Abstract
Pneumococcal surface protein A (PspA) is able to elicit antibodies in mice and humans that can protect mice against fatal infection with Streptococcus pneumoniae. It has been observed that immunization with a single family 1 PspA can protect mice against infections with capsular type 3 or 6B strains expressing PspA family 1 or 2. However, several studies have shown that immunity to PspA is less efficacious against several capsular type 4 strains than against strains of capsular types 3, 6A, and 6B. To determine whether the greater difficulty in protecting against capsular type 4 strains resulted from differences in their PspAs or from differences in their genetic backgrounds, we performed protection experiments using four different challenge strains: a capsular type 3 strain expressing a family 1 PspA (WU2), a capsular type 4 strain expressing a family 2 PspA (TIGR4), and genetically engineered variants of WU2 and TIGR4 expressing each other's PspAs. Prior to infection, the mice were immunized with recombinant family 1 or family 2 PspA. The results revealed that much of the difficulty in protecting against capsular type 4 strains was eliminated when mice were immunized with a homologous PspA of the same PspA family. However, regardless of which PspA the strains expressed, those on the TIGR4 background were about twice as hard to protect against as WU2 strains expressing the same PspA based on the efficacy rates seen in our experiments. These results point out the importance of including more than one PspA in any PspA vaccines developed for human use.
Streptococcus pneumoniae is among the leading causes of morbidity and mortality in the United States (14). The present vaccine strategy relies on 23-valent capsular polysaccharide vaccine for adults and 7-valent polysaccharide-protein conjugate vaccine for young children. Unfortunately, the 23-valent vaccine is not as efficacious as would be desired, and the 7-valent vaccine is too restricted in the polysaccharides that it contains to be able to protect against all disease, especially in the developing world (4, 13, 15, 19, 20, 31). A number of protein vaccine candidates are currently being investigated. These include PspA, pneumolysin, PsaA, and PspC (2, 6, 12, 24-26, 30, 32, 35).
PspA is a surface protein present on all pneumococci (16, 34). It has been shown to be highly immunogenic in mice and to elicit protection against pneumococcal challenge (6, 24, 27, 29, 32). PspA has also been shown to elicit antibodies in humans that can passively protect mice against pneumococcal sepsis (6). The PspA sequence is variable among pneumococcal strains, especially in the α-helical N-terminal domain, which is exposed on the bacterial surface (17, 18, 22). Based on sequence similarities, PspA sequences have been classified into three main families, with over 95% of strains belonging to either family 1 or family 2 (18, 34). Despite the variability in their α-helical sequences (as much as 60% of amino acids differ between families), immunization with an individual family 1 PspA or family 2 PspA has been observed to elicit antibodies that are protective against capsular group 6 or type 3 strains expressing serologically diverse PspAs (6, 21, 27).
In previous studies it has been noted that pneumococcal strains of capsular types 2, 4, and 5 can be harder to protect against with PspA immunization than strains of other capsular types (9, 23, 24, 29, 32). Most of these prior studies were conducted before PspA families had been described (18). In the case of the studies with the capsular type 4 strains, we know now that most were PspA family 2 and that the immunogens were generally PspA family 1 (9). In one study, however, immunization with family 2 PspA from the capsular type 4 strain EF5668 gave solid protection against infection with the homologous strain and also against strains of capsular types 3 and 6 that expressed family 1 PspAs (21). More recently, when we immunized with fragments of family 2 PspA from the capsular type 4 strain EF3296, we observed statistically significant protection against EF3296, but seldom were all of the challenged mice completely protected against fatal disease (29).
In the present study, we have specifically investigated one of the PspA family 2 capsular type 4 strains and sought to determine whether the difficulty of protecting against this strain, compared to type 3 strain WU2, could be eliminated by using a homologous immunogen or whether there might be something about the capsular type 4 strains' genetic backgrounds that makes them difficult to protect against.
To dissect the roles of the PspA family and the genetic background, two pairs of isogenic strains were prepared. One was BR93.1 (28), a variant of WU2 that makes TIGR4's family 2 PspA instead of the family 1 PspA normally expressed by WU2. The other strain was BR6.1, a variant of TIGR4 that expresses family 1 PspA/Rx1 instead of TIGR4's family 2 PspA. The ability of immunity to family 1 and 2 PspAs to protect against these strains was compared with protection against the parental strains WU2 and TIGR4.
