Abstract
Recently, we reported that the prototypical Staphylococcus aureus strain RN6390 (a derivative of NCTC 8325) had significantly reduced aconitase activity relative to a diverse group of S. aureus isolates, leading to the hypothesis that strain RN6390 has impaired tricarboxylic acid (TCA) cycle-mediated acetate catabolism. Analysis of the culture supernatant from RN6390 confirmed that acetate was incompletely catabolized, suggesting that the ability to catabolize acetate can be lost by S. aureus. To test this hypothesis, we examined the carbon catabolism of the S. aureus strains whose genome sequences are publicly available. All strains catabolized glucose and excreted acetate into the culture medium. However, strains NCTC 8325 and N315 failed to catabolize acetate during the postexponential growth phase, resulting in significantly lower growth yields relative to strains that catabolized acetate. Strains NCTC 8325 and RN6390 contained an 11-bp deletion in rsbU, the gene encoding a positive regulator of the alternative sigma factor σB encoded by sigB. An isogenic derivative strain of RN6390 containing the wild-type rsbU gene had significantly increased acetate catabolism, demonstrating that σB is required for acetate catabolism. Taken together, the data suggest that naturally occurring mutations can alter the ability of S. aureus to catabolize acetate, a surprising discovery, as TCA cycle function has been demonstrated to be involved in the virulence, survival, and persistence of several pathogenic organisms. Additionally, these mutations decrease the fitness of S. aureus by reducing the number of progeny placed into subsequent generations, suggesting that in certain situations a decreased growth yield is advantageous.
The tricarboxylic acid (TCA) cycle is an essential source of energy and biosynthetic intermediates for many organisms. Pathogenic organisms can be divided into three categories based on the TCA cycle. Those in the first group do not possess a TCA cycle and have become dependent upon the host to provide amino acids or intermediates for biosynthesis (e.g., Borrelia burgdorferi and Streptococcus pyogenes). Those in the second group have an incomplete TCA cycle and are auxotrophic for some amino acids (e.g., Yersinia pestis and Haemophilus influenzae). Lastly, the third group is characterized as having a complete TCA cycle (e.g., Pseudomonas aeruginosa and Staphylococcus aureus) but, depending upon other metabolic limitations, can be auxotrophic for certain amino acids. The relative independence of the latter two groups of pathogens on the host for amino acids suggests that the TCA cycle may perform important functions in these organisms during pathogenesis. This supposition is supported by extensive experimental data demonstrating that TCA cycle function is involved in virulence, survival, and persistence (11, 20, 34, 36, 51, 53).
Transcriptional regulation of TCA cycle genes is primarily dependent on the presence of oxygen and the carbon source (10, 21, 22, 54, 55). In gram-negative bacteria, TCA cycle activity is greatest during aerobic growth in a medium containing a carbon source capable of being converted into acetyl-coenzyme A. In contrast, gram-positive bacteria repress the TCA cycle when grown in the presence of a rapidly catabolizable carbon source and glutamate. Derepression of the TCA cycle occurs upon depletion of the readily catabolizable carbon source(s) and/or glutamate and coincides with the depletion of acetate from the culture medium. Acetate enters into the TCA cycle in the form of acetyl-coenzyme A when it is ligated with oxaloacetate to produce citrate through the action of citrate synthase. Genetic inactivation of the TCA cycle prevents the catabolism of acetate (53).
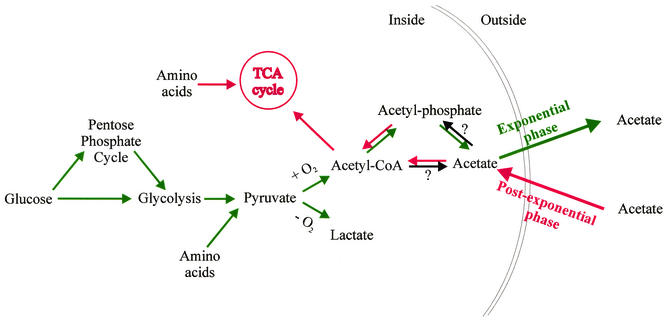
Staphylococcus aureus is a gram-positive pathogen of humans and animals, causing significant morbidity, mortality, and economic loss (49). The organism produces many extracellular virulence factors and cell wall-associated adherence proteins that are important for colonization, tissue invasion, evasion of host defenses, and nutrient acquisition. The expression of many virulence factors is negatively regulated by glucose and is maximal during the postexponential phase of growth (45). S. aureus uses the pentose phosphate and glycolytic pathways to catabolize glucose to pyruvate (Fig. 1) (5). The catabolic fate of pyruvate is determined by the growth conditions. Under anaerobic growth, pyruvate is reduced to lactic acid (30, 31), whereas during aerobic growth, pyruvate undergoes oxidative decarboxylation to produce acetyl-coenzyme A (19). Acetyl-coenzyme A is converted into acetylphosphate, which is then used for substrate-level phosphorylation to generate ATP and acetate. As stated above, acetate accumulates in the culture medium until the concentration of glucose decreases to a level at which it can no longer sustain rapid growth. The exit from the exponential phase of growth corresponds with the catabolism of acetate (53).
FIG. 1.
Schematic representation of glucose catabolism by S. aureus. Green arrows, reactions or pathways used primarily during the exponential phase of growth; red arrows, reactions or pathways used in the postexponential growth phase; black arrows, reactions for which there are insufficient data to determine if the reaction occurs.
