Abstract
Previous studies have demonstrated that female reproductive hormones influence chlamydial infection both in vivo and in vitro. Due to the reduced availability of human genital tissues for research purposes, an alternative hormone-responsive model system was sought to study chlamydial pathogenesis. Mature female swine eliminated from breeding programs were selected as the animals of choice because of the similarity of a sexually transmitted disease syndrome and sequelae in swine to a disease syndrome and sequelae found in humans, because of the near identity of a natural infectious chlamydial isolate from swine to Chlamydia trachomatis serovar D from humans, and because a pig's epithelial cell physiology and the mean length of its estrous cycle are similar to those in humans. Epithelial cells from the cervix, uterus, and horns of the uterus were isolated, cultivated in vitro in Dulbecco's minimum essential medium-Hanks' F-12 (DMEM-F-12) medium with and without exogenous hormone supplementation, and analyzed for Chlamydia suis S-45 infectivity. The distribution of chlamydial inclusions in swine epithelial cells was uneven and was influenced by the genital tract site and hormone status. This study confirmed that, like primary human endometrial epithelial cells, estrogen-dominant swine epithelial cells are more susceptible to chlamydial infection than are progesterone-dominant cells. Further, the more differentiated luminal epithelial cells were more susceptible to infection than were glandular epithelial cells. Interestingly, chlamydial growth in mature luminal epithelia was morphologically more active than in glandular epithelia, where persistent chlamydial forms predominated. Attempts to reprogram epithelial cell physiology and thereby susceptibility to chlamydial infection by reverse-stage, exogenous hormonal supplementation were unsuccessful. Freshly isolated primary pig epithelial cells frozen at −80°C in DMEM-F-12 medium with 10% dimethyl sulfoxide for several weeks can, after thawing, reform characteristic polarized monolayers in 3 to 5 days. Thus, primary swine genital epithelia cultured ex vivo appear to be an excellent cell model for dissecting the hormonal modulation of several aspects of chlamydial pathogenesis and infection.
It is well recognized that genital Chlamydia trachomatis serovars D to K are responsible for epidemic sexually transmitted diseases and sequelae in the United States and worldwide. The chronic nature of these diseases is due, in part, to the fact that C. trachomatis is an obligate intracellular bacterial pathogen; this characteristic requires mammalian cell culture as well as animal models for the study of its infectious process in columnar epithelial cells of the genital tract of the target host.
The majority of stock culture collection and expansion work with human chlamydial isolates has been performed with McCoy cells (fibroblast-like cells), which C. trachomatis does not infect in vivo (21). HeLa cells (epithelial cells from a cervical adenocarcinoma) have been used for most experimental analyses and are highly sensitive to chlamydial infection, but they are less representative of primary, differentiated target epithelial cells (4). As an alternative to the established transformed cell lines, a few studies have been performed with primary, hormone-responsive polarized human endometrial epithelial cells derived from women undergoing a hysterectomy for benign disease (17, 19, 29, 30). Important information about chlamydial attachment and infection which differed, in several aspects, from data obtained from non-hormone-responsive, nonpolarized, chlamydia-infected HeLa cells was obtained: (i) chlamydial inclusion development in primary human cervical and endometrial epithelial cells was patchy and uneven in distribution; (ii) levels of chlamydial attachment to and infectivity of primary human endometrial epithelial cells were significantly greater in estrogen-dominant than in progesterone-dominant cells; (iii) entry of infectious elementary bodies (EBs) into polarized, hormone-responsive, primary human endometrial epithelial cells was mediated predominately by clathrin-coated pit receptor-mediated endocytosis; and (iv) exit of chlamydiae from primary polarized human epithelial cells was pathogenesis directed, i.e., human noninvasive C. trachomatis serovar E progeny exited via the apical surface, whereas invasive C. trachomatis lymphogranuloma venereum-causing progeny were released at the basal domain. More recently, Davis et al. (8) have reported that a component associated with the estrogen receptor complex is associated with C. trachomatis serovar E EBs attached to the apical membrane surface of the polarized human endometrial epithelial cell line HEC-1B.
As a substitute for human tissue, which is of limited availability for research purposes, different animal models have been used to evaluate Chlamydia-host cell interactions; these studies have included mice, guinea pigs, rats, and rabbits. The rat-C. trachomatis mouse pneumonitis model was used for examination of cell-cell interactions to determine the secretion of cytokines as well as the effects of hormones on the immune response and infectivity (13, 14, 20). While the evaluation of normality and sensitivity to hormone supplementation was the main goal achieved by these in vitro and in vivo models, the data from the rat model were essentially opposite to the results from human chlamydial infection data. Once the technique of nested PCR for genus-specific amplification of the Chlamydia omp-1 locus was established, it revealed a large separation between most of the animal and human chlamydial strains. However, new data derived from the 16S-23S ribosomal intergenic spacer signature sequence for the chlamydial groups determined that swine isolates of Chlamydia suis (or swine C. trachomatis-like strains) are highly related to the human C. trachomatis species (99% confidence). Further, the swine C. suis S-45 isolate is virtually identical to human genital C. trachomatis serovar D (100% confidence) (9, 10).
Clinically, chlamydial diseases in swine include conjunctivitis, pneumonia, pericarditis, polyarteritis, and reproductive disorders. In the last named, Chlamydia has been reported to cause abortion in swine and to increase prenatal mortality (1, 26). In young piglets, chlamydiae produce a disease syndrome that is similar to that seen in infants following infection at the time of birth (2).
Since the organ physiology of swine is very similar to that of humans, swine have become the favored model for dermatologists, gastroenterologists, and obstetricians. In addition, polarized porcine epithelial cell model systems have been developed for in vitro analysis of pregnancy paradigms, actions of hormones, prostaglandin secretion, and epithelial cell-stromal cell interactions (5, 6, 24, 32).
This study evaluates the potential for using the C. suis S-45 swine primary genital tract epithelial cell culture model to follow up on previous data obtained from primary human endometrial epithelial cells (17, 19, 30), especially to study the influence of hormones on chlamydial attachment, adhesion-receptor interactions, and entry and development in genital tract cells, as well as microbicidal efficacy.
MATERIALS AND METHODS
Chlamydia strain.
