with BamHI to remove M. catarrhalis DNA insert followed by religation of the pCC1 vector to itself | This study |
| pJTmcaP | O35E-mcaP gene cloned into pBR322 | This study |
| pJTmcaPnpkan | Kanr cassette from pUC18K1 that is introduced into the mcaP ORF carried by the plasmid pJTmcaP | This study |
The human cell lines Chang (conjunctival epithelium; ATCC CCL20.2), A549 (type II alveolar lung epithelium; ATCC CCL85), and HEp-2 (laryngeal epithelium; ATCC CCL-23) were cultured in Ham's F-12 medium (Cellgro) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (GIBCO), 0.15% (vol/vol) sodium bicarbonate (Cellgro), and 4 mM l-glutamine (Cellgro) at a temperature of 37°C and an atmosphere of 92.5% air-7.5% CO2. The human cell line NCIH292 (mucoepidermoid lung epithelium; ATCC CRL-1848) was cultured in the same medium supplemented with 10 mM HEPES (GIBCO), 1 mM sodium pyruvate (Cellgro), and 4.5 g of glucose/liter.
The 16HBE14o− polarized human bronchial cell line (9) was cultured in Eagle's minimum essential medium with Earle's salts (Cellgro) supplemented with 2 mM l-glutamine (Cellgro), 1.5 g of sodium bicarbonate (Cellgro)/liter, 10% (vol/vol) fetal bovine serum (GIBCO), and 0.9 g of glucose/liter in an atmosphere of 92.5% air-7.5% CO2. All plastic ware used for the culture of 16HBE14o− cells was coated with a solution containing human fibronectin (BD BioSciences) and type 1 bovine collagen (BD BioSciences), as previously described (9). When grown under these conditions, these bronchial cells are polarized.
Recombinant DNA techniques.
Standard molecular biology techniques were performed as previously described (52). M. catarrhalis genomic DNA was purified with the Invitrogen Easy-DNA kit. The library of M. catarrhalis O35E.ZCS DNA fragments was generated with the CopyControl Fosmid library production kit (Epicentre) according to the manufacturer's instructions. Plasmid DNA was purified using the QIAprep Spin miniprep system (Qiagen) and mutagenized with a transposon by using the EZ::TN <KAN-2> insertion kit (Epicentre). Plasmid DNA and ligation mixtures were introduced into E. coli by chemical transformation as described previously (52). The method used to electroporate M. catarrhalis cells has been reported elsewhere (Holm and Lafontaine, unpublished). Southern hybridization experiments were performed using the North2South chemiluminescent hybridization and detection system (Pierce). An 0.7-kb SmaI DNA fragment containing the nonpolar Kanr resistance cartridge from the plasmid pUC18K1 (38) was used as a probe in some of these experiments. The 2.1-kb mcaP-specific DNA probe was obtained by PCR with the oligonucleotide primers P1 and P2 (see below).
PCR.
Amplicons used for cloning, construction of isogenic mutants, and sequencing of the M. catarrhalis strain O35E mcaP gene were generated with Pfu DNA polymerase (Stratagene). Other DNA fragments were amplified using Taq DNA polymerase (Invitrogen).
Cloning of the M. catarrhalis O35E mcaP gene in E. coli.
An amplicon of 2.1 kb containing the mcaP gene from strain O35E was generated with the oligonucleotide primers P1 (5′-CGCAATAAAGATCACCATGCTTG-3′) and P2 (5′-CGGGATCCCGCTGACACATTGCATTGATAAA-3′) (BamHI site underlined). This PCR product was digested with BamHI and ligated into the vector pBR322, which had been digested with the endonucleases EcoRV and BamHI. This ligation mixture was introduced into E. coli TOP10, and transformants were selected for resistance to ampicillin. Colonies were then streaked onto LB agar plates supplemented with 1% (vol/vol) Tween 80 to identify clones expressing lipolytic activity. Lipolysis of Tween 80 generates an insoluble precipitate that forms a halo around the colony. This approach yielded the recombinant plasmid pJTmcaP, which expresses the O35E mcaP gene product. Both strands of this mcaP insert were sequenced to verify that no mutations were introduced during PCR.
Construction of M. catarrhalis isogenic mutants.
The plasmid pJTmcaP was linearized with PmeI (New England Biolabs) and ligated with an 0.7-kb SmaI (New England Biolabs) DNA fragment containing the nonpolar Kanr cassette from the plasmid pUC18K1 (38). This ligation mixture was introduced into E. coli TOP10, and transformants were selected for resistance to KAN, thereby yielding the plasmid pJTmcaPnpKAN. A 2.8-kb amplicon, which corresponds to the O35E mcaP gene interrupted by the Kanr cartridge in the middle of the open reading frame (ORF), was generated from pJTmcaPnpKAN with the primers P1 and P2. This PCR product was then electroporated into the M. catarrhalis strains O35E and O35E.ZCS. The resulting Kanr colonies were screened by PCR with primers P1 and P2 to identify potential mcaP isogenic mutants (data not shown). Southern blot experiments were performed to confirm that proper allelic exchange had occurred in the isogenic mutants O35E.M and O35E.ZCSM (data not shown).
Nucleotide sequence analysis.
Plasmid DNA and PCR products were sequenced with an ABI Prism 310 genetic analyzer for automated DNA sequencing (Perkin-Elmer Biosystems). The nucleotide sequence data were analyzed with the GeneTool Lite software (BioTools Incorporated) and the Gapped BLAST 2.0 BLASTX search tool through the National Center for Biotechnology Information service with the nonredundant protein database. The oligonucleotide primer KAN-2 FP1 (Epicentre), which binds to nucleotides (nt) 1127 to 1151 of the EZ::TN <KAN-2> transposon, was used to sequence the plasmid pELFOS190TN6.
Antibodies and detection of selected M. catarrhalis antigens.
The UspA1-specific monoclonal antibody 24B5 (8) and the Hag-specific monoclonal antibody 5D2 (47) have been described elsewhere. Outer membrane vesicles were prepared as described previously (42, 46). Whole-cell lysates of M. catarrhalis strains and E. coli recombinant cells were prepared as previously reported (8, 46). These preparations were heated at 100°C for 15 min, separated by electrophoresis into sodium dodecyl sulfate (SDS)-7.5% (vol/vol) polyacrylamide gels, and either stained with Coomassie blue or transferred to polyvinylidene difluoride membranes (Millipore) for Western blot analysis as previously described (Holm and Lafontaine, unpublished).
Adherence assays.
