Abstract
We previously showed that numerous polymorphonuclear leukocyte (PMN) granule components efficiently kill Borrelia burgdorferi, the agent of Lyme disease. In addition, motile, granule-poor cytoplasts (U-Cyt) from human blood PMN can exert anti-Borrelia activity against opsonized B. burgdorferi independently of oxidative mechanisms. Here we show that lysates of U-Cyt also possess anti-Borrelia activity, a portion of which comes from the abundant cytosolic protein calprotectin. The anti-Borrelia activity of U-Cyt lysates and recombinant calprotectin was partially or completely reversed by specific antibody to calprotectin and by Zn2+, a cation essential for the growth of B. burgdorferi and known to inhibit the antimicrobial activity of calprotectin. Quantitative microscopic and regrowth assays revealed that calprotectin acted in a bacteriostatic fashion against B. burgdorferi. We conclude that calprotectin, a potent bacteriostatic agent from a cell primarily recognized for its oxidative and granular antibacterial mechanisms, may play a modulatory role in infection by the Lyme spirochete, particularly at sites of acute inflammation.
The spirochete Borrelia burgdorferi is the infectious agent of Lyme disease, and its natural life cycle involves a mammalian and an arthropod host (10). Lyme disease usually begins with a characteristic rash, erythema migrans, and persistent infection leads to a range of inflammatory processes that include arthritis, carditis, and a variety of neurological symptoms (10). Polymorphonuclear leukocytes (PMN), important components of both innate immunity and antibody-dependent clearance, are the first immune cells to respond to B. burgdorferi infection in the skin and are heavily represented in the subsequent inflammatory response in joints (10). Experimental studies have indicated that PMN play a critical role in controlling infection by B. burgdorferi; beige mice, which have defects in their PMN granules, have significantly worse arthritis (1). Infected mice have tendonitis, synovial tissue hyperproliferation, and a synovial fluid cellular influx predominated by PMN, abnormalities that resemble those observed in human patients (2, 10). The severity of these symptoms has been correlated with the spirochete burden found in joints (28).
The PMN is a potent killer cell with abundant antimicrobial mechanisms that include contributions from a respiratory burst oxidase and from granule constituents. PMN generally kill target organisms in the controlled environment of phagolysosomes following ingestion (16). We have shown that, unlike macrophages, PMN ingest and kill B. burgdorferi efficiently only in the presence of specific antibody (14). We previously defined the role of particular granule components and developed evidence for extracellular killing of B. burgdorferi by PMN (9, 14). We showed that the PMN granule components elastase, LL-37, bactericidal/permeability-increasing protein, and human neutrophil peptide-1 have anti-Borrelia abilities that are restricted by incubation conditions such as pH and ionic strength (9). In addition, U-cytoplasts (U-Cyt), motile, granule-poor cytoplasts which retain activatable oxidase (12), also reduce the viability of opsonized B. burgdorferi, even without the contribution of reactive oxygen intermediates (9). The remarkable anti-Borrelia activity retained by granule-poor U-Cyt despite scavenging of reactive oxygen intermediates suggests the presence of an additional, previously unconsidered anti-Borrelia mechanism (9).
One PMN antimicrobial agent that is less well studied is the cytosolic protein calprotectin, a heterodimer consisting of one light (11 kDa) and one heavy (14 kDa) chain (5), which has both cellular and extracellular roles. Calprotectin is a particularly abundant protein of the neutrophil, with an estimated cytosolic concentration of 5 to 15 mg/ml, constituting 45% of the cytosolic protein (7, 27). Its effect is exerted primarily by inhibition of growth through competition for zinc (23), although at high concentrations calprotectin may be microbicidal (25). The multifunctional role of calprotectin and its association with a variety of inflammatory diseases have historically created a complex nomenclature; other names given to either the heterodimer or its individual subunits include MRP-8 and MRP-14 (migration-inhibitory factor-related proteins), cystic fibrosis antigen, L1 antigen, calgranulin A and B, and S100A8 and S100A9 (7). Here we show that the cytosolic protein calprotectin is a potent anti-Borrelia component of the neutrophil's armamentarium.
MATERIALS AND METHODS
Preparation of B. burgdorferi culture.
Low-passage, virulent B. burgdorferi strain N40 was grown to logarithmic phase in Barbour-Stoenner-Kelley II medium (BSK) with added neomycin and amphotericin at 33°C (9). For experiments, B. burgdorferi was pelleted (10 min, 3,000 × g, 25°C), resuspended in BSK, and enumerated with a Petroff-Hausser hemacytometer (Hausser Scientific Partnership, Horsham, Pa.) under dark-field microscopy. B. burgdorferi was opsonized by treatment for 30 min at 37°C with 1% heat-inactivated serum (56°C for 30 min) from a well-characterized Lyme disease patient (recognizing bands at 18, 21, 28, 30, 58, and 60 kDa on the B. burgdorferi immunoblot) as described before (9).