The C. suis S-45 strain, originally isolated from fetuses during massive outbreaks of abortion cases in Austria, was provided by J. Storz, Louisiana State University, Baton Rouge, and used for these studies. Although tetracycline resistance is now confirmed for many of these isolates (16), the C. suis S-45 strain that we have is sensitive to tetracycline and penicillin.
The chlamydiae were grown in Buffalo green monkey kidney (BGMK; Viromed Laboratories, Minnetonka, Minn.) cells at 37°C in Dulbecco's minimum essential medium supplemented with Earle's salts and 20% fetal bovine serum (FBS) in an atmosphere of 5% carbon dioxide for 40 h and harvested, and titers were determined as previously described by Moorman et al. (19). The similarity of C. suis S-45 to C. trachomatis allowed the use of the SYVA MicroTrac reagent (Wampole Laboratories, Newark, N.J.), a pool of five fluorescein isothiocyanate-conjugated monoclonal antibodies generated against the C. trachomatis major outer membrane protein, for immunofluorescence detection of swine chlamydial inclusions.
Animals.
Tissues were collected at the local meat-processing factory according to a U.S. Department of Agriculture-approved protocol. All female swine (total number, 7), with a mean body weight 550 to 600 lb, were terminated from breeding programs and had previously produced several litters.
Because of the random sources of animals and the unavailability of anamnesis vita, the stage of the swine estrous cycle of the extracted tissues was assessed visually by a determination of the ovarian follicle status and subsequently confirmed histologically (3, 11, 18).
Histopathology.
After 24 h of fixation in 10% neutral buffered formaldehyde, samples of cervix and uterus and one of the uterine horns were embedded in paraffin by routine methods in the Department of Pathology. The specimen sections were stained with hematoxylin and eosin and examined with a Zeiss Axiovert-100 microscope equipped with a Zeiss AxioCam color digital camera (charge-coupled device; basic resolution, 1.3 megapixels); images were captured by using Zeiss AxioCam PhotoShop software and adjusted in Adobe Photoshop.
Isolation and culture of swine genital tract cells.
The genital tract of each female was separated into cervix, uterus, and horns. Samples were washed in Dulbecco's incomplete phosphate-buffered solution containing 200 μg of penicillin-streptomycin, 100 μg of gentamicin, and 2 μg of amphotericin B (Fungizone) per 1 ml of solution to remove any contaminating microorganisms. The uterus and horns were ligated from both ends with surgical forceps, and a solution of dispase (0.48%) and pancreatin (1.25%) in Hanks' incomplete balanced salt solution (HIBSS) containing antibiotics was injected into the cavity of each tissue. The amount of the solution injected was dependent upon the size of the tissue sample. Tissues were incubated at 25°C for at least 2 h with constant shaking and periodic external tissue palpations for better disruption of epithelial cells. During incubation, the external surfaces of the uterus and horn samples were also covered with Dulbecco's incomplete phosphate-buffered solution containing antibiotics to prevent the tissues from drying.
Luminal epithelial cells, glandular epithelial cells, and stromal cells were isolated aseptically and separated from the three different sites of the genital tract using a combination and some modifications of procedures previously described (5, 6, 28, 31, 32).
For the cervix, luminal epithelial cell isolation was achieved by opening the cervical cavity and incubating the whole (not cut) tissue, fully immersed in dispase-pancreatin solution. After incubation, the dispase-pancreatin solution containing the luminal epithelial cells was released from the organs; cavities were washed four to five times with IBSS supplemented with the same antimicrobial components previously mentioned. All washes were combined and centrifuged in 50-ml tubes at 500 × g for 10 min. Since epithelial cells collected from the uterus and the uterine horn contained many erythrocytes, which may affect epithelial cell attachment and growth, red blood cells were removed with a lysing buffer containing a 8.3-g/liter solution of ammonium chloride in 0.01 M Tris-HCl buffer (pH 7.5; Sigma Chemical Corporation, St. Louis, Mo.). Finally, the luminal epithelial cells were washed several times in HIBSS.
For glandular epithelial and stromal cell isolation, the remaining tissue samples were diced into small pieces (length, 1 cm). Because of the thickness of the mature female cervical tissue, numerous cuts in various directions of the endothelial surface were made through the muscle layer but not completely through the connective external tissue. Samples were mixed with 100 to 200 ml of a collagenase (0.06%)-DNase (0.01%)-trypsin (0.06%) solution containing antibiotics. Minced tissues were incubated for 2 h in a 37°C water bath with constant slow agitation. Subsequently, the samples were filtered sequentially through 425- and 38-μm-pore-size stainless steel sieves to remove large nondigested fragments and to separate out the glandular and stromal cells. Since the isolated glandular epithelial cells tended to form small clumps, they were collected on the 38-μm-pore-size sieve, while stromal and other cells passed through. Glandular epithelial cells, collected on the 38-μm sieve, were washed three times (1 min at 50 × g) with HIBSS containing antibiotics and collected. Stromal cells were incubated with the red blood cell lysing buffer and washed with HIBSS.
For growth, cell pellets were resuspended in DMEM-F-12 medium containing 20% FBS and 100 μg of penicillin-streptomycin, 50 μg of gentamicin, and 1 μg of amphotericin B; adjusted to a concentration 105 to 106 cells/ml; and plated in 25-cm2 tissue culture flasks or in 24-well cluster plates. The medium was changed every 2 to 3 days until the monolayers were near confluence.
Hormone supplementation.
For initial attachment, epithelial or stromal cells were kept undisturbed for 2 days in DMEM-F-12 medium containing 20% charcoal-stripped FBS. Then, half of the medium was discarded and replaced with fresh medium supplemented with various concentrations of 17β estradiol (10−10 or 10−8 M) or 4-pregnene-3,20-dione (progesterone; 10−6 or 10−7 g/liter). At the same time, the concentration of FBS in the medium was decreased to 10%, and the medium was changed every 2 days until the monolayer was nearly confluent. Progesterone at a concentration of 10−5 g/liter was toxic for epithelial cells and induced massive cell mortality. Because the stage of the cycle of the swine was finally determined on the basis of histological changes and the concentration of hormones needed for the epithelial cells was uncertain, all hormone concentrations and combinations were tested individually in each of the respective tissues.
