Abstract
Real-time PCR and enzyme-linked immunosorbent assay were used to evaluate the ability of influenza A virus and Streptococcus pneumoniae opacity variants, either alone or in combination, to induce cytokine and chemokine genes in primary cultures of human middle ear epithelial (HMEE) cells. Following treatment with influenza A virus, the induction of gene expression, which occurred in a dose- and time-dependent manner, was strong for macrophage inflammatory protein 1α (MIP-1α) and MIP-1β; moderate for tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8; and weak for IL-1β and monocyte chemotactic peptide 1 (MCP-1). Except for TNF-α, all the gene products were detected in the cell culture supernatants. In contrast, infection of HMEE cells with S. pneumoniae alone induced low levels of mRNA expression of MIP-1α and MIP-1β and did not significantly induce the transcription of the other cytokines and chemokines examined. However, both S. pneumoniae opacity variants increased mRNA expression of MIP-1α, MIP-1β, IL-6, and MCP-1 in HMEE cells activated by a prior influenza A virus infection compared to levels in cells treated with either agent alone. Up-regulation of IL-6, IL-8, and MCP-1 mRNA expression and production by the virus in combination with opaque S. pneumoniae was two- to threefold higher than that induced by the virus combined with the transparent S. pneumoniae variant. These data indicate that the activation of HMEE cells by influenza A virus enhances the induction of cytokine and chemokine gene transcripts by S. pneumoniae and that this effect appears to be most pronounced when S. pneumoniae is in the opaque phase.
Streptococcus pneumoniae is the primary bacterial pathogen associated with otitis media (OM), accounting for 30% of the cases of this disease (5). The process whereby S. pneumoniae becomes established in the human nasopharynx and effects the transition from a colonized to a disease state in the middle ear has been the focus of intense investigation for years. S. pneumoniae-induced OM is characterized by the presence of numerous bacteria, inflammatory cells, middle ear effusion, and middle ear epithelial cell injury. It is widely accepted that the middle ear epithelium plays a crucial role, serving as a first line of defense in the interaction with invasive pathogens during OM (25). Innate proinflammatory responses induced in host cells by S. pneumoniae infection that may contribute to the OM pathology include the synthesis and release of cytokines, chemokines, and other inflammatory mediators. Extensive evidence suggests that the middle ear epithelium not only is a physical barrier but also has the potential to synthesize a variety of cytokines, including interleukin-1 (IL-1), IL-6, IL-8, and IL-10, which are recognized as being important local mediators in acute inflammation in various animal models of OM (20, 28, 33). Activation of host epithelial and endothelial cells by cytokines results in a shift in S. pneumoniae adherence to new receptors, particularly the platelet-activating factor receptor (10). Moreover, S. pneumoniae also undergoes a spontaneous phase variation between a transparent and an opaque colony phenotype (44). The transparent variant demonstrates an increased ability to adhere to respiratory epithelial cells, while the opaque phenotype is decidedly more virulent (24). Our laboratory recently demonstrated that there is a relationship between an antecedent influenza virus infection and the ability of an S. pneumoniae strain with the opaque phenotype to persist and to induce OM in the chinchilla model (41).
Considerable epidemiologic, clinical, and laboratory evidence suggests that influenza A virus promotes S. pneumoniae-induced OM (6, 16, 21). Several possible mechanisms have been proposed to explain this phenomenon, including viral compromise of eustachian tube mucosal integrity (resulting in an impaired clearance function and the development of negative middle ear pressure) and viral suppression of polymorphonuclear leukocyte function (1, 9, 17, 30, 31). Influenza A virus neuraminidase may also play a potential role in enhancing pneumococcal nasopharyngeal colonization in a murine model (22). A recent report from our laboratory indicates that influenza A virus infection enhances the exposure of asialocarbohydrate moieties in the eustachian tube and may, therefore, increase the accessibility of host cell receptors for S. pneumoniae (40). Despite these advances, much more remains to be learned about the interactions between influenza A virus, S. pneumoniae, and the host during the pathogenesis of OM at the molecular level.
To date, reports of the effects of S. pneumoniae and influenza A virus on the induction of cytokine gene expression in human cells have all focused on bronchial epithelial cells, neutrophils, monocytes, and macrophages. These studies have shown that influenza A virus stimulated the release of IL-6, IL-8, and RANTES from cultured primary human bronchial epithelial cells (2). Influenza A virus-infected monocytes/macrophages secrete macrophage inflammatory protein 1α (MIP-1α), MIP-1β, RANTES, monocyte chemotactic peptide 1 (MCP-1), MCP-3, MIP-3α, and interferon-inducible protein 10 (IP-10), whereas the production of IL-8 appears to be limited (7, 27, 36). S. pneumoniae stimulates tumor necrosis factor alpha (TNF-α) and IL-10 production by human monocytes, whereas S. pneumoniae is a less potent inducer of IL-8 and MIP-1α production in human peripheral blood neutrophils (19, 43). The direct effects of these two pathogens on cytokine and chemokine gene expression and production by human middle ear epithelial (HMEE) cells have never been reported. Our laboratory has examined the relative abilities of nonviable intact nontypeable Haemophilus influenzae (NTHI) parent strain 2019 and its lipooligosaccharide-deficient mutants B29 (htrB gene disruption with alteration of both lipid A and oligosaccharide) and DK-1 (rfaD gene disruption with truncated oligosaccharide) to stimulate HMEE cell cytokine gene expression and protein production during in vitro culture (38). Host cell receptors capable of transducing signals originated by gram-positive bacteria, which are devoid of lipopolysaccharide, have only recently started to be investigated (11, 35, 45), and much less is known regarding the middle ear epithelial cell activation mechanisms initiated by S. pneumoniae or influenza A virus. The purpose of this investigation was to evaluate at the transcriptional level the effect of influenza A virus and S. pneumoniae opaque and transparent variants, either alone or in combination, on the induction of various cytokine and chemokine genes by HMEE cells in vitro. Proinflammatory cytokine and chemokine gene expression in HMEE cells was determined by using a novel, highly accurate, and reproducible real-time-PCR amplification technique. Once the impact of these two pathogens on OM pathogenesis can be defined at a molecular level, potential new targets for future protection and intervention strategies can be identified.
