Abstract
The gram-negative enteric pathogen Yersinia pseudotuberculosis employs a type III secretion system and effector Yop proteins that are required for virulence. Mutations in the type III secretion-translocation apparatus have been shown to cause defects in colonization of the murine cecum, suggesting roles for one or more effector Yops in the intestinal tract. To investigate this possibility, isogenic yop mutant strains were tested for their ability to colonize and persist in intestinal and associated lymph tissues of the mouse following orogastric inoculation. In single-strain infections, a yopHEMOJ mutant strain was unable to colonize, replicate, or persist in intestinal and lymph tissues. A yopH mutant strain specifically fails to colonize the mesenteric lymph nodes, but yopE and yopO mutant strains showed only minor defects in persistence in intestinal and lymph tissues. While no single Yop was found to be essential for colonization or persistence in intestinal tissues in single-strain infections, the absence of both YopH and YopE together almost eliminated colonization of all tissues, indicating either that these two Yops have some redundant functions or that Y. pseudotuberculosis employs multiple strategies for colonization. In competition infections with wild-type Y. pseudotuberculosis, the presence of wild-type bacteria severely hindered the ability of the yopH, yopE, and yopO mutants to persist in many tissues, suggesting that the wild-type bacteria either fills colonization niches or elicits host responses that the yop mutants are unable to withstand.
Yersinia pseudotuberculosis is a gram-negative enteric pathogen that causes gastroenteritis and mesenteric lymphadenitis and can spread systemically in humans and other mammals (31, 49, 60). Infection with Y. pseudotuberculosis usually occurs by ingestion of contaminated food (predominantly pork and milk products) or water (6, 11, 31, 49). Early after oral inoculation of a mouse, Y. pseudotuberculosis is found in the intestines, the lymph follicles called Peyer's patches (PP) that line the small intestine, and the mesenteric lymph nodes (MLN). In general, the bacteria are found extracellularly (63), but the bacteria reach the PP by binding, invading, and traversing through specialized M cells that overlay the PP (3, 13, 38). Late in infection (3 to 5 days postinfection), bacteria can be isolated from the spleen and liver in infection that usually leads to fatality in mice.
All three pathogenic Yersinia species, the enteric pathogens Y. pseudotuberculosis and Y. enterocolitica and the causative agent of plague, Y. pestis, share a tropism for lymph tissues and a 70-kb virulence plasmid called pYV that is essential for virulence (24, 52, 53). The pYV plasmid encodes a type III secretion system and effector proteins called Yops. The type III secretion system functions to secrete Yops from the bacterial cytoplasm and translocate them into mammalian cells, where they interact with specific host targets, altering host cell function. YopB and YopD are essential for translocation of the effector Yops into mammalian cells but not for secretion of effector Yops into the extracellular milieu (28, 48, 70).
In general, Yersinia Yops are thought to modulate the host immune defenses and allow bacteria to replicate extracellularly in lymph tissues and organs (14, 63). At least six effector Yops have been identified in Yersinia: YopH, YopE, YopM, YopO (YpkA), YopJ (YopP in Y. enterocolitica), and YopT. Biochemical activities, host protein targets, and effects on cultured cells have been described for most Yops. For instance, YopH, YopE, YopO, and YopT play roles in preventing phagocytosis of the bacteria by macrophages, neutrophils, and/or epithelial cells; however, the deletion of any one of these Yops does not eliminate the antiphagocytic activity of the bacteria (1, 2, 5, 18, 26, 55-57). YopE and YopT cause cytotoxicity of epithelial cells (5, 32). Finally, YopJ induces apoptosis of macrophages (43, 45, 59). YopH is a tyrosine phosphatase (9, 27) which targets many proteins, leading to a variety of different effects in cultured cells. YopH localizes to focal adhesions and affects integrin signaling, thereby preventing phagocytosis (1, 2, 18, 50, 51, 55). YopH also affects the oxidative burst of macrophages (7) and inhibits T- and B-cell signaling (71), T-cell proliferation (61), and Ca2+ signaling in neutrophils (2). YopE is a Rho/Rac GTPase-activating protein (GAP) that destabilizes actin filaments and thereby prevents phagocytosis (5, 26, 56, 58, 69). YopT inactivates RhoA (74); however, YopT is not produced in all Y. pseudotuberculosis strains, including the one used in the study described here (51). YopO affects the actin cytoskeleton of epithelial cells (16, 28, 34) and binds to Rho and Rac in either the GTP- or GDP-bound state (4, 28). However, the cellular targets of YopO and its exact mechanism and function in virulence are still unknown. YopM is the only Yop that localizes to the nucleus of eukaryotic cells after translocation (35, 64) and was recently found to interact with two mammalian kinases, PRK2 and RSK1 (39).
Mutations in most of the Yops of Yersinia spp. attenuate virulence in animal infections. In most cases, the roles of the Yops in virulence were identified by assaying for the ability of the yop mutant strain to cause death of infected mice. Such experiments identified YopH, YopE, YopO, YopJ, and YopM as virulence factors (9, 10, 19, 23, 36, 44, 46). Other studies have analyzed the ability of yop mutants to colonize specific tissues after oral infection. Following oral infection, yopE and yopO mutant strains colonize the PP early in infection but the mutants fail to survive to day 4 or colonize the spleen (5, 22, 23, 30, 65). Additionally, a yopJ mutant shows decreased colonization of the spleen and MLN at 4 days after oral infection (44). However, the considerable variation in how the animal experiments were conducted (i.e., different strains of inbred mice, different Yersinia species, and different routes of infection) makes it difficult to compare the relative importance of each Yop for colonization and persistence of the bacteria in various tissues.
A role for the Yops in the gastrointestinal (GI) tissues was revealed by a previous signature-tagged mutagenesis study (40). Mutants with defects in the plasmid-encoded type III secretion system (ysc) were outcompeted by wild-type bacteria for colonization of the cecum. However, these results did not indicate which, if any, of the known effector Yops are important for survival in the GI tract. It is conceivable that the type III secretion apparatus also transports other factors or has some role in addition to that of protein translocation. In this study, we have used a set of isogenic yop mutant strains in two types of mouse infections with BALB/c mice to determine the importance of YopE, YopH, YopO, and YopB in the colonization of the GI tract and lymph tissues and in causing weight loss and other signs of disease. Using single yop mutant strains, we determined that YopH is crucial for colonization of the MLN and that YopE and YopO play more modest roles in persistence in the ileum and PP. Using multiple-yop mutant strains, we found that YopH and YopE play essential roles in survival within the GI tract and lymph tissues within 48 h after infection. Additionally, we analyzed the effects of changes in dosage and the presence of wild-type bacteria on the ability of yop mutants to colonize, replicate, and persist.
MATERIALS AND METHODS
Bacterial strains.
Escherichia coli SM10 λpir and SY327 λpir were grown in L (10 g of tryptone peptone, 5 g of yeast extract, and 5 g of NaCl per liter) broth or plates at 37°C. Y. pseudotuberculosis strains were grown in L or Luria-Bertani broth (10 g of tryptone peptone, 5 g of yeast extract, and 10 g of NaCl per liter) or on L plates at 26°C. Two wild-type Y. pseudotuberculosis strains were used in this work: one unmarked mouse-passaged strain (YPIII pIB1) and an isogenic kanamycin-resistant (Kanr) strain that carries a kanamycin marker in a neutral site on the chromosome with homology to tonB (40). The Kanr strain is as virulent as its unmarked parent in single-strain and competition infections (data not shown and see Fig. 4).
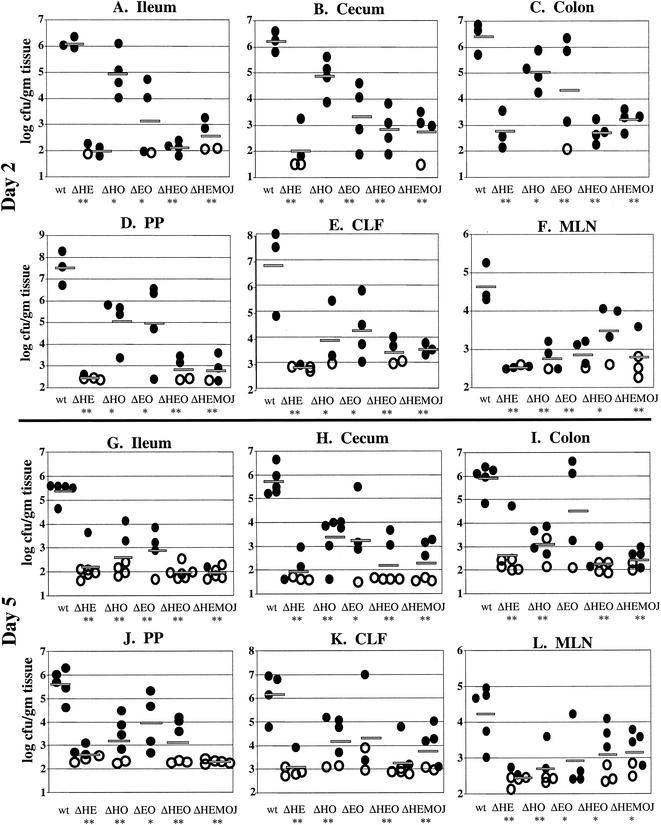
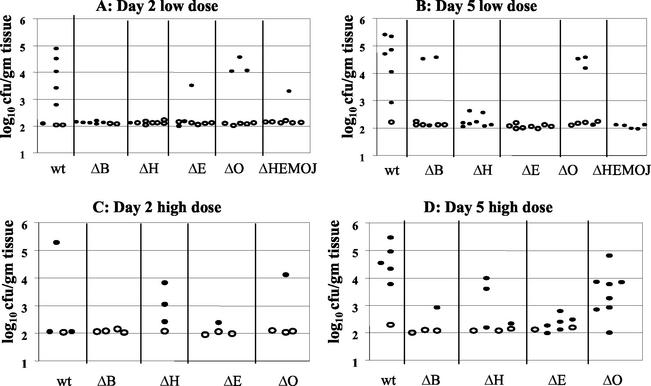
FIG. 4.