Infection.
Nearly confluent and confluent monolayers of primary cells were infected with C. suis S-45 on days 9 or 10 after initiation of culture. Because chlamydiae are sensitive to penicillin, this antibiotic was removed from the medium and replaced with kanamycin (50 μg/ml) at least 3 days before infection. Infection was performed in the same manner as described above for BGMK cells. The infectivity of chlamydial strain S-45 in the primary cells was determined by counting the number of inclusions in 15 microscopic fields at a ×400 magnification (in a 1-mm2 field, no more than ∼42 to 45 epithelial cells) in triplicate for each experimental parameter or hormone, and the sizes of the inclusions were measured and compared by using Quantity One software (Bio-Rad Laboratories, Inc).
TEM.
BGMK cells were grown in petri dishes (diameter, 60 mm) to confluence, infected with C. suis S-45, and processed for transmission electron microscopy (TEM) at 12, 24, 36, 48, and 56 h postinfection (hpi). Swine primary luminal and glandular epithelial cells were grown on Transwell clear 0.45-μm-pore-size inserts and in 25-cm2 flasks (Corning Costar Corporations, Cambridge, Mass.) to confluence or until they were polarized. We then studied their morphology or infected them with C. suis S-45 and then processed them for electron microscopy. Briefly, infected cell monolayers were fixed with a solution of 2% glutaraldehyde-0.5% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for at least 2 h at 37°C. The fixed insert or the cell pellet was agar enrobed and postfixed in 1% osmium tetroxide and then in alcoholic uranyl acetate. Samples were dehydrated in increasing concentrations of ethanol, infiltrated, embedded in fresh pure Epon-Araldite 812 resin, and cured at 60°C for 48 h. Silver-gold thin sections were cut on a Reichert Ultracut (Leica) microtome, counterstained with uranyl acetate and then with lead citrate, and examined in a Tecnai-10 electron microscope (FEI) at 60 kV.
Statistical analysis.
The infectivity of chlamydial S-45 strains in BGMK and primary epithelial cells was determined in a minimum of three independent experiments per assay parameter per animal. For each experiment, triplicate samples of epithelial cells were used for each hormone treatment and the numbers of inclusion-containing cells in 15 microscopic fields chosen at random were calculated. Standard deviation was calculated, and Student's t test was used for statistical analysis.
RESULTS
Chlamydial growth and infectivity in the BGMK cell line.
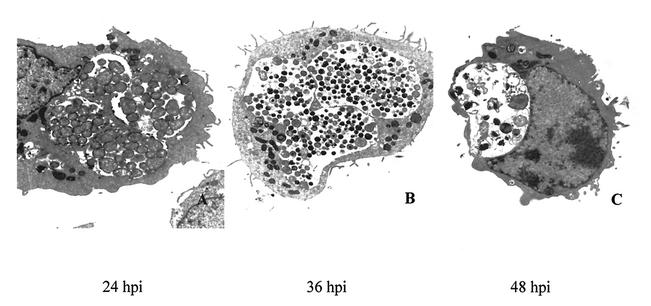
Previous morphological studies have shown that C. suis S-45 forms multiple inclusions in infected cells (12), and these findings were confirmed in our TEM analysis of infected BGMK cells (Fig. 1). Since the highest number of EBs was found in infected BGMK cells after 36 h, the estimated optimal time to determine the titer of the level of S-45 infectivity was determined to be 40 hpi. Crude stock of C. suis S-45 harvested from the BGMK cells grown in tissue culture flasks produced 60% infectivity at a dilution of 1:30 in the same cell line. Because of the unknown sensitivity of normal porcine epithelial cells to chlamydial infection, dilutions of the original crude S-45 stock used on the primary epithelial cell cultures ranged from 1:10 to 1:80.
FIG. 1.
Ultrastructural analysis of C. suis S-45 growth in infected BGMK cells. Transmission electron photomicrographs of BGMK cells infected with C. suis S-45 at 24 (A), 36 (B), and 48 (C) hpi. (A) RBs predominated in multiple inclusions at 24 hpi. (B) By 36 hpi, inclusions contained a mixture of RBs and mature EBs. (C) Essentially empty or partially empty inclusions were visualized at 48 hpi, and the remaining EBs and RBs in these inclusions appeared aberrant or damaged. Magnification, ×8,000.
Determination of the estrous stage.
On the basis of morphological and histological examination, three of seven swine tissues were excluded from the study due to abnormalities in the ovaries and uterus. The remaining four animals were considered to be in the following stages of the cycle: estrus (day 1), early diestrus (days 3 to 5), late diestrus (days 12 to 15), and proestrus (days 16 to 21). The conclusions were based on ovary morphology and histological differences in the appearance of luminal and glandular epithelia in the uterus during the estrous cycle (Fig. 2). The peak of progesterone activity characterizes the late diestrous stage in the swine cycle; estrus is the estrogen-dominant phase of the cycle, and early diestrus and proestrus are the intermediate phases.
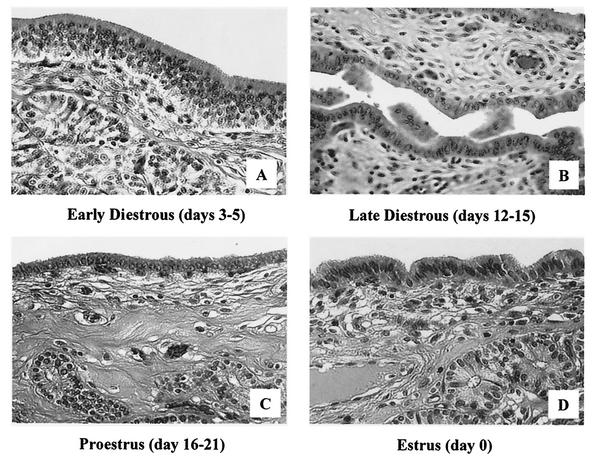
FIG. 2.