MATERIALS AND METHODS
Virus.
Influenza virus A/Alaskan (6/77) (H3N2) was propagated and its titers were determined by a plaque assay as was previously described in detail and previously used by our laboratory (9, 31, 39, 41). Our primary cultures of HMEE cells were infected with influenza A virus at the multiplicities of infection (MOIs) of 0.01 and 0.1 in serum-free alpha minimal essential medium (MEMα) (Gibco BRL, Gaithersburg, Md.) with 250 μg of bovine serum albumin (BSA)/ml, 10 μg of streptomycin/ml, and 5 U of penicillin/ml. The MOI is defined as the number of PFU of the virus per HMEE cell.
Bacteria.
S. pneumoniae type 6A (EF 3114), kindly provided by B. Andersson, Department of Clinical Immunology, University of Gröteborg, Gröteborg, Sweden, has been described in detail previously (3). This strain has been used in previous reports from our laboratory (39, 40). The isogenic opaque and transparent variants of S. pneumoniae type 6A were also used in a previous report from our laboratory (41) and originally isolated by Jeffery Weiser, Children's Hospital of Philadelphia. The opacity phenotype was confirmed prior to infection according to the method established by Weiser et al. (44). S. pneumoniae type 6A variants with the opaque and transparent phenotypes were cultured overnight on Columbia agar plates. The bacteria were harvested by centrifugation at 6,700 × g for 10 min, washed twice in phosphate-buffered saline (pH 7.2), and resuspended in serum-free MEMα containing 250 μg of BSA/ml. The concentration of S. pneumoniae was adjusted by densitometry to a concentration of 107 cells per ml for each experiment and confirmed by serial dilution and quantitative culture.
Cell culture.
The primary culture of HMEE cells had been established in our laboratory and was reported previously (38). Cells were cultured in MEMα (Gibco BRL) buffered with 155 mg of NaHCO3 and 3.6 g of HEPES/liter supplemented with 5 mg of insulin/liter, 2 mg transferrin/liter, 500 μg of hydrocortisone/liter, 25 ng of epithelial growth factor/ml, 10 μg of streptomycin/ml, 5 U of penicillin/ml, 250 ng of amphotericin B/ml, and 10% fetal bovine serum at 37°C. All medium additives were from Collaborative Research (Boston, Mass.) except low-level-endotoxin fetal bovine serum, which was from HyClone (South Logan, Utah).
Stimulation of HMEE cells.
HMEE cells were seeded at a concentration of 2.5 × 105 cells per 150-cm2 tissue culture flask (Costar Corp., Cambridge, Mass.) and grown to approximately 80% confluence. The cells were washed, and the medium was replaced with the serum-free MEMα containing 250 μg of BSA/ml and antibiotics and incubated for 24 h prior to infection with the virus. The cells were infected with influenza virus A/Alaska (6/77) (H3N2) suspended in MEMα with 250 μg of BSA/ml and antibiotics at MOIs of 0.01 and 0.1. Control cell cultures were incubated with the medium alone. After a 1-h incubation period at 37°C, the virus was removed and the infected cells were washed with medium and incubated in fresh serum-free medium containing 250 μg of BSA/ml and antibiotics for 4, 8, 12, and 24 h at 37°C. Culture media were collected, and the infected cells were harvested at different times after virus infection. In the experiments involving HMEE cells infected with the combination of both influenza A virus and either the opaque or transparent S. pneumoniae variant, HMEE cells were cultured in supplemented MEMα and infected with the influenza A virus at an MOI of 0.01 or 0.1 as described above. At 24 h after virus infection, HMEE cell monolayers were washed with culture medium and the cells were incubated for 4 h at 37°C with a suspension of either opaque or transparent S. pneumoniae at an MOI of 10 (10 CFU per HMEE cell) in fresh serum-free culture media with 250 μg of BSA/ml. After a 4-h incubation, the supernatants from the cell cultures were collected, the monolayers were washed three times with phosphate-buffered saline (pH 7.2), and the cells were harvested for total RNA extraction. Based on our preliminary studies, the selected range of MOIs for the virus and S. pneumoniae did not significantly affect HMEE cell viability based on trypan blue exclusion analysis. This experiment included a control group (treated with culture medium only), an influenza A virus-only group, an S. pneumoniae-only group, and a group treated with influenza A virus in combination with either the S. pneumoniae opaque or transparent variant.
Quantitation of HMEE cell cytokine transcripts by real-time PCR.
Real-time PCR is a novel method that allows for a rapid, accurate, and precise quantitation of gene transcripts (15). Real-time-PCR assays were performed to specifically quantitate human cytokine (TNF-α, IL-1β, and IL-6) and chemokine (IL-8, MCP-1, MIP-1β, and MIP-1α) transcripts as we have described previously (38). Briefly, total cellular RNA was extracted using an RNeasy Mini kit (QIAGEN, Valencia, Calif.), and cDNAs were synthesized using the Superscript preamplification system (Gibco BRL). Each cDNA sample was used as a template for a real-time-PCR amplification mixture containing forward and reverse primers and probes for the target cytokine and chemokine genes and for 18S rRNA (internal control) and 2× TaqMan Universal PCR Master Mix obtained from Applied Biosystems (Foster City, Calif.). Real-time-PCR amplifications were performed on an Applied Biosystems Prism 7700 sequence detector according to the manufacturer's instructions. Predicted cycle threshold values were exported directly into Excel worksheets for analysis. Relative changes in gene expression were determined by the 2−ΔΔCΤ method as described previously (26) and reported as the difference (n-fold) relative to the value for a calibrator cDNA (control, unstimulated HMEE cells) prepared in parallel with the experimental cDNAs.