Colonization of intestinal and lymph tissues at day 2 and day 5 postinfection with 2 × 108 CFU of wild-type, yopHE (ΔHE), yopHO (ΔHO), yopEO (ΔEO), yopHEO (ΔHEO), or yopHEMOJ (ΔHEMOJ) Y. pseudotuberculosis. Data from four to six mice from two to three experiments were pooled. Each symbol represents the log10 CFU/gram of tissue for the tissue sample from one mouse. Shaded bars represent the average log10 CFU/gram of tissue. Open symbols indicate that less than 10 CFU were recovered/tissue. Asterisks indicate statistical significance of difference between colonization levels of yop mutants and wild-type bacteria. *, P = 0.1 to 0.01; **, P < 0.01.
Bacterial mutants.
Isogenic, unmarked yop deletions were constructed by deleting the majority of the coding region of each yop protein. The yopE deletion eliminated the yopE and sycE promoters and the first 175 residues of the YopE protein. The yopH deletion has previously been described (71). yopB and yopO are both carried in polycistronic operons; therefore, in-frame deletions of the coding regions were constructed. For these deletions, the initial 18 residues for yopB and 20 residues for yopO and the last 55 residues for yopB and 20 residues for yopO were not deleted to ensure expression of the downstream genes (yopD and yopJ, respectively). The yop deletions were constructed using the suicide vector pCVD442 by allelic exchange (15). Fragments containing 250 to 500 bp of flanking regions on each side of the deletion were constructed by PCR with primer pair 1 and 2 and primer pair 3 and 4 (Table 1) and were cloned into pCVD442 by using SalI, SphI, and SacI for the yopB and yopO strains and SalI, SacI, and SphI for the yopE strain. Plasmids were originally isolated in E. coli SY327, introduced into E. coli SM10 λpir, and then introduced into Y. pseudotuberculosis YPIII pYV (pIBI) by conjugation, as previously described (41).
TABLE 1.
Primers used to construct yop deletions
| Primer | Sequence |
|---|---|
| YopE 1 | GCACATGTCGACGAGCGTTGTATCTAATCCTG |
| YopE 2 | CGTCAGGAGCTCAGCATCCTGTCGGGCAAATA |
| YopE 3 | ACTGACGAGCTCGTATTCCCTTCTCGCAGTGG |
| YopE 4 | CTGATGCCCGGGCGAGTGCTGCATCAATCCATAG |
| YopO 1 | TCATGCGTCGACTCACATCCATTCCCGCTC |
| YopO 2 | GACGATGCATGCCACATAAGCACCTGGAAACGC |
| YopO 3 | CGTTACGCATGCAAGATTAGGATGTTTGCCCGC |
| YopO 4 | CTGCATGAGCTCGAGCGCATCAGCCAACATTGG |
| YopB 1 | CTATGCGTCGACTACGAGGATGCTCACAAGGTC |
| YopB 2 | AGCTGCGCATGCAGGCGCTGGTGTCTCGAC |
| YopB 3 | GTACGTGCATGCAAGGCAGACATGGCAGCG |
| YopB 4 | ATCTGCGAGCTCTTCTCGCGCTTTACGTGCCAG |
| YopM 1 | GTACCGTCGACTCAGCAGTAATACATTGGAC |
| YopM 2 | AGCGTGCATGCGACTGTAGATACATTTCTTGGATTTATG |
| YopM 3 | CAGTCGCATGCACGCTTTTGAGTAGTACGCAAGAG |
| YopM 4 | GATCCGAGCTCTGGTTCGTCCAGAAAAACG |
To confirm loss of the cloning vector and normal maintenance of the pIBI virulence plasmid, colonies were tested for resistance to ampicillin and irgasan and for their phenotypes on plates containing Congo red. Yersinia strains are naturally resistant to irgasan, while E. coli is sensitive to the antibiotic. The loss of the pYV virulence plasmid results in white colonies on L plates containing 0.005% Congo red, 20 mM sodium oxalate, and 20 mM magnesium chloride (54). Colonies that were Amps, Irgr, and red on plates containing Congo red were analyzed by PCR and Southern blotting to determine whether they had acquired the appropriate deletions. Yop secretion was tested using type III secretion-inducing conditions by growing strains in low-Ca2+medium containing the Ca2+ chelator sodium oxalate (20 mM) and MgCl2 (20 mM) for 2 h at 26°C followed by 2 h at 37°C, by trichloroacetic acid precipitation of the supernatants and by analysis of secreted proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (19). Additionally, the deletion strains were tested for growth defects at both 26 and 37°C in L broth, low-Ca2+ medium (20 mM Na oxalate, 20 mM MgCl2), and high-Ca2+ medium (5 mM CaCl2) and were found to behave similarly to the wild-type strain.
Strains were further analyzed in cell culture assays, including assays for bacterial uptake (gentamicin protection assay), host cell cytotoxicity, and tyrosine phosphatase activity that have been previously described (41, 45, 73), to verify that mutant strains behaved as expected. For instance, the yopH mutant strain does not produce YopH, and thus, HeLa cells infected with the yopH stain do not exhibit elevated tyrosine phosphatase activity; however, the strain still produces all other Yops and thus behaves like the wild-type strain in assays for other Yop activities. The deleted yopH was replaced with a wild-type copy of yopH. This rescued strain was able to compete with wild-type Y. pseudotuberculosis in competition experiments (data not shown and see Fig. 6).
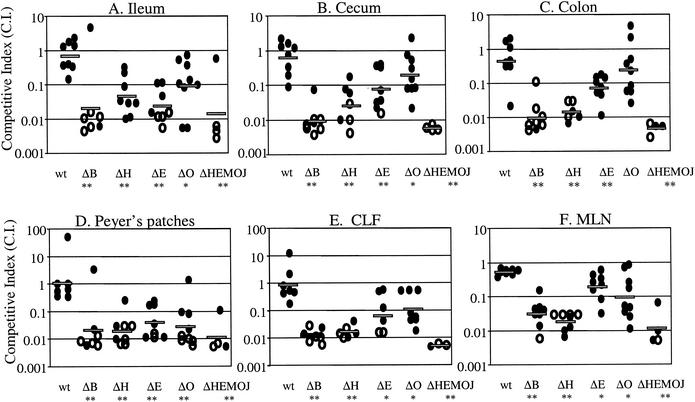
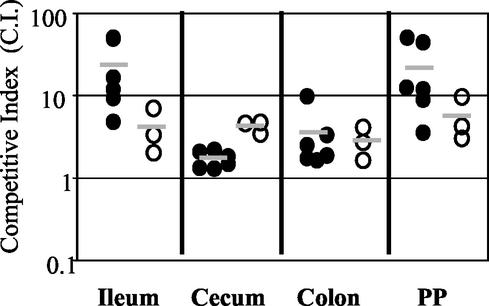
FIG. 6.
Competition between wild-type Y. pseudotuberculosis and yop mutants at 5 days postinfection with 2 × 109 CFU of a mixture of equal amounts of the wild-type strain and either yopB, yopH, yopE, yopO, or yopHEMOJ. Data from six to eight mice from three to four experiments were pooled. Each point represents the C.I. [(mutant/wild-type output ratio)/(mutant/wild-type input ratio)] value for the tissue sample from one mouse. Shaded bars represent the geometric means of the C.I. values. Open symbols indicate that the yop mutant was below the limit of detection, which was usually 200 bacteria. Asterisks indicate the yop mutants that were significantly outcompeted by wild-type bacteria (*, P = 0.1 to 0.01; **, P < 0.01).
The multiple-yop mutant strains were constructed by allelic exchange using the appropriate pCVD442 deletion constructs and sequentially adding additional mutations to the Yersinia strains.
Mouse infections.