Cytoplast and lysate preparations.
Blood donations from healthy volunteers were obtained in accordance with the guidelines of the Human Investigation Committee of Yale University School of Medicine. PMN were isolated from fresh heparinized blood of healthy volunteers that was allowed to sediment in 3% dextran at an angle of 45° for 1 h, followed by hypotonic lysis of contaminating erythrocytes (12). U-Cyt were prepared from purified PMN on a discontinuous Ficoll gradient as described previously (12). Lysates of U-Cyt and PMN were prepared immediately before use by multiple freeze-thaw cycles in liquid N2 and a 37°C water bath.
[3H]adenine B. burgdorferi regrowth assay.
Changes in B. burgdorferi number were quantified with a regrowth assay which measures the uptake of [3H]adenine by B. burgdorferi as we have described previously (9). The [3H]adenine uptake is linear from 104 to 106 B. burgdorferi organisms/ml over 48 h. Opsonized spirochetes (5 × 106 opsonized B. burgdorferi organisms per ml) were incubated in the presence of intact U-Cyt (5 × 104 to 5 × 106/ml) for 1 h at 37°C in phosphate-buffered saline (pH 7.4) with 5.4 mM glucose and 2% human serum albumin with agitation; this buffer maintains the viability of B. burgdorferi throughout the incubation period (9). Triplicate aliquots (50 μl) were plated in 96-well plates in the presence of 200 μl of BSK containing 5 μCi of [3H]adenine, incubated for 48 h at 33°C, and harvested. Incorporation of [3H]adenine by treated B. burgdorferi was compared with that of control samples under the same incubation conditions. The data were fit to a first-order exponential decay equation to determine the 50% inhibitory concentration (IC50) obtained with the assay conditions employed. Limits are expressed as standard errors of the mean.
Microscopic assessment of B. burgdorferi number.
For studies with lysates of U-Cyt and recombinant calprotectin, we employed a direct visual quantitation of spirochetes under dark-field microscopy at 1, 24, and 48 h (9). The microscope assay has proven especially useful both for the study of B. burgdorferi in the presence of components with limited availability, as results can be obtained from smaller experimental volumes, and for the comparison of immediate versus later effects from the same sample. In this assay, 5 × 106 B. burgdorferi organisms/ml are incubated with lysates of U-Cyt or recombinant calprotectin as described below, and the percent cell survival is assessed immediately and after 48 h from 5-μl aliquots of the incubated samples diluted with 10 μl of BSK and enumerated by dark-field microscopy. Spirochete motility and morphology are determined in 10 random fields in a double-blind fashion, and cells are considered killed when complete loss of motility and refractivity is observed (15). The earlier time points were used to distinguish growth inhibition from direct killing, since either effect will result in reduced numbers at 48 h. We have previously shown excellent concordance of the dark-field microscopy assay with the [3H]adenine regrowth assay for determination of B. burgdorferi numbers (15).
Lysates of U-Cyt were incubated with B. burgdorferi (5 B. burgdorferi organisms to 1 U-Cyt equivalent) in 50 μl of assay buffer (50 mM HEPES [pH 7.55], 150 mM NaCl, 5.4 mM glucose, and 1 mM CaCl2) for 1 h at 37°C prior to threefold dilution with BSK and further incubation at 33°C for 48 h of regrowth. Recombinant human calprotectin (MRP 8/MRP 14) (5) was the generous gift of Craig Vanderkooi and Walter Chazin (Vanderbilt University, Nashville, Tenn.). For studies with multiple concentrations of calprotectin, B. burgdorferi was directly incubated in BSK with 20% (vol/vol) assay buffer at 33°C for 1 to 48 h. The IC50 and error were calculated as described above.
For antibody blocking studies, 4 μg of monoclonal anticalprotectin antibody Mac 387 (Dako Corporation, Carpinteria, Calif.) was incubated with 10 μg of U-Cyt lysate protein, as determined by the Bradford assay, or calprotectin (125 μg/ml) in a 45-μl volume for 30 min at 37°C and then for 30 min at 4°C (20) before addition of B. burgdorferi (5 × 106 organisms/ml). The final experimental volume (50 μl) was incubated directly in BSK with 20% (vol/vol) assay buffer at 33°C for 48 h. Control incubations of B. burgdorferi alone with either Mac 387 or a control monoclonal antibody, mouse anti-CD15 (4 μg), a PMN surface marker (Dako Corporation), showed no effect on spirochete growth. Antibody buffers were exchanged into assay buffer immediately before use. Significance was assessed by analysis of variance and paired t tests, one-tailed.