For M. catarrhalis strains, quantitative and visual adherence assays were performed as reported by Holm and Lafontaine (unpublished). For E. coli recombinant bacteria, quantitative adherence assays were conducted in 24-well TC plates (Cellstar) seeded with 2.5 × 105 human cells in 0.5 ml of TC medium. Each monolayer was inoculated with one E. coli colony, incubated at 37°C for 3 h, and centrifuged for 5 min at a speed of 165 × g to facilitate contact between bacteria and human cells. Following a second incubation and centrifugation, the nonadherent bacteria were obtained by removing the TC medium covering each monolayer and gently washing each well five times with 0.5 ml of phosphate-buffered saline (PBS) supplemented with 0.15% gelatin (PBSG; pH 7.0). For each monolayer, the TC medium was combined with the washes, serially diluted with PBSG, and spread onto agar plates in order to determine the number of nonadherent bacteria. Epithelial cells with bound bacteria were released from the plastic support of the TC plate with a polyvinylpyrrolidone-EGTA-trypsin solution containing 1% (vol/vol) polyvinylpyrrolidone (BioSource International), 0.02% (vol/vol) EGTA (BioSource International), 0.02% (vol/vol) trypsin, and 0.02% (vol/vol) EDTA (Cellgro). The contents of each well were suspended in 0.5 ml of PBSG, serially diluted, and plated onto agar plates in order to determine the number of bacteria bound per monolayer of human cells. In Table 2, the results of our adherence assays are expressed as the percentages (± standard errors) of recombinant E. coli bacteria that bound to monolayers. Adherence assays were performed in triplicate on at least three independent occasions. In Fig. 5 and Table 4, the percent adherence of the M. catarrhalis wild-type strain O35E was set at 100%, and the adherence of isogenic mutants is expressed as the percentage (± standard error) relative to that of O35E.
TABLE 2.
Adherence to human cells and lipolytic activities of recombinant E. coli cells
| Construct | McaP expression | Adherence to cell linea
|
Lipolytic activityb | ||
|---|---|---|---|---|---|
| Chang | A549 | 16HBE14o− | |||
| pELCCF1 | − | 0.2 ± 0.03 | 0.06 ± 0.01 | NDe | − |
| pELFOS190 | + | 10.4 ± 2.9c | 9.0 ± 1.6c | ND | + |
| pELFOS190TN6 | − | 0.2 ± 0.04 | 0.1 ± 0.02 | ND | − |
| pBR322 | − | 0.2 ± 0.03 | 0.2 ± 0.06 | 0.1 ± 0.05 | − |
| pJTmcaP | + | 22.3 ± 3.7d | 4.9 ± 1.4d | 4.9 ± 2.1d | + |
Adherence is expressed as the percentage of bacteria binding to monolayers (± standard error).
As determined by the presence of halos surrounding bacteria streaked onto LB medium-1% Tween 80 agar plates.
The difference in the P value compared to the value for the negative control pELCCF1 was found to be statistically significant using a Mann-Whitney test.
The difference in the P value compared to the value for the negative control pBR322 was found to be statistically significant using a Mann-Whitney test.
ND, not determined.
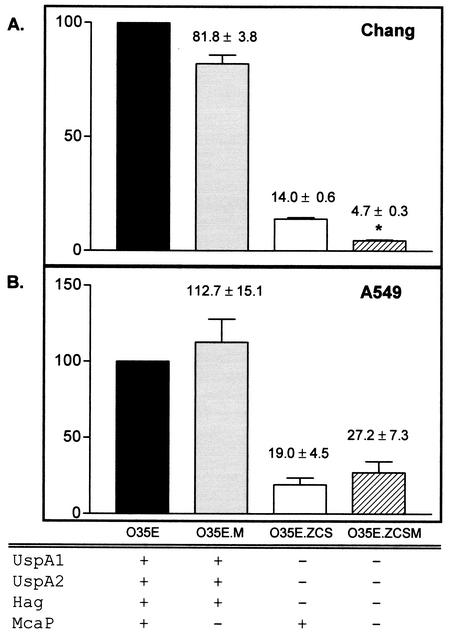
FIG. 5.
Adherence of M. catarrhalis strains to human cell lines in vitro. The adherence of the M. catarrhalis mutants O35E.M, O35E.ZCS, and O35E.ZCSM is expressed as the percentage (± standard error) of that of the parent strain O35E. The asterisk indicates that the decrease in O35E.ZCSM adherence, compared to the adherence of O35E.ZCS, is statistically significant. Expression (+) or lack thereof (−) of selected proteins is indicated.
TABLE 4.
Adherence of M. catarrhalis strains to human cells
| Strain | Adhesin expressed
|
Adherence to cell linea
|
|||||
|---|---|---|---|---|---|---|---|
| UspA1 | UspA2 | Hag | McaP | 16HBE14o− | HEp-2 | NCIH292 | |
| O35E | + | + | + | + | 100 | 100 | 100 |
| O35E.ZCS | − | − | − | + | 27.9 ± 6.4 | 35.3 ± 7.8 | 58.4 ± 12.7 |
| O35E.ZCSM | − | − | − | − | 10.7 ± 0.9b | 7.2 ± 3.6b | 25.9 ± 2.4b |
The adherence of strains O35E.ZCS and O35E.ZCSM is expressed as the percentage of that of the wild-type strain O35E (± standard error), which was set at 100%.
The difference in the P value compared to the value for strain O35E.ZCS was found to be statistically significant using a Mann-Whitney test.
Enrichment for adherent E. coli recombinant clones.
We enriched for recombinant clones that had gained the ability to bind to human cells by modifying a previously reported quantitative adherence assay (32; Holm and Lafontaine, unpublished). Briefly, 2.5 × 105 human cells were seeded into each well of a 24-well TC plate (Cellstar) and incubated overnight before use. E. coli recombinant cells, harboring our plasmid library of M. catarrhalis O35E.ZCS DNA fragments, were also spread onto appropriate agar plates and grown overnight. The next day, bacterial cells were scraped off agar plates and aseptically resuspended to an optical density of 230 Klett units in 5 ml of sterile PBS. Portions of these bacterial suspensions (25 μl containing 107 CFU) were inoculated in duplicate into the wells of a 24-well TC plate containing monolayers of human cells. The TC plate was centrifuged at 165 × g to facilitate contact between bacteria and human cells and then incubated for 3 h at 37°C. Following a second low-speed centrifugation step, nonadherent bacteria were removed by gently rinsing the monolayers five times with PBS. At that point, the wells were seeded with fresh TC medium and incubated at 37°C for an additional 3 h. The TC plate was again centrifuged for 5 min at 165 × g, and monolayers were subsequently washed five times to remove unbound bacteria. Human cells with bound E. coli recombinant clones were released from the plastic support of the TC plate by adding 100 μl of a polyvinylpyrrolidone-EGTA-trypsin solution (see above). The released human cells were suspended in 500 μl of PBS buffer, serially diluted in PBS, and spread onto agar plates. After overnight incubation at 37°C, bacteria were scraped off these agar plates and the enrichment was repeated. After three enrichment steps, E. coli colonies were randomly selected and plasmid DNA was purified. These plasmids were analyzed by restriction analysis, identifying the representative construct pELFOS190.