Female BALB/c mice (Taconic, Germantown, N.Y.) (7 to 8 weeks old) were used for all animal experiments. Mice were subjected to fasting for 16 h prior to infection; meanwhile, bacteria were grown in Luria-Bertani broth for 16 h to stationary phase. Values of optical density at 600 nm were used to determine cell density, and cultures were adjusted to an appropriate concentration in sterile phosphate-buffered saline. Mice were inoculated with 0.2 ml of bacteria orogastrically through a 20-gauge feeding needle, after which mice were provided with food and water ad libitum. Bacteria were plated on L plates containing irgasan (2 μg/ml) to determine the actual dose administered. For competition experiments, plated bacteria (input) were counted and then patched or replica plated onto kanamycin plates (50 μg/ml) to determine the ratio of mutant (Kans) to wild-type (Kanr) bacteria in the inoculum. At 1, 2, or 5 days postinfection, mice were sacrificed by CO2 asphyxiation and tissues were harvested and placed into preweighed tubes with 1 ml of sterile phosphate-buffered saline-15% glycerol. For most experiments, harvested tissues included the spleen, the MLN, the cecal lymph follicle (CLF) located at the apex of the cecum, PP, and the intestinal contents of the ileum (terminal third of the small intestine), the cecum, and the ascending colon (first third of the large intestine). To collect intestinal contents, intestines were cut into pieces approximately 1 inch long and, using forceps, contents were squeezed into the collection tube. Tissues were weighed and then mechanically homogenized using a Tissue Tearor apparatus (Biospec Products Inc., Bartlesville, Okla.). Dilutions of tissue homogenate were plated on L plates containing 2 μg of irgasan/ml to determine CFU/gram of tissue. For most experiments, two to four mice were infected with each Y. pseudotuberculosis strain being tested and experiments were repeated two to four times.
For single-strain infections, all data were transformed logarithmically and expressed in graphs as log10 CFU/gram of tissue. Averages, ratios, and P values were determined from the logarithmically transformed values. When less than 1 bacterium was recovered at a dilution of 10−1, a value of 1 CFU was used to determine the minimal CFU/gram of tissue. P values were determined by a two-tailed, unpaired Student's t test by comparing colonization by the wild-type bacteria to colonization by the yop mutants. Data with P values of <0.01 were considered to be statistically significant.
For competition experiments, after determination of the total CFU/gram of tissue (mutant and wild-type bacteria), colonies were patched or replica plated onto kanamycin plates to determine the ratio of mutant (Kans) to wild-type (Kanr) bacteria in each tissue (output). Data for the competition experiments are expressed as a competitive index (C.I.) as follows: C.I. = (mutant/wild-type output ratio)/(mutant/wild-type input ratio). For competition experiments, tissues containing less than 50 total bacteria in the 10−1 dilution were not included in the results. When less than 1 mutant bacterium was recovered from the tissue homogenate, a value of 1 was used to determine the minimum C.I. for the tissue. In most cases, the limit of detection was 1 mutant bacterium:200 wild-type bacteria. Competition data were transformed logarithmically to determine the geometric means of the C.I. and P value. P values were determined in a two-tailed, unpaired Student's t test comparing the C.I. for mice infected with the two wild-type strains with that for mice infected with a yop mutant and the wild-type Kanr strain. A P value of <0.01 was used as the critical value for significance.
Competitions between yopHE and yopHEMOJ and between yopHEO and yopHEMOJ were conducted with Kanr yopHE and yopHEO strains and Kans yopHEMOJ. C.I. values were determined as follows: [yopHE(O)/yopHEMOJ output ratio]/[yopHE(O)/yopHEMOJ input ratio].
The Institutional Animal Care and Use Committee of Tufts University approved all animal procedures.
Weight and morphology studies.
Mice were weighed daily during single-strain experiments. Percent weight loss or gain was determined by dividing the final weight at day 5 by the initial weight at day 1. Morphology observations were initially made during nonblind experiments; however, results were confirmed in two blind experiments. Tissue morphology was ranked as follows: 0, healthy tissues; 1, moderate signs of disease; and 2, severe signs of disease. The following characteristics were used to assign a morphology rank: for the ileum and cecum, when the intestines were full of green contents they were ranked 0 (healthy tissue); when the contents were not full and a mixture of green and clear was seen, they were ranked 1 (moderate disease); and when the intestines were not full and any contents were clear, they were ranked 2 (severe disease). For the ascending colon, healthy tissue (0) was indicated by the presence of pellets throughout the colon, moderate disease (1) was indicated by presence of pellets in the transverse and descending colon but the absence of pellets in the ascending colon, and severe disease (2) was indicated by the absence of pellets throughout the colon.
Histology.
Mice were infected with 5 × 108 CFU of wild-type Y. pseudotuberculosis, yopB, yopE, yopH, or yopO. At 2 and 5 days postinfection, mice were sacrificed and tissues were harvested for histology. The terminal PP located closest to the cecum and a MLN were isolated. Tissues were fixed in 4% formalin for 2 h, washed in ethanol, and embedded in paraffin. Sections (10 μm thick) were cut and stained with hematoxylin and eosin.
RESULTS
Effector Yops are required for colonization in intestinal and lymph tissues.
Previously, a signature-tagged mutagenesis study identified five mutants in the plasmid-encoded type III secretion-translocation system as unable to compete with wild-type Y. pseudotuberculosis for colonization of the cecum (40). This result suggested that either the type III secretion-translocation apparatus itself or one or more of the effector Yops play an essential role in colonization and/or persistence in the intestines. To determine whether known effector Yops are required for colonization of the intestines, a yopHEMOJ mutant strain, lacking five Yops, was tested in BALB/c mice (Fig. 1). To determine whether other as-yet-unidentified effector Yops or the translocation apparatus itself is required for colonization, the behavior of a yopBHEMOJ strain, which is lacking five effector yops and is defective for protein translocation, was compared to that of both the yopHEMOJ strain and wild-type Y. pseudotuberculosis (Fig. 1). Mice were sacrificed at 1 or 2 days postinfection, and the numbers of CFU/gram of tissue in the lumen of the ileum, cecum, and ascending colon and the PP, MLN, and CLF were determined. At day 1, both the yopHEMOJ and yopBHEMOJ mutants showed 10- to 100-fold defects (P < 0.01) in colonization of the ileum, ascending colon, and PP compared to wild-type Y. pseudotuberculosis, suggesting that at least one of the five effector Yops absent from the yopHEMOJ strain is important for initial colonization of the ileum, ascending colon, and PP.
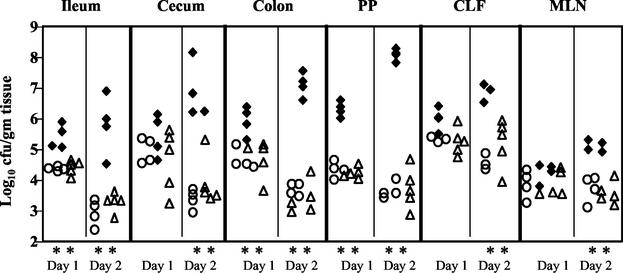
FIG. 1.
Colonization of intestinal and lymph tissues at day 1 and day 2 postinfection with 2 × 109 CFU of wild-type Y. pseudotuberculosis (filled diamonds), yopHEMOJ (open circles), or yopBHEMOJ (open triangles). Data from four to six mice from two experiments were pooled. Each symbol represents the log10 CFU/gram of tissue from one mouse colonized with the appropriate strain. All points were above the limit of detection of 10 CFU/gram of tissue. At day 1, colonization by yopHEMOJ and yopBHEMOJ was statistically different (**; P < 0.01) from that by the wild-type strain in the ileum, colon, and PP. At day 2, colonization by yopHEMOJ and yopBHEMOJ are statistically different from the wild-type strain (P < 0.01) in all tissues.
Between 1 and 2 days postinfection, the average number of wild-type Y. pseudotuberculosis bacteria increased 10- to 100-fold in all tissues, while yopHEMOJ and yopBHEMOJ mutants decreased 3- to 40-fold in all tissues except the CLF and MLN, where the numbers remained constant. The colonization of the yopHEMOJ and yopBHEMOJ strains at day 2 ranged from 25-fold to 2 × 104-fold lower than that of the wild-type strain (P < 0.01). These results indicate that at least one of the five effector Yops is crucial for persistence and replication in the intestines and PP and for replication in the CLF and MLN. No differences were detected between the yopHEMOJ and yopBHEMOJ mutants (P > 0.1), suggesting that neither the translocation of other effectors nor the translocation apparatus itself has any additional major role in colonization of the GI tract and lymph tissues in the absence of YopH, YopE, YopM, YopO, and YopJ.
Analysis of yop mutants in single-strain oral inoculations.
To determine whether any one Yop is required for colonization and persistence in intestinal and lymph tissues, infections with yop deletion strains were compared to infections with wild-type Y. pseudotuberculosis in single-strain infections. Mice were orogastrically inoculated with 2 × 109 CFU (high dose) or 2 × 108 CFU (low dose) of wild-type, yopB, yopH, yopE, or yopO bacteria. These yop mutants were chosen because previously published data (44) and our unpublished results indicate that YopJ and YopM do not play a role in colonization of the intestines. At 2 or 5 days postinfection, mice were sacrificed and the numbers of CFU/gram of ileum, cecal, and ascending colon exudates and PP, MLN, and CLF were determined (Fig. 2 and Table 2). At day 2 postinfection in both high- and low-dose experiments, wild-type Y. pseudotuberculosis was recovered from intestinal exudates, PP, and CLF at (on average) 105 to 107 CFU/g of tissue (Fig. 2 and Table 2). In the low-dose infections, the levels of colonization were 5- to 10-fold lower and more variation of CFU/gram of tissue was observed. In the high-dose experiments, the number of bacteria recovered from all tissues decreased from day 2 to day 5, while in the low-dose experiment, the levels of wild-type Y. pseudotuberculosis remained relatively constant in the intestines but decreased in the PP and MLN. The decrease in CFU in the high-dose infections from day 2 to day 5 suggested that those mice infected with the higher dose might have had a stronger immune response or more pronounced gastroenteritis between day 2 and 5 and, thus, that the ability of Y. pseudotuberculosis to persist and/or replicate was hampered by host defenses.