For Zn2+ reversal studies, B. burgdorferi was incubated directly in the presence or absence of calprotectin (90 and 125 μg/ml) or U-Cyt lysates (5 B. burgdorferi organisms to 1 U-Cyt equivalent) and 3 μM ZnCl2 in 50% BSK with 15 mM HEPES (pH 7.5). BSK medium contains 28 μM Zn2+, as determined by inductively coupled plasma-mass spectrometry (Chemical Analysis Laboratory, University of Georgia, Athens). Significance was assessed by paired t tests, one-tailed.
Microscopic assessment of B. burgdorferi viability was performed to distinguish between growth inhibition and direct killing. B. burgdorferi (107 organisms/ml) was incubated for 1 h in the presence of highly inhibitory levels of either calprotectin (225 μg/ml in assay buffer) or LL-37 (200 μg/ml in phosphate-buffered saline-5.4 mM glucose), the active fragment of the PMN granule component cathelicidin, the kind gift of Birgitta Agerberth (Karolinska Institutet, Stockholm, Sweden) (6). After 1 h at 37°C, the B. burgdorferi was labeled for viability with a supravital stain (Live/dead kit; Molecular Probes, Inc., Eugene, Oreg.). Cells were examined on a Zeiss Axiovert microscope with a 510 long-pass filter under a 100× oil immersion lens.
Western blot analysis of cellular calprotectin levels.
U-Cyt and PMN lysates were separated by sodium dodecyl sulfate-20% polyacrylamide gel electrophoresis (SDS-20% PAGE) and electroblotted to nitrocellulose membranes. The membranes were blocked for 1 h at room temperature in phosphate-buffered saline with 5% bovine serum albumin and then incubated with monoclonal anti-human MAC 387 antibody, specific for both subunits of calprotectin, at 1:500 for 3 h, washed, and incubated with alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Vector Laboratories, Burlingame, Calif.) for 30 min at 1:500. The blots were developed with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (KPL, Gaithersburg, Md.).
RESULTS
U-Cyt and lysates of U-Cyt reduce B. burgdorferi number.
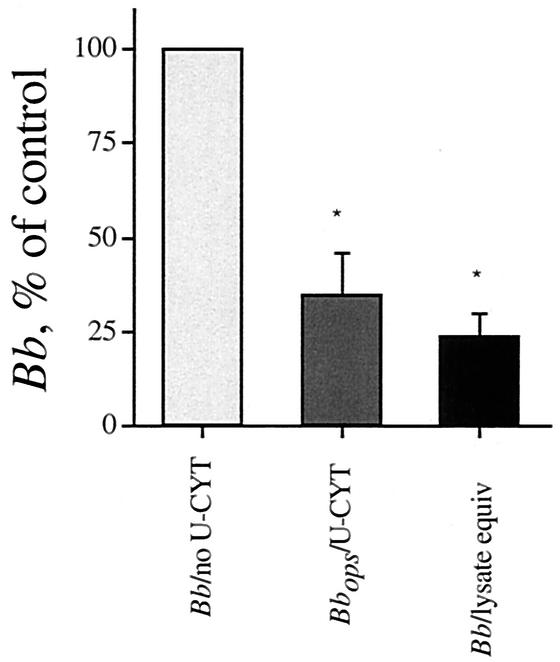
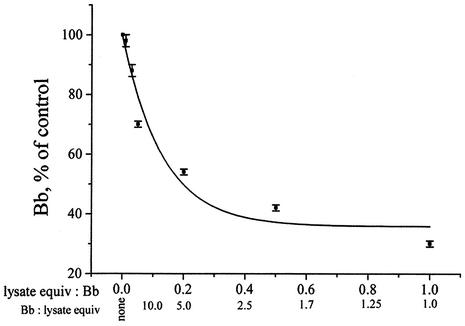
We undertook the present study in order to elucidate the anti-Borrelia activity of U-Cyt. We showed previously that even after 48 h of incubation, intact U-Cyt significantly reduced the number of B. burgdorferi organisms only when the spirochetes had been opsonized with specific antibody (9). A similar reduction in the number of B. burgdorferi organisms was now apparent when either intact U-Cyt or their lysates were employed (n = 3) (Fig. 1). Lysates of U-Cyt reduced the number of opsonized B. burgdorferi organisms over 48 h by 50% when incubated at a ratio of 8 B. burgdorferi organisms to 1 U-Cyt equivalent; the maximum inhibition in B. burgdorferi number observed was 35% of the control value (n = 5) (Fig. 2) under the experimental conditions employed. In granule-poor cytoplasts, these findings suggest the presence of a previously unrecognized cytosolic anti-Borrelia activity.
FIG. 1.
