Abstract
Recently, it was shown that Yersinia outer protein T (YopT) belongs to a new family of cysteine proteases containing invariant C, H, and D residues that are crucial for its activity. YopT cleaves RhoA, Rac, and Cdc42 at their C termini, thereby releasing them from the membrane. Moreover, YopT inhibits the Rho-rhotekin and Rho-guanine nucleotide dissociation inhibitor interactions. To characterize the active domain of YopT, we constructed N- and C-terminal truncations and expressed them as glutathione S-transferase fusion proteins in Escherichia coli. The toxin fragments were tested for stability by trypsin digestion. The activity of the proteins was studied by membrane release assay, rhotekin pulldown experiments, and microinjection. Whereas deletion of the first 74 N-terminal amino acids did not influence the activity of YopT, deletion of 8 amino acids from the C terminus led to complete loss of activity. N-terminal deletion of 100 amino acids led to an inactive protein, although it still contained the amino acids C139, H258, and D274, which are essential for catalysis. Loss of activity of the N-terminal deletions corresponded to the block of interaction with RhoA, indicating that residues 75 to 100 of YopT are essential for binding to the GTPase. By contrast, when up to 15 amino acids of the C terminus were deleted, the protein had no activity but was still able to interact with RhoA, suggesting a role for the C terminus in the enzyme activity of YopT.
Pathogenic yersiniae have the ability to resist the nonspecific immune defense of their eukaryotic host by inhibition of phagocytosis, cytokine release, and oxidative burst (5). These properties are dependent on the presence of a 70-kb virulence plasmid (pYV) that encodes a complex type III secretion machinery (Ysc), as well as effector and translocator Yops (Yersinia outer proteins) (5, 7). After type III secretion into the cytoplasm of the host cell, the effector proteins affect crucial signaling pathways with the aim to subvert the host cell defense. Six effector Yops of Yersinia enterocolitica are known, including YopM, YopP (YopJ in Y. pseudotuberculosis) YopH, YopO (YpkA in Y. pseudotuberculosis), YopE, and YopT (4, 10). No cellular phenotype has been reported for YopM. YopP (YopJ) is involved in induction of apoptosis, probably by inhibition of NF-κB activation (12). Interestingly, four of these six known effector proteins, YopH, YopO, YopE, and YopT, have been shown to affect the actin cytoskeleton (for a review, see reference 8). The rationale of these findings is probably the crucial role of actin in innate and acquired immunity, including phagocytosis, chemotaxis, and gene transcription.
Low-molecular-mass GTPases of the Rho family function as molecular switches in a large array of signaling processes, including organization of the actin cytoskeleton (for a review, see reference 17). Activation occurs by GDP-GTP exchange induced by guanine nucleotide exchange factors and inactivation by hydrolysis of the bound GTP, a process that is facilitated by GAPs (GTPase-activating proteins). Cycling between the two nucleotide-bound states of Rho is accompanied by its cycling between the cytosol and the cell membranes, a process that involves guanine nucleotide dissociation inhibitors (GDIs). GDIs bind to posttranslationally modified (isoprenylated) Rho proteins in the cytosol, keep them in the GDP-bound form, and inhibit nucleotide exchange. Finally, activated Rho proteins can interact with many effector proteins to mediate downstream signaling (3, 6).
Small GTPases of the Rho family are preferred targets of various bacterial toxins and exoenzymes that modify eukaryotic proteins. Some of these bacterial toxins covalently inactivate (e.g., C3-like ADP-ribosyltransferases and large clostridial cytotoxins) or activate (e.g., Escherichia coli cytotoxic necrotizing factors and Bordetella dermonecrotic toxin) Rho proteins, whereas others modulate the function of Rho GTPases in a guanine nucleotide exchange factor (e.g., SopE from Salmonella enterica serovar Typhimurium)- and GAP (e.g., exoenzyme S from Pseudomonas aeruginosa)-like manner (for a review, see reference 1).
It has been shown that YopE, -H, -O/P, and -T engage in Rho signaling pathways. YopE acts as a GAP on RhoA, Rac1, and Cdc42, leading to inactivation and thereby to depolymerization of actin stress fibers in eukaryotic cells (18). YopH is a tyrosine phosphatase that leads to disruption of peripheral focal complexes in HeLa cells by dephosphorylating p130Cas and FAK (11). YopO (YpkA), which has significant homology with eukaryotic Ser/Thr protein kinases, has been reported to interact with the small GTPases RhoA and Rac1 (2).