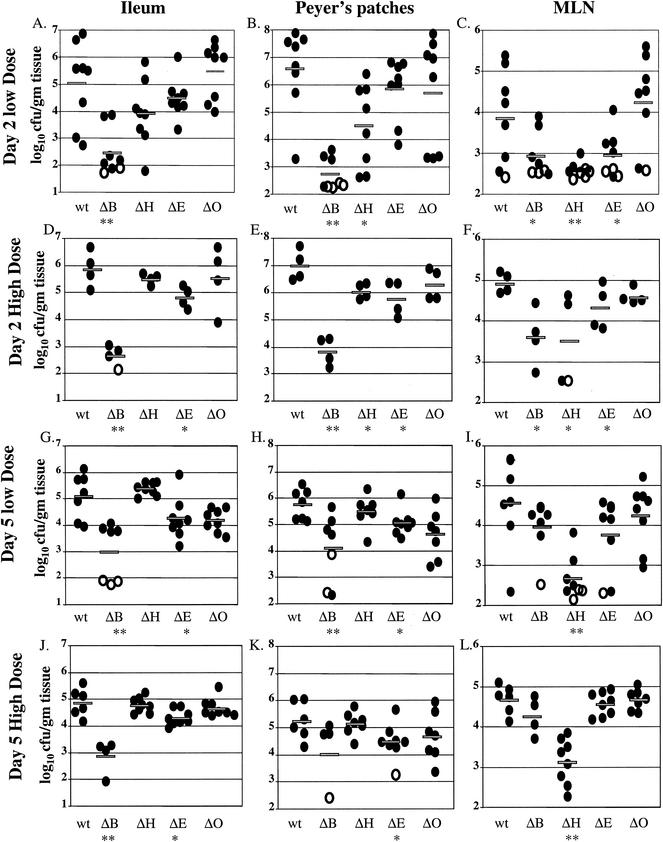
FIG. 2.
Colonization of ileum, PP, and MLN at day 2 and day 5 postinfection with either 2 × 109 CFU or 2 × 108 CFU of wild-type (wt), yopB (ΔB), yopH (ΔH), yopE (ΔE), or yopO (ΔO) Y. pseudotuberculosis. Data from four to eight mice from two to four experiments were pooled. Each symbol represents the log10 CFU/gram of tissue for the tissue sample from one mouse. Shaded bars represent the average log10 CFU/gram of tissue. Open symbols indicate that less than 10 CFU were recovered/tissue. Asterisks indicate statistical significance of difference between colonization levels of yop mutants and wild-type bacteria as follows: *, P = 0.1 to 0.01; **, P < 0.01.
TABLE 2.
Average log10 CFU/gram of tissue at day 2 and day 5 postinfection
| Dose level and tissue | Avg log10 CFU/g of tissue and log10 ratio on:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 postinfection
|
Day 5 postinfection
|
|||||||||||||||||
| wt avga,d | ΔyopB
|
ΔyopH
|
ΔyopE
|
ΔyopO
|
wt avge | ΔyopB
|
ΔyopH
|
ΔyopE
|
ΔyopO
|
|||||||||
| Avgd | Ratiob,c | Avgd | Ratio | Avgd | Ratio | Avgd | Ratio | Avgd | Ratiob,c | Avge | Ratio | Avge | Ratio | Avge | Ratio | |||
| High | ||||||||||||||||||
| Tissues | ||||||||||||||||||
| Ileum | 5.8 | 2.6 | 0.0006 | 5.5 | 0.44 | 4.8 | 0.09 | 5.5 | 0.48 | 4.9 | 2.8 | 0.01 | 4.7 | 0.79 | 4.3 | 0.26 | 4.6 | 0.62 |
| Cecum | 6.2 | 3.9 | 0.005 | 5.5 | 0.2 | 5.0 | 0.06 | 5.9 | 0.52 | 5.1 | 3.4 | 0.02 | 5.1 | 0.96 | 5.3 | 1.49 | 4.8 | 0.56 |
| Colon | 6.2 | 3.2 | 0.001 | 5.0 | 0.07 | 5.2 | 0.09 | 6.2 | 0.95 | 5.4 | 3.1 | 0.005 | 5.4 | 0.96 | 5.8 | 2.35 | 5.5 | 1.28 |
| PP | 7.0 | 3.8 | 0.0007 | 6.0 | 0.11 | 5.8 | 0.06 | 6.3 | 0.2 | 5.2 | 4.2 | 0.1 | 5.1 | 0.8 | 4.4 | 0.17 | 4.6 | 0.27 |
| CLF | 6.8 | 4.1 | 0.002 | 5.1 | 0.02 | 6.2 | 0.23 | 6.1 | 0.17 | 5.0 | 4.1 | 0.14 | 3.3 | 0.02 | 4.4 | 0.23 | 4.3 | 0.21 |
| MLN | 4.9 | 3.6 | 0.05 | 3.5 | 0.04 | 4.3 | 0.25 | 4.7 | 0.6 | 4.7 | 4.2 | 0.39 | 3.1 | 0.03 | 4.5 | 0.77 | 4.7 | 0.98 |
| Spleen | 2.9 | 2.1 | 0.17 | 2.8 | 0.95 | 2.1 | 0.17 | 2.6 | 0.52 | 4.2 | 2.3 | 0.01 | 2.6 | 0.02 | 2.3 | 0.012 | 3.4 | 0.15 |
| Low | ||||||||||||||||||
| Tissues | ||||||||||||||||||
| Ileum | 5.0 | 2.5 | 0.003 | 3.9 | 0.07 | 4.5 | 0.29 | 5.5 | 2.85 | 5.1 | 3.0 | 0.008 | 5.4 | 1.9 | 4.2 | 0.14 | 4.2 | 0.12 |
| Cecum | 5.8 | 2.6 | 0.0007 | 4.7 | 0.08 | 4.8 | 0.1 | 5.8 | 1 | 5.6 | 2.8 | 0.002 | 5.1 | 0.37 | 5.6 | 1.15 | 5.2 | 0.38 |
| Colon | 5.5 | 2.9 | 0.002 | 4.5 | 0.1 | 5.1 | 0.34 | 5.8 | 1.91 | 5.7 | 3.4 | 0.005 | 5.5 | 0.62 | 5.9 | 1.47 | 5.5 | 0.65 |
| PP | 6.6 | 2.7 | 0.0001 | 4.5 | 0.008 | 5.8 | 0.18 | 5.7 | 0.14 | 5.8 | 4.1 | 0.02 | 5.5 | 0.53 | 5.1 | 0.2 | 4.6 | 0.07 |
| CLF | 5.9 | 3.5 | 0.004 | 4.8 | 0.08 | 5.2 | 0.2 | 6.5 | 4.16 | 5.6 | 4.3 | 0.05 | 4.2 | 0.04 | 5.5 | 0.86 | 4.9 | 0.22 |
| MLN | 3.9 | 2.9 | 0.12 | 2.6 | 0.05 | 2.9 | 0.12 | 4.2 | 2.47 | 4.5 | 4.0 | 0.25 | 2.7 | 0.01 | 3.8 | 0.16 | 4.2 | 0.48 |
| Spleen | 3.2 | 2.1 | 0.08 | 2.1 | 0.08 | 2.3 | 0.11 | 2.9 | 0.45 | 4.2 | 2.7 | 0.03 | 2.2 | 0.01 | 2.0 | 0.007 | 3.0 | 0.06 |
avg, average of log10 CFU/gram of tissues. wt, wild type.
Ratio of average log10 (CFU of mutant/gram of tissue)/(average log CFU of wild type/gram of tissue).
Significant differences from wild type are indicated as follows: boldface, P < 0.01; underlined values, P value is between 0.01 and 0.1.
n = four mice pooled from two experiments.
n = six to eight mice pooled from three to four experiments.
The translocation mutant, yopB, colonized the intestinal tissues, PP, and CLF at a level 200- to 7,000-fold lower than the wild-type Y. pseudotuberculosis at day 2 (P < 0.01), regardless of the inoculation dose (Fig. 2 and Table 2). In fact, the yopB mutant was not recovered from some tissues (Fig. 2), indicating that less than 10 bacteria were present (Table 2). The levels of the yopB mutant remained consistent or increased between day 2 and day 5. Most notably, in the lymph tissues at day 5 of the low-dose infections, the yopB mutant was recovered at levels 7- to 42-fold higher than at day 2, indicating that the yopB mutant could replicate in these tissues. The combination of an increase in the number of yopB mutant bacteria and a decrease in the number of wild-type bacteria in the lymph tissues meant that at day 5, some of the differences between colonization of the yopB mutant and wild-type strains in the MLN, PP, and CLF were not statistically significant (Fig. 2E, F, K, and L and Table 2). The difference in the course of infection between the wild-type strain and the yopB mutant could have been due to a slower rate of replication of the yopB mutant during infection, although no defect in replication was observed at 37°C in L broth or tissue culture medium with any yop mutant strain (data not shown). Alternatively, the yopB mutant may have colonized or seeded tissues less efficiently than the wild-type strain but, once established, was capable of replication in some tissues.