Protein sequencing by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Proteins present in M. catarrhalis outer membrane vesicles were resolved by SDS-PAGE and subsequently stained with Coomassie brilliant blue. The band corresponding to McaP was excised and further destained with 30% methanol at room temperature for 3 h. This gel piece was washed with 150 μl of acetonitrile-0.1 M ammonium bicarbonate buffer (1:1, pH 8.0), diced into 1-mm cubes, and rehydrated in 30 μl of 0.1 M ammonium bicarbonate buffer containing 0.4 μg of sequencing-grade, modified trypsin (Promega). An additional 15 μl of digestion buffer without trypsin was added after 10 min. Digestion was carried out for 16 h at 37°C. It should be noted that another aliquot of trypsin (0.2 μg) was added after 12 h, and the sample was incubated for an additional 4 h. Peptides were extracted with 150 μl of 60% acetonitrile containing 0.1% trifluoroacetic acid at 30°C for 30 min and concentrated to a final volume of 15 μl under vacuum. A 2-μl aliquot of this digest was then separated on an Aquasil C18 reverse-phase column (75-mm inside diameter by 15-mm tip by 5 cm; New Objectives) by using an acetonitrile-1% acetic acid gradient system (5 to 75% acetonitrile over 30 min followed by 95% acetonitrile for 3 min) at a flow rate of 250 nl/min. Eluted peptides were directly introduced into an ion-trap mass spectrometer (LCQ Deca XP Plus; Finnigan) equipped with a nanospray source. The mass spectrometer was operated on a double play mode where the instrument was set to automatically acquire a full MS scan (400 to 2,000 m/z) and collision-induced dissociation (CID) spectra on the most abundant ion from the full MS scan (relative collision energy, ∼30%). Dynamic exclusion was set to acquire three CIDs on the most abundant ion and exclude it for 3 min. The CIDs were compared with the in silico fragmentation pattern of McaP protein generated using the MS-Digest provision of Protein Prospector http://prospector.ucsf.edu.
Lipolytic activity assay.
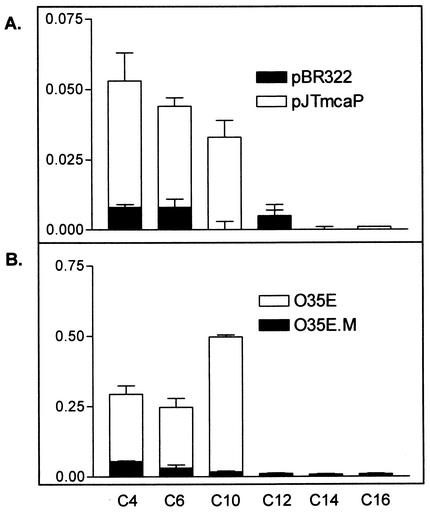
Lipolytic assays were performed as previously described (64). Briefly, 10 mM stock solutions of the p-nitrophenyl esters (pNPs; Sigma) of butyrate (C4), caproate (C6), caprate (C10), laurate (C12), myristate (C14), and palmitate (C16) were prepared using 2-propanol (Fisher) as the solvent. These pNP stocks were subsequently diluted to a final substrate concentration of 1 mM in PBS (pH 8.0) supplemented with 2.3 mg of deoxycholic acid (Sigma)/ml and 1.1 mg of gum arabic (Acros)/ml. Plate-grown bacteria were suspended in either PBS (pH 8.0; recombinant E. coli cells) or PBSG (pH 8.0; M. catarrhalis cells) to a density of 100 Klett units (E. coli) or 25 Klett units (M. catarrhalis), and 950 μl of these suspensions was subsequently mixed with 50 μl of the indicated 1 mM pNP substrate. The absorbance of each sample was then measured at wavelengths of 410 and 580 nm with a spectrophotometer (Spectronic Instruments) in order to estimate lipolytic activity and bacterial cell density, respectively. These optical measurements were taken immediately after the addition of the pNP substrates as well as after 15 min of incubation at room temperature. Lipolytic assays were performed in triplicate on at least three separate occasions. The results presented in Fig. 3 are expressed as the mean (± standard deviation) of the [(OD410 at 15 min − OD410 at 0 min)/OD580 of the sample]/15 min, where OD410 is optical density at 410 nm.
FIG. 3.
Lipolytic activities of E. coli recombinant cells (A) and M. catarrhalis strains (B). Lipid substrates composed of 4 (C4)- to 16 (C16)-carbon chains are described in Materials and Methods. Units = [(OD410 at 15 min − OD410 at 0 min)/OD580]/15 min, where OD410 is the optical density at 410 nm.
Preparation of McaP-enriched protein extracts.
Overnight cultures (100 ml) of E. coli recombinant cells were centrifuged, and the pelleted bacteria were suspended in 10 ml of sterile PBS. These suspensions were incubated at 60°C for 30 min and subsequently centrifuged at 6,000 × g to remove cellular debris. The resulting supernatants were subjected to a second centrifugation for 15 min at 39,000 × g and concentrated using Amicon Centriprep 50 columns (Millipore) to a final volume of ∼1 ml.
Phospholipase assays.
The following enzymes were used to study the ability of McaP to cleave phospholipids: McaP-enriched extract obtained from E. coli cells harboring pJTmcaP (60 μl), extract prepared from E. coli cells containing the control plasmid pBR322 (60 μl), porcine pancreas PLA2 (100 U; Sigma), and Vibrio species PLB (1 U; Sigma). Egg-yolk type XIII-E phosphatidylcholine (PC; 1 mg; Sigma) or lysophosphatidylcholine (LPC; 150 μg; Sigma) was combined with the indicated enzyme in 500 μl of buffer solution (5.0 mM Tris, 1.0 mM CaCl2, 1 mM ZnCl2, pH 7.2) and incubated overnight at 37°C with shaking. After extraction with 3.0 ml of a chloroform-methanol solution (4:1), the chloroform layer was dried under vacuum. The products were resuspended in 20 μl of a chloroform-methanol solution (2:1) and spotted on a Silica Gel 60 plate (Whatman), along with 100 μg of PC, 200 μg of LPC, 150 μg of egg yolk glycerophosphorylcholine (GPC; Sigma), 100 μg of diacylglycerol (DAG; Sigma), or 100 μg of phosphatidic acid (Sigma). To increase the amounts of products resulting from the digestion of PC and improve our ability to visualize them after staining, we combined the extracted products of four individually digested samples containing 1 mg of PC each and resolved them in Fig. 4A. Using the solvent system of Matsuzawa and Hostetler (33), we developed the plate to a height of 7 cm from the origin in a chloroform-methanol-water solution (65:35:5), allowed the plate to dry, and developed to 20 cm from the origin in a solution containing heptane-diethylether-formic acid (90:60:4). Iodine staining was used for the visualization of phospholipids and their digestion products.