When delivered by type III secretion, YopT disrupts the actin cytoskeleton of mammalian cells and causes redistribution of membrane-bound RhoA toward the cytosol. Furthermore, it leads to an increase in electrophoretic mobility and an acidic shift of RhoA, as tested by isoelectric focusing (19). Moreover, recombinant YopT has the ability to release RhoA from isolated cell membranes and artificial vesicles, suggesting that YopT acts on the actin cytoskeleton by inactivating RhoA (19). Interestingly, cytotoxic necrotizing factor 1 (CNF-1), which constitutively activates RhoA by covalent modification, is not able to prevent or to reverse YopT-induced cell rounding (16). This and the finding that YopT blocks the interaction between RhoA and its effector, rhotekin, in precipitation assays suggest that YopT leads to uncoupling of the RhoA-rhotekin interaction (16). Additionally, YopT influences the Rho-RhoGDI interaction. While YopT treatment of control HeLa lysates and cytosols results in a slight increase in RhoGDI binding to Rho (16), incubation with the toxin strongly decreases the RhoGDI binding of activated RhoA, Rac, and Cdc42 (14). Recently, Shao et al. identified YopT as a member of a new family of cysteine proteases that contain invariant Cys, His, and Asp residues. Moreover, they demonstrated that YopT cleaves the posttranslationally modified Rho GTPases near the carboxyl terminus, thereby releasing them from the membrane (14). In a second study, they demonstrated by specific radiolabeling experiments and mass spectrometry that YopT cleaves the posttranslationally modified Rho GTPases RhoA, Rac, and Cdc42 directly in front of the C-terminal cysteine (15). Moreover, the studies suggest that YopT recognizes a sequence of basic amino acids in concert with the lipid-modified cysteine.
In this study, we characterized the active portion of YopT by using different fragments of the toxin expressed as glutathione S-transferase (GST) fusion proteins and analyzed their effects on RhoA.
MATERIALS AND METHODS
Cloning and purification of YopT fragments and mutant forms.
Fragments of the yopT gene with flanking BamHI and SmaI sites were generated by PCR from virulence plasmid pYV from Y. enterocolitica JB580v (9) and cloned in frame into expression vector pGEX2TGL. This vector was previously designed in our laboratory and is a modification of the pGEX2T vector from Amersham Biosciences, Inc. The vector contains an additional oligonucleotide in the multiple cloning site that codes for a glycine linker between the GST residue and the inserted gene, enabling better removal of the GST protein by thrombin cleavage.
Mutagenesis of pGEX2TGL-YopT was performed with a round-circle PCR-based site-directed mutagenesis kit (QuikChange; Stratagene) and the primers described in Table 1.
TABLE 1.
Primers used in this study
| Primer | Sequence 5′ → 3′ |
|---|---|
| YopTsens | GGA TCC ATG GAC AGT ATT CAC GGA CAC TAC C |
| YopTanti | CCC GGG TTA AAC CTC CTT GGA GTC AAA TG |
| YopTΔ1-31sens | GGA TCC GGC GCA CAC CGA GTG AAA GT |
| YopTΔ1-74sens | GGA TCC GCT AAC CAA CGA AGC AGC TTT ACC T |
| YopTΔ1-100sens | GGA TCC GCG GTG AGA GAG TCT GTT GC |
| YopTΔ1-121sens | GGA TCC GGG GCT TTT CTT CAT CAA ATA ATA |
| YopTΔ301-322anti | CCC GGG AGA ATT TTC CAA GAA TGA GTT AGT AAA |
| YopTΔ308-322anti | CCC GGG CAC CCC CAA AGG ATA ATG ATA CAT A |
| YopTΔ315-322anti | CCC GGG TTA AAC GCT AAA ACT CTG CCC CAC CCC |
| YopTΔ319-322anti | CCC GGG TTA GTC AAA TGT AAA AAC GCT AAA ACT CTG C |
| YopT C139Asens | GGG GGT CGC TGA GGC TTT ATG |
| YopTC139Aanti | CAT AAA GCC TCA GCG ACC CCC |
| YopTD274Nsens | GTT ACT TTC TTC AAT CCC AAT TTC GGT G |
| YopTD274Nanti | CAC CGA AAT TGG GAT TGA AGA AAG TAA |
The proper constructs and mutant forms were checked by DNA sequencing. Expression of GST fusion proteins in E. coli TG1 growing at 37°C was induced by addition of 0.2 mM (final concentration) isopropyl-β-d-thiogalactopyranoside at an optical density of 0.6. Four hours after induction, cells were collected and lysed by sonication in lysis buffer (20 mM Tris-HCl [pH 7.4], 10 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 5 mM dithiothreitol) and purified by affinity chromatography with glutathione-Sepharose (Amersham Pharmacia Biotech). Loaded beads were washed once with washing buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl) and additionally five times with lysis buffer (without phenylmethylsulfonyl fluoride) at 4°C. The GST-YopT fusion protein was eluted from the beads with glutathione (10 mM glutathione, 50 mM Tris-HCl [pH 7.4]) twice, for 10 min each time, at room temperature.
Microinjection.
For microinjection, embryonic bovine lung (EBL) cells were seeded subconfluently on glass coverslips (CELLocate; Eppendorf) and cultivated for 24 h in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum in humidified 5% CO2 at 37°C. Wild-type GST-YopT and fragments thereof (500 ng/μl) in 50 mM Tris-HCl (pH 7.4) were microinjected into EBL cells with an Eppendorf 5242 microinjector. For identification of injected cells, fluorescein-labeled anti-mouse immunoglobulin G (600 ng/μl) was coinjected with wild-type GST-YopT or fragments thereof; control cells were injected with fluorescein-labeled anti-mouse immunoglobulin G alone. After the indicated times of incubation at 37°C, cells were visualized by fluorescence microscopy and images were recorded with an AxioCam HRm (Zeiss).