Histological analysis by light microscopy of hematoxylin-and-eosin-stained sections of swine endometrial tissue during the estrus cycle. (A) Tissue at early diestrus (days 3 to 5) shows a high columnar luminal epithelium, a large number of glandular epithelial cell clusters that are close to the luminal epithelium, and increased epithelial mitotic activity. (B) Tissue at late diestrus (days 12 to 15) reflects shedding of many luminal epithelium cells and a transition from high columnar epithelia to the low columnar form. (C) In proestrus (days 16 to 21), single cubical epithelia dominate the luminal surface and glandular clusters are located deep in the stromal layer. (D) Tissue at estrus (day 0) shows a high columnar luminal epithelium and a large number of glandular epithelial cell clusters that are close to the luminal epithelium.
Morphology of polarized primary pig epithelial cells.
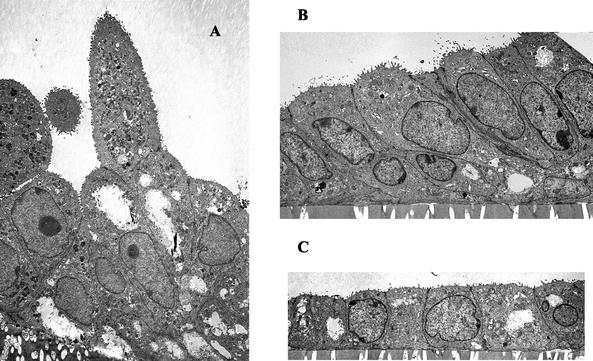
TEM analysis of polarized swine glandular epithelial cells cultured in vitro revealed a close similarity to their appearance in vivo (Fig. 3). Depending on the stage of the cycle, the polarized cell morphology transitioned from being simply cuboidal (progesterone-dominant phase) (Fig. 3C) to high columnar (intermediate phases) (Fig. 3B). Cells isolated during the estrogen-dominant phase of the cycle continued to establish higher miotic activity in vitro than in vivo and to form organoid shapes (Fig. 3A) similar to the shapes of the glands from which they were derived.
FIG. 3.
Ultrastructural analysis of polarized primary swine glandular epithelial cells. (A) Tissue at early diestrus (days 3 to 5) illustrates tall columnar epithelial cells with high miotic activity and the formation of characteristic gland-like (organoid) structures. (B) Tissue at late diestrus (days 12 to 15) reflects high columnar epithelia. (C) In tissue at proestrus (days 16 to 21), the monolayer is representative of single cuboidal epithelial cells. Magnification, ×2,900.
C. suis S-45 growth and infectivity in primary pig epithelial cells.
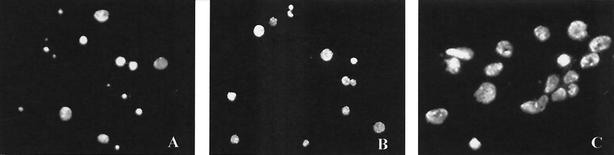
Compared to the growth of C. suis S-45 in the BGMK cell line, which was characterized by an even distribution of chlamydial inclusions throughout the monolayer, the distribution of chlamydial inclusions in the primary luminal and glandular epithelial cells from all genital tract sites was patchy and not predictable (Fig. 4). These results confirm the findings of Moorman et al. (19) relating to the uneven distribution of C. trachomatis inclusions in primary human endometrial epithelial cells compared with that in McCoy cells. This uneven distribution resulted in a high standard deviation with regard to the level of infectivity. Stromal cells were not susceptible to infection; no formation of chlamydial inclusions was observed.
FIG. 4.
Immunofluorescence microscopy of the distribution of C. suis inclusions in swine primary cervical and uterine epithelia cultured in vitro. There was an uneven distribution of chlamydia-infected cells in cervical (A) and uterine (B and C) epithelial cell confluent monolayers; inclusions appeared to be located in certain zones of the epithelial cell monolayer population. Magnifications, ×50 (A and B) and ×350 (C).
The percentages of inclusion-containing cells in both types of swine epithelia averaged from 0 to 2% depending upon the stage of the cycle and the tissue taken from the genital tract. Such a low level of infectivity prevented any observation of dramatic differences among tissue samples, i.e., cervix versus uterus versus horns. Even when the highest dilution (1:10) of the original chlamydial stock was used for inoculation, the percentage of inclusion-containing cells still did not exceed more than 10%. However, despite such a low rate of infectivity, a distinction in chlamydial-inclusion formation in primary cell cultures was obvious and varied from stage to stage (Table 1). Luminal and glandular epithelial cells isolated from the three swine genital tract sites at different stages of the hormonal cycle and grown in medium with no hormone supplementation did yield differences in rates of susceptibility to chlamydial infection. For example, cervical luminal epithelial cells, the first target cell for bacterial infection, isolated in the early diestrous and proestrous stages, were 10 times more susceptible (P ≤ 0.05) to infection than cervical cells obtained from swine at the peak of progesterone activity (days 12 to 15). This result is likely a reflection of a higher estrogen activity during these pre- and postprogesterone peak stages.
TABLE 1.
Levels of C. suis S-45 infectivity for luminal and glandular epithelial cells isolated at the different stages of the estrous cycle
| Epithelial cells | Sample | Mean % showing infection ± SD on:
|
|||
|---|---|---|---|---|---|
| Day 0 | Days 3-5 | Days 12-15 | Days 16-21d | ||
| Luminal | Cervix | 2.1 ± 4.04c | 10.4 ± 14.99c | 1.0 ± 4.04 | 7.8 ± 4.52c |
| Uterus | 7.5 ± 9.80a,c | 5.2 ± 2.19a,c | 0.1 ± 0.32a | 3.2 ± 2.73c | |
| Horn of uterus | 8.9 ± 10.0a,c | 6.9 ± 4.73a,b,c | 0.1 ± 0.41a | 4.2 ± 1.21a,c | |
| Glandular | Cervix | 3.1 ± 4.68c | 2.3 ± 4.09c | 0.1 ± 0.40 | NT |
| Uterus | 3.6 ± 5.20c | 4.8 ± 5.62a,c | 0.1 ± 0.18 | NT | |
| Horn of uterus | 3.5 ± 4.92c | 5.6 ± 5.26a,c | 0.1 ± 0.21 | NT | |
Significantly different (P ≤ 0.05) from the value for epithelial cells isolated from cervix.
Significantly different (P ≤ 0.05) from the value for epithelial cells isolated from uterus.
Significantly different (P ≤ 0.05) from the value for the progesterone-dominant stage of the cycle (days 12 to 15).