ELISAs.
The cell culture supernatants were collected at each time point prior to harvesting of the cells, centrifuged at 500 × g, and frozen at −70°C. Cytokines and chemokines were measured with commercial enzyme-linked immunosorbent assay (ELISA) kits (Quantikine; R&D Systems, Minneapolis, Minn.) according to the manufacture's instructions. The cytokine and chemokine concentrations per milliliter of the culture supernatants were interpolated from standard curves generated by using human recombinant cytokines and chemokines.
Statistical analysis.
The arithmetic means ± the standard errors of the means (SEM) of increases (n-fold) in cytokine and chemokine mRNA expression over the corresponding values from resting cells, based on results of three separate experiments, were calculated at each sample time. Statistics were determined using Student's t test, and a P of <0.05 was used as the level of significance for all analyses.
RESULTS
Cytokine and chemokine gene expression in HMEE cells induced by influenza A virus.
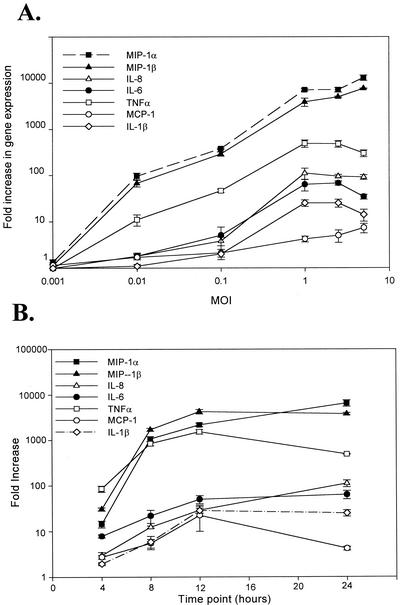
To study influenza A virus-induced cytokine and chemokine gene expression, confluent HMEE cells were infected with influenza A virus at MOIs of 0.001 to 5.0 in serum-free MEMα for 1 h at 37°C. The medium was removed, and the cells were washed and incubated with fresh culture medium for 24 h. Stimulation of HMEE cells with influenza A virus resulted in the activation of all seven cytokine and chemokine genes examined in this study. The induction of these genes in HMEE cells by influenza A virus was dose dependent (Fig. 1A). Influenza A virus did not induce cytokine and chemokine gene expression at an MOI of 0.001. However, at an MOI of 0.01, influenza A virus increased MIP-1α and MIP-1β mRNA expression 100- and 70-fold, respectively, over levels of expression in HMEE cells not infected with virus. TNF-α gene expression increased only 10-fold. Overall, chemokines MIP-1α and MIP-1β were the most highly activated proinflammatory gene products induced in HMEE cells by influenza A virus. The virus induced maxima of 13,000- and 7,600-fold increases in the expression of MIP-1α and MIP-1β transcripts, respectively, at an MOI of 5.0. Influenza A virus also induced moderate increases in IL-6, IL-8, and TNF-α mRNA expression. Levels of IL-1β and MCP-1 gene expression were lower and also dependent on the concentration of the virus added to the cells.
FIG. 1.
Effect of influenza A virus on cytokine and chemokine gene expression in HMEE cells. (A) Dose responses to MOIs of influenza A virus versus gene expression by HMEE cells at 24 h after virus infection; (B) kinetics of cytokine and chemokine gene expression in HMEE cells infected with influenza A virus at an MOI of 1.0. Results are mean increases (n-fold) in gene transcript levels (± SEM) from three separate experiments.
Additional real-time-PCR experiments were performed to determine the kinetics of cytokine and chemokine gene expression in HMEE cells induced by influenza A virus (Fig. 1B). HMEE cells were collected at 4, 8, 12, and 24 h after virus infection at an MOI of 1. Influenza A virus stimulated a rapid and sustained increase in MIP-1α, MIP-1β, and TNF-α mRNA that was detected at 4 h and peaked at 12 or 24 h. Influenza A virus also induced a moderate up-regulation of IL-6 and IL-8 gene expression at 8 h and of IL-1β and MCP-1 gene expression at 12 h. The increase of these cytokine and chemokine transcripts plateaued by 24 h. Similar gene expression kinetics data were obtained from cells induced by virus infection at an MOI of 0.1 (data not shown).
Cytokine and chemokine secretion in influenza A virus-infected HMEE cells.
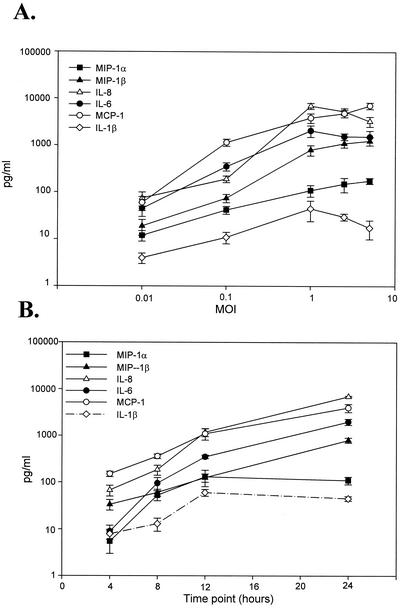
As described in the previous report from our laboratory (38), HMEE cells maintained with culture medium only (control group) constitutively produce low levels of IL-6, IL-8, and MCP-1 but not IL-1β, MIP-1β, and TNF-α in their supernatants.