Preparation of rat brain membranes.
Collected rat brains were homogenized in an appropriate volume of homogenization buffer (100 mM NaCl-50 mM HEPES [pH 7.2] containing 10 μg of leupeptin per ml, 80 μg of benzamidine per mg, 40 μg of aprotinin per ml, and 0.5 mM phenylmethylsulfonyl fluoride).
The homogenate was centrifuged for 10 min at 1,000 × g to remove nuclei and connecting tissue. The supernatant was centrifuged for 60 min at 30,000 × g and subsequently separated into cytosolic and membrane fractions by ultracentrifugation for 60 min at 100,000 × g. Membranes were washed once with homogenization buffer and stored at −20°C.
Before use in the membrane release assay, rat brain membranes were washed three times in buffer B (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 2.5 mM dithiothreitol, 40 μg of aprotinin per ml, 80 μg of benzamidine per ml). All steps were carried out at 4°C.
Membrane release assay.
Rat brain membranes were washed in buffer B and incubated with different concentrations of wild-type GST-YopT or fragments or mutant forms thereof for 30 min at 37°C. After incubation, membranes and supernatants were isolated by ultracentrifugation (60 min at 100,000 × g), separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and analyzed by immunoblotting.
Rhotekin pulldown.
The Rho-binding region, encoding the N-terminal 90 amino acids, of rhotekin was expressed as a GST fusion protein in E. coli BL21. Overnight cultures were diluted 1:100 and grown for 1 h at 37°C, and then 0.1 mM (final concentration) isopropyl-β-d-thiogalactopyranoside was added. Two hours after induction, cells were collected and lysed by sonication in rhotekin lysis buffer (20% sucrose, 10% glycerol, 50 mM Tris-HCl [pH 8.0], 0.2 mM sodium bisulfite, 2 mM MgCl2, 0.5 mM phenylmethylsulfonyl fluoride, 2 mM dithiothreitol, 40 μg of aprotinin per ml, 80 μg of benzamidine per ml) and purified by affinity chromatography with glutathione-Sepharose (Amersham Pharmacia Biotech). Loaded beads were washed three times with rhotekin lysis buffer and once with buffer A (10% glycerol, 50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1% Igepal, 2 mM MgCl2,0.5 mM phenylmethylsulfonyl fluoride).
For activation of Rho proteins, HeLa cells were treated with CNF-1 (400 ng/ml) for 2 h, lysed in buffer A, and then incubated for 30 min at 37°C with different GST-YopT fusion proteins as indicated. The lysates were then incubated with rhotekin-loaded beads for 1 h at 4°C by head-over-head rotation. After incubation, beads were washed once with buffer A. SDS sample buffer was then added, samples were boiled and separated by SDS-PAGE, and RhoA was analyzed by immunoblotting.
GDI pulldown.
GDI (GDI-1) was expressed and purified as a GST fusion protein. Loaded beads were washed three times with buffer B (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 2.5 mM dithiothreitol, 40 μg of aprotinin per ml, 80 μg of benzamidine per ml).
HeLa cells pretreated with CNF-1 (400 ng/ml) were lysed in buffer B, and the cytosols were incubated with GST-YopT for 30 min at 37°C. After incubation, 1/15 of the volume was taken as input and the rest was incubated with GST-RhoGDI beads for 1 h at 4°C with head-over-head rotation. After one washing with buffer B, SDS sample buffer was added and the samples were boiled and separated by SDS-PAGE. RhoA was analyzed by immunoblotting.
Overlay.
Full-length YopT C139A and YopT C139A fragments were expressed and purified as GST fusion proteins. Equal amounts of the GST fusion proteins were subjected to SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membrane. The membrane was incubated for 30 min at room temperature with isoprenylated, 125I-labeled RhoA in buffer C (10 mM HEPES [pH 7.5], 50 mM NaCl, 1% Triton X-100, 2 mM EDTA, 5 mM MgCl2).
Isoprenylated RhoA was expressed in Spodoptera frugiperda (Sf9) insect cells and purified from a Triton X-100-soluble fraction by affinity chromatography. GST fusion protein was cleaved with thrombin. Before 125I labeling, the isoprenylated RhoA had been preincubated with 1 mM GTPγS (30 min at 37°C). The labeling was performed with Iodo-beads (Pierce) in accordance with the manufacturer's directions. After incubation, the blot was washed extensively with TBS-T (200 mM NaCl, 0.25 mM Tris-HCl [pH 7.4], 0.05% Tween 20) and then analyzed and quantified by phosphorimaging.
Immunoblot analysis.
Proteins were separated on SDS-12.5% polyacrylamide gels, transferred onto PVDF membrane, and then blocked with 5% (wt/vol) nonfat dried milk for 1 h. Blots were incubated overnight at 4°C with a monoclonal antibody raised against RhoA (26C4; Santa Cruz) and then for 1 h with a horseradish peroxidase-conjugated secondary antibody. For detection of YopT, a polyclonal YopT antibody generated in rabbit against thrombin-cleaved full-length YopT was used.