NT, not tested.
Luminal epithelial cells isolated from the uterus and horn were most susceptible to chlamydial infection on day 1 of the cycle, which is the estrogen-dominant phase, and the subsequent rate of infectivity of these cells was correlated with fluctuations in the hormonal level. Minimal susceptibility to infection was observed during the progesterone-dominant phase. Similarly, the infectivity of glandular epithelial cells was greater during the estrogen-dominant phase of the cycle in all three sampling sites (P ≤ 0.05) but was noticeably lower during the peak of progesterone activity.
C. suis S-45 infectivity in primary pig epithelial cells following hormone supplementation.
The levels of influence of exogenously supplied hormones on the epithelial cells extracted from the swine genital tract at various stages of the cycle were compared, and the results clearly showed that luminal and glandular epithelial cells isolated during the progesterone-dominant stage of the cycle (days 12 to 15) had a low sensitivity to infection even when they were supplemented with exogenous estrogen. Moreover, no differences in the levels of C. suis S-45 infectivity between exogenously stimulated and control cells were found during progesterone supplementation of the medium (data not shown).
Luminal epithelial cells.
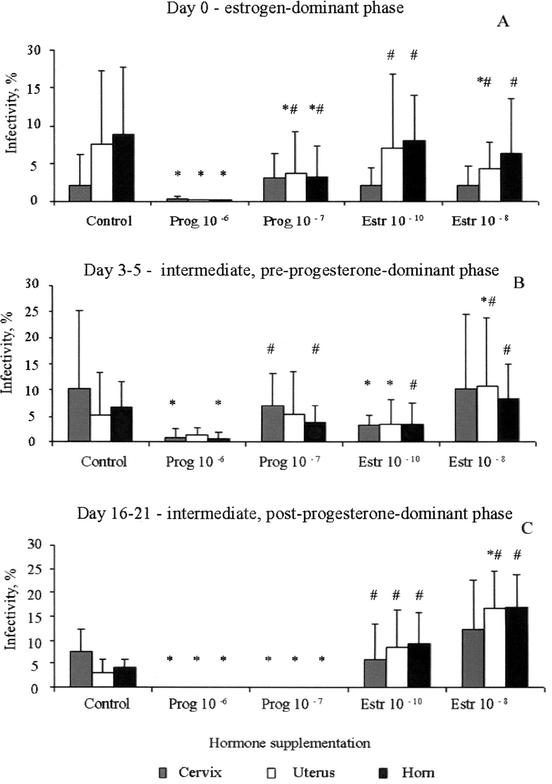
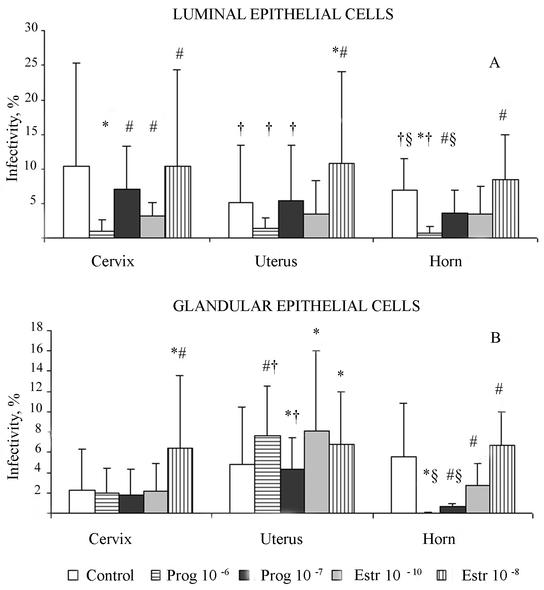
The effect of hormonal stimulation on the sensitivity of luminal epithelial cells, isolated from pig genital tracts during stages of the cycle when the progesterone concentration was low, varied depending upon the sampling site (Fig. 5). In all cases, progesterone (10−6 g/liter) resulted in a decrease (2- to 10-fold) in the number of chlamydial inclusions observed (P ≤ 0.05). Lower concentrations of progesterone had a less noticeable influence on chlamydial inclusion formation, but the infectivity in this epithelial cell stage was obviously different from that of control cells grown in the basic medium (P ≤ 0.05).
FIG. 5.
Influence of hormone supplementation on C. suis S-45 infectivity in luminal epithelial cells isolated from swine genital tracts at different stages of the menstrual cycle. (A) Day 0, estrogen-dominant phase; (B) days 3 to 5, preprogesterone peak, intermediate phase; (C) days 16 to 21, postprogesterone peak, intermediate phase. Pr, progesterone; Estr, estrogen; ∗, significantly different (P ≤ 0.05) from the value for the control nontreated group; #, significantly different (P ≤ 0.05) from the value for epithelial cells treated with progesterone at a concentration of 10−6 g/liter. Calculations were by Student's t test.
Effects of estrogen on the infectivity of C. suis S-45 in the luminal epithelium varied according to the site of the genital tract and the stage of the hormonal cycle. In cells sampled from swine during the estrogen-dominant stage (day 0), supplementation of the medium with both concentrations of estrogen (10−8 and 10−10 M) resulted in a slight decrease in the number of chlamydial inclusions in uterus and horn epithelial samples but was equivalent to control inclusion counts in cervical samples. The same phenomenon was noticed in the samples from days 3 to 5 (pre-progesterone-dominant phase) of the swine cycle for cells grown in the presence of 10−10 M estrogen (P ≤ 0.05). Higher concentrations of estrogen resulted in a slight increase in the infectivity of epithelial cells isolated from all three sites (cervix, uterus, and horn).
The most noticeable effect of hormone supplementation on chlamydial infectivity in luminal epithelial cells was in the epithelial cells isolated at the end of the cycle—just prior to the peak of estrogen activity. At this physiological stage, the addition of progesterone at both concentrations (10−6 and 10−7 g/liter) prevented inclusion formation (P ≤ 0.05). Viewed microscopically, there were high numbers of EBs attached to the epithelial cell surfaces, but not a single inclusion was formed. In marked contrast, estrogen stimulated chlamydial development and increased the level of chlamydial infectivity (at least fivefold) in the uterus and horn samples (P ≤ 0.05).