FIG. 4.
Thin-layer chromatography analysis of McaP phospholipase activity. (A) PC substrate. Lanes 1, unprocessed fatty acids (FA) and DAG. Lane 2, unprocessed PC. Lanes 3 and 4, digestion products of PC incubated with pBR322 or pJTmcaP extracts, respectively. Lanes 5 to 8, unprocessed LPC, GPC, DAG, and phosphatidic acid (PA), respectively. Lanes 5 to 8 also represent the digestion products of PLA, PLB, PLC, and PLD, respectively (as indicated by the brackets). (B) LPC substrate. Lane 1, unprocessed LPC. Lanes 2 and 3, digestion products of LPC incubated with pBR322 or pJTmcaP extracts, respectively. Lanes 4 and 5, products of LPC digestion with commercial PLA2 or PLB. Arrowheads indicate the locations of GPC bands (A) and LPC bands (B). Arrows denote the chloroform-methanol-water solvent front.
Statistical methods.
All statistical analyses were performed using the Mann-Whitney test and GraphPad Prism 2.01 software. P values of <0.05 were considered statistically significant.
Nucleotide sequence accession number.
The nucleotide sequence of the M. catarrhalis O35E mcaP gene has been deposited in GenBank under the accession number AY291294.
RESULTS
Isolation of E. coli recombinant clones expressing a novel M. catarrhalis adhesin.
Previous studies demonstrated that the lack of UspA1 expression considerably reduced the adherence of M. catarrhalis strain O35E to Chang human conjunctival cells, while UspA1 expression by normally nonadherent recombinant bacteria substantially increased binding to these monolayers (1, 32, 47; Holm and Lafontaine, unpublished). We also recently reported that a mutant of M. catarrhalis O35E with a transposon in hag shows greatly decreased binding to A549 human lung cells (Holm and Lafontaine, unpublished). These results imply that UspA1 is the major adhesin for Chang conjunctival cells, whereas Hag is the main adherence factor for A549 lung cells.
While binding was significantly reduced, we observed that the M. catarrhalis uspA1 and hag mutants still attached to Chang and A549 cells. We hypothesized that a molecule that has yet to be identified mediated this adherence. To test this, we constructed a plasmid library of M. catarrhalis DNA fragments, introduced this library into the nonadherent E. coli cloning strain EPI300, and selected for recombinant clones that had gained the ability to bind A549 and Chang cells. To avoid the possibility of isolating clones expressing the previously characterized adhesins UspA1, UspA2, and Hag, the chromosomal DNA used to construct our library was obtained from the uspA1-uspA2-hag mutant O35E.ZCS (47). A total of 6,505 E. coli recombinant clones carrying DNA inserts of 25 to 35 kb were generated. Twenty-five randomly chosen clones were digested with restriction endonucleases and were found to contain random inserts.
Recombinant clones were mixed with A549 or Chang cells and enriched for adherent clones. Using this approach, we independently isolated E. coli recombinant cells that expressed the same adherence factor. Figure 1B illustrates the binding of the representative recombinant clone pELFOS190 to Chang cells. Quantitative assays also revealed that this plasmid considerably increased the binding of E. coli recombinant cells to both Chang and A549 cells (Table 2).
FIG. 1.
Binding of E. coli recombinant cells to Chang monolayers in a visual adherence assay.
Identification and features of the gene product specifying the candidate M. catarrhalis adhesin.
The plasmid pELFOS190 presumably contained the structural gene for an adhesin. This plasmid, however, harbored a 30-kb M. catarrhalis O35E.ZCS DNA fragment. To locate the relevant region of this large clone, we mutagenized pELFOS190 with the EZ::TN <KAN-2> transposon (Epicentre), which contains a Kanr marker for selecting mutants on agar plates. Kanr colonies were directly used to inoculate monolayers of Chang cells in order to perform visual adherence assays. This approach yielded the plasmid pELFOS190TN6 (Fig. 1C), which no longer conferred adherence to human cells (Table 2) and likely contained a transposon in or upstream of the gene encoding the candidate adhesin.
To determine the entire sequence of the disrupted gene, which we designated mcaP for M. catarrhalis adherence protein, we first sequenced out of the transposon with transposon-specific primers and then used “primer walking” to extend this sequence. The candidate mcaP ORF was found to be 1,950 nt in length and predicted to specify a protein of 650 amino acids (aa) with a molecular mass of 71,483 Da. Analysis through the SignalP V1.1 World Wide Web server (www.cbs.dtu.dk/services/SignalP) predicted McaP to contain a signal sequence with a potential cleavage site between aa 50 and 51 (e.g., ATA-Q, where the dash illustrates the cleavage site), based on the algorithm developed by Nielsen and colleagues (44). Interestingly, this putative signal sequence cleavage site resembles that of the M. catarrhalis adhesin UspA1 (ASA-Q [8]). Cleavage of McaP at this position would result in a mature protein with a molecular mass of 66,001 Da. Potential ORFs were also located 109 nt upstream and 149 nt downstream of mcaP, and these ORFs exhibited similarities to biotin synthase (75%) and a hypothetical membrane protein (82%), respectively.
When the predicted amino acid sequence of the McaP protein was analyzed with the Gapped BLAST 2.0 search tool through the National Center for Biotechnology service, the most similar prokaryotic protein was the Moraxella bovis PLB (44% identity and 60% similarity). McaP also appeared to be related to a family of lipolytic enzymes due to the presence of a highly conserved motif, Gly-Asp-Ser-Leu (GDSL) (62), located in the N-terminal region of each family member (residues 60 to 63 in McaP). This group of lipolytic enzymes includes M. bovis PLB (13) and the Pseudomonas aeruginosa surface-bound esterase EstA (64) (29% identical and 42% similar to McaP). Additionally, the C-terminal portion of McaP (aa 371 to 650) exhibited substantial similarity to members of the autotransporter family of proteins (20, 21), more specifically to the predicted integral membrane β-barrel domain that is involved in the transport of these proteins across cell membranes.
Given this similarity to members of the GDSL family, we decided to test whether McaP exhibited lipolytic activity. This was accomplished by growing E. coli recombinant cells on medium supplemented with 1% (vol/vol) Tween 80. This water-soluble substrate yields an insoluble precipitate upon cleavage by a lipolytic enzyme, resulting in easily observed halos around lipolytically active bacteria. Recombinant E. coli cells harboring pELFOS190 had lipolytic activity when grown on Tween 80 agar plates, whereas cells harboring pELFOS190TN6, which contained a transposon in the mcaP ORF, did not (Table 2). Thus, expression of the mcaP gene appeared to confer lipolytic activity.