None of the effector yop mutant strains yopH, yopE, or yopO was as defective as the yopB mutant in colonizing intestinal or lymph tissues, with one exception (Fig. 2 and Table 2). The yopH mutant colonized at levels comparable to or even lower than those of the yopB mutant in the MLN in all experiments and in the CLF at day 5 (Fig. 2 and Table 2). In all other tissues, the yopH mutant had 2- to 15-fold defects at day 2, which were at most moderately significant (P = 0.01 to 0.1). These defects were usually not apparent at day 5, because in the low-dose experiment the levels of colonization by the yopH mutant increased 2- to 30-fold from day 2 to day 5 and in the high-dose experiment the levels of yopH mutant decreased less than those of the wild-type strain. Thus, colonization by a yopH mutant was comparable to that by wild-type Y. pseudotuberculosis in most tissues at 5 days postinfection (Table 2). These results indicate that a yopH mutant can persist and replicate in the GI tract and PP but not in the MLN and CLF. As with the yopB mutant, the increase in the levels of bacterial recovery between day 2 and day 5 suggests a defect in initial colonization or seeding of tissues.
In both low- and high-dose infections, the yopE mutant was recovered at levels 3- to 20-fold lower than wild-type Y. pseudotuberculosis in all tissues at day 2 and many of these defects were at least moderately significant (P = 0.01 to 0.1). By day 5, moderately significant, 4- to 5-fold differences between wild-type Y. pseudotuberculosis and yopE were observed only in the ileum and PP at both infectious doses. In the ileum and PP, the levels of the yopE mutant decreased from day 2 to day 5, while in most other tissues the levels of the yopE mutant increased. Thus, the yopE mutant appeared to initially colonize all tissues less well than the wild-type strain but was capable of persisting and replicating in all tissues except the ileum and PP.
Of the yop effector mutants, the yopO strain appeared most similar to wild-type Y. pseudotuberculosis. The yopO mutant, like the wild-type strain, failed to persist in all tissues except the MLN at the high dose, presumably due to heightened host defenses to infection (Fig. 2 and Table 2).
At 5 days postinfection, most mice infected with wild-type Y. pseudotuberculosis were colonized with about 104 CFU/g in the spleen (Fig. 3). In contrast, the yopH and yopE mutants were not recovered from the spleens of many infected mice; when bacteria were recovered, the amount was lower than that of the wild-type strain. The reduced ability of the yopH and yopE mutants to colonize the spleen is consistent with previous studies using oral and intravenous inoculations (5, 9, 30, 65). The yopO mutant was able to colonize the spleen at day 5 at levels similar to or slightly lower than that of the wild-type strain. These data are in contrast to those of Galyov et al. (23) in which a yopO mutant failed to colonize the spleens of mice following oral inoculation. However, the strain used by Galyov also contains a mutation in yadA which may have had an additional detrimental effect on colonization of the spleen. Colonization of the liver was not examined in these experiments; in other experiments, however, colonization of the spleen correlated with colonization of the liver (unpublished data).
FIG. 3.
Colonization of the spleen at 2 and 5 days postinfection with either 2 × 109 CFU or 2 × 108 CFU of wild-type (wt), yopB (ΔB), yopH (ΔH), yopE (ΔE), yopO (ΔO) or ΔHEMOJ Y. pseudotuberculosis. Data from four to eight mice from two to four experiments were pooled. Each symbol represents the log10 CFU/gram of tissue for the spleen from one mouse. Open symbols indicate that less than 10 CFU/tissue were recovered.
In summary, the effector yop mutants, yopH, yopE, and yopO, had modest defects in colonization, persistence, and/or replication in the GI tract and most lymph tissues. With the exception of the yopH mutant in the MLN and CLF, no one Yop effector was responsible for the lack of colonization and persistence of the yopB and yopHEMOJ mutants. These results suggest either that two or more Yops have redundant functions in colonization or that multiple-Yop-dependent mechanisms are used by Y. pseudotuberculosis to ensure colonization.
Host signs of disease in response to single yop mutants.
Although the yopH and yopE mutants had at most sevenfold defects in colonization of intestinal exudates and PP at day 5, the appearance of the intestinal tissues during dissection was noticeably different from that of tissues colonized with wild-type Y. pseudotuberculosis. Intestinal exudates of mice infected with wild-type Y. pseudotuberculosis or the yopO mutant showed obvious changes in color and consistency compared to exudates of uninfected mice.
Additionally, these mice lost on average 11 to 15% of their initial body weight during the course of the 5-day infection. In contrast, mice infected with the yopH or yopE mutants showed changes in the ileal exudates only and did not lose weight during the infection (data not shown). These changes were distinct from the changes observed in mice that were subjected to fasting, suggesting that anorexia caused by illness was not solely responsible but rather that the colonizing bacteria were also inducing changes in intestinal tissues. Thus, weight loss correlated with changes in the cecal and colonic exudates and with colonization of the spleen by Y. pseudotuberculosis.
In mice infected with wild-type Y. pseudotuberculosis or with the yopH, yopE, and yopO mutants, histological examination of the PP and the MLN revealed inflammation (data not shown). The structural integrity of the germinal center of the lymph node in the PP was compromised, as the distinct border between the germinal center and the regions of B and T cells was destroyed. In some mice, furthermore, the influx of macrophages and granulocytes (predominantly eosinophils) in the PP and the lamina propria of neighboring intestinal villi was apparent at day 5, although the villi remained intact. In the MLN, abscesses and apoptotic cells were visible in mice infected with the wild-type strain or the yopH, yopE, or yopO mutants. Thus, in these preliminary experiments, no obvious differences were seen in the pathology of the MLN, the PP, or the surrounding ileal tissues of mice infected with wild-type, yopH, yopE, or yopO bacteria. The histological results support the visible changes seen in the ileum of all infected mice and indicate that inflammation in the PP and MLN does not correlate with weight loss or colonization of the spleen in infected mice.
yopHE and yopHEO mutants are defective for intestinal colonization and lymph tissues.
Since no single Yop accounted for the inability of the yopHEMOJ mutant to colonize intestinal tissues and PP in single-strain infections, a series of multiple-yop mutant strains were tested for colonization. Mice were infected orogastrically with 2 × 108 CFU of the wild-type strain, the yopHEMOJ mutant, or one of four multiple-yop mutant strains: yopHE, yopHO, yopEO, or yopHEO (Fig. 4). At both day 2 and day 5, the yopHEMOJ mutant strain generally colonized the intestines and PP at a level 1,000-fold lower than did wild-type Y. pseudotuberculosis and was not detected at all in many tissue samples. The yopHEMOJ strain colonized the MLN at a level 72- and 12-fold lower on day 2 and day 5, respectively.
The yopHE, yopHO, and yopEO mutant strains were recovered at lower levels than wild-type Y. pseudotuberculosis in most tissues; however, only the yopHE and yopHEO strains were as defective as the yopHEMOJ strain in all tissues at both day 2 and day 5. These results indicate that the absence of both YopH and YopE renders Y. pseudotuberculosis unable to effectively colonize, persist, and replicate in intestinal and lymph tissues (Fig. 4). The yopHO mutant colonized the intestines and PP at levels between those of the wild-type strain and the yopHEMOJ mutant strain (15- to 300-fold lower than the wild-type strain) and colonized the CLF and MLN as poorly as did the yopHEMOJ mutant at day 2 postinfection. This latter observation was consistent with the results from the single-strain infections with the yopH mutant, which showed that YopH is crucial for colonization of the MLN and CLF (Table 2). By day 5, the yopHO mutant had decreased to a level nearly as low as that of the yopHEMOJ strain in intestinal exudates and the PP. This result indicates that the yopHO strain is initially able to colonize these tissues (albeit less efficiently than wild-type Y. pseudotuberculosis) but, in contrast to the yopH mutant (Fig. 2), is unable to persist and replicate to day 5. The yopEO strain colonized at levels intermediate between those of the wild-type and the yopHEMOJ strains at both day 2 and day 5. At 5 days postinfection, the differences between the yopEO mutant and wild-type Y. pseudotuberculosis were only moderately significant (P = 0.01 to 0.1). Thus, although the yopEO strain colonized less well than the wild-type strain, its defects were more modest than those of the yopHE and yopHO strains. Additionally, the yopEO strain was recovered at similar levels at day 2 and 5 days postinfection, indicating that unlike the yopHE and yopHO strains, the yopEO stain was able to persist from day 2 to day 5.
