Abstract
Arcanobacterium pyogenes is an opportunistic pathogen associated with suppurative diseases in economically important food animals such as cattle, pigs, and turkeys. A. pyogenes adheres to host epithelial cells, and adhesion is promoted by the action of neuraminidase, which is expressed by this organism. However, a neuraminidase-deficient mutant of A. pyogenes only had a reduced ability to adhere to host epithelial cells, indicating that other factors are involved in adhesion. Far Western blotting revealed the presence of an approximately 120-kDa A. pyogenes cell wall protein that binds collagen type I. The 3.5-kb gene that encodes the 124.7-kDa CbpA protein was cloned, and sequence analysis indicated that CbpA contains a typical MSCRAMM protein domain structure. Recombinant, six-His-tagged CbpA (HIS-CbpA) was capable of binding collagen types I, II, and IV but not fibronectin. In addition, CbpA was involved in the ability of A. pyogenes to adhere to HeLa and 3T6 cells, as a cbpA knockout strain had 38.2 and 57.0% of wild-type adhesion, respectively. This defect could be complemented by providing cbpA on a multicopy plasmid. Furthermore, HIS-CbpA blocked A. pyogenes adhesion to HeLa or 3T6 cells in a dose-dependent manner. cbpA was only present in 48% of the A. pyogenes strains tested (n = 75), and introduction of plasmid-encoded cbpA into a naturally cbpA-deficient strain increased the ability of this strain to bind to HeLa and 3T6 cells 2.9- and 5.7-fold, respectively. These data indicate that CbpA, a collagen-binding protein of A. pyogenes, plays a role in the adhesion of this organism to host cells.
Arcanobacterium pyogenes is an opportunistic pathogen of economically important food animals that causes liver abscesses in feedlot cattle (18), pneumonia (8) and arthritis in pigs (41), and osteomyelitis in turkeys (3). Infection is often autogenous, as A. pyogenes is also a common commensal of the upper respiratory, gastrointestinal, and genital tracts of cattle and swine (4, 12, 21). As a commensal, A. pyogenes must be able to adhere to host mucosal surfaces, and recent work has identified two neuraminidases, NanH and NanP, the action of which promotes the adherence of this organism to host epithelial cells (14, 15).
Host cell adhesion is the critical first step in bacterial colonization, subsequently leading to infection, in the case of pathogens. Adhesion results from the interaction of a number of surface-exposed or secreted bacterial proteins with host cells and molecules. One such class of adhesins is cell surface-expressed proteins that can bind to components of the host extracellular matrix (ECM). In gram-positive genera, many of these proteins belong to the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) family, a class of cell surface-anchored proteins that bind to one or more components of the host ECM, such as fibronectin, laminin, or collagen (26). MSCRAMMs have in common a conserved architecture, consisting of an N-terminal signal sequence required for secretion, a ligand-binding domain, one or more sets of polypeptide repeats, and a C-terminal region involved in cell wall anchoring (26). It is hypothesized that the ECM component forms a molecular bridge between the bacterial MSCRAMMs and host cell integrins (10, 32), resulting in bacterial adhesion to the host.
Furthermore, these molecules act as virulence factors, as adhesion mediated by fibronectin-binding MSCRAMMs promotes invasion of host cells (reviewed in reference 10). The collagen adhesin of Staphylococcus aureus, Cna, is a virulence factor in several experimental animal models, including murine osteomyelitis (6) and rabbit keratitis (31). Similarly, Ace, the collagen adhesin of Enterococcus faecalis, is important for adherence to dentin in tooth root canals (9).
This report describes the identification of the first A. pyogenes ECM-binding MSCRAMM, CbpA, which is capable of binding collagen types I, II, and IV. CbpA mediates adhesion of A. pyogenes to collagen-expressing cell lines, and this adhesion can be inhibited, in a dose-dependent manner, by the presence of exogenous CbpA.
MATERIALS AND METHODS
Bacteria and growth conditions.
A. pyogenes strain BBR1 was isolated from a bovine abscess, and strain 424 is a bovine isolate obtained from the Colorado State Veterinary Diagnostic Laboratory. The other A. pyogenes strains were obtained from veterinary diagnostic laboratories or personal collections. A. pyogenes strains were grown on brain heart infusion (BHI; Difco) agar plates supplemented with 5% bovine blood at 37°C and 5% CO2 or in BHI broth supplemented with 5% newborn calf serum (Omega Scientific, Inc.) at 37°C. Escherichia coli DH5αMCR strains (Gibco-BRL) were grown on Luria-Bertani (Difco) agar or in Luria-Bertani broth at 37°C. Antibiotics were added as follows: for A. pyogenes strains, erythromycin at 15 μg/ml and kanamycin at 30 μg/ml; for E. coli strains, ampicillin at 100 μg/ml, chloramphenicol at 30 μg/ml, erythromycin at 200 μg/ml, and kanamycin at 50 μg/ml.
DNA techniques.
E. coli plasmid DNA extraction, transformation, DNA restriction, ligation, agarose gel electrophoresis, and Southern transfer of DNA to nylon membranes were performed essentially as previously described (2). Electroporation-mediated transformation of A. pyogenes strains was performed as previously described (11). A. pyogenes genomic DNA was isolated by the method of Pospiech and Neumann (29). A library of A. pyogenes BBR1 genomic DNA was constructed in λGEM-12 in accordance with the manufacturer's (Promega) instructions. The methods used for bacteriophage growth and DNA purification were essentially as previously described (2). Preparation of DNA probes with oligonucleotide primers internal to specific genes, DNA hybridization, and probe detection were performed with the DIG DNA Labeling and Detection Kit (Roche) as recommended by the manufacturer. PCR DNA amplification was performed with Taq DNA polymerase (Promega) and the supplied reaction buffer for 35 cycles consisting of 1 min at 94°C, 1 min at 55°C, and 1 min/kb at 72°C, with a final extension step of 72°C for 5 min.