Glandular epithelial cells.
Interestingly, glandular epithelial cells supplied with exogenously added hormones produced a different level of sensitivity to C. suis, which was more dependent on the tissue site than on the stage of the hormonal cycle. The best example is illustrated in glandular epithelia isolated from swine on days 3 to 5 (declining levels of estrogen) of the cycle (Fig. 6). Neither progesterone nor estrogen (10−7 M) affected the amount of chlamydial infectivity in cells isolated from the cervix. However, once again, supplementation with 10−8 M estrogen induced a threefold increase in infectivity (P ≤ 0.05).
FIG. 6.
Influence of hormone supplementation on the sensitivity of luminal and glandular epithelial cells, isolated from different sites of the swine genital tract, at the early diestrus stage (days 3 to 5) to C. suis S-45 infection. (A) Luminal epithelial cells; (B) glandular epithelial cells. Prog, progesterone; Estr, estrogen; ∗, significantly different (P ≤ 0.05) from the value for the control nontreated group; #, significantly different (P ≤ 0.05) from the value for epithelial cells treated with progesterone at a concentration of 10−6 g/liter; †, significantly different (P ≤ 0.05) from the value for cervix samples; §, significantly different (P ≤ 0.05) from the value for uterus samples. Calculations were by Student's t test.
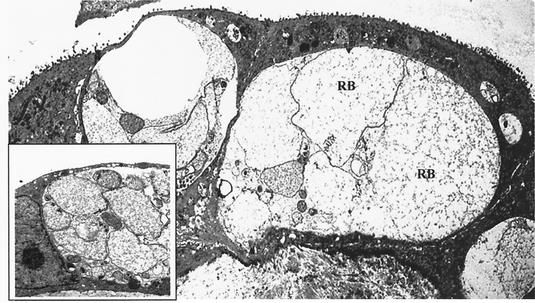
Uterine glandular epithelial cells demonstrated more unpredictable reactions when the medium was supplemented with progesterone. Higher concentrations of progesterone (10−6 g/liter) induced an increase in the level of C. suis S-45 infection in the glandular epithelial cell cultures, whereas lower concentrations of progesterone resulted in a slight decrease in the level of epithelial cell infectivity (P ≤ 0.05). In contrast, both 10−10 and 10−8 M estrogen supplementation induced increasing numbers of inclusions found in the uterine glandular epithelial cell monolayers. These inclusions were filled primarily with abnormally enlarged C. suis S-45 reticulate bodies (RBs), characteristic of a persistent morphology; only a few EBs were detected per inclusion (Fig. 7). These findings may be a reflection of the less differentiated state of glandular epithelial cells.
FIG. 7.
Ultrastructural analysis of C. suis S-45 in estrogen-dominant primary swine uterine glandular epithelial cells. Shown are transmission electron photomicrographs of primary glandular epithelial cells infected with C. suis S-45 at 40 hpi; abnormally enlarged RBs predominated in inclusions formed in these cells. Magnification, ×2,100. (Inset) Portion of an inclusion containing a mixture of enlarged as well as normal RBs and some mature EBs. Magnification, ×4,200.
Sensitivity to chlamydial infection of glandular epithelial cells isolated from the horn was similar to that described for luminal epithelial cells; there was a decrease in the rate of infectivity during progesterone exposure but an increase in infectivity as a result of estrogen exposure. Overall, these comparative infectivity data suggest that the uterine horn epithelia are the best host cells for the study of C. suis infectivity. Also, after isolated uterine horn epithelia were frozen at −80°C in medium with 10% dimethyl sulfoxide and then thawed, there was good recovery of the epithelial cells and nice polarized monolayers could be obtained in 3 to 5 days on Transwell inserts (Corning Incorporated, Corning, N.Y.).
In addition to the levels of infectivity of epithelial cells with C. suis S-45 being affected by hormones, the size of the chlamydial inclusions was also affected by hormone supplementation. Estrogen induced at most a ninefold increase in the size of chlamydial inclusions (P ≤ 0.05) in both luminal and glandular epithelial cells, with the difference being more noticeable in glandular epithelial cells (Fig. 8). Progesterone had no significant influence on the size of chlamydial inclusions.
FIG. 8.
Immunofluorescence microscopy of C. suis inclusions in confluent glandular epithelial cells isolated from swine cervix at the stage of early diestrus (intermediate stage). Chlamydial inclusion size in glandular epithelial cells cultured in DMEM/F-12 medium (A), medium supplemented with progesterone (10−7 g/liter) (B), and medium supplemented with estrogen (10−10 M) (C). Magnification, ×350.
DISCUSSION
The fact that ascending C. trachomatis infection can have particularly devastating reproductive consequences for females, such as pelvic inflammatory disease, tubal factor infertility, and ectopic pregnancy, has been known for many years (25). It is, therefore, somewhat perplexing that so little attention has been given to the influence of reproductive hormones on the chlamydial infection of genital epithelia, both from a clinical perspective and from an experimental perspective.
More than 2 decades ago, a primary human endometrial epithelial cell model was established for the study of C. trachomatis pathogenesis (19). Subsequently, it was shown that epithelial cells were more sensitive to C. trachomatis serovar E in the estrogen-dominant (proliferative) stage of the human menstrual cycle than at other stages (17, 30). When estrogen-dominant-phase cells were grown and maintained in the normal human physiological concentration of estrogen (10−10 M), chlamydial attachment and infectivity was enhanced ≥80%. In contrast, in endometrial epithelial cells obtained from the middle to late progesterone-dominant phases, chlamydial attachment and infectivity was decreased from 50 to 35 and 20%, respectively. These findings have led to an increase in interest of the influence of estrogen and progesterone on chlamydial interaction with host genital epithelial cells.