U-Cyt and lysates of U-Cyt reduce B. burgdorferi number. Intact U-Cyt incubated with opsonized B. burgdorferi (Bbops) cells were compared with paired freeze-thawed lysates of U-Cyt incubated with B. burgdorferi (Bb; 5 B. burgdorferi organisms to 1 U-Cyt, intact or lysate) (n = 3). B. burgdorferi number was determined by either [3H]adenine uptake or dark-field microscopic assay after 48 h. Experimental significance was assessed by analysis of variance (degrees of freedom = 2, P = 0.0012); individual differences were assessed with a paired t test. Both intact U-Cyt and paired lysates of U-Cyt reduced B. burgdorferi number significantly (n = 3). The asterisk indicates that the difference in values for paired U-Cyt or lysates of U-Cyt and B. burgdorferi alone is statistically significant (P = 0.014 and 0.004, respectively). U-Cyt did not have an appreciable anti-Borrelia effect unless the B. burgdorferi was opsonized (9). Lysates of U-Cyt given B. burgdorferi were at least as effective as intact U-Cyt given opsonized B. burgdorferi, but the two groups were not significantly different from each other (n = 3, P = 0.1).
FIG. 2.
The inhibitory effect of lysates of U-Cyt on B. burgdorferi number is dose dependent. Lysates of U-Cyt were incubated with B. burgdorferi (Bb) at 5 × 106 B. burgdorferi organisms/ml for 1 h at 37°C and then at 33°C for 48 h for regrowth. The IC50 (50% inhibition) was 8 B. burgdorferi organisms to 1 U-Cyt equivalent. The B. burgdorferi number was determined microscopically at 48 h. Inhibition is expressed as a percentage of the value for the control, B. burgdorferi incubated in buffer alone.
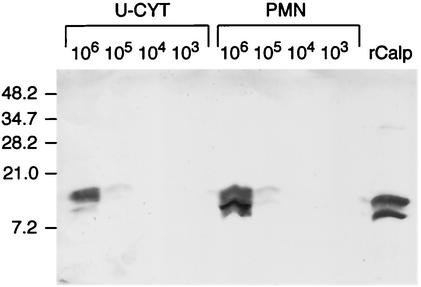
U-Cyt and PMN contain similar amounts of calprotectin.
We assessed the levels of calprotectin in serial 10-fold dilutions (106 to 103) of U-Cyt and PMN by Western blotting. Despite their reduced size, U-Cyt cell lysates and whole PMN contained similar quantities of the heterodimeric calprotectin (Fig. 3). Repeated Western blots of multiple U-Cyt preparations demonstrated equivalent distributions of the two bands in U-Cyt prepared from PMN from other donors (data not shown).
FIG. 3.
PMN and U-Cyt contain similar amounts of calprotectin. A total of 106 to 103 cell equivalents of PMN and U-Cyt were separated on an SDS-20% PAGE gel and electroblotted to a nitrocellulose membrane. Cellular recombinant human calprotectin (rCalp) was visualized with the monoclonal anti-human Mac 387 antibody, specific for both subunits of calprotectin; the control antibody showed no bands (not shown). The bands visualized after 10-fold dilutions of PMN and U-Cyt were similar. Calprotectin (2.5 μg) is shown at the right. Molecular weight standards are indicated on the left.
Anti-Borrelia effect of calprotectin.
We incubated B. burgdorferi cells with calprotectin to determine whether they were sensitive to this protein. We found a dose-dependent reduction in the number of B. burgdorferi organisms when the spirochetes were incubated with physiologically relevant concentrations of calprotectin (5 μg/106 PMN) (Table 1), corresponding to cytosolic concentrations of 5 to 15 mg/ml (4, 7, 27). At 48 h, the IC50 was 140 μg of calprotectin per ml; the maximum inhibition was 72%, i.e., only 28% ± 2.2% of the control remained when B. burgdorferi cells were incubated with 300 μg of calprotectin per ml (Table 1).
TABLE 1.
Effect of calprotectin on growth of spirochetes at 48 ha
| Calprotectin (μg/ml) | No. of B. burgdorferi organisms (% of control) ± SEM (n = 2-4) |
|---|---|
| 0 | 100 |
| 18 | 68 ± 0.5 |
| 50 | 65 ± 4.0 |
| 150 | 50 ± 4.2 |
| 300 | 28 ± 2.2 |
Cells were incubated in BSK with 20% assay buffer (50 mM HEPES [pH 7.55], 150 mM NaCl, 5.4 mM glucose, and 1 mM CaCl2) at 33°C for 48 h. The IC50 of calprotectin was determined to be 140 μg/ml.
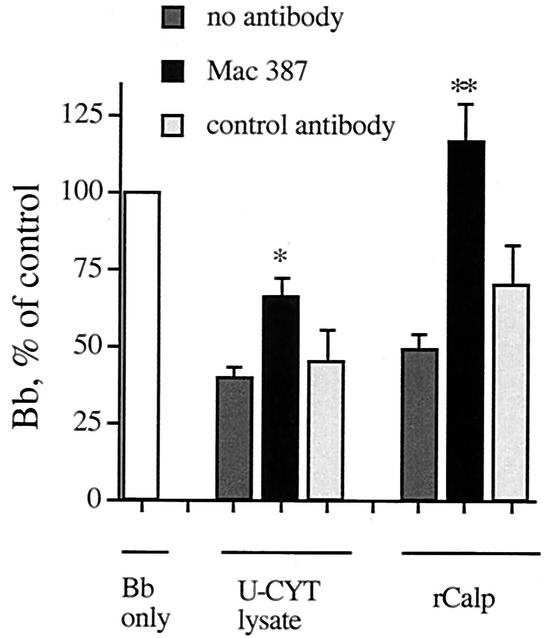
Anticalprotectin antibody reduces the anti-Borrelia activity of lysates of U-Cyt and of calprotectin.