Nucleotide sequence determination.
The sequence of cbpA was determined from overlapping subclones of λJGS27 by automated DNA sequencing. Sequencing was performed on both strands, crossing all restriction sites, with KS, SK, T7, M13 universal, or M13 reverse sequencing primers or oligonucleotide primers designed to the sequence of the cbpA gene region. Sequencing reactions were performed by the University of Arizona Genomic Analysis and Technology Core with a 377 DNA sequencer (Applied Biosystems Inc.).
Computer sequence analysis.
Nucleotide sequence data were compiled with the Sequencher program (GeneCodes). Database searches were performed with the BlastX and BlastP algorithms (1). Sequence analysis was performed with the suite of programs available through the Genetics Computer Group, Inc. (University of Wisconsin). Signal sequence prediction was performed with SignalP (23). Transcriptional terminators were identified with mfold (36). Multiple-sequence alignments were performed with CLUSTAL W (40).
Cloning and purification of recombinant, six-His tagged CbpA (HIS-CbpA).
A portion of the cbpA gene, lacking the coding region for the signal sequence, was amplified from A. pyogenes BBR1 genomic DNA by PCR with 5′ primer 5′-CGCCGCGCACGCTAGCGGCAAAGATT-3′, containing an in-frame mutation encoding an NheI site (underlined), and 3′ primer 5′-TACGATACGGTCTTTCTTCTGG-3′. These primers amplified an 884-bp product stretching from base 83 to base 966 of the cbpA gene. The PCR fragment was digested with NheI-BglII and cloned into NheI-BglII-digested pTrcHisB (Invitrogen) to generate pJGS583. A 3.5-kb BglII-EcoRV fragment, containing the 3′ end of cbpA, was cloned into pJGS583 digested with BglII and PstI (blunted with T4 DNA polymerase) to generate pJGS591. pJGS591 encodes HIS-CbpA, a 1,137-amino-acid (aa) protein comprising 1,123 aa of the mature CbpA protein with an N-terminal extension of 14 aa encoded by pTrcHisB, including a six-His sequence.
Cultures for preparation of HIS-CbpA were grown to an optical density at 600 nm of 0.6 prior to induction with 2.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. Cells were harvested by centrifugation at 5,000 × g, and the cell pellet was resuspended in 20 mM Tris-HCl-100 mM NaCl, pH 8.0. The cells were disrupted by two passages through a French pressure cell (Aminco) at 138 MPa, and the insoluble material was removed by centrifugation at 12,000 × g. HIS-CbpA was purified from the soluble fraction with TALON metal affinity resin (Clontech), in accordance with the manufacturer's instructions. HIS-CbpA was eluted from the resin with 50 mM imidazole-20 mM Tris-HCl-100 mM NaCl, pH 8.0. Total protein concentration was determined with Bradford protein assay reagent (Bio-Rad).
The HIS-CbpA used in adhesion assays was dialyzed against 400 volumes of Iscove's modified Dulbecco's medium (IMDM; Life Technologies) supplemented with 10% fetal bovine serum (FBS; Omega Scientific, Inc.) for 18 h at 4°C.
Preparation of goat antiserum to HIS-CbpA.
A female goat was immunized with 500 μg of HIS-CbpA in Ribi Adjuvant System (Corexa) intramuscularly in the hind leg at two sites. A similar booster immunization of 500 μg of HIS-CbpA in Ribi Adjuvant System was administered on days 14 and 28. Blood was collected on day 54, and serum was harvested from the clotted blood by centrifugation at 400 × g. Preimmune serum was prepared in a similar manner prior to immunization.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting techniques.
Prior to electrophoresis, whole A. pyogenes cells were treated with mutanolysin and lysozyme (Sigma) essentially as previously described (42). Enzyme-treated whole A. pyogenes cells, whole E. coli cells, or HIS-CbpA was mixed 1:1 with sample buffer (0.2 M Tris-HCl [pH 6.8], 2.5% SDS, 10% β-mercaptoethanol, 20% glycerol, 0.013% bromophenol blue) and heated in a boiling water bath for 10 min prior to electrophoresis in an SDS-10% (wt/vol) polyacrylamide gel (2). The separated proteins were stained with Coomassie brilliant blue or transferred to nitrocellulose essentially as previously described (2). Western blots were immunostained with 1/100 goat anti-HIS-CbpA and 1/500 rabbit anti-goat immunoglobulin G (IgG; heavy and light chains)-peroxidase conjugate (KPL) as the primary and secondary antibodies, respectively.
Far Western blotting was performed the same way as Western blotting, with the following exceptions. Membranes were blocked by incubation with 5% bovine serum albumin (BSA) in 0.01 M phosphate-buffered saline (PBS), pH 7.2, for 30 min at room temperature with shaking and subsequent incubation with 50 μg of bovine collagen type I (Sigma) per ml in PBS for 2 h as described above and immunostained with 1/100 rabbit anti-bovine collagen I (Chemicon) and 1/500 goat anti-rabbit IgG (heavy and light chains)-peroxidase conjugate (KPL) as the primary and secondary antibodies, respectively.
In order to assess the ECM-binding specificity of CbpA, 5 μg each of bovine collagen type I, bovine collagen type II (Sigma), murine collagen type IV (BD Biosciences), bovine fibronectin (Sigma), and BSA and trypsin inhibitor (irrelevant control proteins) was spotted onto nitrocellulose membranes in a 5-μl volume and allowed to air dry for 10 min. Membranes were incubated with 50 μg of HIS-CbpA per ml for 2 h at room temperature with shaking and immunostained with 1/100 goat anti-HIS-CbpA and 1/500 rabbit anti-goat IgG (heavy and light chains)-peroxidase conjugate (KPL), respectively.