The post-exponential phase of growth is characterized by increased extracellular virulence factor production and derepression of genes encoding the enzymes of TCA cycle. Many secondary metabolites (e.g., acetate in the form of acetyl-coenzyme A) are catabolized by the TCA cycle. Recently, we have shown that inactivation of the TCA cycle enzyme aconitase prevents post-exponential-phase catabolism of acetate, induces a premature stationary phase, and significantly reduces virulence factor production (53). Surprisingly, the commonly used S. aureus strain RN6390 (a derivative of NCTC 8325) has significantly reduced aconitase activity relative to a genetically diverse group of recent clinical isolates (52). These findings led us to hypothesize that strain RN6390 has impaired acetate catabolism and that S. aureus secondary metabolite catabolism can be altered or lost. Our hypothesis would seem to contradict the data obtained by site-directed mutagenesis and in vivo mutagenesis screens that have identified components of the TCA cycle as being important for S. aureus pathogenesis (9, 38, 53). These studies demonstrated that inactivation of the TCA cycle enzymes aconitase (citB/acnA), α-ketoglutarate dehydrogenase (odhA), or dihydrolipoamide succinyltransferase (odhB) could alter the host-pathogen interaction.
Genotypic variation within the S. aureus species has been studied extensively (16, 17, 42-44), with particular interest in the agr operon (12, 28, 29, 37, 52). Phenotypic studies of S. aureus have primarily focused on amino acid requirements (14, 35, 47, 48, 56) or exponential-phase carbon catabolism (1, 13, 27, 41, 47, 50). However, analysis of postexponential growth phase catabolism in S. aureus has been largely ignored (53), and variation in postexponential growth phase catabolism has not been studied. These issues are important to study because most secreted virulence factors are expressed during the postexponential phase of growth (45). Hence, the aims of this study were to determine if variation exists in S. aureus postexponential growth phase catabolism and to assess the physiological consequences, if any, of such variation. To address these aims, we chose to examine the growth, catabolism, and virulence factor production of eight S. aureus strains whose genomes have been sequenced. These strains represent the “wild-type” strains used in S. aureus research for the last 30 years and presented an excellent opportunity to examine phenotype-genotype correlations in this organism.
MATERIALS AND METHODS
Bacterial strains, materials, and growth conditions.
The strains used in this study are listed in Table 1. S. aureus strains were grown in tryptic soy broth (TSB) containing 0.25% glucose (BD Biosciences, Sparks, Md.) or on TSB containing 1.5% agar (TSA). All bacterial cultures were inoculated 1:200 from an overnight culture (normalized for growth) into TSB, incubated at 37°C, and aerated at 225 rpm, with a flask-to-medium ratio of 10:1. Bacterial growth was assessed by measuring the optical density at 600 nm.
TABLE 1.
Strains used in this study
| Strain | Institution or relevant characteristic(s) | Website or reference |
|---|---|---|
| NCTC 8325 | University of Oklahoma | http://www.genome.ou.edu/ |
| N315 | National Institute of Technology and Evaluation | http://www.bio.nite.go.jp/dogan/genome-list-e.html |
| Mu50 | National Institute of Technology and Evaluation | http://www.bio.nite.go.jp/dogan/genome-list-e.html |
| MW2 | National Institute of Technology and Evaluation | http://www.bio.nite.go.jp/dogan/genome-list-e.html |
| MRSA252 | The Wellcome Trust Sanger Institute | http://www.sanger.ac.uk/ |
| MSSA476 | The Wellcome Trust Sanger Institute | http://www.sanger.ac.uk/ |
| COL | The Institute for Genomic Research | http://www.tigr.org/ |
| RF122 | University of Minnesota | http://www.cbc.umn.edu/ResearchProjects/AGAC/Pm/index.html |
| RN6390 | Derivative of NCTC 8325 cured of prophage, 11-bp deletion in rsbU | R. P. Novick |
| SH1000 | RN6390 with intact rsbU | 24 |
Measurement of acetate, glucose, and ammonia in culture supernatants.
Aliquots of bacteria (1.5 ml) were centrifuged for 5 min at 20,800 × g at 4°C, and supernatants were removed and stored at −20°C until use. Acetate, glucose, and ammonia concentrations were determined with kits purchased from R-Biopharm, Inc. (Marshall, Mich.) and used according to the manufacturer's directions.
Determination of beta-hemolytic titers.
To determine beta-hemolytic activity, twofold serial dilutions of culture supernatants were mixed with an equal volume of 2% washed rabbit erythrocytes in U-bottomed microtiter plates. The plates were incubated at 37°C for 60 min and then at 4°C overnight. The hemolytic titer is defined as the inverse of the highest dilution at which 50% of the erythrocytes remained intact after the overnight incubation (16).
RNA isolation and Northern blot analysis.
Bacterial cultures were grown as described above. Cells were harvested by centrifugation, and total RNA was isolated with the FastPrep system (Qbiogene, Carlsbad, Calif.). RNA samples (10 μg) were electrophoresed in a 1.5% agarose-0.66 M formaldehyde gel with a morpholinepropanesulfonic acid (MOPS) running buffer. Blotting of RNA onto a Hybond N+ membrane (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) was performed with the VacuGene XL blotting apparatus (Pharmacia). The transfer was performed with 20× SSC (3 M NaCl, 0.3 M sodium citrate [pH 7.0]) for 2 h. Membranes were hybridized overnight with a PCR-amplified probe derived from RNAIII with primers RNAIIIF (GAAGTAGAACAGCAACGCG) and RNAIIIR (GATCACAGAGATGTGATGG).
Detection of specific transcripts was done with the enhanced chemiluminescence detection kit (Amersham). As an internal control, all Northern blots were probed for 16S rRNA.
Western immunoblot analysis.
S. aureus strains were grown for 7 h, and culture supernatants (15 ml) were harvested by centrifugation and concentrated with Millipore Ultrafree-15 centrifugal filters (Millipore Corporation, Bedford, Mass.). The protein samples (30 μl) were mixed with 10 μl of sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (33). Proteins were transferred onto a nitrocellulose membrane with the Bio-Rad Mini Trans Blot Cell at 23 V overnight at 4°C. The membrane was incubated for 1 h in blocking buffer (0.5% Tween 20, 0.5 M NaCl, 10 mM Tris [pH 8.2]) and incubated for 1 h with a primary antibody against alpha-toxin or protein A (Accurate Chemical & Scientific Corporation, Westbury, N.Y.). Development of the Western immunoblot was performed with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin secondary antibody and developed with 3,3′-diaminobenzidine tetrahydrochloride dihydrate (DAB) solution (phosphate-buffered saline, 0.5 mg of DAB per ml, and 0.006% H2O2).