RESULTS
Cloning and purification of fragments and mutant forms of YopT.
Recently, it was shown that microinjected recombinant YopT causes rounding of EBL cells and redistribution of the actin cytoskeleton. Moreover, YopT leads to release of RhoA from purified cell membranes. Incubation of lysate or cytosol with YopT causes inhibition of the RhoA-rhotekin interaction and affects the RhoA-RhoGDI interaction (15).
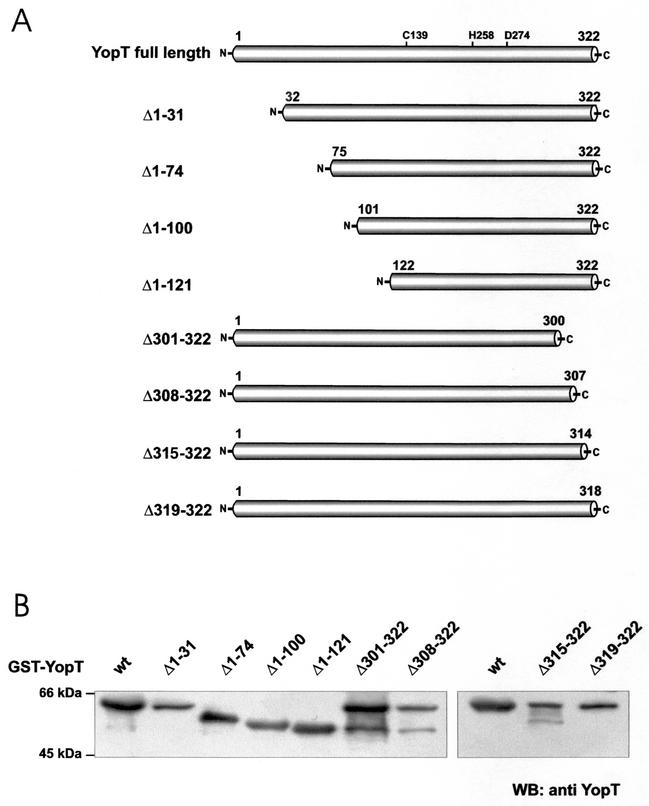
To study the biological relevance of the observed effects and characterize the catalytic domain of YopT, we constructed N- and C-terminal fragments and the point mutations C139A and D274N of YopT (Fig. 1A) and expressed them in E. coli TG1 cells. The purity and molecular mass of the expressed fragments were checked by SDS-PAGE and immunoblotting (Fig. 1B). All fragments and mutant forms were expressed with the right size. The protein samples contained further proteins, which have been identified as GST and the chaperone DnaK by matrix-assisted laser desorption ionization-time of flight mass spectrometry. All of the toxin fragments and mutant forms described have been checked in the assays mentioned above.
FIG. 1.
(A) YopT fragments. The first and last amino acids of each fragment are indicated. N-terminal truncations were named Δ1-31, Δ1-74, Δ1-100, Δ1-121, and Δ301-322, whereas C-terminally truncated fragments were designated Δ301-322, Δ308-322, Δ315-322, and Δ319-322. All fragments contain the amino acids cysteine 139, histidine 258, and aspartate 274, which are crucial for the activity of full-length YopT. (B) Immunoblot of GST-YopT fragments. All of the fragments used in this study were expressed as GST fusion proteins in E. coli TG1. Expression of the different GST fusion proteins was checked by immunoblot analysis with a YopT antibody. wt, wild type; WB, Western blot.
Activity of YopT fragments and mutant forms in microinjection experiments.