Construction of a cbpA mutant and a complementing plasmid.
Construction of a cbpA mutant was done with an allelic-exchange plasmid in which the coding region for the collagen-binding domain was completely replaced with an erm(X) cassette. A 4.9-kb EcoRV fragment containing the entire cbpA gene was cloned into pHSS19 (22) to generate pJGS524. The 0.7-kb BglII-NruI fragment that encodes the CbpA collagen-binding domain was replaced with a 1.6-kb erm(X) cassette (38), resulting in recombinant plasmid pJGS531. pJGS531, based on a ColE1 replicon, acted as a suicide vector in A. pyogenes (13). pJGS531 plasmid DNA was introduced into A. pyogenes BBR1 cells by electroporation, and recombinants were selected on BHI-blood agar containing erythromycin. The 4.9-kb EcoRV fragment containing the entire cbpA gene was cloned into the PvuII site in pEP2 (30) to construct the complementing plasmid, pJGS557.
Tissue culture cell adhesion assays.
HeLa (human epithelial) cells and 3T6 cells, a collagen-secreting murine fibroblast cell line (7), were cultured in IMDM-10% FBS with 100 μg of gentamicin (Sigma) per ml in a humidified 5% CO2 atmosphere at 37°C. For adhesion assays, cells in IMDM-10% FBS, without gentamicin, were seeded into 24-well plates at 4 × 105 cells per well in 1-ml volumes. The cells were incubated at 37°C in 5% CO2 for 18 h prior to the addition of stationary-phase A. pyogenes bacteria. Bacterial adhesion was assessed after 2 h of incubation at 37°C in 5% CO2. Cell monolayers were washed three times with PBS to remove nonadherent bacteria. Adherent bacteria were recovered by treatment of the cell monolayers with 1 ml of 0.1% Triton X-100 for 10 min on ice, and viable bacteria were enumerated by dilution plating.
Assays to determine whether HIS-CbpA can competitively inhibit adhesion of wild-type A. pyogenes were performed as described above, except that HIS-CbpA in IMDM-10% FBS was added to the cell monolayers at 0 to 50 μg/ml (final concentration) and they were incubated for 1 h prior to the addition of A. pyogenes BBR1 bacteria, also resuspended in the appropriate concentration of HIS-CbpA in IMDM-10% FBS. All adhesion assays were performed in triplicate on at least two separate occasions.
Detection of collagen expression by tissue culture cells.
HeLa and 3T6 cells were grown to a confluent monolayer in chamber slides (Lab-Tek), fixed in 10% buffered formalin, and stained by the Picrosirus Red method (16).
Statistical analysis.
A one-way analysis of variance was performed on the data from the tissue culture adhesion assays with Excel statistical software (Microsoft, Inc.).
Nucleotide sequence accession number.
The cbpA sequence data obtained in this study were submitted to the DDBJ/EMBL/GenBank databases and assigned accession number AY223543.
RESULTS
A. pyogenes expresses a collagen adhesin.
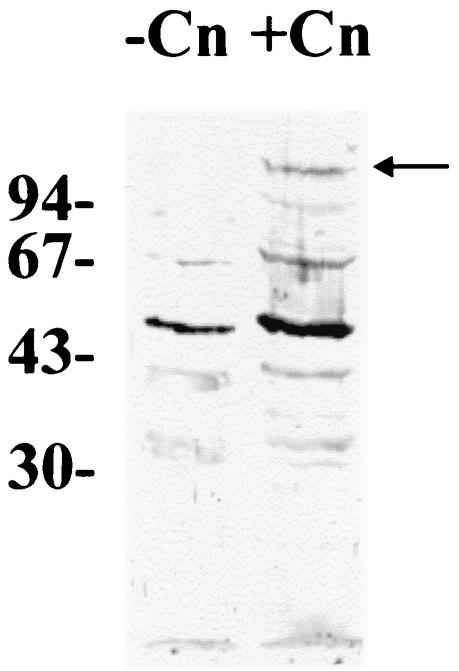
During an unrelated project, a portion of an A. pyogenes gene was cloned, the translation of which showed similarity to the S. aureus collagen adhesin, Cna (GenBank accession no. M81736). To determine whether A. pyogenes is capable of binding collagen, Far Western blotting with bovine collagen type I as the ligand was performed with whole A. pyogenes BBR1 cells. In the absence of collagen, several A. pyogenes antigens were recognized by the primary and/or secondary antibodies. This is not unexpected, as its ubiquitous nature results in A. pyogenes-specific antibodies in many animal species. However, a unique, approximately 120-kDa band was detected in whole A. pyogenes cells incubated with collagen type I (Fig. 1). The same 120-kDa band was also detected in an A. pyogenes cell wall extract tested by Far Western blotting (data not shown). This adhesin was designated CbpA.
FIG. 1.
A. pyogenes BBR1 binds collagen type I. Whole A. pyogenes BBR1 cells were subjected to SDS-10% (wt/vol) PAGE. Separated proteins were transferred to nitrocellulose, and Far Western blotting was performed either in the absence (−Cn) or in the presence (+Cn) of collagen type I. The positions of molecular size standards (sizes are in kilodaltons) are shown on the left. The arrow indicates the position of an approximately 120-kDa A. pyogenes protein that is able to bind collagen I.
Cloning and sequencing of the cbpA gene.