Results from this study indicate that the hormone-responsive female swine genital epithelial cell-C. suis S-45 model may be a valid one for dissecting sex hormone modulation of chlamydial pathogenesis and infectivity. Anatomically, the pig uterus is bifurcated, and physiologically, the estrogen-dominant phase in swine is of shorter duration than in humans (7). However, in confirmation of the findings of Maslow et al. (17), estrogen-dominant-phase primary swine epithelial cells, both luminal and glandular, were found to be more susceptible to chlamydial infection than were progesterone-dominant-phase cells. EBs were able to attach to the surfaces of epithelial cells when progesterone was at its peak but did not enter the cells or form inclusions. Exposing progesterone-dominant cells to estrogen could not reverse the cell physiology. Even during intermediate estrogen phases, when the progesterone concentration was fluctuating up and down, i.e., in the diestrous and proestrous stages, the epithelial cells were still susceptible to chlamydial infection and large numbers of well-formed inclusions were observed in the monolayers. These findings correlate with data obtained from the guinea pig model, where the prolongation and severity of chlamydial infection was higher in the animals treated with estradiol than in untreated animals and the animals receiving progesterone failed to show any clinical signs of chlamydiosis (22, 23).
In the studies involving primary human endometrial epithelial cell cultures, it was shown that the concentration of exogenously supplemented estrogen is critical and needs to be similar to concentrations in the normal human physiological range of ca. 10−10 M in order to enhance chlamydial infection (17). In the present study, estrogen at this concentration had a less noticeable effect on the rate of infectivity than did a concentration of 10−8 M. According to data pertaining to the monitoring of peripheral blood hormonal concentration during the swine estrous cycle, the level of estrogen varies from 10−10 to 10−11 M and the level of progesterone varies from 10−7 to 10−9 g/liter (11, 27). However, the investigators used supplementations of 10−8 M estrogen and 10−6 g of progesterone per liter in order to study the effect on apical and basal protein secretion in a polarized porcine uterine epithelial model system (5). Neither sex hormone concentration affected the proteins secreted by the polarized cells. However, during a study of hormonal influence on expression of the pregnancy-associated gene encoding antileukoproteinase in swine glandular epithelial cells, enhancement of expression was found after supplementation with estrogen at 10−8 M compared with expression with 10−10 M (24). Assuming that the hormone concentration in the uterus is slightly higher than in the peripheral blood, the concentration that we used in our study is probably in the normal physiological in situ swine range.
Interestingly, luminal epithelial cells, isolated from all three sites of the genital tract, were more susceptible to S-45 infection than glandular epithelial cells, even when they were cultured in hormone-free medium. This finding is probably due to the fact that the luminal epithelium represents more differentiated, mature cells. Thus, polarized luminal epithelia should provide an excellent model for analyzing apical membrane receptors involved in EB attachment in estrogen- and progesterone-dominant phases as well as for microbicidal intervention, whereas glandular epithelia appear to offer an opportunity to investigate less differentiated but sensitive epithelial cells as a nidus for the chronic persistence of chlamydiae.
Despite initial problems with contamination of the cervix from environmental and external microorganisms, which can be eliminated by the incorporation of several antibiotics in the early culture medium, primary cervical epithelial cells offer a better opportunity to investigate factors contributing to initial chlamydial infection and the contributions of innate immune responses and whether or not there is organ or site specificity with regard to immunological surveillance in the lower “nonsterile” genital tract versus the upper “sterile” genital tract as proposed by Kelly et al. (15) and Quayle (25).
Large numbers of epithelial cells can be obtained from swine uterine horns, and more information on factors that influence this tissue in regard to swine reproduction is available, especially in the era of artificial insemination. The most obvious challenge at the ex vivo-in vitro level will be characterizing the epithelial cells in the population of cells that do promote chlamydial inclusion development, given only a maximum 10% infectivity and the uneven or patchy redistribution in vitro of these susceptible cells. A combination of fluorescence-activated cell sorting and laser capture microdissection should help with this problem. There is some evidence that this pattern of chlamydial infectivity is the norm in vivo.
Acknowledgments
This work was supported by Public Health Service grants AI13446 and PO1 AI37829, which were awarded to the Milton S. Hershey Medical Center, from the National Institutes of Health, NIAID.
We acknowledge the helpful discussions and advice of Bernhard Kaltenboeck and Arthur Andersen on the use of C. suis and the swine model for analyzing hormone effects on chlamydial infection. We especially thank B. Tober-Meyer, director of the Division of Laboratory Animal Resources, East Tennessee State University, Johnson City, and John R. Diehl, Department of Animal and Veterinary Sciences, Clemson University, Clemson, S.C., for help in obtaining the swine tissues and in determining the staging of the swine hormone cycle. Special appreciation is also extended to L. A. Miller, Department of Pathology, J.-H. Quillen College of Medicine, East Tennessee State University, Johnson City, for paraffin processing of the swine tissues and preparation of histological slides for more accurate determination of the hormonal stage of the tissues. Finally, we thank Heather Lane for help with preparation of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Andersen, A. A. 1994. Chlamydial diseases in swine, p. 259-263. In Proceedings of the American Association of Swine Practitioners, 25th Annual Meeting. American Association of Swine Practitioners, Perry, Iowa.
- 2.Andersen, A. A., and D. G. Roberts. 1996. Experimental C. trachomatis infection in 4-7 day old pigs, p. 110. In A. Stary (ed.), Proceedings of the 3rd European Society for Chlamydia Research. Società Editrice Esculapio, Bologna, Italy.
- 3.Bischof, R. J., M. R. Brandon, and C.-S. Lee. 1994. Studies on the distribution of immune cells in the uteri of prepubertal and cycling gilts. J. Reprod. Immunol. 26:111-129. [DOI] [PubMed] [Google Scholar]
- 4.Bose, S. K., and P. C. Goswami. 1986. Enhancement of adherence and growth of Chlamydia trachomatis by estrogen treatment of HeLa cells. Infect. Immun. 53:646-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen, J. A., G. R. Newton, D. W. Weise, F. W. Bazer, and R. C. Burghardt. 1996. Characterization of a polarized porcine uterine epithelial model system. Biol. Reprod. 55:613-619. [DOI] [PubMed] [Google Scholar]
- 6.Braileanu, G. T., J. Hu, and M. A. Mirando. 2000. Directional secretion of prostaglandin F 2α by polarized luminal epithelial cells from pig endometrium. Prostaglandins Other Lipid Mediat. 60:167-174. [DOI] [PubMed] [Google Scholar]
- 7.Coffey, R. D., G. R. Parker, and K. M. Laurent. 2000-2002. Manipulation of the estrous cycle in swine. Cooperative Extension Service, College of Agriculture, University of Kentucky, Lexington. [Online.] www.ca.uky.edu/agc/pubs/asc/asc152/asc152.htm.