MATERIALS AND METHODS
Reagents.
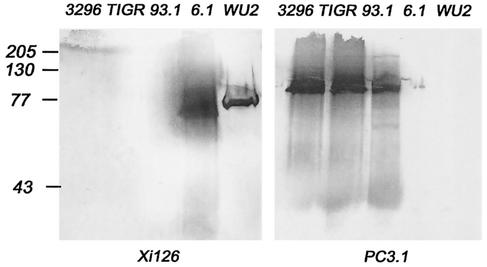
Alexa Fluor 488-conjugated streptavidin was from Molecular Probes Inc. (Eugene, Oreg.). Alkaline phosphatase-conjugated streptavidin, biotin-conjugated goat anti-mouse, and biotin-conjugated goat anti-rabbit antibodies were from Southern Biotechnology Associates (Birmingham, Ala.). Protein markers and Ready gels were from Bio-Rad Laboratories (Hercules, Calif.). Monoclonal anti-PspA antibody (Xi126) was produced as described previously (22). Monoclonal antibody PC3.1 was elicited by immunization with PspA/EF3296 and was a gift from Aventis Inc., Toronto, Ontario, Canada.
Bacteria.
Bacterial strains and plasmids used are described in Table 1. The pneumococcal strains were stored at −80°C in 12% glycerol, transferred to blood agar plates, and incubated at 37°C in a 5% CO2 atmosphere overnight. Colonies from blood agar were used to inoculate Todd-Hewitt medium containing 0.5% yeast extract. Bacteria were harvested in early stationary phase at 1,500 × g for 15 min and suspended in 60 mM phosphate-buffered saline (PBS, pH 7.2). The bacterial concentration was estimated from the absorbance at 600 nm and confirmed by viable counts on blood agar.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristic
|
Source or reference(s) | ||||
|---|---|---|---|---|---|---|
| Origin | Capsule | Genetic background | PspA family | PspA origin | ||
| Strains | ||||||
| WU2 | Wild type | 3 | WU2 | 1 | WU2 | 10 |
| EF3296 | Wild type | 4 | EF3296 | 2 | EF3296a | 3 |
| TIGR4 | Wild type | 4 | TIGR4 | 2 | TIGR4a | 1, 33 |
| BR93.1 | WU2 pspA::pREN4 | 3 | WU2 | 2 | TIGR4a | 28 |
| JY2078 | Rx1 derivative, Ermr | Rx1 | 1 | Rx1 | 36 | |
| BR6.1 | JY2078 × TIGR4, Ermr | 4 | TIGR4 | 1 | WU2 | This study |
| Plasmids | ||||||
| pJY4164 | Ermr; oriR Escherichia coli | 36 | ||||
| pREN4 | pJY4164::PCR fragment SKH73-LSM2 from TIGR4 | 28 | ||||
Expressed mature PspAs of EF3296 and TIGR4 have identical α-helical domains (the N-terminal surface-exposed half of PspA).
Construction of PspA switch variants.
S. pneumoniae TIGR4 was included because it is transformable and expresses a family 2 PspA whose α-helical region is identical to that of PspA/EF3296 (18, 33). Cloned and expressed PspA fragments were already available from PspA/EF3296 (29). Strain WU2 was chosen as a transformable family 1 PspA capsular type 3 strain, which is readily protected against by immunization with family 1 PspA/Rx1 (8). PspA/Rx1 was important to the study because we have used it more than any other PspA for immunization and challenge studies.
The construction of the WU2 variant BR93.1, in which PspA/WU2 was replaced with PspA/TIGR4, has been described elsewhere (28). The TIGR4 variant expressing only family 1 PspA was prepared by a similar procedure. TIGR4 was transformed with the chromosomal DNA of an Rx1 derivative, JY2078 (36), carrying an erythromycin marker downstream of the pspA gene. An erythromycin-resistant transformant in which crossover of genetic material had occurred in the pspA region was selected and backcrossed three times with TIGR4 parental DNA, each time selecting for erythromycin resistance. The resultant strain was designated as BR6.1. The change of the pspA sequence in BR6.1 was confirmed by PCR and Southern blotting (data not shown).