To confirm that a portion of the anti-Borrelia effect of lysates of U-Cyt was a result of the action of calprotectin, we incubated lysates of U-Cyt or calprotectin with the blocking monoclonal antibody Mac 387 or a control antibody (anti-CD15). Incubation of B. burgdorferi alone with either Mac 387 or control did not significantly affect B. burgdorferi growth (n = 5, not significant). The reduction in B. burgdorferi number resulting from incubation with lysates of U-Cyt was significantly blocked by specific anticalprotectin antibody but not control antibody (Fig. 4). In addition, as expected, Mac 387 completely removed the anti-Borrelia effect of calprotectin (Fig. 4); the control antibody did not.
FIG. 4.
Anticalprotectin antibody reduces the anti-Borrelia activity of lysates of U-Cyt and of calprotectin itself. B. burgdorferi (Bb) was incubated with lysates of U-Cyt at a ratio of 5 B. burgdorferi organisms per U-Cyt equivalent or with calprotectin (rCalp) at 125 μg/ml. The average number of B. burgdorferi cells relative to the control after 48 h is shown for lysates of U-Cyt (n = 7) and calprotectin (n = 6). The anti-Borrelia activity of lysates of U-Cyt and calprotectin was inhibited when either was incubated with specific antibody (n = 6 to 7). A control PMN monoclonal antibody did not significantly affect B. burgdorferi growth (n = 3 to 5). These two antibodies incubated with B. burgdorferi alone did not significantly affect B. burgdorferi growth (n = 5, not significant). Experimental significance was assessed by analysis of variance for lysate of U-Cyt (degrees of freedom = 3, P = 0.0016) and calprotectin (degrees of freedom = 3, P = 0.0015); individual differences were assessed with a paired t test. The asterisk indicates that the differences in values for lysates of U-Cyt with specific antibody and lysates of U-Cyt alone or lysates of U-Cyt with control antibody were significant (P = 0.001 and 0.04, respectively). The double asterisk indicates the significance of the corresponding differences for calprotectin (P = 0.0006 and 0.03, respectively).
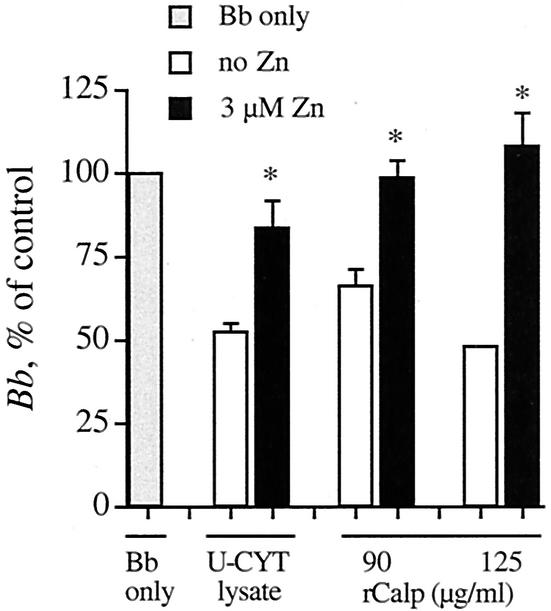
Zn2+ reverses the anti-Borrelia activity of lysates of U-Cyt and of calprotectin.
The antimicrobial effects of calprotectin on Candida albicans are reversible by 10 to 30 μM Zn2+ (3, 23). We examined the effect of Zn2+ on the anti-Borrelia activity of lysates of U-Cyt and of calprotectin. The incremental concentration of only 3 μM Zn2+ partially reversed the anti-Borrelia activity of lysates of U-Cyt and completely reversed the ability of calprotectin to reduce B. burgdorferi number (Fig. 5). The BSK medium, used at 50% (vol/vol) in this assay, contributes 14 μM Zn2+ (BSK contains 28 μM Zn2+, as determined by mass spectrometry; see Materials and Methods). This final concentration (17 μM) is in the range employed for reversal of anti-Candida activity (above), suggesting that, as with Candida (3, 21, 22), chelation of Zn2+ may be responsible for the anti-Borrelia effect. Additions of higher concentrations of Zn2+ did not increase the reversal of anti-Borrelia activity by U-Cyt lysates (data not shown).
FIG. 5.