To determine whether the yopHE and yopHEO mutants were as attenuated as the yopHEMOJ mutant, the yopHE and yopHEO strains were tested in competition with the yopHEMOJ strain. Since all three strains showed drastic colonization defects at day 2 in single-strain infections (Fig. 4), mice were sacrificed at 1 day postinfection and the C.I. values were determined (Fig. 5). The yopHE and yopHEO mutants out-competed the yopHEMOJ strain in all tissues, indicating that other Yops play minor roles in initial colonization. It was notable, however, that the yopHE mutant outcompeted the yopHEMOJ mutant by more than 10-fold in the ileum and PP and that the yopHEO mutant outcompeted the yopHEMOJ mutant by less than 10-fold, suggesting that YopO aids in the colonization of the ileum and PP.
FIG. 5.
Competition of yopHE versus yopHEMOJ (closed circles) and yopHEO versus yopHEMOJ (open circles), 1 day postinfection with 2 × 109 CFU of an equal mixture of yopHE and yopHEMOJ or yopHEO and yopHEMOJ. Data from three to six mice from two experiments were pooled. Data are graphed as C.I. {[yopHE(O)/yopHEMOJ output ratio]/[yopHE(O)/yopHEMOJ input ratio]} values for the tissue samples from one mouse. Shaded bars represent geometric means of the C.I. values. All points were above the limit of detection.
yop mutant phenotypes are more severe in competition infections.
Competition infections were performed to determine whether the presence of wild-type Y. pseudotuberculosis affects the ability of the effector yop mutants to colonize tissues. Wild-type bacteria could adversely affect yop mutants by competing for colonization sites or by inducing host defenses that the yop mutants cannot overcome. Alternatively, the presence of wild-type Y. pseudotuberculosis could aid the yop mutants, since Yop proteins act outside of the bacteria to alter host cell behavior. Mice were orogastrically infected with 2 × 109 bacteria in an inoculum comprised of an equal mixture of the Kanr wild-type strain and Kans yop mutant strains. A high infectious dose was used to ensure consistent, high levels of wild-type bacteria early in infection (compare low- and high-dose levels of colonization as shown in Fig. 2A to C and 2D to F). Mice were sacrificed at 5 days postinfection, tissues were harvested, and the C.I. was determined (Fig. 6). As a control and for comparison in statistical analysis (see Materials and Methods), the Kanr wild-type strain was competed against the isogenic Kans wild-type strain. As expected, the experimental C.I. values for these strains hovered around 1 for all tissues (Fig. 6). When the yopHEMOJ or yopB mutants were competed against wild-type Y. pseudotuberculosis, the mutants were not recovered from the majority of tissues samples, as shown in Fig. 6 (limit of detection, approximately 1:200), and were out-competed by the wild-type strain by at least 16- to 160-fold (P < 0.01).
The three effector yop mutants each had a distinct pattern of survival, but all showed greater deficiencies in colonization in the presence of wild-type Y. pseudotuberculosis than were observed in the single-strain infections. The yopH mutant had the most drastic phenotype; it was below the limit of detection (1:200) in many tissue samples and was outcompeted by the wild-type strain at least 15- to 55-fold (P < 0.01) in all tissues (Fig. 6 and Table 3). The inability to recover the yopH mutant at day 5 in competition studies was in stark contrast to the relatively high levels of the yopH mutant seen in the high-dose single-strain infections at day 5. The ratios of the yopH mutant to wild-type Y. pseudotuberculosis in the PP and all intestinal tissues were equal to or greater than 0.79 in the high-dose single-strain infection (Table 2), whereas the average C.I. values in these tissues were less than 0.05 (Table 3). The yopE mutant also survived less well in the presence of the wild-type strain, with 3- to 30-fold defects (P < 0.01) in all tissues. The most severe yopE defects were observed in the ileum and PP, the same tissues as showed the day 5 defects in the single-strain infections. The average C.I. values for the yopE mutant in the ileum and PP were 0.017 and 0.024, respectively (Table 3), while the ratios of recovered yopE mutant versus the wild-type strain in the high-dose single-strain infections were 0.26 and 0.17 in the ileum and PP, respectively (Table 2). In the cecum and colon, the yopE mutant was significantly outcompeted by wild-type bacteria. This is in contrast to the results of the single-strain infections, in which the yopE mutant was recovered at levels comparable to those of the wild-type strain (Table 2). The yopO mutant was outcompeted by the wild-type strain at least 39-fold in the PP and 6- to 8-fold in the MLN, CLF, and ileum (P < 0.01), while no defects were seen in the cecum and colon (Table 3).
TABLE 3.
Geometric mean of C.I. at day 2 and day 5a
| Tissue | C.I. geometric mean
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt/wt Kan
|
ΔyopB/wt Kan
|
ΔyopH/wt Kan
|
ΔyopE/wt Kan
|
ΔyopO/wt Kan
|
ΔyopHEMOJ/wt Kan day 5 | ||||||
| Day 2 | Day 5 | Day 2 | Day 5 | Day 2 | Day 5 | Day 2 | Day 5 | Day 2 | Day 5 | ||
| Ileum | 0.7 | 0.021 | 0.047 | 0.024 | 0.094 | 0.015 | |||||
| Cecum | 0.85 | 0.63 | 0.008 | 0.009 | 0.19 | 0.026 | 0.26 | 0.078 | 0.58 | 0.2 | 0.006 |
| Colon | 0.45 | 0.01 | 0.014 | 0.073 | 0.24 | 0.005 | |||||
| PP | 0.70 | 1.12 | 0.006 | 0.022 | 0.15 | 0.02 | 0.16 | 0.04 | 0.65 | 0.03 | 0.012 |
| CL | 0.89 | 0.013 | 0.017 | 0.085 | 0.11 | 0.005 | |||||
| MLN | 0.81 | 0.54 | 0.051 | 0.032 | 0.07 | 0.02 | 0.19 | 0.2 | 0.82 | 0.097 | 0.012 |
Geometric mean of the C.I. from six to eight mice pooled from three to four experiments. C.I. for mutant/wild type that were statistically significant from the C.I. for wild type/wild type are indicated as follows: boldface, P < 0.01; underlined characters, P value is between 0.01 and 0.1. wt, wild type.
To determine whether the failure of the yop mutants to compete with wild-type Y. pseudotuberculosis was due to events occurring early in infection, the yop mutants were tested for their ability to compete with wild-type Y. pseudotuberculosis in the cecum, PP, and MLN at day 2. In general, the C.I. values for the cecum, PP, and MLN at day 2 were remarkably similar to the ratios of yop mutant to wild-type bacteria recovered in these tissues in the high-dose single-strain infections (Table 3 and Table 2). For instance, in the PP the ratio of the yopH mutant to the wild-type strain in single-strain infections (Table 2) and the C.I. (Table 3) were 0.11 and 0.15, respectively, and in the cecum, the ratio was 0.2 and the C.I. was 0.19. The C.I. values for the yopE mutant in the cecum and PP (0.26 and 0.16) were actually higher than the ratios between yopE and the wild-type strain in single-strain infections (0.09 and 0.06), indicating that at day 2 the presence of wild-type Y. pseudotuberculosis may be slightly advantageous to the yopE mutant in colonizing the cecum and PP. Therefore, we conclude that although the yop mutants colonize at lower levels than the wild-type strain at day 2, the presence of wild-type Y. pseudotuberculosis did not further impede the ability of the yop mutants to initially seed and colonize the cecum, MLN, and PP. Between day 2 and day 5, however, wild-type Y. pseudotuberculosis severely inhibited the ability of the yop mutants to persist.
DISCUSSION
Oral inoculation of mice with a lethal dose of Y. pseudotuberculosis is a multifaceted process that involves bacterial colonization and replication in a variety of tissues followed by spread to organs concomitant with a rise in the host inflammatory response and disease symptoms. At 24 h after ingestion of wild-type Y. pseudotuberculosis, the bulk of the inoculating dose has passed through the GI tract or has been killed; however, some bacteria survive and colonize the ileum, cecum, ascending colon, PP and MLN. By 48 h after infection, bacterial numbers increase in these tissues and seeding of the spleen and liver is observed. In addition, there is a clear host response to infection as the numbers of granulocytes and macrophages increase in the PP and MLN. Between day 2 and day 5, the numbers of Y. pseudotuberculosis continue to increase in the spleen and liver but the numbers of bacteria recovered from many of the initial sites of colonization decrease and weight loss and other signs of disease are observed. Given these parameters of wild-type Y. pseudotuberculosis infection, we have examined a set of isogenic yop mutant strains for their ability to colonize, persist, and replicate in the GI tract, lymph tissues, and the spleen in single-strain infections and in the context of infection with wild-type Y. pseudotuberculosis. As reported here, a strain lacking the five effector Yops failed to colonize any of these tissues in either single-strain or competition studies. Deletion of either yopH or yopE generally caused lower levels of colonization at day 2 but allowed normal levels in intestinal and lymph tissues in single-strain infections by day 5. Nonetheless, the absence of YopH prevented colonization of the MLN. In contrast to the yopH and yopE mutants, a yopHE strain was as defective as the yopHEMOJ strain at both day 2 and day 5, indicating that YopH and YopE play crucial roles in the first 24 h of infection.