Western blot.
Bacterial cells were grown to an optical density of 0.6 at 600 nm, washed twice in PBS, and treated with 2 mg of hen egg lysozyme/ml for 2 h at 37°C. Sodium dodecyl sulfate (1%) was added, and the suspension was vortexed until clear. The lysates were stored at −20°C. The lysates were run on polyacrylamide gels (Bio-Rad Ready gels), which were electroblotted to a 0.45-μm-pore-size nitrocellulose membrane (Bio-Rad) in Tris-glycine buffer (20% methanol, 25 mM Tris, 192 mM glycine, pH 8.1 to 8.4) at 100 V for 1 h at 4°C. The blotted membrane was blocked with 1% bovine serum albumin in PBS-T (PBS containing 0.05% Tween 20) for 1 h at room temperature and washed three times (5 min each) with PBS-T. The membranes were overlaid with the monoclonal antibodies to PspA (antibody PC3.1 for detection of PspA/TIGR4 or Xi126 for detection of PspA/WU2 and PspA/Rx1) for 30 min at 37°C, washed three times in PBS-T, and further incubated with a mix of biotinylated goat anti-mouse antibody (1:1,000 in PBS-T) and alkaline phosphatase-conjugated streptavidin (1:500 dilution in PBS-T) for 30 min at 37°C. After washing, the membrane was developed using 0.1 mg of nitroblue tetrazolium/ml and 0.5 mg of 5-bromo-4-chloro-3-indolylphosphate/ml in 0.15 M Tris-HCl, pH 8.8.
Binding of anti-PspA serum antibodies to the bacterial surface.
Bacteria were grown on blood agar plates or in Todd-Hewitt medium with yeast extract and were suspended in PBS at a concentration of 108 bacteria/ml. A bacterial pellet of 107 bacteria (quantitated by absorbance at 420 nm) was suspended in Ringer's solution containing monoclonal anti-PspA PC3.1 antibodies (20 μg/ml) or anti-PspA Xi126 (undiluted hybridoma supernatant) for 30 min at room temperature and washed by centrifugation at 1,500 × g for 5 min in PBS. Biotinylated goat anti-mouse antibodies (1:100 dilution in PBS) were added for an additional 30 min at room temperature. After a second wash in PBS, Alexa Fluor 488-conjugated streptavidin was added (1:100 dilution) for 30 min at room temperature, and the cells were washed and inspected by epifluorescence microscopy with a Leitz upright microscope (Leitz, Wetzlar, Germany). The binding was quantitated by flow cytometry with a FACSCalibur flow cytometer (Becton Dickinson Biosciences, San Jose, Calif.).
PspA fragments for immunization.
PspA/Rx1 fragment JAS218 was constructed and expressed as described previously (17). Fragment SW111 of PspA/EF3296 constituting amino acids (aa) 1 to 478 was kindly provided by Aventis Inc. Fragment HR108 constituting aa 314 to 418 from PspA/EF3296 was constructed and expressed as described previously (29). The PspA/EF3296 fragments were used for immunization, since the DNA sequences of pspA/EF3296 and pspA/TIGR4 are identical except for 1 bp resulting in a Phe-to-Leu replacement at aa position 16 of the leader sequence, making the mature PspA proteins EF3296 and TIGR4 identical. SW111 and HR108 are therefore referred to throughout the text as TIGR4 sequences even though they are derived from the identical EF3296 sequence.
Mouse immunization and challenge.