Exposure of HMEE cells to different MOIs of influenza A virus for 24 h demonstrated that influenza A virus stimulated secretion of cytokines and chemokines in a dose-dependent manner (Fig. 2A). The three constitutively produced inflammatory mediators (IL-6, IL-8, and MCP-1) were secreted at higher concentrations than those of MIP-1α and IL-1β, which were not constitutively produced by HMEE cells. The peak response of MCP-1 (7,000 pg/ml) detected at an MOI of 5 was 117 times higher than that induced at an MOI of 0.01, whereas the peak concentrations of IL-8 (7,000 pg/ml) and IL-6 (2,100 pg/ml) induced at an MOI of 1 were 93 and 43 times higher, respectively, than those induced at an MOI of 0.01. The peak secretions of MIP-1α and MIP-1β at an MOI of 5 were 15 and 66 times higher than that induced by the virus at an MOI of 0.01. Following influenza A virus infection, IL-1β expression occurred in a dose-dependent manner up to an MOI of 1, but the concentration levels slightly decreased at MOIs of 2.5 and 5. In this study, TNF-α was not detectable in supernatants from the virus-infected or control group.
FIG. 2.
Effects of influenza A virus MOIs on cytokine and chemokine secretion in HMEE cells. (A) Dose responses to MOIs of influenza A virus infection on cytokine and chemokine secretions 24 h after virus infection; (B) kinetics of cytokine and chemokine secretion in HMEE cells infected with influenza A virus at an MOI of 1.0. Results are mean concentrations (± SEM) in cell supernatants from two duplicate wells from ELISAs of three separate experiments.
To investigate the kinetics of cytokine and chemokine production in HMEE cells exposed to influenza A virus at MOIs of 0.1 and 1.0, the amounts of cytokines and chemokines that were released at 4, 8, 12, and 24 h after the introduction of the virus were measured by ELISA. Control HMEE cells constitutively secreted IL-6 (3 to 10 pg/ml), IL-8 (20 to 35 pg/ml), and MCP-1 (23 to 126 pg/ml) in the cell culture supernatants over the 24-h experiment period. Figure 2B shows that HMEE cells secreted IL-6, IL-8, MIP-1β, and MCP-1 in a time-dependent manner following infection with the virus. As early as 4 h after stimulation with the virus at an MOI of 1.0, significantly elevated levels of IL-8 (68 pg/ml), MCP-1 (150 pg/ml), and MIP-1β (34 pg/ml) were found in the supernatants. The secretion of IL-6, IL-8, MCP-1, and MIP-1β peaked at 24 h following virus infection. The concentrations of MIP-1α and IL-1β peaked at 12 h (130 and 60 pg/ml, respectively) and then slightly decreased at 24 h. Similar kinetics for cytokine and chemokine secretion patterns were obtained following induction by the virus at an MOI of 0.1 (data not shown).
The effect of an antecedent influenza A virus infection on cytokine and chemokine gene expression in HMEE cells induced by S. pneumoniae opacity variants.
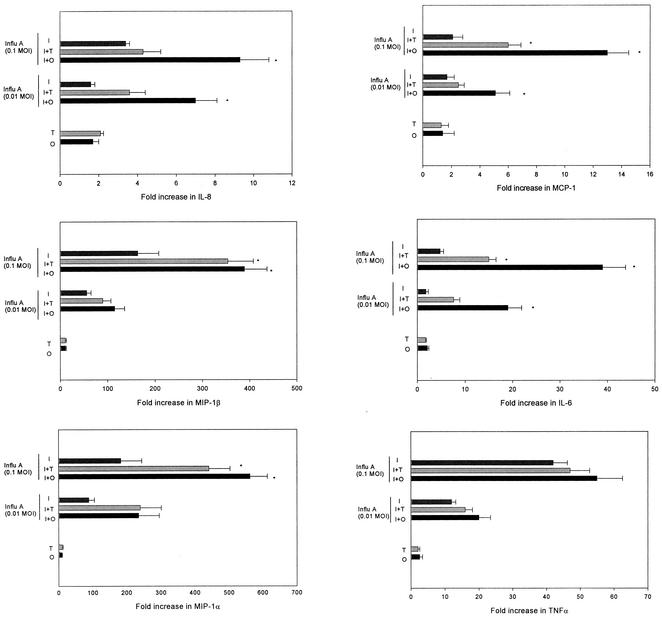
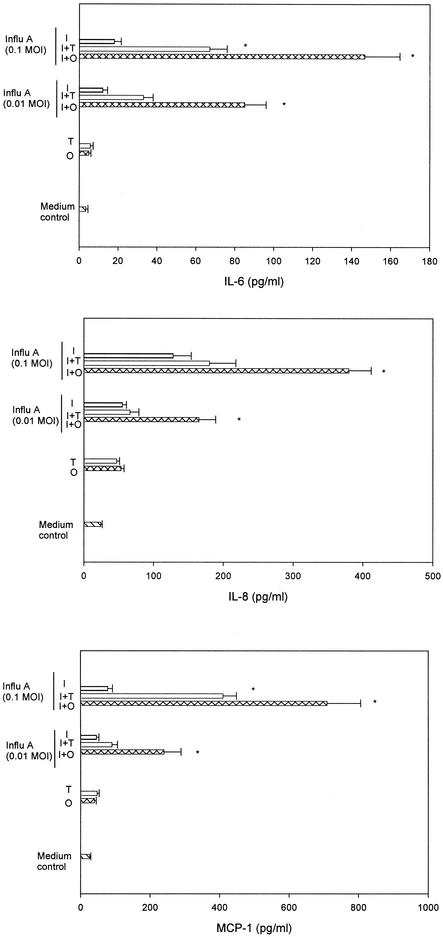
To further analyze the molecular mechanisms of the synergistic effect of influenza A virus and S. pneumoniae in the pathogenesis of OM, we analyzed whether an antecedent influenza A virus infection played a role in cytokine and chemokine gene expression in HMEE cells induced by an S. pneumoniae type 6A opaque or transparent variant. HMEE cells infected with influenza A virus at MOIs of 0.01 and 0.1 were treated with the live S. pneumoniae opacity variant at an MOI of 10 for 4 h, and cytokine and chemokine gene expression was evaluated by real-time PCR. During the 4-h incubation period, the concentration of both the opaque and transparent S. pneumoniae variants increased slightly and exhibited a <1-log-unit increase by the end of the experiment. S. pneumoniae alone induced low levels of mRNA expression of MIP-1α and MIP-1β and did not significantly induce other cytokine gene expression or secretion by HMEE cells under these experimental conditions. Influenza A virus alone at these low MOIs induced a limited up-regulation of these gene transcripts. However, the combination of influenza A virus and the S. pneumoniae opaque variant significantly induced mRNA expression of IL-8, MCP-1, and IL-6 