Abstract
To evaluate the role of major histocompatibility complex (MHC) genes in the resistance to Cryptococcus neoformans, we conducted infection experiments in MHC-congenic strains of mice. Significant MHC-dependent susceptibility differences were found among homozygotes and heterozygotes. This study is the first experimental demonstration of MHC-dependent susceptibility to C. neoformans infections in mice and indicates that MHC genes can be important in host resistance.
Major histocompatibility complex (MHC) genes are unique in their general importance for conferring susceptibility or resistance to infectious and autoimmune diseases (5). It is unclear what keeps these demonstrably harmful genes from being eliminated by natural selection unless they provide some advantage, presumably against some other disease. The only way to unravel these interactions is to characterize the effects of MHC genes on a variety of pathogens and autoimmune diseases. The goal of this study was to experimentally test for an MHC-dependent susceptibility pattern during chronic Cryptococcus neoformans infections.
C. neoformans is a common, opportunistic pathogen that causes disease in immunocompromised individuals. Previous studies have found that various components of immunity are important in clearing a C. neoformans infection. These components include interleukin-12 (11), interleukin-18 (17), inducible nitric oxide synthase (2), gamma interferon (16), B cells (3), and T cells (7). Previous attempts to determine if MHC genes influence C. neoformans infections in mice (22) and humans (20) have had conflicting results.
MHC-congenic mice (C57BL/10SnJ-H-2b, B10.D2-H-2d, B10.M-H-2f, B10.BR-H-2k, B10.Q-H-2q) and BALB/c mice were obtained from Jackson Laboratories and bred thereafter under specific-pathogen-free conditions. The inclusion of BALB/c mice allowed a comparison between strains that have identical MHC genes but differ for other genes that may affect resistance to C. neoformans. All animals used for the infection experiments were either F2 segregants or first-generation progeny of F2 segregants. MHC F2 segregants were created by intercrossing the F1 heterozygotes (b/q, d/q, d/k, f/k) in order to randomize any genetic mutations that might have become differentially fixed in the backgrounds of these strains of mice. The differential accumulation of mutations in congenic strains separated by a substantial period of time (for example, over 30 years for these MHC B10 congenics) is a serious problem that can lead to erroneous conclusions (reference 8 and references therein). Generating F2 segregants randomizes any background mutations and therefore solves this problem in a much simpler way than rederiving the lines (8, 13, 25). Mice were MHC genotyped by PCR by means of two different microsatellite loci within the MHC region (a tetranucleotide repeat [23] and d17Mit34 [6]). These reactions were analyzed with denaturing gel electrophoresis, and the genotypes were scored by band size. All animal use complied with federal regulations and the guidelines of the University of Utah's Institutional Animal Care and Use Committee.
Infection experiments were conducted five times, with five of the seven genotypes being tested three or more times. (BALB/c and d/k mice were tested twice.) Mice were infected via intraperitoneal injection with 2 × 107 CFU of the wild-type H99 strain of C. neoformans (21) per ml. An error caused inocula for experiments 3 and 4 to be 4 × 108 and 3 × 106 CFU/ml, respectively. These dosage differences did not result in any significant load differences for female mice and were not correlated with final loads in males. Initially, the spleen, liver, and brain were collected to determine which organ had the most consistent loads of C. neoformans. Brain loads varied widely during this chronic infection, while spleen and liver loads were highly correlated. The liver was chosen as the optimal organ for collection because it is a site of primary infection with an intraperitoneal injection and because it had consistent loads. Thus, the liver was the only organ collected in subsequent infection experiments. Although our results could be considered liver specific, MHC-dependent clearance generally operates in the same way in different tissues, as, for example, with numbers of Salmonella organisms cleared from the spleen and liver (14).