Since the biochemical activities of YopH, a tyrosine phosphatase (9, 27), and YopE, a Rac-GAP (5, 69), are different, these two Yops may ensure bacterial colonization by inactivating the same cell types via different mechanisms. Thus, in the absence of one Yop, the presence of the other would still be sufficient to at least partially block host cell function. While this hypothesis is theoretically attractive, several laboratories have observed that in the absence of either YopH or YopE, Yersinia becomes susceptible to phagocytosis by neutrophils and macrophages in cell culture, indicating that the remaining Yops are not sufficient to completely thwart phagocytosis (1, 2, 9, 26). Thus, either this hypothesis is not correct or, in an animal infection model, Y. pseudotuberculosis yopH and yopE mutants can still block phagocytosis by cells encountered during infection. A second possibility is that YopH and YopE have distinct roles in colonizing tissues and thus that in the absence of one Yop, the other Yop can still promote colonization, although less efficiently than when both Yops are present. In this scenario, one might postulate that the bacteria colonize multiple different niches. Future experiments directed at determining the locations of the wild-type strain, yopH and yopE mutants, and the host cell targets of YopH and YopE in the GI tract and PP should distinguish between these two possibilities.
While it is likely that loss of the YopE Rac-GAP activity, which renders Y. pseudotuberculosis susceptible to phagocytosis by host cells, is the reason why the yopE mutant colonizes less efficiently than the wild-type strain, it has also been shown that in the absence of YopE, Y. pseudotuberculosis acquires novel phenotypes. Specifically, an increase in host cell death is observed after infection with a yopE mutant, apparently because YopE activity blocks leakage from the pore formed by YopB and the translocation apparatus (5, 68). In the mouse, this leakage could result in more tissue damage and inflammation. However, these assays were done in cell culture at a multiplicity of infection of 100 bacteria per host cell, which is likely to be considerably higher than that occurring in a natural infection of the mouse. Nonetheless, it is conceivable that some of the properties of mice infected with strains lacking yopE are due to cytotoxicity caused by the translocation apparatus.
It is interesting that the yopB mutant is able to persist in the lymph tissues to day 5 postinfection while yopH and yopJ (44) mutant strains cannot. The results with the yopB mutant are consistent with previous reports and unpublished data (J. M. Balada and J. Mecsas, unpublished data) showing that mutants lacking the type III secretion system colonize the MLN (25, 37). The presence of a type III secretion-translocation apparatus may alert the host to the presence of the bacteria via the innate immune response. Alternatively, an imbalance of Yop activities on host cells (due to deletion of some effector yops) may enhance bactericidal host defenses.
Regardless of the precise biochemical and phenotypic defect of a particular yop mutant, the observation that a given yop mutant behaves differently in different tissues suggests that there are distinct mechanisms by which the bacteria colonize and persist in different tissues. For instance, the yopH mutant strain was unable to colonize the MLN but was proficient in colonization of the PP in single-strain infections. In contrast, the yopE and yopO mutants showed modest defects in colonizing the PP but colonized the MLN as well as did wild-type Y. pseudotuberculosis. These results indicate that the environment of the MLN is significantly different than that of the PP, despite both being lymph nodes containing similar cell types. In contrast to differences between colonization of the PP and MLN, phenotypes in the PP generally mirrored phenotypes in the ileum but not in the cecum or ascending colon. This observation might reflect continual reseeding of the PP from the ileum (or vice versa) but also demonstrates that the environment of the ileum is distinct from those of the cecum and ascending colon with regard to the ability of the yop mutants to colonize.
The fact that YopH and YopE are necessary for colonization and replication of the GI tract is somewhat surprising in light of the observations that YopH and YopE prevent internalization of Yersinia by epithelial cells in cell culture (1, 2, 5, 8, 18, 26, 41, 55-57). If YopE and YopH prevent phagocytosis or internalization of bacteria during infection, how is Y. pseudotuberculosis internalized by M cells (3, 13, 29, 38, 42)? There are several possible explanations. First, YopH and YopE may not be expressed when Y. pseudotuberculosis attaches to and transverses through M cells. This explanation implies that Y. pseudotuberculosis invades M cells prior to Yop expression and that once Yops are expressed, no additional PP colonization occurs via M cells. A second possibility is that YopH and YopE are not delivered into M cells. However, a study of Yersinia infection in a cell culture system that involved generation of M-like cells demonstrated that Yops reduce transcytosis of bacteria through the M-like cells, suggesting that Yops are translocated into these M-like cells (62). There are however, differences between the M-like cells derived in cell culture and M cells found in the small intestine of mice. Most notably, the M-like cells bound by Yersinia are largely UEA1−, while M cells in the ileum are UEA1+ (13, 38). Additionally, differences in lectins expressed on cells may alter the ability of Yersinia to translocate Yops. In fact, it has been shown that translocation of YopE is sensitive to the types of proteoglycans present on the surface of mammalian cells (12). Therefore, it remains an open question as to whether Yersinia Yops are translocated into M cells in the ileum of a mouse. A third possible explanation is that M cells may be able to resist the actions of YopH and YopE. This possibility implies biochemical differences between M cells and other epithelial cells that have not been identified to date.
While this is the first report that specific effector Yops are necessary for initial colonization, persistence, and replication in the GI tract, type III secretion systems and their effector proteins of other enteric pathogens (including EspG of enteropathogenic E. coli [17] and proteins regulated by hilA in Salmonella) have been identified as important in colonization or in provoking diarrheal symptoms in the GI tract. In Salmonella enterica serovar Typhimurium, mutations in hilA (which encodes a transcriptional activator of the SPI-1 type III secretion system) result in decreased colonization of the small intestine in both single and competition experiments after oral inoculation of mice (47). In a calf oral inoculation model, an hilA mutant was not as virulent as wild-type S. enterica serovar Typhimurium, as measured by death of the animals and severity of diarrhea (66). Additionally, the type III effector proteins SopA, SopB, SopD, SopE2, and SipA of S. enterica serovar Typhimurium and S. dublin are important in causing fluid accumulation in calves (33, 72). It is interesting that Salmonella contains an hilA-regulated two-domain effector protein, SptP, that has both tyrosine phosphatase and Rac-GAP activities similar to those of YopH and YopE (20, 21). However, an sptP mutant colonizes the ileum, PP, and MLN and causes diarrhea in calves at levels comparable to wild-type S. enterica serovar Typhimurium (67). Thus, although SptP has biochemical activities similar to those of YopH and YopE, SptP is not playing a similar role in S. enterica serovar Typhimurium infection of calves.
The differences in the levels of colonization by the yopH and yopE mutants in the single-strain and the competition experiments indicate that the presence of wild-type Y. pseudotuberculosis affects the ability of the mutants to persist to day 5. There are two likely explanations for the negative impact of the wild-type bacteria on the yop mutants. First, the wild-type bacteria may outcompete the yop mutants for sites of colonization. We noted that the presence of wild-type bacteria did not affect the ability of the yop mutants to colonize at day 2, indicating that the yopH and yopE mutants were able to find sites of colonization in the presence of wild-type bacteria. However, over the course of 5 days, wild-type Y. pseudotuberculosis may continually seed new niches more efficiently than yop mutants and thus overtake the mutants. Second, the wild-type bacteria may elicit a strong immune response that the yop mutants cannot survive. The second possibility is consistent with the weight and morphology results of the single-strain infections in which yopE and yopH did not appear to cause disease. In the presence of wild-type bacteria, the yop mutants were presumably encountering host defenses that were lacking in the single-strain infections. Additionally, these two possibilities are not mutually exclusive—the yop mutants may fail to survive in the presence of the wild-type strain because of a combined inability to efficiently colonize niches and to survive the host immune response. Future work involves distinguishing between these two possibilities and exploiting the competition assay to investigate seeding patterns of different yop mutants and to determine host factors that specifically target yop mutants.
Acknowledgments
We thank members of the Mecsas lab for thoughtful discussion, Andrew Camilli, Jennifer Coburn, Ralph Isberg, and Abraham L. Sonenshein for critical reading of the manuscript, and Alvar Gustafson for expert advice on histological samples.
This work was supported in part by a Natalie V. Zucker Award, a Charles H. Hood Foundation grant, National Institute of Health grant R21-AI49348, the Center for Gastroenterology Research on Absorptive and Secretory Processes, Tufts-New England Medical Center (NIDDK, P30 DK34928), and startup funds from Tufts University awarded to J.M. L. K. Logsdon was supported by National Institutes of Health training grant T32-AI 07422.