compared to that induced by S. pneumoniae alone or virus alone at both MOIs, 0.01 and 0.1. The combination of the virus and the S. pneumoniae transparent variant only significantly induced IL-6 and MCP-1 mRNA expression compared to that induced by S. pneumoniae alone or virus alone at an MOI of 0.1. Treatment of influenza A virus-infected HMEE cells with either the S. pneumoniae opaque or transparent variant induced up-regulation of MIP-1β and MIP-1α transcripts compared to levels induced by S. pneumoniae alone or influenza A virus alone at an MOI of 0.1 but had no effect on TNF-α (Fig. 3) or IL-1β (data not shown) mRNA synthesis compared with levels in the cells infected with virus alone. Moreover, infection with a combination of influenza A virus and the opaque variant of S. pneumoniae resulted in a significant increase in the induction (two- to threefold-higher up-regulation) of IL-6, IL-8, and MCP-1 mRNA expression in HMEE cells compared to levels induced by the virus and the S. pneumoniae variant with the transparent phenotype. To validate the gene expression data, the products of these three cytokine and chemokine genes in the supernatants were determined by ELISA. The concentrations of IL-6, IL-8, and MCP-1 released after a 4-h incubation period were compared. The combination of influenza A virus and either the S. pneumoniae opaque or transparent variant significantly increased the levels of IL-6 and MCP-1 in the culture supernatants compared to levels produced by the virus alone at an MOI of 0.1. However, the concentrations of IL-6 and MCP-1 produced by the combination of the virus and the S. pneumoniae variant with the opaque phenotype were two- to threefold higher than concentrations produced by the transparent variant (Fig. 4). Moreover, influenza A virus-infected HMEE cells treated with the S. pneumoniae variant with the opaque phenotype induced significantly more IL-8 secretion than the virus-only group. In contrast, there was no significant effect of the virus in combination with the transparent phenotype on the secretion of IL-8. The products of the MIP-1α, MIP-1 β, IL-1β, and TNF-α genes were not detectable in the supernatants from the cells treated with both pathogens.
FIG. 3.
Induction of cytokine and chemokine genes in HMEE cells by opaque S. pneumoniae alone (O), transparent S. pneumoniae alone (T), influenza A virus (Influ A) at MOIs of 0.01 and 0.1 alone (I), or a combination of a previous infection with influenza A virus and opaque S. pneumoniae (I+O) or transparent S. pneumoniae (I+T). Results are mean increases (n-fold) in the gene transcript levels (± SEM) from three separate experiments. *, P < 0.05, compared to results of treatment with influenza A virus alone.
FIG. 4.
Secretions of IL-6, IL-8, and MCP-1 by HMEE cells stimulated with opaque S. pneumoniae alone (O), transparent S. pneumoniae alone (T), influenza A virus alone (I), or a combination of a prior infection of influenza A virus (Influ A) at MOIs of 0.01 and 0.1 and opaque S. pneumoniae (I+O) or transparent S. pneumoniae (I+T). Results are mean concentrations (± SEM) in cell supernatants from duplicate wells from ELISAs of three separate experiments. *, P < 0.05, compared to results of treatment with influenza A virus alone.
Preliminary data indicated that a 4-h incubation of HMEE cells with live S. pneumoniae at an MOI of 10 was optimal. Incubation with live bacteria beyond 4 h impacted cell viability; therefore, in order to maintain HMEE cell viability for longer incubation periods with S. pneumoniae, we stimulated HMEE cells with a heat-killed S. pneumoniae opacity variant at an MOI of 100 for up to 24 h. Gene expression and the protein products were evaluated. In contrast to what occurred with killed NTHI as previously reported from our laboratory (38), heat-killed S. pneumoniae opaque and transparent variants did not induce significant, high-level gene expression by HMEE cells. There was also no significant induction of gene expression in influenza A virus-infected HMEE cells treated with a killed S. pneumoniae opacity variant compared to that induced by either pathogen alone (data not shown).