Zn2+ reverses the anti-Borrelia activity of lysates of U-Cyt and of calprotectin. B. burgdorferi (Bb) was incubated with U-Cyt lysates (5 B. burgdorferi organisms to 1 U-Cyt equivalent) and calprotectin (rCalp; 90 or 125 μg/ml). Average numbers of B. burgdorferi relative to controls after 48 h are shown. The anti-Borrelia activity of U-Cyt lysates and calprotectin at either concentration was reversed by 3 μM Zn2+. In three experiments, the asterisks indicate statistical significance of the reversals when Zn2+ was added (P = 0.04, 0.03, and 0.01, respectively).
Calprotectin is bacteriostatic.
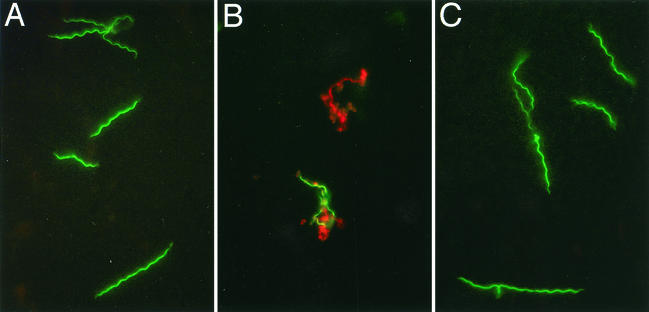
To clarify the mechanism of the anti-Borrelia effect of calprotectin, we examined treated spirochetes microscopically. When enumerated under dark-field microscopy, numbers of B. burgdorferi organisms treated with calprotectin remained essentially constant at 1, 24, and 48 h. During the same interval, spirochete controls increased two- to threefold (data not shown). In contrast, spirochetes treated with LL-37, the active fragment of the PMN granule protein cathelicidin (6), employed here for comparison, showed an immediate reduction in the number of viable B. burgdorferi organisms by 1 h (Fig. 6), followed by proliferation of the remaining viable B. burgdorferi organisms over the subsequent 48 h. With fluorescence microscopy and a supravital stain, we found that after 1 h, B. burgdorferi with calprotectin remained viable; B. burgdorferi with LL-37 was being killed (Fig. 6). When examined after 48 h of incubation, the numbers of spirochetes treated with LL-37 were 9% of the control numbers (9); those treated with calprotectin were 28% of the control numbers. We conclude that the reduction in the number of B. burgdorferi organisms incubated with calprotectin observed at 48 h was the result of bacteriostasis rather than a direct lethal effect, such as that achieved by LL-37.
FIG. 6.
Calprotectin has no immediate effect on B. burgdorferi viability. B. burgdorferi was incubated with LL-37, the active fragment of the PMN microbicidal granule component cathelicidin, or with calprotectin for 1 h before being labeled with a vital stain and examined by fluorescence microscopy, with a 100× objective and a 510-nm long-pass filter. Live organisms stain green; killed organisms stain red. (A) Buffer control; (B) LL-37 (200 μg/ml); (C) calprotectin (300 μg/ml). After 1 h in LL-37, B. burgdorferi is being killed; B. burgdorferi controls and those treated with calprotectin remain viable.
DISCUSSION
The extensive antimicrobial mechanisms of PMN include contributions from oxygen and nitrogen intermediates and granule constituents that can act both intra- and extracellularly (16). We previously identified the importance of the PMN granule components elastase, bactericidal/permeability-increasing protein, LL-37, and human neutrophil peptide-1 for efficient killing of B. burgdorferi and found that killing can also occur extracellularly (9, 14). U-Cyt, anucleate PMN fragments depleted of granules but retaining motility and respiratory burst activity, have proven to be a useful tool for identifying contributions from various portions of the web of cellular antimicrobial mechanisms (9, 11, 24). For example, with cytoplasts we were able to show that nitric oxide, not previously detected in human PMN, contributed to killing of Staphylococcus aureus (11) and that targeted oxidant generation by PMN did not kill Candida albicans hyphae unless granule constituents were added back (24).
When intact U-Cyt were studied in the presence of inhibitors or scavengers of O2−, H2O2, and OH•, no reduction in their killing of B. burgdorferi was found (9). We have now shown that lysates of U-Cyt were also able to reduce the number of B. burgdorferi organisms, as well as intact U-Cyt given opsonized B. burgdorferi, confirming the lack of contribution of membrane-bound oxidative mechanisms seen previously (Fig. 1) (9). Dose-dependent reduction of B. burgdorferi number by lysates of U-Cyt (Fig. 2) established that an additional anti-Borrelia mechanism was present, and we now identify it, at least in part, as the cytosolic protein calprotectin. The susceptibility of B. burgdorferi to calprotectin was confirmed with recombinant calprotectin (Table 1). Its presence in U-Cyt, in amounts comparable to those found in whole PMN, was confirmed by Western blot analysis (Fig. 3). Finally, the anti-Borrelia activity of lysates of both U-Cyt and calprotectin was significantly reversed, in part or completely, by incubation with the calprotectin-specific antibody Mac 387 (Fig. 4).