Cloning and expression of the M. catarrhalis O35E mcaP ORF in E. coli.
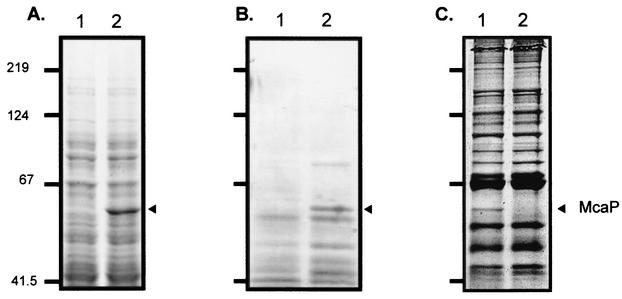
The plasmid pELFOS190TN6 contained a large M. catarrhalis DNA fragment that could specify several gene products. To rule out polar effects and conclusively determine whether McaP functions as an adhesin as well as a lipolytic enzyme, a 2.1-kb amplicon containing the mcaP ORF was cloned and expressed in the nonadherent E. coli cloning strain TOP10. The resulting construct, which was designated pJTmcaP, expressed a distinct 62-kDa protein (Fig. 2A, lane 2) that conferred a 25- to 100-fold increase in adherence to human cells compared to that conferred by the negative vector control pBR322 (Table 2). These results clearly demonstrated that the mcaP gene product corresponded to our candidate adhesin. Furthermore, McaP expression by E. coli cells harboring pJTmcaP conferred lipolytic activity (Table 2). We next tested recombinant E. coli cells for lipolytic activity by using pNPs. In these tests, the lipolytic cleavage of pNPs releases nitrophenol, which can be measured spectrophotometrically. As shown in Fig. 3A, E. coli cells harboring pJTmcaP preferentially cleaved short-chain substrates. In general, esterases can cleave only short-chain substrates (4 to 10 carbons) whereas lipases have a preference for longer-chain substrates (12 to 18 carbons). Even when incubated overnight with long-chain pNPs, E. coli cells harboring pJTmcaP were unable to cleave these substrates. In contrast, Staphylococcus aureus cells, which exhibit lipase activity (50), readily digested these long-chain ester derivatives (data not shown). Thus, McaP may be classified as an esterase.
FIG. 2.
SDS-PAGE analysis of M. catarrhalis strains and E. coli recombinant cells. (A) Whole-cell lysates prepared from E. coli TOP10 recombinant cells harboring the plasmid pBR322 (lane 1) or pJTmcaP (lane 2), resolved by SDS-PAGE, and stained with Coomassie blue. (B) Ten microliters of heat extract prepared from E. coli cells harboring pBR322 (lane 1) or pJTmcaP (lane 2). (C) Proteins present in outer membrane vesicles that were prepared from strain O35E (lane 1) and its isogenic mcaP mutant O35E.M (lane 2). The arrowheads indicate the position at which the mcaP gene product migrates. Positions of molecular mass markers are shown to the left in kilodaltons.
It has been reported elsewhere that autotransporter proteins can be released from bacterial outer membranes upon incubation at 60°C (5, 61). Since sequence analysis suggested that McaP might be an autotransporter, we used this heating method to obtain protein extracts enriched for McaP. The extracts were resolved by SDS-PAGE and stained with Coomassie blue. As shown in lane 2 of Fig. 2B, the extract obtained from E. coli cells harboring pJTmcaP contained a protein of the size expected for McaP. This protein was absent from an extract of E. coli cells containing the plasmid control pBR322 (Fig. 2B, lane 2). These samples were assayed for their ability to cleave pNP substrates, and only the extract enriched for McaP was found to be lipolytically active (data not shown).
Since the bacterial gene product most similar to McaP was the M. bovis PLB, we tested whether McaP functioned as a phospholipase. This was accomplished by using thin-layer chromatography to separate the products that result from digestion of PC with the aforementioned extracts. Lane 4 in Fig. 4A demonstrates that the McaP-enriched extract cleaved PC, while the control extract did not (lane 3 in Fig. 4A). Thus, McaP is a phospholipase.
Phospholipases can be further classified based on the substrate bond(s) that they cleave (53). Figure 4A shows some of the products generated by digestion of PC with different phospholipases. Briefly, digestion with PLA or PLB releases fatty acids (lane 1). Digestion with PLA also results in the production of LPC (lane 5), whereas cleavage by PLB yields GPC (lane 6). Digestion with PLC and PLD yields DAG (lane 7) and phosphatidic acid (lane 8), respectively. Lane 4 in Fig. 4A indicates that digestion of PC with the McaP-enriched extract yielded fatty acids but not DAG or phosphatidic acid. Thus, McaP did not exhibit PLC or PLD activity. The presence of GPC and free fatty acids in the McaP digestion sample, however, indicated that McaP was a PLB (arrowhead in lane 4 of Fig. 4A).
Due to the faintness of the indicated GPC band, we also analyzed the products of LPC digestion by McaP. It should be noted that PLB cleaves LPC whereas PLA does not hydrolyze this substrate. As shown by the arrowheads in Fig. 4B, LPC is present in the products of digestion with commercial PLA2 (lane 4) and the pBR322 control extract (lane 2). The substrate, however, is absent from the digestion products with commercial PLB (lane 5). Therefore, the absence of LPC in the McaP digestion products (lane 3) confirms that McaP cleaved this substrate and has PLB activity.
Construction and characterization of M. catarrhalis isogenic mcaP mutants.
The experiments described above were performed in E. coli. To study the functional properties of McaP in its native host M. catarrhalis, we constructed an isogenic mcaP mutant (designated O35E.M). As shown in lane 2 of Fig. 2C, this mutant lacked expression of a 62-kDa OMP. Western blot analyses also showed that O35E.M expressed wild-type levels of UspA1, UspA2, and Hag (data not shown). To confirm that this 62-kDa OMP corresponded to the mcaP gene product, outer membrane vesicles prepared from M. catarrhalis O35E were resolved by SDS-PAGE. The protein indicated by the arrowhead in Fig. 2C was subjected to in-gel digestion with trypsin. The resulting peptide fragments were sequenced by LC-MS/MS and were found to correspond to peptides spanning the entire length of the deduced amino acid sequence of McaP (Table 3). These results demonstrate that the OMP indicated by the arrowhead in Fig. 2C was McaP.
TABLE 3.