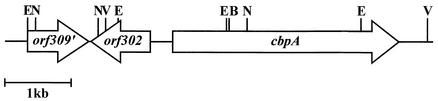
In order to clone the entire gene encoding the collagen adhesin, a digoxigenin-labeled PCR probe was generated from a region spanning bases 2,934 to 3,120 of the cbpA gene and used to probe a λGEM-12 library of A. pyogenes BBR1 genomic DNA. One hybridizing plaque, λJGS27, contained a 13.9-kb partial Sau3AI insert, and from this, a series of BamHI, EcoRI, EcoRV, and NotI subclones were constructed in pBCKS (Stratagene). The DNA sequence of the cbpA gene region was deduced from these overlapping clones, and the 3,456-bp cbpA gene was identified (Fig. 2). This gene encodes a protein with a predicted molecular mass of 124.7 kDa, consistent with that identified by Far Western blotting (Fig. 1). No E. coli σ70-like promoter sequences were apparent upstream of cbpA, and 14 bp downstream of the stop codon was a putative rho-independent terminator (ΔG = −18.4 kcal/mol). Immediately upstream of cbpA, and transcribed in the opposite direction, was an open reading frame, orf302 (Fig. 2), encoding a protein with amino acid similarity to a Streptomyces coelicolor putative transcriptional regulatory protein (GenBank accession no. NP_625638; 23.2% identity, 45.9% similarity). Orf302 possesses a C-terminal helix-turn-helix motif, suggesting that orf302 encodes a DNA-binding protein. Upstream of orf302 was orf309′ (Fig. 2), a partially sequenced open reading frame, the translated product of which has amino acid similarity to the high-affinity choline transport proteins, with the most similarity to that of Erwinia amylovora (GenBank accession no. AF264948; 34.1% identity and 54.2% similarity over the available region of Orf309′). The translation of orf309′ contains three predicted transmembrane domains, suggesting membrane localization, consistent with the possibility that Orf309′ is a transporter. The orientation of orf302, transcribed in the opposite direction to cbpA (Fig. 2), and the presence of a strong transcriptional terminator suggest that cbpA is monocistronic.
FIG. 2.
Map of the A. pyogenes BBR1 cbpA gene region. The BglII (B), EcoRI (E), EcoRV (V), and NruI (N) sites used to clone portions of the cbpA gene are shown.
Analysis of the primary structure of CbpA.
The primary amino acid sequence of CbpA demonstrates a typical MSCRAMM domain structure (Fig. 3). CbpA contains an N-terminal signal sequence with a cleavage site between Ala 27 and Arg 28, an A domain (aa 28 to 544) containing a putative collagen-binding subdomain (aa 155 to 329), four repetitive B domains (aa 545 to 637, 638 to 734, 735 to 827, and 828 to 918), an LPxTG-like motif (aa 1118 to 1122), a membrane-spanning domain (aa 1123 to 1146), and a positively charged C terminus (aa 1147 to 1150). The mature CbpA protein is 1,124 aa long and has a predicted molecular mass of 121.9 kDa.
FIG. 3.
Deduced amino acid sequence of CbpA. The predicted signal peptide cleavage site is denoted by the vertical arrow. The putative collagen-binding subdomain is underlined. The B domains, B1, B2, B3, and B4, are double underlined, with the start of each repeat indicated by bent arrows. The LPXTG-like motif and the charged C terminus are boxed in black, and the intervening membrane-spanning domain is boxed. Amino acid numbers are on the right.
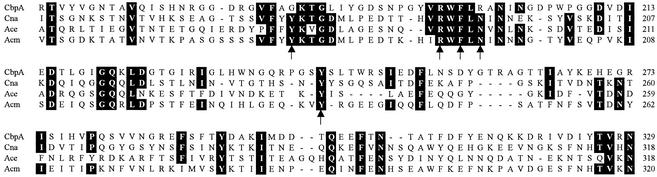
CbpA showed similarity to other collagen-binding proteins, with the most similarity to Cna from S. aureus (GenBank accession no. M81736; 30.9% identity, 50.4% similarity) and Acm from Enterococcus faecium (GenBank accession no. AY135217; 16.9% identity, 31.8% similarity). When the putative CbpA collagen-binding subdomain was aligned with those from Cna, Acm, and Ace (GenBank accession no. AF260872), no increased similarity was observed (Fig. 4). However, CbpA contained three of the five residues important for collagen binding in Cna, including tyrosine 233 (Fig. 4) (27, 39).
FIG. 4.
Alignment of the amino acid sequences of the putative collagen-binding subdomains of A. pyogenes CbpA, S. aureus Cna (GenBank accession no. M81736), E. faecalis Ace (GenBank accession no. AF260872), and E. faecium Acm (GenBank accession no. AY135217). Where three or more amino acids are identical, they are boxed in black. The five amino acids thought to be critical for collagen binding of Cna (39) are indicated by arrows. Amino acid numbers for each protein are on the right.
CbpA contained four B domains, which varied from 90 to 96 aa in length. These repeats were shorter and more variable than the 187-aa B domains from Cna, which had at least 98.4% identity (28). Pairwise comparisons indicated that the most divergent CbpA B domains had 30.2% identity, while the most similar domains had 95.7% identity (Fig. 3).
At the C terminus of CbpA was a sequence similar to the cell wall sorting signals found in surface-expressed proteins of gram-positive bacteria (37). Like all of the other A. pyogenes cell surface-expressed proteins sequenced to date, CbpA contained a variant LPxTG motif (Fig. 3). The prediction that CbpA is a cell surface-expressed protein is consistent with its identification in the A. pyogenes cell wall (data not shown) and its function as an adhesin.
Determination of the prevalence of the cbpA gene by DNA dot blotting.
Genomic DNA was prepared from 75 A. pyogenes strains and subjected to hybridization at high stringency with a probe spanning bases 254 to 965 of cbpA, which included the putative collagen-binding domain. The DNA from 36 A. pyogenes isolates hybridized to the probe (data not shown), indicating that cbpA is found in 48.0% of the A. pyogenes isolates tested. Twenty-two (48.9%) of 45 bovine A. pyogenes isolates carried cbpA, while 12 (40%) of 20 porcine A. pyogenes isolates were positive for the gene. In contrast, six (100%) of six avian isolates, including five from cases of turkey osteomyelitis, carried cbpA. The four remaining isolates, two canine, one feline, and one of unknown origin, did not carry cbpA.