Competition for iron is a well-documented mechanism of antimicrobial defense; B. burgdorferi is unusual in that it requires Zn2+ for growth, not Fe2+ (17). This is in keeping with our previous demonstration that B. burgdorferi is not susceptible to the iron chelator lactoferrin (9). In general, bacteria are reported to require less zinc than fungi (26), so it is not surprising that susceptibility of fungi to calprotectin, which binds zinc, has received the most attention (25). Studies have shown that the antifungal ability of calprotectin is reversible upon addition of Zn2+ (13, 19, 20, 23). Zn binding to calprotectin leads to conformational changes that alter its ability to bind arachidonic acid (8), but it is unclear how this affects its antimicrobial functions. Multiple studies have shown that the mechanism of the antimicrobial action of calprotectin is sequestration or deprivation of Zn (3, 21, 22). Our studies show that B. burgdorferi is as susceptible to calprotectin as such fungi as Candida albicans and Cryptococcus neoformans, for which the MIC is 4 to 128 μg/ml (25), versus a MIC of 18 to 300 μg/ml for B. burgdorferi. This susceptibility to calprotectin was reversed upon addition of 3 μM Zn2+ (Fig. 5), suggesting that Zn2+ deprivation is the probable mechanism of action, as has been shown for Candida albicans (3).
In contrast to the previously studied individual granule components, which require specific controlled conditions for optimal activity (9), calprotectin exerted its anti-Borrelia activity in the complex growth medium BSK. Thus, unlike granule proteins, many of which are active only in the environment of the phagolysosome or have decreased activity in other physiologically relevant conditions, calprotectin can function as an extracellular antimicrobial factor. Indeed, ambient calprotectin levels correlate with PMN death and lysis (27). In bacterial infections, plasma levels as high as 11 μg/ml are seen; in 41 patients with rheumatoid arthritis, synovial fluid contained a median of 18 μg/ml (range, 2 to 375 μg/ml) (plasma controls generally contain ≤1 μg/ml) (7). A disorder of calprotectin regulation resulting in increased plasma levels has recently been reported; these patients also experience arthritis (18). Our data show that a portion of the anti-B. burgdorferi activity of lysates of U-Cyt was reversible by a calprotectin-specific antibody (Fig. 4) and by 3 μM Zn2+ (Fig. 5), indicating that cellular calprotectin contributes to anti-Borrelia PMN activity. The absence of early killing of B. burgdorferi (Fig. 6) indicates that calprotectin activity against B. burgdorferi is bacteriostatic in nature. The possible modulatory role of calprotectin at inflammatory sites in Lyme disease remains to be elucidated.
These studies indicate that physiologic concentrations of the cytosolic protein calprotectin can contribute to the anti-Borrelia activity of PMN. Future studies may identify further roles for this abundant cytosolic protein in a cell primarily recognized for its oxidative and granular antibacterial mechanisms, particularly where zinc dependence proves to be important.
Acknowledgments
We are grateful to Rita Palmarozza for technical assistance in the preparation of U-Cyt.
This work was supported in part by grants from the NIH (AI 43558, AR 10493, AR 48513, and AR 07107), the Mathers and Arthritis foundations, and the Eshe Fund. D.L. is a Fellow of the Arthritis Foundation. The production of recombinant light and heavy chains of calprotectin in the Chazin laboratory was supported by NIH grant GM62112.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Barthold, S. W., and M. de Souza. 1995. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172:778-784. [DOI] [PubMed] [Google Scholar]
- 2.Barthold, S. W., M. de Souza, E. Fikrig, and D. H. Persing. 1992. Lyme borreliosis in the laboratory mouse, p. 223-242. In S. E. Schutzer (ed.), Lyme disease: molecular and immunologic approaches. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Clohessy, P. A., and B. E. Golden. 1995. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand. J. Immunol. 42:551-556. [DOI] [PubMed] [Google Scholar]
- 4.Edgeworth, J., M. Gorman, R. Bennett, P. Freemont, and N. Hogg. 1991. Identification of p8, 14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 266:7706-7713. [PubMed] [Google Scholar]
- 5.Hunter, M. J., and W. J. Chazin. 1998. High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J. Biol. Chem. 273:12427-12435. [DOI] [PubMed] [Google Scholar]