Mice were sacrificed 39 days postinfection. This end date was chosen because the chronic infection needed to proceed long enough for the MHC genes to have an effect but not long enough to risk death of the mice. At the time of sacrifice, there were no obvious signs of clinical disease, as measured by significant weight loss and neurological symptoms, though some of the mice had ruffled fur and many had lost 5% of their body weight. C. neoformans loads were determined from platings of homogenized livers. Briefly, the livers were collected under antiseptic conditions and then homogenized in 10 ml of phosphate-buffered saline. Ten microliters of the homogenate was diluted in 90 μl of phosphate-buffered saline in serial dilutions to 10−5. For each dilution, 10 μl was plated on yeast extract-peptone-glucose agar containing 10,000 U of penicillin/ml, 1 mg of gentamicin/ml, and 0.1 mg of chloramphenicol/ml. Plates were incubated at 35°C for 36 h, after which time C. neoformans colonies from two dilutions were counted and averaged.
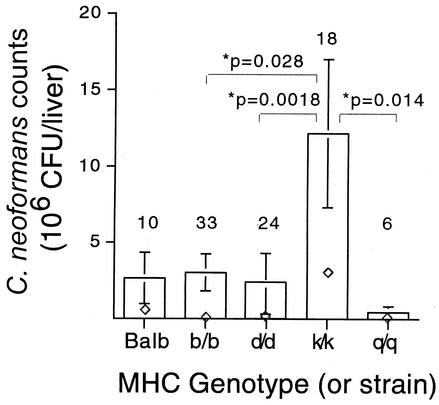
The loads from female mice across the five infection experiments were similar, so the data were pooled. Figure 1 depicts the C. neoformans loads for five different strains of mice. The MHC-congenic strains with haplotypes b/b, d/d, and q/q and BALB/c mice were relatively resistant, while k/k mice were relatively susceptible (P < 0.028). Though male mice were also infected, the loads were significantly different between experiments (analysis of variance, P = 0.001) and relative susceptibilities between genotypes were sometimes reversed, making interpretation difficult (data not shown).
FIG. 1.
Female MHC-dependent susceptibility to C. neoformans in F2 segregants. Counts are mean numbers of CFU per whole liver ± standard errors. ⋄, median values. Sample sizes are indicated above each bar. All P values are from a Wilcoxon rank sum test. Results from all other comparisons were insignificant.
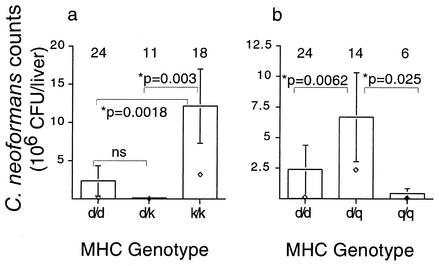
To evaluate resistance patterns in heterozygotes, four MHC heterozygotes (b/d, b/q, d/k, and d/q) were also infected. Unfortunately, three heterozygote combinations (b/d, b/q, and d/q) were uninformative with respect to dominant or recessive patterns because the loads among the b/b, d/d, and q/q homozygotes were not significantly different (Fig. 1). In the case where the loads of the d/d and k/k homozygotes were significantly different (Fig. 1), the d/k heterozygote inherited resistance in a dominant fashion (Fig. 2a). In addition, the d/q heterozygote displayed a pattern of underdominance where the heterozygote did worse than either homozygote (heterozygote disadvantage) (Fig. 2b).
FIG. 2.
Resistance to C. neoformans in F2 segregants is inherited in both a dominant (a) and an underdominant (b) fashion. Counts are mean numbers of CFU per whole liver ± standard errors. ⋄, median values. Sample sizes are indicated above each bar. All P values are from a Wilcoxon rank sum test. Results from all other comparisons were insignificant.
C. neoformans loads in female MHC-congenic mice showed that the k/k genotype was 4- to 10-fold more susceptible than three other MHC genotypes and the BALB/c strain (H-2d) (P < 0.028). Previous studies have disagreed on the role of MHC in C. neoformans infections. Two studies which assayed humoral responses in mice (12) and disease correlations with the HLA phenotype in humans (24) suggested that the MHC genotype influenced the response to a C. neoformans infection. However, two studies that assayed T-cell proliferation (20) and survival in mice (22) suggested that these measures of the immune response were not influenced by the MHC genotype. The study that assayed T-cell proliferation (20) was not very comparable to our study because a different strain of C. neoformans (an acapsular mutant) was used in an in vitro assay with human T lymphocytes.