Amino acid sequences of peptides derived from the SDS-PAGE-purified McaP protein from M. catarrhalis O35E
| Sequencea | Corresponding residues in the McaP deduced amino acid sequence |
|---|---|
| LVQIGK | 69-74 |
| SYGHTADANDGTTLTGTNYAVGGAR | 104-128 |
| YLTLNNHQADPK | 159-170 |
| ILAQYYR | 338-344 |
| IDRPTIK | 402-408 |
| HDFDHLR | 452-458 |
| ISANLGVDHLSTQSLR | 459-474 |
| FFENQPNLSTALSFK | 527-541 |
| TVETAVLSNR | 582-591 |
| LNLLAGLGYQHEFK | 564-577 |
As determined by LC-MS/MS.
To study the contribution of McaP to binding of M. catarrhalis to A549 and Chang cells, we compared the adherence of the isogenic mutant O35E.M to that of the wild-type strain O35E. No significant reduction was observed (Fig. 5). One possible explanation for this lack of effect was that other adhesins provided a high background of adherence. To test this hypothesis, we constructed a quadruple mutant (O35E.ZCSM) that did not express McaP or the adhesins UspA1, UspA2, and Hag. The adherence of O35E.ZCSM was then compared to that of the triple isogenic strain O35E.ZCS, which lacked expression of UspA1, UspA2, and Hag but expressed McaP. The mutant O35E.ZCSM exhibited a significant decrease in binding to Chang (Fig. 5A), 16HBE14o−, and NCIH292 as well as HEp-2 cells (Table 4) but not to A549 cells (Fig. 5B). The slight increase in the adherence of O35E.M and O35E.ZCSM to A549 cells was not statistically significant. These results demonstrated that McaP contributed to M. catarrhalis adherence. To verify that this observed loss of adherence of strain O35E.ZCSM was not due to a growth defect, we monitored the growth of O35E, O35E.M, O35E.ZCS, and O35E.ZCSM in broth as well as TC media. We found that all strains grew at the same rate.
Finally, we tested the ability of McaP to function as an esterase in M. catarrhalis by using the chromogenic pNP substrates. The data presented in Fig. 3B clearly show that lack of McaP expression abolished the esterase activity of M. catarrhalis O35E cells. The lack of McaP expression by O35E.ZCSM also abolished the esterase activity of O35E.ZCS cells (data not shown).
DISCUSSION
Although M. catarrhalis is one of the leading causes of otitis media and exacerbations in patients with COPD, little is known about pathogenesis by this gram-negative organism. For many bacteria, adherence plays a crucial role in colonization and the subsequent development of infection (4, 25, 59). To study the repertoire of adherence factors expressed by M. catarrhalis, we used a functional approach that identified the OMP McaP. Even though the enrichment protocol that we used involved two distinct human cell types, virtually all of the adherence-positive E. coli recombinant clones that we tested contained the mcaP gene. Nineteen of these plasmids were digested with restriction endonucleases, confirming that they were not siblings. Since our library was obtained from DNA of cells lacking expression of UspA1, UspA2, and Hag, clones expressing these three adhesins were not expected to be enriched. Furthermore, our plasmid library contained nonoverlapping inserts. Thus, the high frequency of isolation of McaP-expressing recombinant E. coli is not likely the result of a biased library.
Although the enrichment of mcaP clones in nearly all cases does not exclude the existence of additional unidentified M. catarrhalis adhesin(s), our results suggest that clones expressing McaP preferentially bind to A549 and Chang cells in our assays. Interestingly, lack of McaP expression did not appear to decrease the binding of strain O35E.ZCSM to A549 cells (Fig. 5B), even though our data clearly showed that McaP contributes to M. catarrhalis adherence to Chang, 16HBE14o−, HEp-2, and NCIH292 cells (Fig. 5A and Table 4). These observations raise the possibility that M. catarrhalis expresses an adhesin other than UspA1, UspA2, Hag, or McaP, which would be involved in adherence to A549 cells. We are currently testing this hypothesis.
Our present lack of antibodies to McaP limits our ability to conclusively determine whether this protein contains surface-exposed epitopes or to evaluate its level of expression by M. catarrhalis cells. Nevertheless, McaP can be visualized upon Coomassie blue staining of OMPs resolved by SDS-PAGE (lane 2 in Fig. 2C). Furthermore, the esterase activity of whole M. catarrhalis cells conferred by McaP (Fig. 3B) suggests that at least a portion of this protein is surface exposed or exported. This esterase activity toward the substrate tributyrin has been extensively used as a diagnostic tool to differentiate M. catarrhalis from Neisseria sp. (56, 58). The identity of the responsible lipolytic protein, however, was previously unknown. Our data clearly demonstrate that McaP confers this phenotype. Sequence analysis also indicates that McaP may belong to the family of outer membrane autotransporter proteins. Taken together, these observations suggest that McaP is associated with the outer membrane of M. catarrhalis.
In addition to its ability to function as an esterase, McaP is a PLB that cleaves PC as well as LPC. While esterases and phospholipases are both categorized as lipolytic enzymes, their substrate specificities differ, as does the present understanding of their respective contribution to microbial pathogenesis. To the best of our knowledge, little is known about the role of esterases in pathogenesis or adherence. In contrast, the contribution of phospholipases to the development of infections has been delineated to a greater extent, particularly that of PLA and PLC (18, 53, 57). For example, phospholipases contribute to destruction of lung surfactant (16, 23), increased vascular permeability (39), colonization of host tissues (11, 65), stimulation of the inflammatory response (31), and generation of signal transducers (48). Interestingly, the PLB secreted by Candida albicans (PLB1) has been shown to increase invasion and virulence in a mouse model of infection (40). The ability of McaP to function as both a phospholipase and an adhesin led us to question whether it also plays a role in invasion of human cells. However, the expression of McaP by recombinant E. coli did not increase invasion, nor did the isogenic mcaP mutant strain O35E.M exhibit decreased invasion (data not shown). Thus, the adherence phenotype that we observed in our assays is not the result of invasion, which in turn indicates that McaP is indeed an adhesin.
It is tempting to speculate about the connection between the adherence and lipolytic properties of McaP. While one function may be dependent upon the other, it is possible that these two biologically important functions are separable. Our sequence analysis suggests that McaP is a member of the family of autotransporter proteins, classified by their ability to utilize a C-terminal α-barrel to secrete an internal passenger domain across the outer membrane. After reaching the extracellular milieu, the passenger domains are capable of performing one or more virulence-associated functions (20, 21). For example, the Haemophilus influenzae protein Hap functions as a serine protease as well as an adhesin. Although separate domains of the protein confer these activities, they interact in such a way that loss of proteolytic cleavage results in increased adherence to human epithelial cells (22). With this in mind, we are currently investigating the classification of McaP as an autotransporter protein as well as the relationship between its various functions.