- 6.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 7.Johne, B., M. K. Fagerhol, T. Lyberg, H. Prydz, P. Brandtzaeg, C. F. Naess-Andresen, and I. Dale. 1997. Functional and clinical aspects of the myelomonocyte protein calprotectin. J. Clin. Pathol. Mol. Pathol. 50:113-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerkhoff, C., T. Vogl, W. Nacken, C. Sopalla, and C. Sorg. 1999. Zinc binding reverses the calcium-induced arachidonic acid-binding capacity of the S100A8/A9 protein complex. FEBS Lett. 460:134-138. [DOI] [PubMed] [Google Scholar]
- 9.Lusitani, D., S. E. Malawista, and R. R. Montgomery. 2002. Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J. Infect. Dis. 185:797-804. [DOI] [PubMed] [Google Scholar]
- 10.Malawista, S. E. 2001. Lyme disease, p. 2629-2648. In W. J. Koopman (ed.), Arthritis and allied conditions: a textbook of rheumatology, 14th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 11.Malawista, S. E., R. R. Montgomery, and G. Van Blaricom. 1992. Evidence for reactive nitrogen intermediates in killing of staphylococci by human neutrophil cytoplasts: a new microbicidal pathway for polymorphonuclear leukocytes. J. Clin. Investig. 90:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malawista, S. E., and G. Van Blaricom. 1987. Cytoplasts made from human blood polymorphonuclear leukocytes with or without heat: preservation of both motile function and respiratory burst oxidase activity. Proc. Natl. Acad. Sci. USA 84:454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mambula, S. S., E. R. Simons, R. Hastey, M. E. Selsted, and S. M. Levitz. 2000. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect. Immun. 68:6257-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery, R. R., D. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185:1773-1779. [DOI] [PubMed] [Google Scholar]
- 15.Pal, U., R. R. Montgomery, D. Lusitani, P. Voet, V. Weynants, S. E. Malawista, Y. Lobet, and E. Fikrig. 2001. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J. Immunol. 166:7398-7403. [DOI] [PubMed] [Google Scholar]
- 16.Philips, M. R., and B. N. Cronstein. 2001. Structure and function of neutrophils, p. 358-382. In W. J. Koopman (ed.), Arthritis and allied conditions: a textbook of rheumatology, 14th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17.Posey, J. E., and F. C. Gherardini. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651-1653. [DOI] [PubMed] [Google Scholar]
- 18.Sampson, B., M. K. Fagerhol, C. Sunderkotter, B. Golden, P. Richmond, N. Klein, I. Z. Kovar, J. H. Beattie, B. Wolska-Kusnierz, Y. Saito, and J. Roth. 2002. Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet 360:1742-1745. [DOI] [PubMed] [Google Scholar]
- 19.Santhanagopalan, V., B. L. Hahn, B. E. Dunn, J. H. Weissner, and P. G. Sohnle. 1995. Antimicrobial activity of calprotectin isolated from human empyema fluid supernatants. Clin. Immunol. Immunopathol. 76:285-290. [DOI] [PubMed] [Google Scholar]
- 20.Sohnle, P. G., C. Collins-Lech, and J. H. Wiessner. 1991. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J. Infect. Dis. 163:187-192. [DOI] [PubMed] [Google Scholar]
- 21.Sohnle, P. G., B. L. Hahn, and R. Karmarkar. 2001. Effect of metals on Candida albicans growth in the presence of chemical chelators and human abscess fluid. J. Lab. Clin. Med. 137:284-289. [DOI] [PubMed] [Google Scholar]
- 22.Sohnle, P. G., B. L. Hahn, and V. Santhanagopalan. 1996. Inhibition of Candida albicans growth by calprotectin in the absence of direct contact with the organisms. J. Infect. Dis. 174:1369-1372. [DOI] [PubMed] [Google Scholar]
- 23.Sohnle, P. G., M. J. Hunter, B. Hahn, and W. J. Chazin. 2000. Zinc-reversible antimicrobial activity of calprotectin (migration inhibitory factor-related proteins 8 and 14). J. Infect. Dis. 182:1272-1275. [DOI] [PubMed] [Google Scholar]
- 24.Stein, D. K., S. E. Malawista, G. Van Blaricom, and R. D. Diamond. 1995. Cytoplasts generate oxidants and kill Staphylococcus aureus but require added neutrophil granule constituents for fungicidal activity against Candida albicans hyphae. J. Infect. Dis. 172:511-520. [DOI] [PubMed] [Google Scholar]
- 25.Steinbakk, M., C. F. Naess-Andresen, E. Lingaas, I. Dale, P. Brandtzaeg, and M. K. Fagerhol. 1990. Antimicrobial actions of calcium binding leucocyte L1 protein calprotectin. Lancet 336:763-765. [DOI] [PubMed] [Google Scholar]
- 26.Sugarman, B. 1983. Zinc and infection. Rev. Infect. Dis. 5:137-147. [DOI] [PubMed] [Google Scholar]
- 27.Voganatsi, A., A. Panyutich, K. T. Miyasaki, and R. K. Murthy. 2001. Mechanism of extracellular release of human neutrophil calprotectin complex. J. Leukoc. Biol. 70:130-134. [PubMed] [Google Scholar]
- 28.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]