The study by Rhodes et al. (22) is the one most comparable to our study because MHC differences in B10-congenic strains were examined. However, their failure to find an MHC effect is inconsistent with both our data and the MHC-dependent humoral responses found by Dromer et al. (12). Possible explanations for this inconsistency include the following factors: (i) survival was assayed rather than pathogen loads, (ii) a different strain of C. neoformans was used, and (iii) B10 k/k mice were not infected, and B10k/k is the only MHC genotype that showed significant susceptibility according to our data. However, two recombinant strains [B10.A and B10.A (2R)] which express k alleles at the class I K locus and both class II loci were infected. These two strains showed no significant pattern of susceptibility. These data combined with our own suggest that the Dk allele may confer susceptibility to C. neoformans infection. This involvement of a class I gene is consistent with evidence that Th1 and cellular immunity are important in clearing C. neoformans infections (1).
Our results might seem inconsistent with the results of Dromer et al. (12), where the k haplotype was a high antibody responder. However, high antibody titers are difficult to interpret because they can have opposite implications with regard to immunocompetence: they may indicate either high responsiveness or trouble clearing the infection. A Th2 response to a C. neoformans infection often results in high antibody titers and a lack of clearance, leading to a chronic infection (18).
How resistance is inherited (dominant or recessive) appears to be haplotype dependent (Fig. 2). These different allele-specific dominant or recessive patterns are not unique as similar findings have been seen for both Streptococcus pyogenes (9) and Salmonella enterica serovar Typhimurium (19). The molecular basis of these differences is presumably due to the influence of the MHC genotype on which T-cell epitope becomes immunodominant. The relatively rare pattern of underdominance seen in the d/q heterozygotes might arise if the important immunodominant T cells of the homozygotes are deleted during thymic education of the heterozygote. T cells used by one MHC allele can be deleted during negative selection in the thymus when another MHC allele is present (15). Alternatively, the addition of new MHC specificities can alter which T-cell epitope becomes immunodominant (26).
MHC molecules are important in disease resistance for many infectious agents, including other fungal pathogens such as Candida albicans (4, 10). This study is the first demonstration that MHC can have a role in the clearance of C. neoformans infections and suggests that MHC-mediated immune recognition can be an important variable in susceptibility to C. neoformans infections. However, it must be noted that, as is the case with all studies using MHC-congenic strains, an observed effect may be due to other genes linked to the MHC.
Acknowledgments
We thank Linda Morrison, Kyle Gardner, and Maureen Wilkinson for technical help and mouse husbandry.
This work was supported by an NIH grant to W.K.P. (GM39578) and partially by a Technology Innovation grant to D.L.G. from the University of Utah.