Determination of stationary-phase survival.
Single bacterial colonies were inoculated into 1-liter flasks containing 100 ml of TSB, grown at 37°C, and aerated by shaking at 225 rpm for 8 days. Aliquots (200 μl) were removed at 24-h intervals, and the CFU per milliliter were determined with TSA. Sterile deionized water was added as needed to offset the evaporative loss of water.
α-Ketoglutarate dehydrogenase activity assays.
α-Ketoglutarate dehydrogenase activity was assayed in cell-free lysates of S. aureus prepared as follows. Aliquots (3 ml) were harvested at the indicated times and centrifuged, and bacteria were suspended in 1.5 ml of lysis buffer containing 100 mM Tris (pH 7.0), 0.1 mM dithiothreitol, 2 mM MgCl2, and 50 μg of lysostaphin per ml (Sigma). The bacteria were incubated at 37°C for 10 min and ruptured twice with a French press at 15,000 lb/in2. The lysate was centrifuged for 5 min at 20,800 × g at 4°C. α-Ketoglutarate dehydrogenase activity was assayed in the cell-free lysate with the method described by Fisher (15).
Nucleotide sequencing and alignments.
DNA nucleotide sequences, deposited in the publicly available S. aureus genomic DNA sequence databases (Table 1), were aligned and analyzed with Lasergene (DNAStar, Madison, Wis.). DNA sequencing of strain Mu50 open reading frame SA1149 was performed as described before (52) with primers sdhB for-1 (5′-GAAGAAACATTTGAAATTCCATATCG) and sdhB rev-1 (5′-TGGTCCCGGACCTAAATCATACGTTC).
RESULTS
Carbon catabolism in S. aureus strain RN6390.
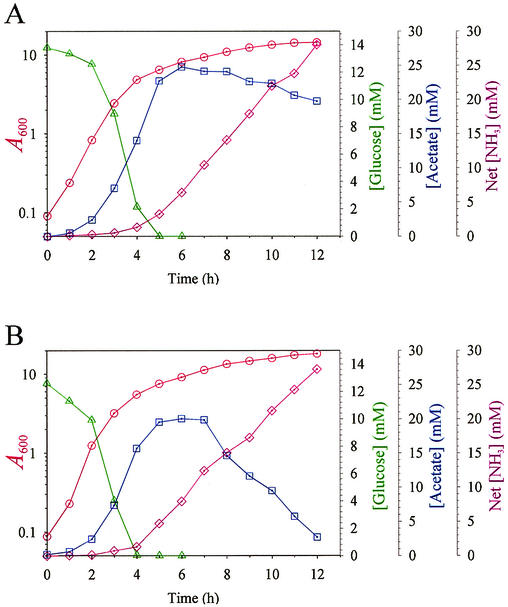
Recently we reported that S. aureus strain RN6390, a strain used extensively for genetic and virulence studies, has significantly reduced aconitase activity relative to a genetically diverse group of recent clinical isolates (52). This observation led us to hypothesize that acetate catabolism was impaired in strain RN6390. To test this hypothesis, the concentration of acetate in the culture supernatant was assayed throughout the growth cycle (Fig. 2A). Consistent with this hypothesis, the strain did not substantially deplete acetate from the culture medium.
FIG. 2.
Growth characteristics of S. aureus strains RN6390 and SH1000. Strains (A) RN6390 (rsbU mutant) and (B) SH1000 were grown in TSB. At 1-h intervals, an aliquot (1.5 ml) was removed, the absorbance at 600 nm was measured, and the glucose, acetate, and ammonia concentrations in the culture supernatants were determined. The results presented are representative of at least two independent experiments.
Carbon catabolism by strains whose genomes have been sequenced.
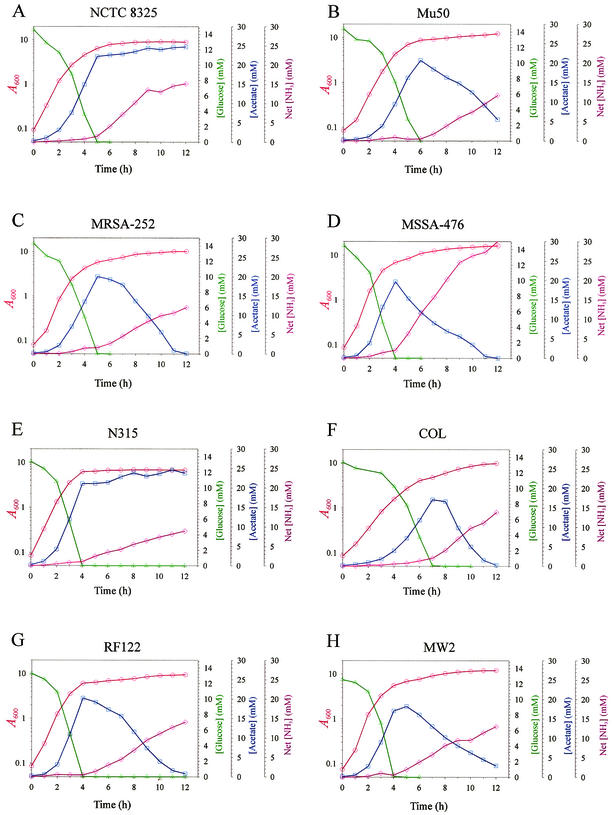
The inability of strain RN6390 to catabolize acetate suggested that S. aureus secondary metabolite catabolism could be altered or lost. To examine this question, the carbon catabolism of additional S. aureus strains was studied. We used eight strains whose genome sequences are publicly available (Table 1) to permit the analysis of phenotype-genotype correlations. The concentration of glucose and acetate in the culture supernatants of these eight strains grown in the presence 0.25% (wt/vol) glucose were determined. All strains depleted glucose and accumulated acetate in the culture medium, confirming a common path for the catabolism of glucose (Fig. 3). However, two strains (NCTC 8325 and N315) failed to catabolize acetate (Fig. 3), even after 27 h in culture (data not shown). These data demonstrated that S. aureus strains vary in their ability to catabolize acetate, a secondary metabolite.