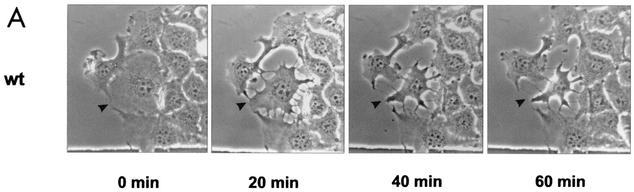
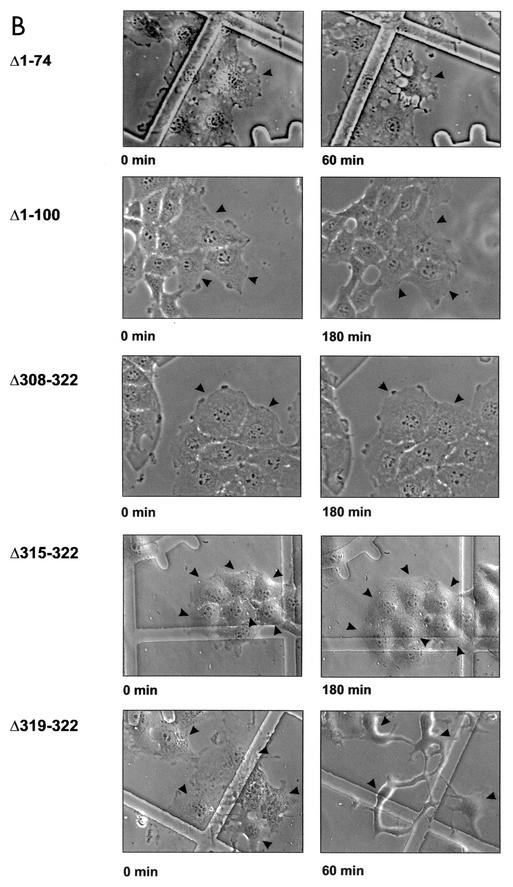
To test the activity of fragments and mutant forms of YopT, we microinjected the purified proteins into EBL cells. As shown in Fig. 2A, GST-YopT already caused retraction of microinjected cells after 20 min, with cell rounding after about 60 to 90 min, while cell-cell contacts were still present. Deletion of the C-terminal 22, 15, or even 8 amino acids led to total loss of YopT activity in the microinjection assay, whereas truncation of 4 amino acids had no effect on its activity. Even 3 h after microinjection, the cells showed no morphological changes in comparison to control cells. This indicates that the C terminus of YopT is crucial for toxin activity. In contrast, deletion of 31 or 74 amino acids of the N terminus had no effect on YopT activity. Deletion of 100 amino acids of the N terminus resulted in an inactive toxin fragment (Fig. 2B; shown are the crucial truncations). This indicates that residues 75 to 100 of the toxin are important for YopT action. Recently, Shao et al. (14) showed that cysteine 139, histidine 258, and aspartate 274 of YopT are crucial for cytotoxicity. We also observed no morphological changes in EBL cells after microinjection of these YopT mutant forms (not shown).
FIG. 2.
Microinjection of YopT into EBL cells. (A) Time course of EBL cells after microinjection of GST-YopT. (B) N-terminal (Δ1-31, Δ1-74, Δ1-100, and Δ1-121) and C-terminal (Δ301-322, Δ308-322, Δ315-322, and Δ319-322) GST-YopT fragments were microinjected into EBL cells. Shown are pictures of crucial GST-YopT fragments, which were taken 60 and 180 min after microinjection, respectively. Injected cells are indicated by arrowheads. The experiment was repeated at least three times with similar results. wt, wild type.
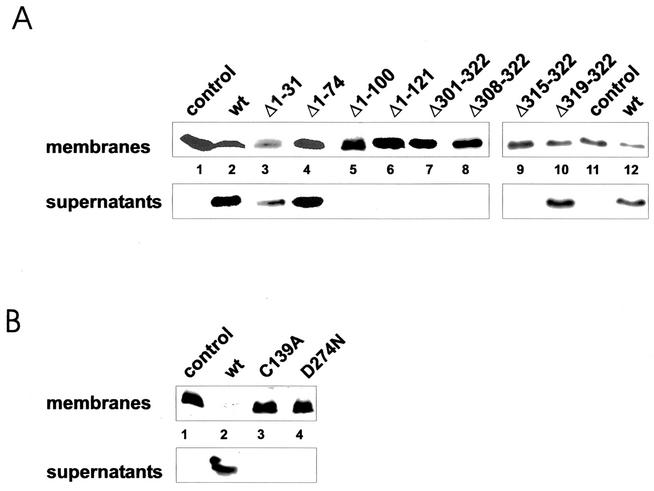
Membrane release of RhoA.
To further analyze the activity of the toxin fragments, we studied the membrane release of RhoA after incubation of purified rat brain membranes with the isolated proteins. As shown for the activity in the living cell, the C-terminal truncations Δ301-322, Δ308-322, and Δ315-322, as well as the N-terminal truncations Δ1-100 and Δ1-121, were inactive in the membrane release assay. By contrast, the forms with N-terminal deletions of 31 and 74 amino acids and that with a C-terminal deletion of 4 amino acids were fully active in comparison with the full-length protein (Fig. 3A). Additionally, the YopT mutant forms C139A and D274N were not able to release RhoA from membranes. This was in agreement with the results obtained in the microinjection studies (Fig. 3B).
FIG. 3.
Membrane release of RhoA. Isolated rat brain membranes were incubated with fragments (A) or mutant forms (B) of GST-YopT, as indicated. After separation of membranes and corresponding supernatants by ultracentrifugation, the samples were analyzed for RhoA by Western blotting (WB). Shown is a typical result of at least four independent experiments. wt, wild type.