Five- to eight-week-old CBA/CAHN/XID (CBA/N) or BALB/cByJ (BALB/c) mice (Jackson Laboratory, Bar Harbor, Maine) were immunized subcutaneously with purified recombinant PspA fragments. The procedure and fragments have been described elsewhere (17, 29). Mice were immunized subcutaneously with 5 μg of SW111 (aa 1 to 478, PspA/EF3296), HR108 (aa 314 to 418, PspA/EF3296), or JAS218 (aa 170 to 288, PspA/Rx1) with alum as an adjuvant (100 μg/ml). The mice were boosted subcutaneously 2 weeks later with the same dose of immunogen and alum. A fortnight after the boost, they were challenged with the recombinant strain BR93.1 or BR6.1 or with wild-type strain WU2 or TIGR4 at a dose that was 2 logs higher than the 50% lethal dose. The mice were then monitored for death over 21 days; the mice that survived the challenge were scored as protected against infection.
Statistical analyses.
Mouse survival was recorded daily and monitored for 21 days, and values were compared by the two-tailed Mann-Whitney U test (nonparametric, two-sample rank) unless indicated otherwise. Comparisons of live and dead animals in survival experiments were evaluated with 2 × 2 contingency tables and Fisher's exact test. P values in the text refer to results from the Mann-Whitney test unless otherwise noted.
RESULTS
Characterization of the strains.
Two PspA switch variants were constructed (Table 1) to help determine whether the PspA structure or the genetic background was responsible for the difficulty in protecting against the capsular type 4 strain TIGR4 by immunization with PspA. In recombinant strain BR93.1, the pspA sequence of WU2 was replaced by pspA/TIGR4. In the recombinant strain BR6.1, the pspA sequence in TIGR4 was replaced with pspA/Rx1. In each case, the expression of the transformed PspA and not the strain's original PspA was shown by Western blot analysis (Fig. 1). Lysates of TIGR4, EF3296, and mutant BR93.1 produced PspA that reacted strongly with the monoclonal antibody PC3.1, specific for the family 2 PspAs of EF3296 and TIGR4, but not with monoclonal antibody Xi126, specific for family 1 PspA. Lysates of WU2 and BR6.1 reacted strongly with the monoclonal antibody Xi126 specific for the family 1 PspA/Rx1 but not with monoclonal antibody PC3.1.
FIG. 1.
PspA reactivity in pneumococcal strains. Bacterial lysates were run in sodium dodecyl sulfate-12% polyacrylamide gels and immunoblotted using monoclonal anti-PspA antibodies reactive with PspA/Rx1 (Xi126) or PspA/EF3296 (PC3.1). These two monoclonal antibodies were chosen because neither reacts with nonhomologous PspA. The samples run were EF3296 lysate (3296), TIGR4 lysate (TIGR), BR93.1 lysate (93.1), BR6.1 lysate (6.1), and WU2 lysate (WU2). The left panel shows the pattern of reactivities for the strains with Xi126 antibodies, and the right panel shows reactivities with PC3.1. EF3296, TIGR4, and BR93.1 (WU2 expressing PspA/TIGR4) lysates all showed reactivity with PC3.1 antibodies but did not react with Xi126, showing that the strains expressed only family 2 PspA. WU2 and BR6.1 (TIGR4 expressing PspA/Rx1) lysates reacted only with Xi126 antibodies, and no reactivity with PC3.1 antibodies was detected, showing that these strains expressed only the family 1 PspA. Numbers at left are molecular masses in kilodaltons.