Cloning and expression of HIS-CbpA.
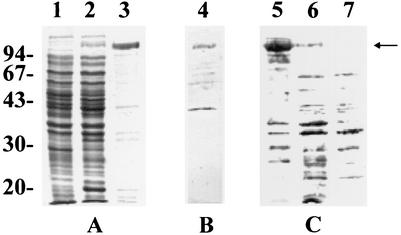
In order to prepare recombinant CbpA for the production of antibodies and ECM-binding specificity studies, cbpA lacking the coding sequence for the signal peptide was cloned into the six-His tag vector pTrcHisB to generate pJGS591 as described in Materials and Methods. SDS-PAGE and Coomassie brilliant blue staining of IPTG-induced cultures of DH5αMCR(pJGS591) identified the presence of an approximately 123-kDa band, which corresponded to the predicted size of HIS-CbpA (Fig. 5A). Purified HIS-CbpA retained collagen-binding activity, as determined by Far Western blotting (Fig. 5B).
FIG. 5.
Overexpression and purification of HIS-CbpA. Whole-cell lysates of IPTG-induced cultures of DH5αMCR(pTrcHisB) (lane 1), DH5αMCR(pJGS591) (lane 2), and 150 ng of purified HIS-CbpA (lane 3) were subjected to SDS-PAGE and Coomassie brilliant blue staining (A). One hundred fifty nanograms of purified HIS-CbpA (lane 4) was subjected to Far Western blotting with collagen type I as the ligand (B). One hundred fifty nanograms of purified HIS-CbpA (lane 5), whole A. pyogenes BBR1 cells (lane 6), or whole CbpA-1 cells (lane 7) was subjected to Western blotting with anti-HIS-CbpA diluted 1/100 (C). The positions of molecular size standards (sizes are in kilodaltons) are shown on the left. The arrow indicates the position of CbpA or HIS-CbpA.
Reaction of CbpA with specific antibodies.
Antiserum prepared against HIS-CbpA detected a protein of approximately 120 kDa in whole A. pyogenes cells (Fig. 5C). Preimmune serum did not react with CbpA or HIS-CbpA (data not shown).
The presence of CbpA in whole-cell samples of A. pyogenes BBR1 harvested at 10 time points throughout the cell cycle was determined. CbpA was detected in approximately equivalent amounts at all time points (data not shown). While this result does not indicate whether expression of cbpA is constitutive, it indicates that, like Cna (17), CbpA is always present on the A. pyogenes cell surface.
ECM-binding specificity of HIS-CbpA.
The ECM-binding specificity of CbpA was determined by Far Western dot blotting. ECM proteins, collagen types I, II, and IV and fibronectin, were spotted onto nitrocellulose, and the binding of HIS-CbpA was determined (Fig. 6). HIS-CbpA bound all of the collagen types tested but did not bind fibronectin. HIS-CbpA was also unable to bind the irrelevant control proteins BSA and trypsin inhibitor (Fig. 6), indicating the specificity of HIS-CbpA for collagen. Like Cna (39), CbpA appears to recognize the triple-helical structure of collagen, as HIS-CbpA was unable to bind to denatured collagen molecules (data not shown).
FIG. 6.
HIS-CbpA binds collagen types I, II, and IV but not fibronectin. Five micrograms of each protein was spotted onto nitrocellulose and incubated with 50 μg of HIS-CbpA per ml. The binding of HIS-CbpA was detected by immunostaining with anti-HIS-CbpA diluted 1/500. 1, BSA; 2, trypsin inhibitor; 3, bovine collagen type I; 4, bovine collagen type II; 5, murine collagen type IV, 6: bovine fibronectin. Immunostaining with preimmune serum diluted 1/500 resulted in no reactivity (data not shown).
Characterization of a cbpA mutant.
The cbpA gene was disrupted by replacement of an internal fragment with an erm(X) cassette in allelic-exchange plasmid pJGS531. Following introduction of this plasmid into A. pyogenes by electroporation, Emr Kms colonies were chosen for further analysis. A. pyogenes BBR1 and cbpA mutant (CbpA-1) genomic DNAs were hybridized with cbpA-, erm(X)- and pHSS19-specific probes following Southern blotting. These experiments confirmed that the integration of the erm(X) cassette within the cbpA locus had occurred by a double-crossover event in CbpA-1 (data not shown). Furthermore, CbpA-1 did not express CbpA, as determined by Western blotting with antiserum against HIS-CbpA (Fig. 5C).
Adhesion of A. pyogenes to epithelial and fibroblast cell lines.
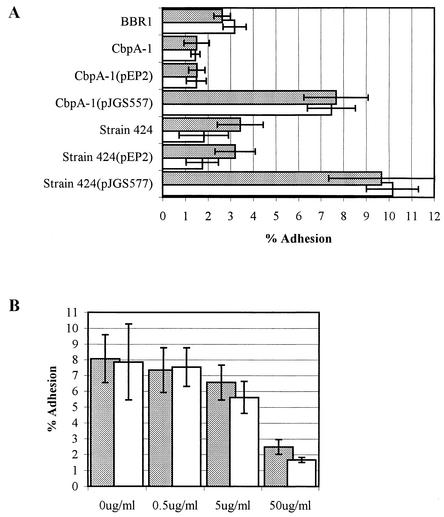
In order to confirm that 3T6 and HeLa cells expressed collagen under the culture conditions used, Picrosirus Red staining was performed. Both cell lines expressed collagen, with 3T6 cells expressing more collagen than HeLa cells (data not shown). Wild-type A. pyogenes strain BBR1 adhered similarly to HeLa and 3T6 cells, with average adhesions of 2.6 and 3.2%, respectively (Fig. 7A). The CbpA-1 mutant had an impaired ability to bind to HeLa and 3T6 cells, with an average adhesion of 1.5% for both cell lines (Fig. 7A). The differences in adhesion between the wild-type and cbpA mutant strains were significant, with P < 0.001 in both cases. Complementation of the CbpA-1 mutant with pJGS557 was able to reverse the adhesion defect (average adhesions of 7.7 and 7.5% for HeLa and 3T6 cells, respectively; Fig. 7A). The substantially increased adhesion of the complemented mutant over that of the wild type is possibly due to a gene dosage effect, as the complementing plasmid carrying cbpA is multicopy. Introduction of pEP2 into CbpA-1 had no effect on adhesion (Fig. 7A). These findings suggest that CbpA mediates binding of A. pyogenes to collagen-expressing cell lines, since its absence results in significantly reduced adhesion and the binding defect can be complemented in trans.