Editor: J. T. Barbieri
REFERENCES
- 1.Andersson, K., N. Carballeira, K. E. Magnusson, C. Persson, O. Stendahl, H. Wolf-Watz, and M. Fallman. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol. Microbiol. 20:1057-1069. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, K., K. E. Magnusson, M. Majeed, O. Stendahl, and M. Fallman. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285-294. [DOI] [PubMed] [Google Scholar]
- 4.Barz, C., T. N. Abahji, K. Trulzsch, and J. Heesemann. 2000. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482:139-143. [DOI] [PubMed] [Google Scholar]
- 5.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 6.Black, R. E., R. J. Jackson, T. Tsai, M. Medvesky, M. Shayegani, J. C. Feeley, K. I. MacLeod, and A. M. Wakelee. 1978. Epidemic Yersinia enterocolitica infection due to contaminated chocolate milk. N. Engl. J. Med. 298:76-79. [DOI] [PubMed] [Google Scholar]
- 7.Bliska, J. B., and D. S. Black. 1995. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 61:3914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliska, J. B., K. L. Guan, J. E. Dixon, and S. Falkow. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA 88:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolin, I., and H. Wolf-Watz. 1988. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol. Microbiol. 2:237-245. [DOI] [PubMed] [Google Scholar]
- 11.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd, A. P., M. P. Sory, M. Iriarte, and G. R. Cornelis. 1998. Heparin interferes with translocation of Yop proteins into HeLa cells and binds to LcrG, a regulatory component of the Yersinia Yop apparatus. Mol. Microbiol. 27:425-436. [DOI] [PubMed] [Google Scholar]
- 13.Clark, M. A., M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Differential surface characteristics of M cells from mouse intestinal Peyer's and caecal patches. Histochem. J. 26:271-280. [PubMed] [Google Scholar]
- 14.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dukuzumuremyi, J. M., R. Rosqvist, B. Hallberg, B. Akerstrom, H. Wolf-Watz, and K. Schesser. 2000. The Yersinia protein kinase A is a host factor inducible RhoA/Rac-binding virulence factor. J. Biol. Chem. 275:35281-35290. [DOI] [PubMed] [Google Scholar]
- 17.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallman, M., K. Andersson, S. Hakansson, K. E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsberg, A., and H. Wolf-Watz. 1988. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol. Microbiol. 2:121-133. [DOI] [PubMed] [Google Scholar]
- 20.Fu, Y., and J. E. Galan. 1998. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol. Microbiol. 27:359-368. [DOI] [PubMed] [Google Scholar]
- 21.Galan, J. E., and Y. Fu. 2000. Modulation of actin cytoskeleton by Salmonella GTPase activating protein SptP. Methods Enzymol. 325:496-504. [DOI] [PubMed] [Google Scholar]
- 22.Galyov, E. E., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361:730-732. [DOI] [PubMed] [Google Scholar]
- 23.Galyov, E. E., S. Hakansson, and H. Wolf-Watz. 1994. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J. Bacteriol. 176:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gemski, P., J. R. Lazere, T. Casey, and J. A. Wohlhieter. 1980. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect. Immun. 28:1044-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant, T., V. Bennett-Wood, and R. M. Robins-Browne. 1998. Identification of virulence-associated characteristics in clinical isolates of Yersinia enterocolitica lacking classical virulence markers. Infect. Immun. 66:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan, K. L., and J. E. Dixon. 1990. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science 249:553-556. [DOI] [PubMed] [Google Scholar]
- 28.Hakansson, S., E. E. Galyov, R. Rosqvist, and H. Wolf-Watz. 1996. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 20:593-603. [DOI] [PubMed] [Google Scholar]
- 29.Hanski, C., U. Kutschka, H. P. Schmoranzer, M. Naumann, A. Stallmach, H. Hahn, H. Menge, and E. O. Riecken. 1989. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect. Immun. 57:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmstrom, A., R. Rosqvist, H. Wolf-Watz, and A. Forsberg. 1995. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infect. Immun. 63:2269-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbert, W. T., C. W. Petenyi, L. A. Glasgow, C. T. Uyeda, and S. A. Creighton. 1971. Yersinia pseudotuberculosis infection in the United States. Speticema, appendicitis, and mesenteric lymphadenitis. Am. J. Trop. Med. Hyg. 20:679-684. [DOI] [PubMed] [Google Scholar]
- 32.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29:915-929. [DOI] [PubMed] [Google Scholar]
- 33.Jones, M. A., M. W. Wood, P. B. Mullan, P. R. Watson, T. S. Wallis, and E. E. Galyov. 1998. Secreted effector proteins of Salmonella dublin act in concert to induce enteritis. Infect. Immun. 66:5799-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juris, S. J., A. E. Rudolph, D. Huddler, K. Orth, and J. E. Dixon. 2000. A distinctive role for the Yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc. Natl. Acad. Sci. USA 97:9431-9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesser, C. F., and S. I. Miller. 2001. Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. EMBO J. 20:1840-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung, K. Y., B. S. Reisner, and S. C. Straley. 1990. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58:3262-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian, C. J., W. S. Hwang, J. K. Kelly, and C. H. Pai. 1987. Invasiveness of Yersinia enterocolitica lacking the virulence plasmid: an in-vivo study. J. Med. Microbiol. 24:219-226. [DOI] [PubMed] [Google Scholar]
- 38.Marra, A., and R. R. Isberg. 1997. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer's patch intestinal epithelium. Infect. Immun. 65:3412-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald, C., P. O. Vacratsis, J. B. Bliska, and J. E. Dixon. 2003. The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 278:18514-18523. [DOI] [PubMed] [Google Scholar]
- 40.Mecsas, J., I. Bilis, and S. Falkow. 2001. Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis. Infect. Immun. 69:2779-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mecsas, J., B. Raupach, and S. Falkow. 1998. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 28:1269-1281. [DOI] [PubMed] [Google Scholar]
- 42.Michiels, T., P. Wattiau, R. Brasseur, J. M. Ruysschaert, and G. Cornelis. 1990. Secretion of Yop proteins by Yersiniae. Infect. Immun. 58:2840-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulder, B., T. Michiels, M. Simonet, M. P. Sory, and G. Cornelis. 1989. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect. Immun. 57:2534-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray, R. A., and C. A. Lee. 2000. Invasion genes are not required for Salmonella enterica serovar typhimurium to breach the intestinal epithelium: evidence that Salmonella pathogenicity island 1 has alternative functions during infection. Infect. Immun. 68:5050-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordfelth, R., and H. Wolf-Watz. 2001. YopB of Yersinia enterocolitica is essential for YopE translocation. Infect. Immun. 69:3516-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paff, J. R., D. A. Triplett, and T. N. Saari. 1976. Clinical and laboratory aspects of Yersinia pseudotuberculosis infections, with a report of two cases. Am. J. Clin. Pathol. 66:101-110. [DOI] [PubMed] [Google Scholar]
- 50.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fallman. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persson, C., R. Nordfelth, K. Andersson, A. Forsberg, H. Wolf-Watz, and M. Fallman. 1999. Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33:828-838. [DOI] [PubMed] [Google Scholar]
- 52.Portnoy, D. A., and S. Falkow. 1981. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J. Bacteriol. 148:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Portnoy, D. A., S. L. Moseley, and S. Falkow. 1981. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect. Immun. 31:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riley, G., and S. Toma. 1989. Detection of pathogenic Yersinia enterocolitica by using Congo red-magnesium oxalate agar medium. J. Clin. Microbiol. 27:213-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosqvist, R., I. Bolin, and H. Wolf-Watz. 1988. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect. Immun. 56:2139-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 57.Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruckdeschel, K., A. Roggenkamp, V. Lafont, P. Mangeat, J. Heesemann, and B. Rouot. 1997. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato, K. 1987. Yersinia pseudotuberculosis infection in children. Clinical manifestations and epidemiology. Contrib. Microbiol. Immunol. 9:111-116. [PubMed] [Google Scholar]
- 61.Sauvonnet, N., I. Lambermont, P. van der Bruggen, and G. R. Cornelis. 2002. YopH prevents monocyte chemoattractant protein 1 expression in macrophages and T-cell proliferation through inactivation of the phosphatidylinositol 3-kinase pathway. Mol. Microbiol. 45:805-815. [DOI] [PubMed] [Google Scholar]
- 62.Schulte, R., S. Kerneis, S. Klinke, H. Bartels, S. Preger, J. P. Kraehenbuhl, E. Pringault, and I. B. Autenrieth. 2000. Translocation of Yersinia enterocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to beta1 integrins apically expressed on M-like cells. Cell. Microbiol. 2:173-185. [DOI] [PubMed] [Google Scholar]
- 63.Simonet, M., S. Richard, and P. Berche. 1990. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skrzypek, E., C. Cowan, and S. C. Straley. 1998. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30:1051-1065. [DOI] [PubMed] [Google Scholar]
- 65.Straley, S. C., and M. L. Cibull. 1989. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL− Yersinia pestis in BALB/c mice. Infect. Immun. 57:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Bäumler. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsolis, R. M., L. G. Adams, M. J. Hantman, C. A. Scherer, T. Kimbrough, R. A. Kingsley, T. A. Ficht, S. I. Miller, and A. J. Bäumler. 2000. SspA is required for lethal Salmonella enterica serovar Typhimurium infections in calves but is not essential for diarrhea. Infect. Immun. 68:3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Viboud, G. I., and J. B. Bliska. 2001. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 70.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. The Salmonella enterica serotype typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, Z. Y., J. C. Clemens, H. L. Schubert, J. A. Stuckey, M. W. Fischer, D. M. Hume, M. A. Saper, and J. E. Dixon. 1992. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 267:23759-23766. [PubMed] [Google Scholar]
- 74.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]