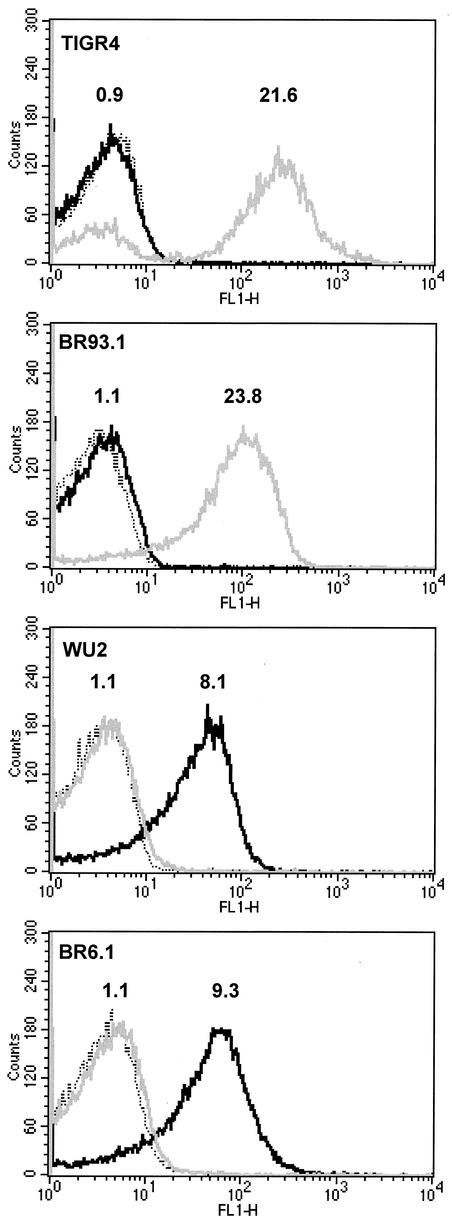
The parental and mutant strains expressed their PspAs on their bacterial surfaces, as detected by antibodies specific for the parental PspAs and quantitated by flow cytometry (Fig. 2). BR93.1 did not bind Xi126 antibodies, and BR6.1 did not bind PC3.1 antibodies. TIGR4 and BR93.1 bound about equal amounts of PC3.1 antibody (21.6 and 23.8 times the values of their respective streptavidin-alone-treated controls), indicating that they expressed similar levels of antibody-accessible PspA on the surface (Fig. 2). The same was true for WU2 and BR6.1, which showed binding of 8.1 and 9.3 times the values of their respective streptavidin-alone-treated controls (Fig. 2).
FIG. 2.
Flow cytometric analysis of PspA expression. S. pneumoniae TIGR4, BR93.1, WU2, and BR6.1 were treated with anti-PspA Xi126 or PC3.1 antibodies and counterstained with biotinylated anti-mouse antibodies and Alexa Fluor 488-conjugated streptavidin. Binding was quantitated by flow cytometry. Dotted trace, streptavidin-alone-treated bacteria; gray trace, binding of Xi126 antibodies; black trace, binding of PC3.1 antibodies. Numerals above the black and gray traces indicate the levels of binding as the fluorescent signal of the sample divided by the fluorescent signal of the streptavidin control. TIGR4 and BR93.1 (WU2 expressing PspA/TIGR4) showed no binding of Xi126 antibodies but showed strong and similar levels of binding of PC3.1, indicating that the strains expressed only PspA/TIGR4. WU2 and BR6.1 (TIGR4 expressing PspA/Rx1) showed no binding with PC3.1 antibodies but showed strong and similar levels of binding with Xi126, indicating that they expressed only family 1 PspA.
No difference in expression of capsular polysaccharides between the transformants and relevant parental strains was observed based on the strength of agglutination by capsular type-specific antibody or colony morphology on blood agar plates (data not shown). This finding is consistent with the fact that the PspA switch variants demonstrated the same virulence as the parental strain of the same capsular type. The capsular type 3 strains WU2 and BR93.1 both showed 100% lethal doses in CBA/N mice of around 200 CFU. The capsular type 4 strains TIGR4 and BR6.1 both had 100% lethal doses in BALB/c mice of about 106. More extensive studies have confirmed that the contribution of the two PspAs to virulence is the same when they are tested on the same genetic background (28).
Relative effects of the genetic backgrounds and PspA family of challenge strains on the ability of recombinant family 2 PspA fragments to elicit protection.