DISCUSSION
In this study, we have focused on the immediate, innate immune response of HMEE cells during experimental infection with influenza A virus or the combination of the virus and an S. pneumoniae opacity variant. HMEE cells retain many features of respiratory epithelial cells and have been previously shown to be susceptible to NTHI lipooligosaccharide (38). The present study demonstrates that HMEE cells are also highly susceptible to influenza A virus. The most strongly induced chemokine genes are those for MIP-1α and MIP-1 β. The rapid induction of expression of these two chemokines in HMEE cells, which preceded or paralleled cytokine induction, suggests that these chemokines were most likely induced directly by influenza A virus rather than indirectly by the virus induction of TNF-α and IL-1. Secretion of the chemokine proteins IL-8 and MCP-1 was also highly induced by the virus. Our data corroborate results in a previous clinical report showing that MIP-1α and -1β, IL-8, and MCP-1 have been detected in nasopharyngeal secretions of influenza A virus-infected individuals (13, 34). IL-8, which belongs to the CXC chemokine subfamily, is chemotactic primarily for neutrophils (and for T cells, NK cells, endothelial cells, basophils, and eosinophils) and stimulates neutrophil degranulation, adhesion, and microbicidal activity (37). MIP-1α, MIP-1β, and MCP-1, which belong to the CC chemokine subfamily, are chemotactic primarily for monocytes/macrophages and distinct populations of lymphocytes (29, 32). In addition, TNF-α is the most strongly induced cytokine, followed by IL-6 and IL-1β. IL-6 has been shown to be essentially protective in infection and is linked to its capacity to induce the release of acute-phase proteins (8). TNF-α contributes to the acute phase of the inflammatory response, primes the immune system for rapid activity, and promotes the release of other cytokines (12). These results are consistent with a highly inflammatory response in influenza A virus-induced OM, with extensive infiltration of neutrophils and monocytes (9). Our results show for the first time that HMEE cells participate in the network of chemokine and cytokine response to influenza A virus infection and provide evidence for a new aspect of innate, mucosal immunity.
Our data demonstrate that S. pneumoniae is a weak inducer of cytokine and chemokine gene expression and production by HMEE cells during the 4-h incubation period. A recent report also shows that S. pneumoniae is a less potent inducer of IL-8 and MIP-1α production in human peripheral blood neutrophils than Salmonella enterica serovar Typhimurium, which suggests that signals in addition to phagocytosis are required for the up-regulation of these two chemokine genes (19). Our results are in line with earlier reports indicating that the presence of pneumococci in a tissue in and of itself is not sufficient to cause an inflammatory response, even when the organism is introduced into a sterile site such as the lung or the subarachnoid space. In such healthy tissues, a challenge with approximately 100,000 bacteria per ml is required to trigger an inflammatory response. In contrast, the pneumococcus becomes invasive with ensuing inflammation with as few as 20 bacteria if there is an antecedent proinflammatory signal such as a cytokine or a viral infection (18).
IL-6, IL-8, and MCP-1 were constitutively produced by HMEE cells as previously reported by our laboratory (38). MIP-1α, MIP-1β, and IL-1β proteins were not detected by ELISA in cell culture supernatants of the control group (cells incubated with MEMα only) but were detected in the cell supernatants from HMEE cells stimulated with influenza A virus. However, the secretion of MIP-1α, MIP-1β, and IL-1β proteins in the culture medium was not detected in HMEE cells infected with both influenza A virus and S. pneumoniae variants with the opaque and transparent phenotypes. TNF-α protein was not detectable in either the control or viral infection groups with the ELISA used in this study. We have reported that the up-regulation of IL-1β, MIP-1β, and TNF-α transcripts is evident in HMEE cells infected with nonviable NTHI but that their products (protein) are not detectable in the culture supernatants (38). The reason for this is not entirely clear. The previous study from our laboratory reported that TNF-α was detected by Western blotting in nuclear extracts of HMEE cells after infection with NTHI, which suggests that HMEE cells may produce a nonsecreted form of TNF-α during the early phase of infection in vitro (4, 38). Anther possible explanation is that the secretory machinery required for excretion may be affected by bacterial cell wall components or cytotoxins. Data are available to suggest that incubation of respiratory epithelial cells with pneumococci causes a progressive change in the morphology of the cells, resulting in cell separation and eventually complete destruction of the monolayer (14). Moreover, pneumococci are known to induce apoptosis in host cells (46). Even though we did not observe any significant cell damage in HMEE cells during infection, pneumococcal cytopathic and apoptotic effects on the cells may affect intracellular processing of cytokine and chemokine secretion.
The present data also demonstrate that HMEE cells which were activated by influenza A virus prior to exposure to the S. pneumoniae opaque variant express significantly higher levels of mRNA and release higher levels of IL-6, IL-8, and MCP-1 than cells exposed to the virus and the transparent variant. However, there was no statistical difference in the levels of induction of MIP-1α, MIP-1β, IL-1β, and TNF-α transcripts in HMEE cells infected with both the influenza A virus and the S. pneumoniae variant with the opaque or transparent phenotype. Previous reports demonstrate that opacity variants do not change the physical characteristics of the peptidoglycan stem peptides but that the opaque phenotype is associated with enhanced production of capsular polysaccharide and pneumococcal surface protein A (24), which inhibits complement activation, thereby reducing the effectiveness of complement receptor-mediated pathways for clearance (42). Our present results indicate that interactions between the influenza A virus-activated HMEE cells and S. pneumoniae opacity variants are complex and remain to be fully elucidated. Several explanations are plausible. Influenza A virus may induce viral glycoproteins and other changes on the host cell membrane that may cause the glycoproteins to function as the new receptor for S. pneumoniae, or the bacteria may produce proteolytic enzymes, which may be involved in the cleavage of influenza A virus hemagglutinin, rending the virus more pathogenic. Nevertheless, the higher level of up-regulation of cytokine and chemokine genes by influenza A virus and opaque S. pneumoniae in HMEE cells may account for the enhanced inflammatory response observed in the middle ear in the chinchilla OM model (41).