FIG. 3.
Growth characteristics of eight S. aureus strains. The growth (A600) and concentrations of ammonia, glucose, and acetate in culture supernatants were measured at 1-h intervals for strains (A) NCTC 8325, (B) Mu50, (C) MRSA-252, (D) MSSA-476, (E) N315, (F) COL, (G) RF122, and (H) MW2. The results presented are representative of at least two independent experiments.
The inability to obtain carbon from the catabolism of acetate could increase the catabolism of other metabolites, such as amino acids. To test this hypothesis, the concentration of ammonia (an indicator of amino acid catabolism) in the culture supernatants was measured throughout the growth cycle (Fig. 3). The amount of ammonia produced by strains N315 and NCTC 8325 was not significantly different from the amount produced by strains MW2, Mu50, MSSA-476, MRSA-252, COL, and RF122. Additionally, the concentration of ammonia correlated well with the growth yields of all strains (ρ = 0.86). Taken together, these data suggested that strains NCTC 8325 and N315 did not compensate for the loss of acetate catabolism by increasing amino acid catabolism. Hence, the loss of acetate catabolism restricted the pool of carbon available for growth. This result suggests that strains NCTC 8325 and N315 would have a diminished growth yield relative to strains that catabolize acetate. To test this hypothesis, the growth yields of the eight strains after 12 h of growth were determined. Consistent with this hypothesis, strains that catabolized acetate had significantly higher growth yields relative to strains that did not catabolize acetate, except for strain MRSA-252 (Table 2). Thus, the loss of acetate catabolism correlates with a diminished growth yield, and the total number of bacteria would be reduced relative to the number of bacteria capable of catabolizing acetate. Taken together, these data suggest that virulence factor production would be lower in strains that lack secondary metabolite catabolism because fewer bacteria would be generated.
TABLE 2.
Growth characteristics of S. aureus strains
| Strain | Catabolizes acetate | Doubling time (min) | A600a |
Pb relative to:
|
Beta-hemolytic titer | Production ofc:
|
||
|---|---|---|---|---|---|---|---|---|
| NCTC 8325 | N315 | Alpha-toxin | Protein A | |||||
| N315 | No | 29 ± 1 | 6.88 ± 0.33 | ND | ND | 0.0 ± 0 | No | Yes |
| NCTC 8325 | No | 29 ± 2 | 9.37 ± 0.21 | ND | ND | 5,120 ± 0 | Yes | No |
| MRSA-252 | Yes | 27 ± 2 | 9.09 ± 0.77 | 0.2239 | 0.0024 | 120 ± 57 | No | No |
| MSSA-476 | Yes | 23 ± 1 | 15.65 ± 0.44 | <0.0001 | <0.0001 | 320 ± 0 | Yes | No |
| COL | Yes | 50 ± 3 | 10.40 ± 0.20 | 0.0012 | <0.0001 | 834 ± 274 | No | Yes |
| RF122 | Yes | 28 ± 3 | 11.77 ± 0.63 | 0.0006 | <0.0001 | 7,680 ± 3,620 | Yes | No |
| Mu50 | Yes | 33 ± 2 | 12.36 ± 0.77 | 0.0004 | 0.0001 | 240 ± 113 | No | Yes |
| MW2 | Yes | 23 ± 0 | 12.19 ± 0.47 | 0.0014 | <0.0001 | 480 ± 226 | Yes | Yes |
Growth yields were determined after 12 h of growth. Values are the means ± standard deviations for five independent determinations.
Statistical significance of the mean A600 relative to value that for strain NCTC 8325 or strain N315 by the Student t test. ND, not determined.
The presence of alpha-toxin and protein A was determined by Western immunoblot analysis of culture supernatants after 7 h of growth.
β-Hemolytic activity and alpha-toxin and protein A production.
S. aureus secretes or has on its cell surface many virulence factors whose expression is growth phase dependent. Cell-associated proteins, such as protein A, are produced primarily in the exponential phase and repressed during the postexponential phase of growth. In contrast, secreted proteins such as alpha-toxin are produced primarily in the postexponential phase and repressed in the exponential phase of growth (8). As noted above, entry into the postexponential phase of growth coincides with the depletion of glucose from the culture medium and the catabolism of acetate. Hence, it is reasonable to postulate that strains that do not catabolize acetate will have less carbon and energy for virulence factor production. Additionally, the decreased growth yields of strains that do not catabolize acetate would be predicted to decrease the total amount of virulence factors made in the postexponential growth phase.
To address these possibilities, beta-hemolytic titers were determined and alpha-toxin (hla) and protein A (spa) protein levels were examined by Western immunoblots (Table 2 and data not shown). Although there was considerable variation in the beta-hemolytic activity of the eight strains, there was no simple correlation between the ability to catabolize acetate and the hemolytic titers of the strains. The presence of alpha-toxin was confirmed by Western immunoblot analysis for all strains with hemolytic activity except those with the lowest hemolytic titers (strains MRSA-252 and Mu50). As expected, protein A production correlated inversely with alpha-toxin production except in strain MRSA-252, which produced neither protein (data not shown). These data demonstrate that virulence factor production occurs independently of the ability to catabolize acetate.
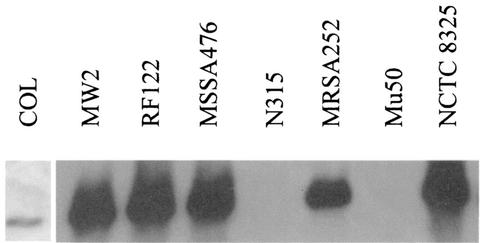
RNAIII transcription.