FIG. 7.
CbpA mediates adhesion of A. pyogenes strains to HeLa or 3T6 cells. (A) A. pyogenes strains were added to monolayers of HeLa (gray bars) or 3T6 (open bars) cells and allowed to adhere for 2 h at 37°C prior to washing and recovery of cell-associated bacteria. (B) HIS-CbpA, at the concentrations indicated, was preincubated with HeLa (gray bars) or 3T6 (open bars) cell monolayers for 1 h prior to addition of A. pyogenes BBR1. Adhesion is expressed as a percentage of the number of bacteria originally added to the cells. Error bars indicate one standard deviation of the mean calculated from the averages of two independent experiments performed in triplicate.
To determine whether CbpA can enhance the adhesiveness of a naturally cbpA negative isolate, the cbpA gene was introduced into A. pyogenes strain 424, which does not carry cbpA (data not shown). Strain 424(pJGS557) had an enhanced ability to adhere to HeLa and 3T6 cells, with average adhesions of 9.7 and 10.2%, respectively, compared with the parental strain, with average adhesions of 3.4 and 1.8%, respectively (P < 0.001 in both cases; Fig. 7A). The adhesion of strain 424(pEP2) was not significantly different from that of the parental strain for either HeLa or 3T6 cells (Fig. 7A).
In order to determine whether exogenous CbpA could block the adhesion of wild-type BBR1, 0.5 to 50 μg of HIS-CbpA per ml was added 1 h prior to, and simultaneously with, the addition of A. pyogenes BBR1. A dose-dependant response was observed, with 50 μg of HIS-CbpA per ml significantly reducing the ability of BBR1 to adhere to HeLa or 3T6 cells (P < 0.001 in both cases; Fig. 7B).
DISCUSSION
A prerequisite for bacterial colonization, and hence subsequent infection, is adherence to host cells, often via ECM proteins such as collagen and fibronectin. The finding that a neuraminidase-deficient mutant was only reduced in its ability to adhere to HeLa cells (15) led us to investigate whether A. pyogenes expresses other adhesins. This report describes the identification of CbpA, a 124.7-kDa collagen-binding protein from A. pyogenes that mediates adhesion to epithelial and fibroblast cells.
CbpA was most similar to Cna of S. aureus (28) and, like that protein, also contained the domain structure typical of MSCRAMM proteins, including a signal peptide, a collagen-binding domain, repetitive B domains, and a cell wall anchoring domain. Recombinant HIS-CbpA bound collagen types I, II, and IV but not fibronectin and appears to recognize the triple-helical structure of collagen, as it was unable to bind to denatured collagens.
Collagen binding has been ascribed to a subdomain of the nonrepetitive A domain of Cna, Ace, and Acm (20, 34, 39). Within this subdomain, these three proteins have in common the conservation of five amino acids that have been shown to be important for collagen binding in Cna (27, 39) (Fig. 4). CbpA contained substitutions for two of these amino acids, glycine for tyrosine at position 179 and arginine for asparagine at position 198 (residues 175 and 193 in Cna, respectively). The replacement of asparagine 198 with arginine is of interest, as mutational analysis of Cna indicated that replacement of asparagine 193 with lysine, another positively charged amino acid, substantially reduced the affinity of the mutant protein for collagen (39). It is possible that CbpA has a reduced affinity for collagen compared with Cna or that other substitutions in this subdomain or the entire A domain result in compensatory structural changes allowing collagen binding. The latter hypothesis is supported by the finding that, while the Ace and Cna subdomains bind collagen, the entire A domains do so with greater affinity, indicating that regions flanking the subdomain probably contribute to the formation of an optimal collagen-binding structure (32, 34).
Variability in the number of B domains has been reported for Cna (24), Ace (19), and Acm (20). Although the B domains from the different collagen-binding proteins are not similar, within a protein species they are highly conserved with respect to both length and sequence. In contrast, the B domains of CbpA are variable in length (90 to 96 aa) and have significant sequence divergence (30.2 to 95.7% identity; Fig. 3). The role of the B domains has not been established, although it is known that a recombinant Cna A domain binds collagen in the absence of any B domains (33). Similarly, a truncated form of HIS-CbpA, containing only the A subdomain (aa 29 to 391), bound all of the collagen types at wild-type levels (data not shown).
CbpA mediates adhesion to host cells, as the cbpA mutant, CbpA-1, exhibits reduced adhesion to both HeLa and 3T6 cells. This defect could be complemented in trans by provision of cbpA on a replicating plasmid. Introduction of cbpA into a strain naturally lacking this gene resulted in increased adhesion to HeLa and 3T6 cells, further supporting the hypothesis that CbpA is a host cell adhesin. The mechanism of MSCRAMM binding to host cells is still somewhat unclear. However, structural analyses of Cna and human α1β1 integrin revealed that both proteins contain collagen-binding “trenches,” which could allow collagen to act as a molecular bridge between the bacterial MSCRAMM and the host cell integrin (32). Similar analyses of the Ace A subdomain revealed that it also formed a trench-like structure (34). The finding that exogenous HIS-CbpA blocks adherence of A. pyogenes to host cells in a dose-dependent manner is consistent with this mechanism of action.