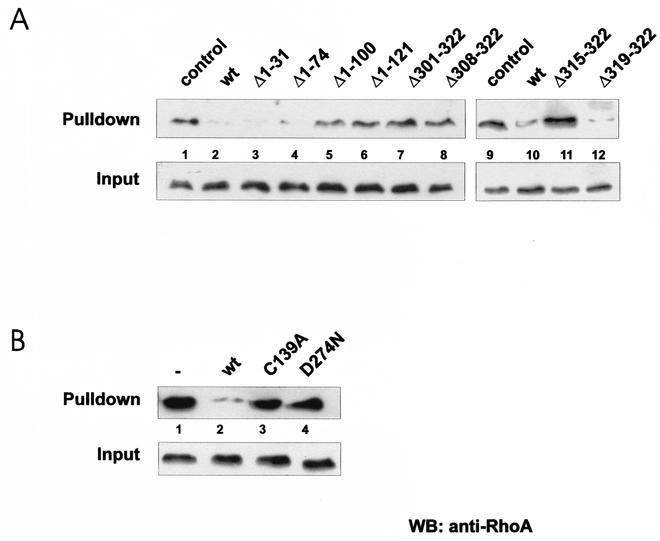
Interaction of YopT-treated RhoA with rhotekin.
We tested whether the active YopT fragments are also able to block the interaction of RhoA with its effector, rhotekin. As observed before, only the N-terminal YopT truncations Δ1-31 and Δ1-74 and the deletion of four C-terminal amino acids revealed activity, whereas the N-terminal truncations Δ1-100 and Δ1-121 and the C-terminal truncations Δ301-322, Δ308-322, and Δ315-322 were not able to block the Rho-rhotekin interaction (Fig. 4A). These findings indicate that the same regions of the toxin are crucial for YopT-induced retraction and rounding of cells after microinjection, release of RhoA from purified membranes, and blocking of the RhoA-rhotekin interaction and that all of these effects are likely results of the same modification. In agreement with the microinjection studies and the membrane release assay, no effect of the YopT mutant forms C139A and D274N on the Rho-rhotekin interaction was observed (Fig. 4B).
FIG. 4.
Rhotekin pulldown assay. Lysates of CNF-1-treated HeLa cells were incubated as indicated with wild-type (wt) GST-YopT and fragments (A) or mutant forms (B) thereof for 30 min at 37°C. After incubation with GST-YopT, 1/15 of the volume was taken as input. The rest of the lysates were incubated with GST-rhotekin beads for 60 min at 4°C. The beads were then washed, and the rhotekin-bound RhoA and the input control were separated by SDS-PAGE and detected by Western blotting (WB) with a RhoA-specific antibody. Shown is a typical result of at least four independent experiments.
Isoprenylation of Rho is required for the YopT effect.
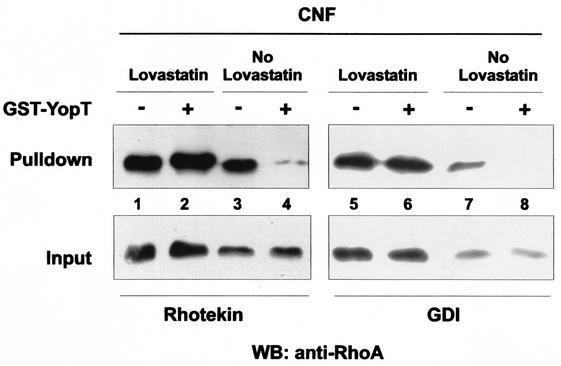
Next we tested whether isoprenylation of RhoA is necessary for the YopT effect on the interaction of constitutively active RhoA and its effector rhotekin, as well as on the interaction between activated Rho and RhoGDI. Therefore, HeLa cells were first treated for 16 h with 30 μM lovastatin, a β-3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitor, to block isoprenylation of RhoA. CNF-1 (400 ng/ml), which activates RhoA by deamidation of Gln63 (13), was then added, and the mixture was incubated for 2 h. While lovastatin treatment causes rounding of cells, subsequent treatment with CNF-1 leads to flattening and increased stress fiber formation because of constitutive activation of RhoA. After incubation with GST-YopT for 30 min at 37°C, cell lysates were subjected to a rhotekin or GDI pulldown assay, respectively. Whereas YopT strongly decreased the interaction between Rho and its effector, rhotekin, in cells treated with CNF-1 only, it had no effect when the cells had previously been treated with lovastatin (Fig. 5, lanes 1 to 4). In the same way, lovastatin blocked the YopT-induced inhibition of the Rho-RhoGDI interaction (Fig. 5, lanes 5 to 8). Independently of YopT treatment, RhoA from lovastatin- and CNF-1-treated cells could be precipitated with RhoGDI beads (lanes 5 and 6). By contrast, when cells had not been treated with lovastatin, incubation of the lysates with YopT completely blocked the interaction between RhoA and GDI (lanes 7 and 8).
FIG. 5.
Influence of lovastatin on the effect of YopT. Subconfluent HeLa cells were treated with 30 μM lovastatin for 16 h and then incubated for 2 h with CNF-1 (400 ng/ml) or only treated with CNF-1. Cytosols were prepared and incubated with or without GST-YopT for 30 min at 37°C. After incubation with GST-YopT, 1/15 of the probes was taken as input while the rest was incubated with either GST-rhotekin or GST-GDI beads for 60 min at 4°C. The beads were then washed. Bound RhoA and the input control were separated by SDS-PAGE and detected by Western blotting (WB) with a RhoA-specific antibody. Shown is a typical result of at least four independent experiments.
Binding of YopT fragments to RhoA.