Although our studies focused on the role of influenza A virus and S. pneumoniae opacity variants in the induction of seven chemokine and cytokine genes in HMEE cells, the molecular mechanisms of intracellular signaling pathways involved in the gene regulations in HMEE cells have yet to be described. Nuclear factor κB (NF-κB), activating protein 1, interferon regulatory factors, and signal transducers and activators of transcription are all involved in the induction of chemokine and cytokine gene expression and have been shown to be activated by influenza A virus infection (23). Future studies of signaling intermediates and pathways may identify important targets for therapeutic intervention in OM.
In conclusion, these data indicate that the activation of HMEE cells by influenza A virus enhances the induction of cytokine and chemokine gene transcripts by S. pneumoniae and that this effect appears to be most pronounced when S. pneumoniae is in the opaque phase.
Acknowledgments
This study was supported, in part, by a grant from the NIDCD/NIH (R01 DC3105-06).
Editor: J. N. Weiser
REFERENCES
- 1.Abramson, J. S., G. S. Giebink, and P. G. Quie. 1982. Influenza A virus-induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect. Immun. 36:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi, M., S. Matsukura, H. Tokunaga, and F. Kokubu. 1997. Expression of cytokines on human bronchial epithelial cells induced by influenza virus A. Int. Arch. Allergy Immunol. 113:307-311. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, B., J. Dahmen, T. Frejd, H. Leffler, G. Magnusson, G. Noori, and C. S. Eden. 1983. Identification of a disaccharide unit of glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cell. J. Exp. Med. 158:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basolo, F., P. G. Conaldes, L. Fiore, S. Calvo, and A. Toniolo. 1993. Normal breast epithelial cells produce interleukin 6 and 8 together with tumor necrosis factor: defective IL6 expression in mammary carcinoma. Int. J. Cancer 55:926-930. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone, C. D., J. S. Stephenson, and L. M. Martin. 1992. Ten-year review of otitis media pathogens. Pediatr. Infect. Dis. J. 11(Suppl. 8):S7-S11. [DOI] [PubMed] [Google Scholar]
- 6.Buchman, C. A., W. J. Doyle, D. P. Skoner, J. C. Post, C. M. Alper, J. T. Seroky, K. Anderson, R. A. Preston, F. G. Hayden, P. Fireman, and G. D. Ehrilich. 1995. Influenza A virus-induced acute otitis media. J. Infect. Dis. 172:1348-1351. [DOI] [PubMed] [Google Scholar]
- 7.Bussfeld, D., A. Kaufmann, R. G. Meyer, D. Gemsa, and H. Sprenger. 1998. Differential mononuclear leukocyte attracting chemokine production after stimulation with active and inactivated influenza A virus. Cell. Immunol. 186:1-7. [DOI] [PubMed] [Google Scholar]
- 8.Cavaillon, J. M. 1999. Pathophysiological role of pro- and anti-inflammatory cytokines in sepsis. Sepsis 2:127-140. [Google Scholar]
- 9.Chung, M. H., S. R. Griffith, K. H. Park, D. J. Lim, and T. F. DeMaria. 1993. Cytological and histological changes in the middle ear after inoculation of influenza A virus. Acta Otolaryngol. 113:81-87. [DOI] [PubMed] [Google Scholar]
- 10.Cundell, D. R., N. P. Gerard, C. Gerard, I. Idanpaan-Heikkila, and E. Tuomanen. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 11.Dziarski, R., A. J. Ulmer, and D. Gupta. 2000. Interactions of CD14 with components of Gram-positive bacteria. Chem. Immunol. 74:83-107. [DOI] [PubMed] [Google Scholar]
- 12.Fong, Y., and S. F. Lowry. 1990. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin. Immunol. Immunopathol. 55:157-170. [DOI] [PubMed] [Google Scholar]
- 13.Fritz, R. S., F. G. Hayden, D. P. Calfee, L. M. Cass, A. W. Peng, W. G. Alvord, W. Strober, and S. E. Straus. 1999. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J. Infect. Dis. 180:586-593. [DOI] [PubMed] [Google Scholar]
- 14.Geelen, S., C. Bhattacharyya, and E. Tuomanen. 1993. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect. Immun. 61:1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, U. E., C. A. Heid, and P. M. Williams. 1996. A novel method for real time quantitative RT-PCR. Genome Res. 6:995.. [DOI] [PubMed] [Google Scholar]
- 16.Giebink, G. S., I. K. Berzins, S. C. Marker, and G. Schiffman. 1980. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect. Immun. 30:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giebink, G. S., M. L. Ripley, and P. F. Wright. 1987. Eustachian tube histopathology during experimental influenza A virus infection in the chinchilla. Ann. Otol. Rhinol. Laryngol. 96:199-206. [DOI] [PubMed] [Google Scholar]
- 18.Gosink, K., and E. Tuomanen. 2000. Streptococcus pneumoniae: invasion and inflammation, p. 214-224. In V. A. Fischetti et al. (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 19.Hachicha, M., P. Rathanaswami, P. H. Naccache, and S. R. McColl. 1998. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J. Immunol. 160:449-454. [PubMed] [Google Scholar]
- 20.Hebda, P. A., C. M. Alper, W. J. Doyle, G. J. Burckart, W. F. Diven, and A. Zeevi. 1998. Upregulation of messenger RNA for inflammatory cytokines in middle ear mucosa in a rat model of acute otitis media. Ann. Otol. Rhinol. Laryngol. 107:501-507. [DOI] [PubMed] [Google Scholar]
- 21.Heikkinen, T. 2001. The role of respiratory viruses in otitis media. Vaccine 19:S51-S55. [DOI] [PubMed] [Google Scholar]