Virulence factor production in S. aureus is regulated in part by the agr loci (46). Two divergently transcribed RNAs are made from the agr loci. RNAII codes for the components of the agr cell density-dependent transcriptional regulatory system, and RNAIII is the RNA effector molecule. RNAIII reciprocally regulates the synthesis of cell-associated adhesion factors and secreted proteins. Mutation of the agr operon results in the loss of RNAIII and alpha-toxin production and the derepression of protein A expression (46). The lack of detectable alpha-toxin production by strains N315, COL, and Mu50 coupled with post-exponential growth phase production of protein A by these strains (Table 2) suggested that these strains do not synthesize RNAIII. To test this hypothesis, Northern blot analysis was used to determine if RNAIII was made (Fig. 4). Detectable levels of RNAIII were made by all strains except N315 and Mu50. Strain COL had a low level of RNAIII after 9 h of growth. The low level of RNAIII made by strain COL could account for the absence of detectable alpha-toxin and enhanced protein A production; however, it is unclear why the low level of RNAIII did not affect the beta-hemolytic titer.
FIG. 4.
RNAIII Northern blot. Total RNA was isolated from strains MW2, RF122, MSSA-476, N315, MRSA-252, Mu50, and NCTC 8325 after 7 h of growth, transferred to a charged nylon membrane, and probed with an RNAIII-specific probe. Total RNA was isolated from strain COL after 9 h of growth.
Stationary-phase survival.
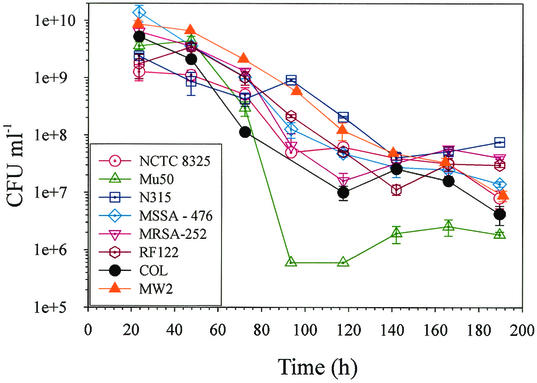
Aconitase inactivation enhances stationary-phase survival of S. aureus (53). The two more likely explanations for this observation are that (i) the metabolic block in the TCA cycle prevents the catabolism of secondary metabolites, thus depriving the bacteria of the necessary energy to enter the death phase, and (ii) aconitase has a direct role in regulating entry into the death phase. The observation that strains NCTC 8325 and N315 had impaired acetate catabolism provided an opportunity to elucidate the basis of the increased stationary-phase survival of an aconitase mutant. To determine if secondary metabolite catabolism is necessary for entry into the death phase, single bacterial colonies were inoculated into TSB and incubated with aeration for 8 days, and the CFU were determined daily (Fig. 5). All eight S. aureus strains reached a maximum cell density within 48 h of inoculation and entered the death phase within 72 h. The rate of loss of viability was approximately equal for all strains except Mu50, consistent with previous observations that strain Mu50 loses viability rapidly (23). Interestingly, the rapid loss of viability by strain Mu50 was followed by stabilization of the cell density and remaining viable after 8 days in culture. Taken together, these data indicated that secondary metabolite catabolism was not required for the entry into the death phase.
FIG. 5.
Stationary-phase survival of S. aureus strains NCTC 8325, Mu50, MRSA-252, MSSA-476, N315, COL, RF122, and MW2. Single colonies were inoculated into TSB, grown at 37°C, and aerated by shaking at 225 rpm for 8 d. At 24-h intervals, aliquots were removed, and CFU were determined in quadruplicate. Time zero on the graph represents the point at which the cultures were inoculated. The data are presented as the average and standard deviation.
Molecular basis of loss of secondary metabolite catabolism in strains NCTC 8325 and N315.
Strains N315 and NCTC 8325 entered the stationary phase of growth prematurely (Fig. 3). Previously, we reported a similar phenotype in an aconitase mutant strain (53), leading us to speculate that one or both strains had a mutation in one or more of the TCA cycle genes. To address this possibility, the nucleotide sequences of all genes encoding TCA cycle enzymes from strains MW2, Mu50, MSSA-476, MRSA-252, COL, NCTC 8325, and N315 were examined for the presence of mutations that could potentially disrupt the TCA cycle (data not shown). Sequence analysis revealed numerous polymorphisms, but the mutations in only two strains (strains Mu50 and N315) would be predicted to result in amino acid deletions or truncations. Strain Mu50 had a single base pair deletion in the gene encoding the succinate dehydrogenase beta subunit (sdhB) (http://w3.grt.kyushu-u.ac.jp/VRSA/, open reading frame SAV1149, between nucleotides 285 and 286), causing a frameshift and resulting in a predicted protein truncation. Strain Mu50 catabolizes acetate, leading us to speculate that the single nucleotide deletion was a sequencing artifact. To address this possibility, we sequenced a region ≈150 bases upstream and downstream of the putative deletion (data not shown). Consistent with the hypothesis, no deletion was present in this region of the sdhB gene.
Strain NCTC 8325.
There were no mutations in the TCA cycle genes of NCTC 8325, suggesting that a metabolic block in the TCA cycle was not the cause of the loss of acetate catabolism. Previously, it was reported that strain NCTC 8325 contains an 11-bp deletion in rsbU (32), a gene encoding a positive regulator of the alternative sigma factor σB encoded by sigB. This mutation is also present in strain RN6390, a derivative of strain NCTC 8325 (24), raising the possibility that impaired acetate catabolism in strains NCTC 8325 and RN6390 was due to the loss of σB function. To test this hypothesis, the concentrations of glucose and acetate in the culture medium were determined for strain RN6390 and the isogenic strain SH1000 (strain RN6390 containing a wild-type rsbU gene) (24) (Fig. 2). Consistent with our hypothesis, strain SH1000 had a significantly enhanced ability to catabolize acetate, resulting in an increased growth yield. These data indicated that wild-type σB function was required for acetate catabolism.