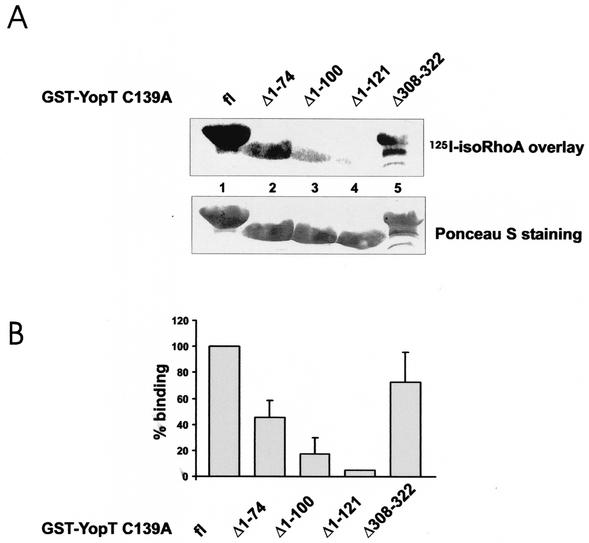
Because all of the catalytically active amino acids identified (14) are present in the YopT fragments studied, we decided to analyze whether the loss of activity corresponds to loss of binding to the YopT target RhoA. The data presented in Fig. 5 show that only isoprenylated Rho is a substrate for YopT. Thus, the following binding studies cannot be performed with recombinant RhoA produced in bacteria. Instead, we used RhoA expressed in Sf9 insect cells for the binding studies. Recently, it was shown that the inactive YopT mutant form YopT C139A can be precipitated with activated RhoA (14). Therefore, we introduced this mutation into all fragments and analyzed the interaction between these YopT C139A fragments and the GTPase in an overlay assay. GST-YopT C139A fragments were subjected to SDS-PAGE and then transferred onto PVDF membrane. The overlay was performed with 125I-labeled isoprenylated RhoA, which was preincubated with 1 mM GTPγS. As shown in Fig. 6, we observed interaction between isoprenylated RhoA and full-length GST-YopT C139A and GST-YopT C139A Δ1-74, as well as between the GTPase and the C-terminal truncation GST-YopT C139A Δ308-322. By contrast, we found only a weak interaction with GST-YopT C139A Δ1-100 and GST-YopT C139A Δ1-121, indicating that the N-terminal part (especially amino acids 75 to 100) of YopT is required for RhoA binding.
FIG. 6.
Binding of YopT fragments to RhoA. The indicated YopT C139A fragments were expressed as GST fusion proteins, and then equal amounts of the proteins were separated by SDS-PAGE and transferred onto PVDF membrane. The membrane was subsequently used for an overlay assay with isoprenylated, 125I-labeled RhoA that had previously been activated with GTPγS. After incubation with RhoA, the membrane was washed extensively and the bound RhoA was analyzed by phosphorimaging. Equal amounts of the GST-YopT proteins were checked by Ponceau S staining (bottom of panel A). In panel A, a typical experiment is shown. Panel B shows the quantification of three independent experiments as means plus standard deviations. fl, full length.
DISCUSSION
Recently, it was shown that Yersinia YopT, which is delivered by type III secretion into mammalian cells, leads to morphological changes and cytotoxic effects in HeLa cells and macrophages, respectively (7). As found for YopT-producing yersiniae (19), recombinant YopT caused release of RhoA from membranes after incubation in vitro (16). It was shown that YopT belongs to a new family of cysteine proteases and cleaves Rho in front of the C-terminal cysteine, thereby leading to release of the GTPase from the membrane and to inactivation of the Rho protein (14). To further characterize the action of recombinant YopT, we generated N- and C-terminal truncations of YopT from plasmid pGEX2TGL-YopT (16). The GST-YopT fragments were expressed in E. coli TG1 cells, purified by affinity chromatography, and checked for activity in different assays. Microinjection studies, the membrane release assay, and the rhotekin and GDI pulldown assays showed the same results; i.e., only the N-terminal YopT truncations Δ1-31 and Δ1-74 and the C-terminal truncation Δ319-322 revealed activity comparable to that of full-length YopT, whereas the N-terminal truncations Δ1-100 and Δ1-121 and the C-terminal truncations Δ301-322, Δ308-322, and Δ315-322 were not able to induce morphological changes after injection into EBL cells or to block the interaction between activated RhoA and rhotekin or GDI, respectively. Moreover, these truncations were not able to release RhoA from membranes, indicating that all of the measured activities are located in the same region of YopT. Interestingly, all of the truncations analyzed in this study contain the amino acids C139, H258, and D274, which were identified recently as being essential for catalysis (14). Nevertheless, some of the YopT fragments studied were inactive in the various assays used. We found comparable stability of these N- and C-terminal toxin truncations and full-length YopT in proteolytic studies with trypsin (data not shown).