Editor: T. R. Kozel
REFERENCES
- 1.Abrahams, I. 1966. Further studies on acquired resistance to murine cryptococcosis: enhancing effect of Bordetella pertussis. J. Immunol. 96:525-529. [PubMed] [Google Scholar]
- 2.Aguirre, K. M., and G. W. Gibson. 2000. Differing requirement for inducible nitric oxide synthase activity in clearance of primary and secondary Cryptococcus neoformans infection. Med. Mycol. 38:343-353. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre, K. M., and L. L. Johnson. 1997. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect. Immun. 65:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashman, R. B. 1987. Mouse candidiasis. II. Host responses are T-cell dependent and regulated by genes in the major histocompatibility complex. Immunogenetics 25:200-203. [DOI] [PubMed] [Google Scholar]
- 5.Baum, H., and N. A. Staines. 1997. MHC-derived peptides and the CD4+ T-cell repertoire: implications for autoimmune disease. Cytokines Cell. Mol. Ther. 3:115-125. [PubMed] [Google Scholar]
- 6.Blake, J. A., J. E. Richardson, C. J. Bult, J. A. Kadin, and J. T. Eppig. 2002. The Mouse Genome Database (MGD): the model organism database for the laboratory mouse. Nucleic Acids Res. 30:113-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan, K. L., and H. A. Doyle. 2000. Requirement for CD4+ T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect. Immun. 68:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll, L. S., and W. K. Potts. 2001. Accumulated background variation among H2 mutant congenic strains: elimination through PCR-based genotyping of F2 segregants. J. Immunol. Methods 257:137-143. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. Y., S. A. Cohen, M. B. Zaleski, and B. Albini. 1992. Genetic control of streptococcus-induced hepatic granulomatous lesions in mice. Immunogenetics 36:28-32. [DOI] [PubMed] [Google Scholar]
- 10.Costantino, P. J., N. F. Gare, and J. R. Warmington. 1995. Humoral immune responses to systemic Candida albicans infection in inbred mouse strains. Immunol. Cell Biol. 73:125-133. [DOI] [PubMed] [Google Scholar]
- 11.Decken, K., G. Köhler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dromer, F., P. Yeni, and J. Charreire. 1988. Genetic control of the humoral response to cryptococcal capsular polysaccharide in mice. Immunogenetics 28:417-424. [DOI] [PubMed] [Google Scholar]
- 13.Gerlai, R. 1996. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 19:177-181. [DOI] [PubMed] [Google Scholar]
- 14.Hormaeche, C. E., K. A. Harrington, and H. S. Joysey. 1985. Natural resistance to salmonellae in mice: control by genes within the major histocompatibility complex. J. Infect. Dis. 152:1050-1056. [DOI] [PubMed] [Google Scholar]
- 15.Kappler, J. W., N. Roehm, and P. Marrack. 1987. T cell tolerance by clonal elimination in the thymus. Cell 49:273-280. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami, K., Y. Kinjo, S. Yara, Y. Koguchi, K. Uezu, T. Nakayama, M. Taniguchi, and A. Saito. 2001. Activation of Vα14+ natural killer T cells by α-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect. Immun. 69:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami, K., Y. Koguchi, M. H. Qureshi, Y. Kinjo, S. Yara, A. Miyazato, M. Kurimoto, K. Takeda, S. Akira, and A. Saito. 2000. Reduced host resistance and Th1 response to Cryptococcus neoformans in interleukin-18 deficient mice. FEMS Microbiol. Lett. 186:121-126. [DOI] [PubMed] [Google Scholar]
- 18.Lovchik, J. A., J. A. Wilder, G. B. Huffnagle, R. Riblet, C. R. Lyons, and M. F. Lipscomb. 1999. Ig heavy chain complex-linked genes influence the immune response in a murine cryptococcal infection. J. Immunol. 163:3907-3913. [PubMed] [Google Scholar]
- 19.McClelland, E. E., D. J. Penn, and W. K. Potts. 2003. Major histocompatibility complex heterozygote superiority during coinfection. Infect. Immun. 71:2079-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody, C. H., C. J. L. Wood, R. M. Syme, and J. C. Spurrell. 1999. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect. Immun. 67:936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perfect, J. R., S. D. Lang, and D. T. Durack. 1980. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am. J. Pathol. 101:177-194. [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes, J. C., L. S. Wicker, and W. J. Urba. 1980. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect. Immun. 29:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha, B. K., and S. E. Cullen. 1986. Molecular mapping of murine I region recombinants: crossing over in the E beta gene. J. Immunol. 136:1112-1119. [PubMed] [Google Scholar]
- 24.Van Dam, M. G., R. A. Seaton, and A. J. Hamilton. 1998. Analysis of HLA association in susceptibility to infection with Cryptococcus neoformans var. gattii in a Papua New Guinean population. Med. Mycol. 36:185-188. [PubMed] [Google Scholar]
- 25.Wolfer, D. P., and H. P. Lipp. 2000. Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp. Physiol. 85:627-634. [PubMed] [Google Scholar]
- 26.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]