Collagen adhesins are thought to act as disease-specific virulence factors, specifically by promoting adhesion to collagen-rich tissues. Introduction of cna into a cna-defective S. aureus strain resulted in an increased ability to adhere to cartilage and cause septic arthritis in mice (25). Furthermore, an S. aureus cna mutant had a significantly reduced ability to cause murine hematogenous osteomyelitis (6). Similarly, Ace mediates adhesion of E. faecalis to dentin in root canals, as an ace mutant had a significantly reduced ability to do so (9).
The presence and/or expression of genes encoding collagen adhesins is not ubiquitous within a bacterial species. The ace gene is carried by all of the E. faecalis isolates tested (n = 165), although these strains were not tested for the ability to bind collagen (5). acm was carried by all of the strains tested (n = 32), but only 34.4% of the isolates expressed the ability to bind collagen, as a result of deletions, mutations, or the presence of insertion sequences in the gene or its promoter region (20). In contrast, only approximately 50% of S. aureus strains carry cna, although this usually correlates with the ability of the isolate to bind collagen (35).
Similarly, the A. pyogenes cbpA gene is present in only 48.0% of isolates, although these isolates were not tested for the ability to bind collagen. CbpA is the second A. pyogenes protein involved in adhesion that is only encoded by a subset of isolates, as the NanP neuraminidase is present in only 64.2% of strains (15). This result highlights the possibility that A. pyogenes isolates involved in different diseases may be differentially equipped with adhesins. Most of the A. pyogenes isolates used in this study were obtained from soft-tissue infections or as normal flora, and generally, little difference was observed in cbpA carriage in isolates from various animal hosts or diseases. The one exception was turkey osteomyelitis isolates, all of which carried cbpA. Although the number of these isolates is small (n = 5), this result suggests that, like Cna (6), CbpA may act as a virulence factor for A. pyogenes osteomyelitis. While it is unlikely that collagen adhesins target bacteria to tissue-specific sites, such as bone or cartilage, its presence on the bacterial cell may promote the colonization of these collagen-rich tissues, enhancing the ability of isolates expressing a collagen-binding protein to initiate infection.
Acknowledgments
We thank Stefani Rudnick for construction of the λGEM-12 library and Hien Trinh and Dawn Bueschel for excellent technical assistance.
Partial support for this work was provided by U.S. Department of Agriculture Hatch ARZT-136724-H-02-123.
Editor: J. T. Barbieri
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology, vol 1. Greene Publishing Associates and John Wiley & Sons, Inc., New York, N.Y.
- 3.Brinton, M. K., L. C. Schellberg, J. B. Johnson, R. K. Frank, D. A. Halvorson, and J. A. Newman. 1993. Description of osteomyelitis lesions associated with Actinomyces pyogenes infection in the proximal tibia of adult male turkeys. Avian Dis. 37:259-262. [PubMed] [Google Scholar]
- 4.Carter, G. R., and M. M. Chengappa. 1991. Essentials of veterinary bacteriology and mycology, 4th ed. Lea & Febiger, Philadelphia, Pa.
- 5.Duh, R. W., K. V. Singh, K. Malathum, and B. E. Murray. 2001. In vitro activity of 19 antimicrobial agents against enterococci from healthy subjects and hospitalized patients and use of an ace gene probe from Enterococcus faecalis for species identification. Microb. Drug Resist. 7:39-46. [DOI] [PubMed] [Google Scholar]
- 6.Elasri, M. O., J. R. Thomas, R. A. Skinner, J. S. Blevins, K. E. Beenken, C. L. Nelson, and M. S. Smelter. 2002. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone 30:275-280. [DOI] [PubMed] [Google Scholar]
- 7.Green, H., B. Goldberg, and G. J. Todaro. 1966. Differentiated cell types and the regulation of collagen synthesis. Nature 212:631-633. [DOI] [PubMed] [Google Scholar]
- 8.Høie, S., K. Falk, and B. M. Lium. 1991. An abattoir survey of pneumonia and pleuritis in slaughter weight swine from 9 selected herds. IV. Bacteriological findings in chronic pneumonic lesions. Acta Vet. Scand. 32:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubble, T. S., J. F. Hatton, S. R. Nallapareddy, B. E. Murray, and M. J. Gillespie. 2003. Influence of Enterococcus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentin. Oral Microbiol. Immunol. 18:121-126. [DOI] [PubMed] [Google Scholar]
- 10.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Höök. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 11.Jost, B. H., S. J. Billington, and J. G. Songer. 1997. Electroporation-mediated transformation of Arcanobacterium (Actinomyces) pyogenes. Plasmid 38:135-140. [DOI] [PubMed] [Google Scholar]
- 12.Jost, B. H., K. W. Post, J. G. Songer, and S. J. Billington. 2002. Isolation of Arcanobacterium pyogenes from the porcine gastric mucosa. Vet. Res. Commun. 26:419-425. [DOI] [PubMed] [Google Scholar]
- 13.Jost, B. H., J. G. Songer, and S. J. Billington. 1999. An Arcanobacterium (Actinomyces) pyogenes mutant deficient in production of the pore-forming cytolysin pyolysin has reduced virulence. Infect. Immun. 67:1723-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jost, B. H., J. G. Songer, and S. J. Billington. 2001. Cloning, expression, and characterization of a neuraminidase gene from Arcanobacterium pyogenes. Infect. Immun. 69:4430-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jost, B. H., J. G. Songer, and S. J. Billington. 2002. Identification of a second Arcanobacterium pyogenes neuraminidase and involvement of neuraminidase activity in host cell adhesion. Infect. Immun. 70:1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junqueira, L. C., G. Bignolas, and R. R. Brentani. 1979. Picrosirus staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 11:447-455. [DOI] [PubMed] [Google Scholar]
- 17.Mascari, L., and J. M. Ross. 2002. Quantifying the temporal expression of the Staphylococcus aureus collagen adhesin. Microb. Pathog. 32:99-103. [DOI] [PubMed] [Google Scholar]
- 18.Nagaraja, T. G., S. B. Laudert, and J. C. Parrott. 1996. Liver abscesses in feedlot cattle. Part I. Causes, pathogenesis, pathology, and diagnosis. Comp. Contin. Educ. Pract. Vet. 18:S230-S241, S256.