To test whether the loss of activity of some YopT fragments was due to loss of interaction between YopT and its substrate, RhoA, we performed an overlay assay with 125I-labeled RhoA that had previously been activated with GTPγS. To allow binding, we introduced the mutation C139A into all of the fragments because it was shown by Shao et al. that only the inactive mutant form of YopT was able to precipitate RhoA in pulldown experiments (14). Whereas RhoA bound to full-length YopT C139A and the corresponding Δ1-74 truncation, we observed no or at least weaker interaction with the N-terminal truncations C139A Δ1-100 and C139A Δ1-121. The interactions between RhoA and the YopT truncations were confirmed by a pulldown assay using cell lysate and the YopT truncations bound to beads (data not shown). Interestingly, deletion of the C terminus did not lead to loss of interaction with RhoA (shown for the fragment Δ308-322 in Fig. 6). The data suggest that the site of interaction between RhoA and the toxin is located between amino acids 75 and 100 of YopT. Structural prediction revealed residues 76 to 100 to be a single α helix. This helix may be involved in RhoA binding.
The C-terminal truncations Δ308-322 and Δ315-322 of YopT were not active, although residues suggested to be necessary for catalysis (14) are present. Furthermore, the C139A mutant form of the shortest C-terminal fragment, Δ308-322, still interacted with RhoA. Therefore, this region may harbor additional residues involved in folding and/or catalysis. One possible explanation is binding of a potential cofactor at the C terminus. Because recombinant YopT acts in vitro without addition of cell lysates or other substances, the potential cofactor could be an ion or a small protein that was copurified from E. coli. A second possible explanation for the loss of activity is incorrect folding of the protein missing C-terminal residues. Further investigation is needed to clarify this issue.
We showed that the effects of YopT on the Rho-rhotekin interaction and on the Rho-GDI interaction, respectively, can be blocked by lovastatin, which inhibits isoprenylation of Rho. These results were in agreement with the data reported recently by Shao and coworkers (14). They demonstrated that interaction between YopT and the Rho GTPases RhoA, Rac, and Cdc42 requires posttranslational modification of the GTPases. Our results also suggest that the isoprenyl moiety of Rho is important for the action of YopT. In contrast to normal posttranslational modification, where the aaX portion of the CaaX box is removed after prenylation, the whole CaaX box is present after lovastatin treatment. The additional aaX residues may block the cleavage of RhoA by YopT. This could explain why activated RhoA containing aaX is not cleaved and still interacts with rhotekin and GDI after lovastatin treatment. In a further study, we showed that the RhoA-GDI interaction is slightly enhanced after treatment of lysates with YopT. In contrast to the present study, RhoA was not activated in the former study. This indicates that the conditions for the interaction of RhoA with GDI may be different for the GDP- and GTP-bound forms of the GTPase. Our data suggest that the C terminus of YopT is crucial for its activity. Further investigation is needed to clarify whether the C-terminal residues of YopT are directly involved in catalysis.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinscraft (SFB 388).
Editor: D. L. Burns
REFERENCES
- 1.Aktories, K., G. Schmidt, and F. Hofmann. 2000. GTPases targeted by bacterial toxins, p. 311-331. In A. Hall (ed.), GTPases. Oxford University Press, Oxford, England.
- 2.Barz, C., T. N. Abahji, K. Trülzsch, and J. Heesemann. 2000. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482:139-143. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, A. L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 348:241-255. [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis, G. R. 2000. Type III secretion: a bacterial device for close combat with cells of their eukaryotic host. Philos. Trans. R. Soc. Lond. B Biol. 355:681-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 7.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29:915-929. [DOI] [PubMed] [Google Scholar]
- 8.Juris, S. J., F. Shao, and J. E. Dixon. 2002. Yersinia effectors target mammalian signalling pathways. Cell. Microbiol. 4:201-211. [DOI] [PubMed] [Google Scholar]
- 9.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene 136:271-275. [DOI] [PubMed] [Google Scholar]
- 10.Palmer, L. E., S. Hobbie, J. E. Galán, and J. B. Blsika. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 11.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fällman. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruckdeschel, K., J. Machold, A. Roggenkamp, S. Schubert, J. Pierre, R. Zumbihl, J.-P. Liautard, J. Heesemann, and B. Rouot. 1997. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J. Biol. Chem. 272:15920-15927. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 14.Shao, F., P. M. Merritt, Z. Bao, R. W. Innes, and J. E. Dixon. 2002. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109:575-588. [DOI] [PubMed] [Google Scholar]
- 15.Shao, F., P. O. Vacratsis, Z. Bao, K. E. Bowers, C. A. Fierke, and J. E. Dixon. 2003. Biochemical characterization of the Yersinia YopT protease: cleavage site and recognition elements in Rho GTPases. Proc. Natl. Acad. Sci. USA 100:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorg, I., U.-M. Goehring, K. Aktories, and G. Schmidt. 2001. Recombinant Yersinia YopT leads to uncoupling of RhoA-effector interaction. Infect. Immun. 69:7535-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takai, Y., T. Sasaki, and T. Matozaki. 2001. Small GTP-binding proteins. Physiol. Rev. 81:153-208. [DOI] [PubMed] [Google Scholar]
- 18.von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 19.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]