This report constitutes the first description of the novel M. catarrhalis adhesin McaP, which also functions as an esterase and a PLB. No other M. catarrhalis lipolytic enzyme has been reported to date, and few microbial PLBs have been characterized, particularly in terms of their contribution to pathogenesis. Considering the frequent classification of adherence and lipolytic activity as virulence factors, further studies of the role of McaP in pathogenesis as well as its vaccinogenic potential are warranted.
Acknowledgments
This study was supported in part by institutional start-up funds from the Medical College of Ohio, a grant from the Thrasher Research Fund (award number 02816-6), and a grant from NIH/NIAID (1 RO1 AI051477-01 A1) to E.R.L.
We thank Eric Hansen at the University of Texas Southwestern Medical Center in Dallas for providing M. catarrhalis strains and antibodies. We also thank Dieter Gruenert and the University of California for providing the human bronchial cell line 16HBE14o−. We also thank Herman Schut, Jieh-Juen Yu, and Chiung Yu-Hung for providing valuable information and materials for the thin-layer chromatography assays. We also thank Eric Hansen, Robert Blumenthal, Herman Schut, Darren Sledjeski, and Mark Wooten for their helpful comments on the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi, C., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 65:4367-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society. 1995. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 152:S77-S121. [PubMed] [Google Scholar]
- 4.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 5.Benz, I., and M. A. Schmidt. 1992. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27). Infect. Immun. 60:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappelletty, D. 1998. Microbiology of bacterial respiratory infections. Pediatr. Infect. Dis. J. 17:S55-S61. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, J. J. 1999. Moraxella (Branhamella) catarrhalis: clinical, microbiological and immunological features in lower respiratory tract infections. APMIS Suppl. 88:1-36. [PubMed] [Google Scholar]
- 8.Cope, L. D., E. R. Lafontaine, C. A. Slaughter, C. A. Hasemann, Jr., C. Aebi, F. W. Henderson, G. H. McCracken, Jr., and E. J. Hansen. 1999. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J. Bacteriol. 181:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cozens, A. L., M. J. Yezzi, K. Kunzelmann, T. Ohrui, L. Chin, K. Eng, W. E. Finkbeiner, J. H. Widdicombe, and D. C. Gruenert. 1994. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 10:38-47. [DOI] [PubMed] [Google Scholar]
- 10.Del Beccaro, M. A., P. M. Mendelman, A. F. Inglis, M. A. Richardson, N. O. Duncan, C. R. Clausen, and T. L. Stull. 1992. Bacteriology of acute otitis media: a new perspective. J. Pediatr. 120:81-84. [DOI] [PubMed] [Google Scholar]
- 11.Dorrell, N., M. C. Martino, R. A. Stabler, S. J. Ward, Z. W. Zhang, A. A. McColm, M. J. Farthing, and B. W. Wren. 1999. Characterization of Helicobacter pylori PldA, a phospholipase with a role in colonization of the gastric mucosa. Gastroenterology 117:1098-1104. [DOI] [PubMed] [Google Scholar]
- 12.Faden, H. 2001. The microbiologic and immunologic basis for recurrent otitis media in children. Eur. J. Pediatr. 160:407-413. [DOI] [PubMed] [Google Scholar]
- 13.Farn, J. L., R. A. Strugnell, P. A. Hoyne, W. P. Michalski, and J. M. Tennent. 2001. Molecular characterization of a secreted enzyme with phospholipase B activity from Moraxella bovis. J. Bacteriol. 183:6717-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald, M., R. Mulcahy, S. Murphy, C. Keane, D. Coakley, and T. Scott. 1997. A 200 kDa protein is associated with haemagglutinating isolates of Moraxella (Branhamella) catarrhalis. FEMS Immunol. Med. Microbiol. 18:209-216. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald, M., R. Mulcahy, S. Murphy, C. Keane, D. Coakley, and T. Scott. 1999. Transmission electron microscopy studies of Moraxella (Branhamella) catarrhalis. FEMS Immunol. Med. Microbiol. 23:57-66. [DOI] [PubMed] [Google Scholar]
- 16.Flieger, A., S. Gongab, M. Faigle, H. A. Mayer, U. Kehrer, J. Mussotter, P. Bartmann, and B. Neumeister. 2000. Phospholipase A secreted by Legionella pneumophila destroys alveolar surfactant phospholipids. FEMS Microbiol. Lett. 188:129-133. [DOI] [PubMed] [Google Scholar]
- 17.Forsgren, A., M. Brant, A. Mollenkvist, A. Muyombwe, H. Janson, N. Woin, and K. Riesbeck. 2001. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J. Immunol. 167:2112-2120. [DOI] [PubMed] [Google Scholar]
- 18.Ghannoum, M. A. 2000. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 13:122-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjorloff Wingren, A., R. Hadzic, A. Forsgren, and K. Riesbeck. 2002. The novel IgD binding protein from Moraxella catarrhalis induces human B lymphocyte activation and Ig secretion in the presence of Th2 cytokines. J. Immunol. 168:5582-5588. [DOI] [PubMed] [Google Scholar]
- 20.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 22.Hendrixson, D. R., and J. W. St. Geme III. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841-850. [DOI] [PubMed] [Google Scholar]
- 23.Holm, B. A., L. Keicher, M. Y. Liu, J. Sokolowski, and G. Enhorning. 1991. Inhibition of pulmonary surfactant function by phospholipases. J. Appl. Physiol. 71:317-321. [DOI] [PubMed] [Google Scholar]
- 24.Hu, W. G., J. Chen, J. C. McMichael, and X. X. Gu. 2001. Functional characteristics of a protective monoclonal antibody against serotype A and C lipooligosaccharides from Moraxella catarrhalis. Infect. Immun. 69:1358-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hultgren, S. J., S. Abraham, M. Caparon, P. Falk, J. W. St. Geme III, and S. Normark. 1993. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell 73:887-901. [DOI] [PubMed] [Google Scholar]
- 26.Karalus, R., and A. Campagnari. 2000. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2:547-559. [DOI] [PubMed] [Google Scholar]
- 27.Klein, J. O. 2000. The burden of otitis media. Vaccine 19(Suppl. 1):S2-S8. [DOI] [PubMed] [Google Scholar]
- 28.Klein, J. O. 1994. Otitis media. Clin. Infect. Dis. 19:823-833. [DOI] [PubMed] [Google Scholar]
- 29.Klein, J. O., D. W. Teele, and S. I. Pelton. 1992. New concepts in otitis media: results of investigations of the Greater Boston Otitis Media Study Group. Adv. Pediatr. 39:127-156. [PubMed] [Google Scholar]