- 8.Davis, C. H., J. E. Raulston, and P. B. Wyrick. 2002. Protein disulfide isomerase, a component of the estrogen receptor complex, is associated with Chlamydia trachomatis serovar E attached to human endometrial epithelial cells. Infect. Immun. 70:3413-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett, K. D. E., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 10.Everett, K. D. E. 2000. Chlamydia and Chlamydiales: more than meets the eye. Vet. Microb. 75:109-126. [DOI] [PubMed] [Google Scholar]
- 11.Kaeoket, K., E. Peterson, and A. M. Dalin. 2001. The sow endometrium at different stages of the estrous cycle: studies on morphological changes and infiltration by cells of the immune system. Anim. Reprod. Sci. 65:95-114. [DOI] [PubMed] [Google Scholar]
- 12.Kaltenboeck, B., and J. Storz. 1992. Biological properties and genetic analysis of the ompA locus in chlamydiae isolated from swine. Am. J. Vet. Res. 53:1482-1487. [PubMed] [Google Scholar]
- 13.Kaushic, C., F. Zhou, A. D. Murdin, and C. R. Wira. 2000. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun. 68:4207-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushic, C., K. Grant, M. Crane, and C. R. Wira. 2000. Infection of polarized primary epithelial cells from rat uterus with Chlamydia trachomatis: cell-cell interaction and cytokine secretion. Am. J. Reprod. Immun. 44:73-79. [DOI] [PubMed] [Google Scholar]
- 15.Kelly, K. A., J. C. Walker, S. H. Jameel, H. L. Gray, and R. G. Rank. 2000. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia trachomatis infection. Infect. Immun. 68:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenart, J., A. A. Andersen, and D. D. Rockey. 2001. Growth and development of tetracycline-resistant Chlamydia suis. Antimicrob. Agents Chemother. 45:2198-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maslow, A. S., C. H. Davis, J. Choong, and P. B. Wyrick. 1988. Estrogen enhances attachment of Chlamydia trachomatis to human endothelial epithelial cells in vitro. Am. J. Obstet. Gynecol. 159:1006-1014. [DOI] [PubMed] [Google Scholar]
- 18.McDonald, L. E., and M. H. Pineda (ed.). 1988. Veterinary endocrinology and reproduction, 4th ed. Lea & Febiger, Philadelphia, Pa.
- 19.Moorman, D., J. W. Sixbey, and P. B. Wyrick. 1986. Interaction of Chlamydia trachomatis with human epithelium in culture. J. Gen. Microbiol. 132:1055-1067. [DOI] [PubMed] [Google Scholar]
- 20.Morre, S. A., and J. M. Lyons. 2000. Murine models of Chlamydia trachomatis genital tract infection: use of mouse pneumonitis strains versus human strains. Infect. Immun. 68:7209-7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paavonen, J., and P. Wolner-Hanseen. 1989. Chlamydia trachomatis: a major threat to reproduction. Hum. Reprod. 4:111-124. [DOI] [PubMed] [Google Scholar]
- 22.Pasley, J. N., R. G. Rank, A. J. Hough, Jr., C. Cohen, and A. L. Barron. 1985. Effects of various doses of estradiol on chlamydial genital infection in ovariectomized guinea pigs. Sex. Transm. Dis. 12:8-13. [DOI] [PubMed] [Google Scholar]
- 23.Pasley, J. N., R. G. Rank, A. J. Hough, Jr., C. Cohen, and A. L. Barron. 1985. Absence of progesterone effects on chlamydial genital infection in female guinea pigs. Sex. Transm. Dis. 12:155-158. [DOI] [PubMed] [Google Scholar]
- 24.Reed, K. L., L. Baginga, D. L. Davis, T. E. Chung, and R. C. M. Simmens. 1996. Porcine endometrial glandular epithelial cells in vitro: transcriptional activities of the pregnancy-associated genes encoding antileukoproteinase and uteroferrin. Biol. Reprod. 55:469-477. [DOI] [PubMed] [Google Scholar]
- 25.Quayle, A. J. 2002. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J. Reprod. Immun. 57:61-79. [DOI] [PubMed] [Google Scholar]
- 26.Schiller, I., R. Koesters, R. Weilenmann, R. Thoma, B. Kaltenboeck, P. Heitz, and A. Pospischil. 1997. Mixed infection with porcine Chlamydia trachomatis/pecorum and infections with ruminant Chlamydia psittaci serovar 1 associated with abortions in swine. Vet. Microbiol. 58:251-260. [DOI] [PubMed] [Google Scholar]
- 27.Stanchev, P., H. Rodriguez-Martinez, L. E. Edqvist, and H. Eriksson. 1990. Characterization of the uterine sex steroid receptors in the pig and their variation during the oestrous cycle. J. Steroid Biochem. 35:689-699. [DOI] [PubMed] [Google Scholar]
- 28.Wang, G., G. A. Johnson, T. E. Spences, and F. W. Bazer. 2000. Isolation, immortalization, and initial characterization of uterine cell lines: an in vitro model system for the porcine uterus. In Vitro Cell. Dev. Biol. Anim. 36:650-656. [DOI] [PubMed] [Google Scholar]
- 29.Wyrick, P. B., J. Choong, C. H. Davis, S. T. Knight, M. O. Royal, A. S Maslow, and C. R. Bagnell. 1989. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect. Immun. 57:2378-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyrick, P. B., C. H. Davis, S. T. Knight, J. Choong, J. E. Raulston, and N. Schramm. 1993. An in vitro human epithelial cell culture system for studying the pathogenesis of Chlamydia trachomatis. Sex. Transm. Dis. 6:248-256. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, Y. L., and D. L. Davis. 2000. Morphology of luminal and glandular epithelial cells from pig endometrium grown on plastic or extracellular matrices. J. Anim. Sci. 78:131-138. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, Z., B. C. Paria, and D. L. Davis. 1991. Pig endometrial cells in primary culture: morphology, secretion of prostaglandins and protein, and effects of pregnancy. J. Anim. Sci. 69:3005-3015. [DOI] [PubMed] [Google Scholar]