Strain N315.
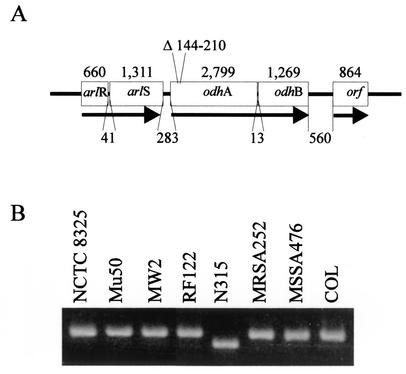
Strain N315 had a 66-bp deletion in the α-ketoglutarate dehydrogenase gene odhA, which encodes the E1 subunit of the α-ketoglutarate dehydrogenase complex (Fig. 6), consistent with the inability of N315 to catabolize acetate. The 66-bp deletion was confirmed by PCR (Fig. 6). Interestingly, the 66-bp deletion retains the open reading frame, raising the possibility that α-ketoglutarate dehydrogenase activity is present in strain N315. To test this possibility, cell lysates of strains MSSA-476, Mu50, and N315 were assayed for α-ketoglutarate dehydrogenase activity. The level of activity varied between the three strains, but all had α-ketoglutarate dehydrogenase specific activity (data not shown). These data demonstrated that the loss of acetate catabolism by strain N315 was not likely due to the mutation in the odhA gene.
FIG. 6.
Confirmation of α-ketoglutarate dehydrogenase mutation in S. aureus strain N315. (A) Schematic representation of the odhAB locus of strain N315, including the flanking genes. (B) PCR confirmation of the 66-bp deletion in the odhA gene of N315.
The absence of an association between the odhA mutation and the loss of acetate catabolism led us to examine the nucleotide sequences of the genes required for sigB expression (rsbU, rsbV, rsbW, and sigB). Unlike NCTC 8325, no mutations were found that would account for the inability to catabolize acetate (data not shown). Additionally, sigB mutants are hypertoxigenic with respect to alpha-toxin production (7), a result inconsistent with our observations (Table 2). Thus, the cause of the loss of acetate catabolism in strain N315 remains unknown.
DISCUSSION
Bacteria normally adapt to distinct environmental niches by altering gene expression, allowing for growth and survival (18). This type of niche adaptation is readily reversible and usually transient. However, niche adaptation can also occur by the accumulation of mutations within the genome of the organism (3, 6, 25). This type of niche adaptation can be permanent but may be reversed by additional mutations. Thus, the loss of secondary metabolite catabolism would represent a permanent niche adaptation, implying that mutations could be found that would account for the loss of function. The whole-genome nucleotide sequencing of the eight S. aureus strains examined in this study presented a unique opportunity to examine phenotype-genotype correlations in a medically important organism.
Origins of the loss of acetate catabolism—laboratory attenuation?
The strains chosen for whole-genome sequencing were originally isolated from human or animal sources. These strains have undergone long-term laboratory propagation, raising the possibility that the loss of acetate catabolism occurred subsequent to their isolation from a host. The loss of acetate catabolism correlated with a decreased growth yield relative to strains that do catabolize acetate (Table 2), resulting in a competitive disadvantage. However, the propagation of S. aureus in either batch or continuous culture caused an increase in the growth yield (4, 52), leading to an increased competitive fitness in vitro. Laboratory propagation of S. aureus has also been associated with mutations in the agr operon (4, 37, 52); however, these mutations are not known to affect acetate catabolism. Interestingly, the serial passage of S. aureus strain SA564 for 6 weeks in batch culture failed to produce any deletions in the genome (52) or sequence alterations in the serine-aspartate repeat region of clumping factor B (clfB) (B. N. Kreiswirth, unpublished data). Taken together, these data suggest that mutations affecting acetate catabolism in strains NCTC 8325 and N315 predate their isolation from a host.
Implications of loss of secondary metabolite catabolism.
Aerobically grown S. aureus cells catabolize glucose and accumulate acetate extracellularly during the exponential phase of growth (Fig. 1). When the concentration of glucose decreases to a level at which it can no longer sustain rapid growth, the bacteria enter the postexponential phase and catabolize acetate (Fig. 1 and 3). Interestingly, two of the eight S. aureus strains (N315 and NCTC 8325) whose genomes have been sequenced have lost the ability to catabolize acetate. The loss of acetate catabolism did not alter stationary-phase survival (Fig. 5) or affect virulence factor production (Table 2), suggesting the absence of an obvious advantage for maintaining acetate catabolism. However, both strains have reduced growth yields relative to strains that do catabolize acetate. One measure of fitness in an organism is its ability to place progeny into the next generation; hence, a reduced growth yield would decrease the fitness of bacterial strains that do not catabolize acetate relative to those that do catabolize acetate.
Evolution of a catabolic pathway?
The evolutionary origin of the TCA cycle has been of considerable research interest for many years and has been used as a paradigm for the study of the origin and evolution of complex metabolic pathways (2, 26, 39, 40, 57). The consensus is that the TCA cycle evolved as two independent pathways for the assimilation of pyruvate into biosynthetic intermediates (an oxidative pathway for the generation of α-ketoglutarate and a reductive pathway for the synthesis of succinyl-coenzyme A) and that it was a complete cycle in proteobacteria (26). Thus, the observation that the predominant form of the TCA cycle in prokaryotes is an incomplete one suggests that the TCA cycle is undergoing reductive evolution (26). We have presented evidence that demonstrates that S. aureus can lose secondary metabolite catabolism, raising the possibility that the S. aureus TCA cycle is undergoing reductive evolution by multiple independent genetic events.
Postgenomic challenges for staphylococcal research.