- 19.Nallapareddy, S. R., K. V. Singh, R.-W. Duh, G. M. Weinstock, and B. E. Murray. 2000. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of Ace during human infections. Infect. Immun. 68:5210-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nallapareddy, S. R., G. M. Weinstock, and B. E. Murray. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733-1747. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan, S., T. G. Nagaraja, J. Staats, M. M. Chengappa, and R. D. Oberst. 1998. Biochemical and biological characterizations and ribotyping of Actinomyces pyogenes and Actinomyces pyogenes-like organisms from liver abscesses in cattle. Vet. Microbiol. 61:289-303. [DOI] [PubMed] [Google Scholar]
- 22.Nickoloff, J. A., and R. J. Reynolds. 1991. Subcloning with new ampicillin- and kanamycin-resistant analogs of pUC19. BioTechniques 10:469-472. [PubMed] [Google Scholar]
- 23.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 24.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Höök. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 25.Patti, J. M., T. Bremmel, D. Krajewska-Pietrasik, A. Abdelnour, A. Tarkowski, C. Ryden, and M. Höök. 1994. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 62:152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patti, J. M., and M. Höök. 1994. Microbial adhesins recognizing extracellular matrix macromolecules. Curr. Opin. Cell Biol. 6:752-758. [DOI] [PubMed] [Google Scholar]
- 27.Patti, J., K. House-Pompeo, J. O. Boles, N. Garza, S. Gurusiddappa, and M. Höök. 1995. Critical residues in the ligand-binding site of the Staphylococcus aureus collagen-binding adhesin (MSCRAMM). J. Biol. Chem. 270:12005-12011. [DOI] [PubMed] [Google Scholar]
- 28.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Höök. 1992. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 29.Pospiech, A., and B. Neumann. 1995. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 30.Radford, A. J., and A. L. Hodgson. 1991. Construction and characterization of a Mycobacterium-Escherichia coli shuttle vector. Plasmid 25:149-153. [DOI] [PubMed] [Google Scholar]
- 31.Rhem, M. N., E. M. Lech, J. M. Patti, D. McDevitt, M. Höök, D. B. Jones, and K. R. Wilhelmus. 2000. The collagen binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect. Immun. 68:3776-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich, R. L., C. C. S. Deivanayagam, R. T. Owens, M. Carson, A. Höök, D. Moore, J. Symersky, V. W.-C. Yang, S. V. L. Narayana, and M. Höök. 1999. Trench-shaped binding sites promote multiple classes of interactions between collagen and the adherence receptors α1β1 integrin and Staphylococcus aureus Cna MSCRAMM. J. Biol. Chem. 274:24906-24913. [DOI] [PubMed] [Google Scholar]
- 33.Rich, R. L., B. Demeler, K. Ashby, C. C. S. Deivanayagam, J. W. Petrich, J. M. Patti, S. V. L. Narayana, and M. Höök. 1998. Domain structure of the Staphylococcus aureus collagen adhesin. Biochemistry 37:15423-15433. [DOI] [PubMed] [Google Scholar]
- 34.Rich, R. L., B. Kreikemeyer, R. T. Owens, S. LaBrenz, S. V. L. Narayana, G. M. Weinstock, B. E. Murray, and M. Höök. 1999. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J. Biol. Chem. 274:26939-26945. [DOI] [PubMed] [Google Scholar]
- 35.Ryding, U., J. I. Flock, M. Flock, B. Soderquist, and B. Christensson. 1997. Expression of collagen-binding protein and types 5 and 8 capsular polysaccharide in clinical isolates of Staphylococcus aureus. J. Infect. Dis. 176:1096-1099. [DOI] [PubMed] [Google Scholar]
- 36.SantaLucia, J., Jr. 1998. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc. Natl. Acad. Sci. USA 95:1460-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneewind, O., D. Mihaylova-Petkov, and P. Model. 1993. Cell wall sorting signals in surface proteins of Gram-positive bacteria. EMBO J. 12:4803-4811. [DOI] [PMC free article] [PubMed]
- 38.Serwold-Davis, T. M., and N. B. Groman. 1986. Mapping and cloning of Corynebacterium diphtheriae plasmid pNG2 and characterization of its relatedness to plasmids from skin coryneforms. Antimicrob. Agents Chemother. 30:69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Symersky, J., J. M. Patti, M. Carson, K. House-Pompeo, M. Teale, D. Moore, L. Jin, A. Schneider, L. J. DeLucas, M. Höök, and S. V. Narayana. 1997. Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat. Struct. Biol. 4:833-838. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner, G. V. 1982. A microbiological study of polyarthritis in slaughter pigs. J. S. Afr. Vet. Assoc. 53:99-101. [PubMed] [Google Scholar]
- 42.Wells, J. M., P. W. Wilson, P. M. Norton, M. J. Gasson, and R. W. Le Page. 1993. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol. Microbiol. 8:1155-1162. [DOI] [PubMed] [Google Scholar]