- 30.Klugman, K. P. 1996. The clinical relevance of in-vitro resistance to penicillin, ampicillin, amoxycillin and alternative agents, for the treatment of community-acquired pneumonia caused by Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis. J. Antimicrob. Chemother. 38(Suppl. A):133-140. [DOI] [PubMed] [Google Scholar]
- 31.Kume, N., M. I. Cybulsky, and M. A. Gimbrone, Jr. 1992. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Investig. 90:1138-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuzawa, Y., and K. Y. Hostetler. 1980. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4′-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J. Biol. Chem. 255:5190-5194. [PubMed] [Google Scholar]
- 34.McMichael, J. C. 2000. Progress toward the development of a vaccine to prevent Moraxella (Branhamella) catarrhalis infections. Microbes Infect. 2:561-568. [DOI] [PubMed] [Google Scholar]
- 35.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 19(Suppl. 1):S101-S107. [DOI] [PubMed] [Google Scholar]
- 36.McMichael, J. C., M. J. Fiske, R. A. Fredenburg, D. N. Chakravarti, K. R. VanDerMeid, V. Barniak, J. Caplan, E. Bortell, S. Baker, R. Arumugham, and D. Chen. 1998. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect. Immun. 66:4374-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier, P. S., R. Troller, I. N. Grivea, G. A. Syrogiannopoulos, and C. Aebi. 2002. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine 20:1754-1760. [DOI] [PubMed] [Google Scholar]
- 38.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muckle, C. A., and C. L. Gyles. 1983. Relation of lipid content and exotoxin production to virulence of Corynebacterium pseudotuberculosis in mice. Am. J. Vet. Res. 44:1149-1153. [PubMed] [Google Scholar]
- 40.Mukherjee, P. K., K. R. Seshan, S. D. Leidich, J. Chandra, G. T. Cole, and M. A. Ghannoum. 2001. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology 147:2585-2597. [DOI] [PubMed] [Google Scholar]
- 41.Murphy, T. F. 1996. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol. Rev. 60:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy, T. F., and M. R. Loeb. 1989. Isolation of the outer membrane of Branhamella catarrhalis. Microb. Pathog. 6:159-174. [DOI] [PubMed] [Google Scholar]
- 43.National Heart, Lung, and Blood Institute. 1998. Morbidity & mortality: chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md. http://www.nhlbi.nih.gov/resources/docs/cht-book.htm. [Online.]
- 44.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 45.Nordstrom, T., A. Forsgren, and K. Riesbeck. 2002. The immunoglobulin D-binding part of the outer membrane protein MID from Moraxella catarrhalis comprises 238 amino acids and a tetrameric structure. J. Biol. Chem. 277:34692-34699. [DOI] [PubMed] [Google Scholar]
- 46.Patrick, C. C., A. Kimura, M. A. Jackson, L. Hermanstorfer, A. Hood, G. H. McCracken, Jr., and E. J. Hansen. 1987. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect. Immun. 55:2902-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearson, M. M., E. R. Lafontaine, N. J. Wagner, J. W. St. Gem III, and E. J. Hansen. 2002. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect. Immun. 70:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prokazova, N. V., N. D. Zvezdina, and A. A. Korotaeva. 1998. Effect of lysophosphatidylcholine on transmembrane signal transduction. Biochemistry (Moscow) 63:31-37. [PubMed] [Google Scholar]
- 49.Reddy, M. S., T. F. Murphy, H. S. Faden, and J. M. Bernstein. 1997. Middle ear mucin glycoprotein: purification and interaction with nontypable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol. Head Neck Surg. 116:175-180. [DOI] [PubMed] [Google Scholar]
- 50.Rosenstein, R., and F. Gotz. 2000. Staphylococcal lipases: biochemical and molecular characterization. Biochimie 82:1005-1014. [DOI] [PubMed] [Google Scholar]
- 51.Ruuskanen, O., and T. Heikkinen. 1994. Otitis media: etiology and diagnosis. Pediatr. Infect. Dis. J. 13:S23-S26. [PubMed] [Google Scholar]
- 52.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Schmiel, D. H., and V. L. Miller. 1999. Bacterial phospholipases and pathogenesis. Microbes Infect. 1:1103-1112. [DOI] [PubMed] [Google Scholar]
- 54.Sethi, S., N. Evans, B. J. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465-471. [DOI] [PubMed] [Google Scholar]
- 55.Sethi, S., and T. F. Murphy. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh, S., K. M. Cisera, J. D. Turnidge, and E. G. Russell. 1997. Selection of optimum laboratory tests for the identification of Moraxella catarrhalis. Pathology 29:206-208. [DOI] [PubMed] [Google Scholar]
- 57.Songer, J. G. 1997. Bacterial phospholipases and their role in virulence. Trends Microbiol. 5:156-161. [DOI] [PubMed] [Google Scholar]
- 58.Speeleveld, E., J. M. Fossepre, B. Gordts, and H. W. Van Landuyt. 1994. Comparison of three rapid methods, tributyrine, 4-methylumbelliferyl butyrate, and indoxyl acetate, for rapid identification of Moraxella catarrhalis. J. Clin. Microbiol. 32:1362-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.St. Geme, J. W., III. 1997. Bacterial adhesins: determinants of microbial colonization and pathogenicity. Adv. Pediatr. 44:43-72. [PubMed] [Google Scholar]
- 60.Stool, S. E., and M. J. Field. 1989. The impact of otitis media. Pediatr. Infect. Dis. J. 8:S11-S14. [PubMed] [Google Scholar]
- 61.Torres, A. G., N. T. Perna, V. Burland, A. Ruknudin, F. R. Blattner, and J. B. Kaper. 2002. Characterization of Cah, a calcium-binding and heat-extractable autotransporter protein of enterohaemorrhagic Escherichia coli. Mol. Microbiol. 45:951-966. [DOI] [PubMed] [Google Scholar]
- 62.Upton, C., and J. T. Buckley. 1995. A new family of lipolytic enzymes? Trends Biochem. Sci. 20:178-179. [DOI] [PubMed] [Google Scholar]
- 63.Wang, H., and B. C. Dowds. 1993. Phase variation in Xenorhabdus luminescens: cloning and sequencing of the lipase gene and analysis of its expression in primary and secondary phases of the bacterium. J. Bacteriol. 175:1665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilhelm, S., J. Tommassen, and K. E. Jaeger. 1999. A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J. Bacteriol. 181:6977-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziprin, R. L., C. R. Young, J. A. Byrd, L. H. Stanker, M. E. Hume, S. A. Gray, B. J. Kim, and M. E. Konkel. 2001. Role of Campylobacter jejuni potential virulence genes in cecal colonization. Avian Dis. 45:549-557. [PubMed] [Google Scholar]