The S. aureus strains chosen for whole-genome sequencing represent a genetically diverse group of organisms with a common feature: they all successfully colonized and caused disease in humans or animals. We have demonstrated that significant variation occurs in S. aureus growth, secondary metabolite catabolism, virulence factor production, and expression of virulence regulators. Taken together, these data suggest that there are multiple physiological characteristics, in addition to genotypic characteristics, that promote successful colonization and pathogenesis. Understanding how intraspecies physiologic diversity contributes to host-pathogen interactions is important to understanding the molecular mechanisms of pathogenesis. For this reason, the whole-genome sequences of these eight S. aureus strains provide an exceptional opportunity to study phenotype-genotype correlations and to begin to understand how intraspecies physiologic diversity impacts host-pathogen interactions.
Acknowledgments
We thank L. J. Reitzer for critical review of the manuscript, M. Otto for strains, and V. Kapur for strain RF122 nucleotide sequence data.
Editor: D. L. Burns
REFERENCES
- 1.Arvidson, S., T. Holme, and T. Wadstrom. 1970. Formation of bacteriolytic enzymes in batch and continuous culture of Staphylococcus aureus. J. Bacteriol. 104:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, J. E., and H. Krebs. 1981. The evolution of metabolic cycles. Nature 291:381-382. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss, C. D., and E. R. Moxon. 2002. Hypermutation and bacterial adaptation. ASM News 68:549-555. [Google Scholar]
- 4.Bjorklind, A., and S. Arvidson. 1980. Mutants of Staphylococcus aureus affected in the regulation of exoprotein synthesis. FEMS Microbiol. Lett. 7:203-206. [Google Scholar]
- 5.Blumenthal, H. J. 1972. Glucose catabolism in staphylococci, p. 111-135. In J. O. Cohen (ed.), The staphylococci. Wiley-Interscience, New York, N.Y.
- 6.Bush, R. M., and K. D. Everett. 2001. Molecular evolution of the Chlamydiaceae. Int. J. Syst. E vol. Microbiol. 51:203-220. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, A. L., Y. T. Chien, and A. S. Bayer. 1999. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect. Immun. 67:1331-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung, A. L., S. J. Projan, and H. Gresham. 2002. The genomic aspect of virulence, sepsis, and resistance to killing mechanisms in Staphylococcus aureus. Curr. Infect. Dis. Rep. 4:400-410. [DOI] [PubMed] [Google Scholar]
- 9.Coulter, S. N., W. R. Schwan, E. Y. Ng, M. H. Langhorne, H. D. Ritchie, S. Westbrock-Wadman, W. O. Hufnagle, K. R. Folger, A. S. Bayer, and C. K. Stover. 1998. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 30:393-404. [DOI] [PubMed] [Google Scholar]
- 10.Cronan, J. E., and D. LaPorte. 1996. Tricarboxylic acid cycle and glyoxylate bypass, p. 206-216. In F. C. Neidhardt, R. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 11.Dassy, B., and J. M. Fournier. 1996. Respiratory activity is essential for post-exponential-phase production of type 5 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 64:2408-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufour, P., S. Jarraud, F. Vandenesch, T. Greenland, R. P. Novick, M. Bes, J. Etienne, and G. Lina. 2002. High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 184:1180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan, J. B., and M. L. Morse. 1965. Carbohydrate transport in Staphylococcus aureus. II. Characterization of the defect of a pleiotropic transport mutant. Biochim. Biophys. Acta 109:172-183. [DOI] [PubMed] [Google Scholar]
- 14.Emmett, M., and W. E. Kloos. 1975. Amino acid requirements of staphylococci isolated from human skin. Can. J. Microbiol. 21:729-733. [DOI] [PubMed] [Google Scholar]
- 15.Fisher, S. H., and B. Magasanik. 1984. Synthesis of oxaloacetate in Bacillus subtilis mutants lacking the 2-ketoglutarate dehydrogenase enzymatic complex. J. Bacteriol. 158:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald, J. R., P. J. Hartigan, W. J. Meaney, and C. J. Smyth. 2000. Molecular population and virulence factor analysis of Staphylococcus aureus from bovine intramammary infection. J. Appl. Microbiol. 88:1028-1037. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald, J. R., D. E. Sturdevant, S. M. Mackie, S. R. Gill, and J. M. Musser. 2001. Evolutionary genomics of Staphylococcus aureus: Insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic. Proc. Natl. Acad. Sci. USA 98:8821-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forng, R. Y., C. Champagne, W. Simpson, and C. A. Genco. 2000. Environmental cues and gene expression in Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. Oral Dis. 6:351-365. [DOI] [PubMed] [Google Scholar]
- 19.Gardner, J. F., and J. Lascelles. 1962. The requirement for acetate of a streptomycin-resistant strain of Staphylococcus aureus. J. Gen. Microbiol. 29:157-164. [DOI] [PubMed] [Google Scholar]
- 20.Gerard, H. C., J. Freise, Z. Wang, G. Roberts, D. Rudy, B. Krauss-Opatz, L. Kohler, H. Zeidler, H. R. Schumacher, J. A. Whittum-Hudson, and A. P. Hudson. 2002. Chlamydia trachomatis genes whose products are related to energy metabolism are expressed differentially in active vs. persistent infection. Microbes Infect. 4:13-22. [DOI] [PubMed] [Google Scholar]
- 21.Goldschmidt, M. C., and D. M. Powelson. 1953. Effect of the culture medium on the oxidation of acetate by Micrococcus pyogenes var. aureus. Arch. Biochem. Biophys. 46:154-163. [DOI] [PubMed] [Google Scholar]
- 22.Guest, J. R. 1992. Oxygen-regulated gene expression in Escherichia coli. The 1992 Marjory Stephenson prize lecture. J. Gen. Microbiol. 138:2253-2263. [DOI] [PubMed] [Google Scholar]
- 23.Hanaki, H., K. Kuwahara-Arai, S. Boyle-Vavra, R. S. Daum, H. Labischinski, and K. Hiramatsu. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42:199-209. [DOI] [PubMed] [Google Scholar]