- 22.Hirano, T., Y. Kurono, I. Ichimiya, M. Suzuki, and G. Mogi. 1999. Effects of influenza A virus on lectin-binding patterns in murine nasopharyngeal mucosa and on bacterial colonization. Otolaryngol. Head Neck Surg. 121:616-621. [DOI] [PubMed] [Google Scholar]
- 23.Julkunen, I., T. Sarenva, J. Pirhonen, T. Ronni, K. Melen, and S. Matikainen. 2001. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 12:171-180. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 25.Lim, D. J., Y. M. Chun, H. Y. Lee, S. K. Moon, K. H. Chang, J. D. Li, and A. Andalibi. 2001. Cell biology of tubotympanum in relation to pathogenesis of otitis media—a review. Vaccine 19:S17-S25. [DOI] [PubMed] [Google Scholar]
- 26.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 27.Matikainen, S., J. Pirhonen, M. Miettinen, A. Lehtonen, C. Govenius-Vintola, T. Sareneva, and I. Julkunen. 2000. Influenza A and Sendai viruses induce differential chemokine gene expression and transcription factor activation in human macrophages. Virology 276:138-147. [DOI] [PubMed] [Google Scholar]
- 28.Melhus, A., and A. F. Ryan. 2000. Expression of cytokine genes during pneumococcal and nontypeable Haemophilus influenzae acute otitis media in the rat. Infect. Immun. 68:4024-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menten, P., A. Wuyts, and J. V. Damme. 2002. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 13:455-481. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi, Y., Y. Nakai, Y. Esaki, Y. Ohno, Y. Sugiura, and H. Okamoto. 1991. Influenza A virus-induced otitis media and mucociliary dysfunction in the guinea pig. Acta Otolaryngol. 486:135-148. [DOI] [PubMed] [Google Scholar]
- 31.Park, K., L. O. Bakaletz, J. M. Coticchia, and D. J. Lim. 1993. Effect of influenza A virus on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann. Otol. Rhinol. Laryngol. 102:551-558. [DOI] [PubMed] [Google Scholar]
- 32.Rollins, S. J. 1992. “Oh, no. Not another cytokine.” MCP-1 and respiratory disease. Am. J. Respir. Cell Mol. Biol. 7:126-127. [DOI] [PubMed] [Google Scholar]
- 33.Sato, K., C. L. Liebeler, M. K. Quartey, C. T. Le, and G. S. Giebink. 1999. Middle ear fluid cytokine and inflammatory cell kinetics in the chinchilla otitis media model. Infect. Immun. 67:1943-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skoner, D. P., D. A. Gentile, A. Patel, and W. J. Doyle. 1999. Evidence for cytokine mediation of disease expression in adults experimentally infected with influenza A virus. J. Infect. Dis. 180:10-14. [DOI] [PubMed] [Google Scholar]
- 35.Spellerberg, B., C. Rosenow, W. Sha, and E. Tuomanen. 1996. Pneumococcal cell wall activates NF-kappa-B in human monocytes: aspects distinct from endotoxin. Microb. Pathog. 20:309-317. [DOI] [PubMed] [Google Scholar]
- 36.Sprenger, H., R. G. Meyer, A. Kaufmann, D. Bussfeld, E. Rischkowsky, and D. Gemsa. 1996. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J. Exp. Med. 184:1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storgaard, M., K. Larsen, S. Blegvad, H. Nodgaard, T. Ovesen, P. L. Anderson, and N. Obet. 1997. Interleukin-8 and chemotactic activity of middle ear effusions. J. Infect. Dis. 175:474-477. [DOI] [PubMed] [Google Scholar]
- 38.Tong, H. H., Y. Chen, M. James, J. Van Deusen, D. B. Welling, and T. F. DeMaria. 2001. Expression of cytokine and chemokine genes by human middle ear epithelial cells induced by formalin-killed Haemophilus influenzae or its lipooligosaccharide htrB and rfaD mutants. Infect. Immun. 69:3678-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong, H. H., L. M. Fisher, G. M. Kosunick, and T. F. DeMaria. 2000. Effect of adenovirus type 1 and influenza A virus on Streptococcus pneumoniae nasopharyngeal colonization and otitis media in the chinchilla. Ann. Otol. Rhinol. Laryngol. 109:1021-1027. [DOI] [PubMed] [Google Scholar]
- 40.Tong, H. H., I. Grants, X. Liu, and T. F. DeMaria. 2002. Comparison of alteration of cell surface carbohydrates of the chinchilla tubotympanum and colonial opacity phenotype of Streptococcus pneumoniae during experimental pneumococcal otitis media with or without an antecedent influenza A virus infection. Infect. Immun. 70:4292-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong, H. H., J. N. Weiser, M. A. James, and T. F. DeMaria. 2001. Effect of influenza A virus on nasopharyngeal colonization and otitis media induced by transparent or opaque phenotype variants of Streptococcus pneumoniae in the chinchilla model. Infect. Immun. 69:602-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu, A. T., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Furth, A. M., E. M. Verhard-Seijmonsbergen, J. A. M. Langermans, J. T. Van Dissel, and R. Van Furth. 1999. Anti-CD14 monoclonal antibodies inhibit the production of tumor necrosis factor alpha and interleukin-10 by human monocytes stimulated with killed or live Haemophilus influenzae or Streptococcus pneumoniae organisms. Infect. Immun. 67:3714-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiser, J. N., B. Austrian, P. K. Sreenivasan, and H. B. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 46.Zysk, G., W. Bruck, J. Gerber, Y. Bruck, H. Prange, and R. Nau. 1996. Anti-inflammatory treatment influences neuronal apoptosis cell death in the dentate gyrus in experimental pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 55:722-728. [DOI] [PubMed] [Google Scholar]