- 24.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter, P. R., and C. A. Fraser. 1990. Application of the theory of adaptive polymorphism to the ecology and epidemiology of pathogenic yeasts. Appl. Environ. Microbiol. 56:2219-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huynen, M. A., T. Dandekar, and P. Bork. 1999. Variation and evolution of the citric-acid cycle: a genomic perspective. Trends Microbiol. 7:281-291. [DOI] [PubMed] [Google Scholar]
- 27.Ivler, D. 1965. Comparative metabolism of virulent and avirulent staphylococci. Ann. N.Y. Acad. Sci. 128:62-80. [DOI] [PubMed] [Google Scholar]
- 28.Jarraud, S., G. J. Lyon, A. M. Figueiredo, L. Gerard, F. Vandenesch, J. Etienne, T. W. Muir, and R. P. Novick. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182:6517-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science. 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 30.Kendall, A. I., T. E. Friedemann, and M. Ishikawa. 1930. Quantitative observations on the chemical activity of “resting” Staphylococcus aureus. J. Infect. Dis. 47:223-228. [Google Scholar]
- 31.Krebs, H. A. 1937. Dismutation of pyruvic acid in Gonococcus and Staphylococcus. Biochem. J. 31:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83-86. [DOI] [PubMed] [Google Scholar]
- 35.Mah, R. A., D. Y. Fung, and S. A. Morse. 1967. Nutritional requirements of Staphylococcus aureus S-6. Appl. Microbiol. 15:866-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 37.McNamara, P. J., and J. J. Iandolo. 1998. Genetic instability of the global regulator agr explains the phenotype of the xpr mutation in Staphylococcus aureus KSI9051. J. Bacteriol. 180:2609-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mei, J. M., F. Nourbakhsh, C. W. Ford, and D. W. Holden. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia with signature-tagged mutagenesis. Mol. Microbiol. 26:399-407. [DOI] [PubMed] [Google Scholar]
- 39.Melendez-Hevia, E., T. G. Waddell, and M. Cascante. 1996. The puzzle of the Krebs citric acid cycle: assembling the pieces of chemically feasible reactions, and opportunism in the design of metabolic pathways during evolution. J. Mol. E vol. 43:293-303. [DOI] [PubMed] [Google Scholar]
- 40.Morowitz, H. J., J. D. Kostelnik, J. Yang, and G. D. Cody. 2000. The origin of intermediary metabolism. Proc. Natl. Acad. Sci. USA 97:7704-7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morse, S. A., and R. A. Mah. 1973. Regulation of staphylococcal enterotoxin B: effect of anaerobic shock. Appl. Microbiol. 25:553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musser, J. M., and V. Kapur. 1992. Clonal analysis of methicillin-resistant Staphylococcus aureus strains from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J. Clin. Microbiol. 30:2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musser, J. M., P. M. Schlievert, A. W. Chow, P. Ewan, B. N. Kreiswirth, V. T. Rosdahl, A. S. Naidu, W. Witte, and R. K. Selander. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. USA 87:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musser, J. M., and R. K. Selander. 1990. Genetic analysis of natural populations of Staphylococcus aureus, p. 59-67. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, Inc., New York, N.Y.
- 45.Novick, R. P. 2000. Pathogenicity factors and their regulation, p. 392-407. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington D.C.
- 46.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nychas, G. J., H. S. Tranter, R. D. Brehm, and R. G. Board. 1991. Staphylococcus aureus S-6: factors affecting its growth, enterotoxin B production and exoprotein formation. J. Appl. Bacteriol. 70:344-350. [DOI] [PubMed] [Google Scholar]
- 48.Onoue, Y., and M. Mori. 1997. Amino acid requirements for the growth and enterotoxin production by Staphylococcus aureus in chemically defined medium. Int. J. Food Microbiol. 36:77-82. [DOI] [PubMed] [Google Scholar]
- 49.Rubin, R. J., C. A. Harrington, A. Poon, K. Dietrich, J. A. Greene, and A. Moiduddin. 1999. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg. Infect. Dis. 5:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sivakanesan, R., and E. A. Dawes. 1980. Anaerobic glucose and serine metabolism in Staphylococcus epidermidis. J. Gen. Microbiol. 118:143-157. [DOI] [PubMed] [Google Scholar]
- 51.Somerville, G., C. A. Mikoryak, and L. Reitzer. 1999. Physiological characterization of Pseudomonas aeruginosa during exotoxin A synthesis: glutamate, iron limitation, and aconitase activity. J. Bacteriol. 181:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somerville, G. A., S. B. Beres, J. R. Fitzgerald, F. R. DeLeo, R. L. Cole, J. S. Hoff, and J. M. Musser. 2002. In vitro serial passage of Staphylococcus aureus: changes in physiology, virulence factor production, and agr nucleotide sequence. J. Bacteriol. 184:1430-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Somerville, G. A., M. S. Chaussee, C. I. Morgan, J. R. Fitzgerald, D. W. Dorward, L. J. Reitzer, and J. M. Musser. 2002. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 70:6373-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonenshein, A. L. 2002. The Krebs citric acid cycle, p. 151-162. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 55.Strasters, K. C., and K. C. Winkler. 1963. Carbohydrate metabolism of Staphylococcus aureus. J. Gen. Microbiol. 33:213-229. [DOI] [PubMed] [Google Scholar]
- 56.Taylor, D., and K. T. Holland. 1989. Amino acid requirements for the growth and production of some exocellular products of Staphylococcus aureus. J. Appl. Bacteriol. 66:319-329. [DOI] [PubMed] [Google Scholar]
- 57.Wachtershauser, G. 1990. Evolution of the first metabolic cycles. Proc. Natl. Acad. Sci. USA 87:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]