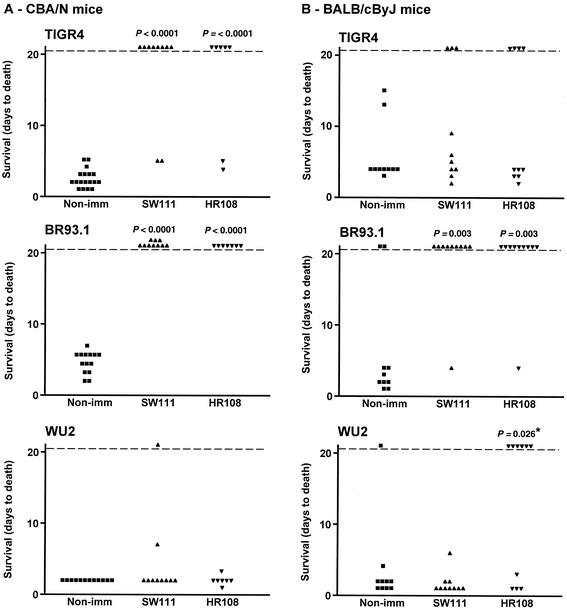
CBA/N and BALB/c mice were immunized with the recombinant family 2 PspA fragments SW111 and HR108. SW111 (aa 1 to 478) encodes the entire α-helical domain of PspA/TIGR4 and a portion of the proline-rich region (29). HR108 is a 105-aa (residues 314 to 418) fragment of the α-helical domain of PspA/TIGR4, which was shown to contain the most important cross-protective epitopes (29). HR108 is homologous to the region of family 1 PspA/Rx1 to which its major cross-protective epitopes have also been mapped (11, 22). The PspA regions homologous to HR108 have been used to classify PspAs into clades and families (18). BALB/c and CBA/N mice were immunized with SW111 or HR108 in alum, or with alum alone. Mice given each immunogen were divided into groups and challenged with WU2, TIGR4, or BR93.1 (Fig. 3).
FIG. 3.
Protection against pneumococcal infection after immunization with PspA/EF3296 fragments. Each point represents the results of a single mouse. P values were calculated from comparison of days to death between immunized and nonimmunized groups by the Mann-Whitney U test. No P values were given if the value was ≥0.05. The only exception is the value marked by an asterisk, which was >0.05 by the Mann-Whitney test. The value provided was calculated by Student's t test, which, when valid (as was the case here), can be more sensitive than the Mann-Whitney U test. (A) CBA/N mice were immunized with either SW111 or HR108. After a booster immunization, the mice were infected with 250 CFU of TIGR4, 240 CFU of BR93.1, or 300 CFU of WU2. (B) BALB/c mice were immunized with either SW111 or HR108. After a booster immunization, the mice were infected with 1.4 × 106 CFU of TIGR4, 1.7 × 106 CFU of BR93.1, or 1.0 × 106 CFU of WU2. Immunization with SW111 and HR108 protected some mice against TIGR4. Although these results were not statistically significant, they may be real, since pooling the data for SW111 and HR108 immunizations showed significance at P = 0.0011.
Protection elicited by SW111.
Immunization of CBA/N mice with SW111 elicited complete protection against BR93.1 (P = 0.0001) and protected 8 of 10 mice against challenge with TIGR4 (P = <0.0001; Fig. 3A). Both of these challenge strains express the family 2 PspA of TIGR4, whose α-helical region is identical to that of the immunizing SW111 PspA. When the same experiment was conducted with BALB/c mice, SW111 immunization protected 9 of 10 mice infected with capsule type 3 strain BR93.1 (P = 0.003) but only 3 of 10 mice infected with the capsular 4 strain TIGR4 (even though both strains expressed the same family 2 PspA). The protection elicited against BR93.1 was significantly better (P = 0.02) than that elicited against TIGR4 (Fig. 3B).
Immunization with SW111 resulted in no protection against WU2 in either CBA/N or BALB/c mice, indicating that cross-protective antibodies reactive with the family 1 PspA of WU2 had not been elicited to a protective level by this family 2 fragment. The failure of SW111 to elicit protection against WU2 was reminiscent of our earlier findings, which demonstrated that fragments of the α-helical region of PspA/EF3296 could not elicit protection against another PspA family 1 capsular type 3 strain, A66.1. In those same prior studies, however, SW111 did elicit protection against a capsular group 6 strain expressing a family 1 PspA (29).
Protection elicited by HR108.
In both CBA/N and BALB/c mice, protection elicited by HR108 was similar to that elicited by SW111 (whose 478-aa sequence includes the 105 aa of HR108). In CBA/N mice, HR108 immunization totally protected against BR93.1 challenge (P < 0.0001) and protected five of seven mice challenged with TIGR4 (P = 0.001) (Fig. 3A). HR108 immunization of BALB/c mice protected 9 of 10 mice against BR93.1 (P = 0.003), whereas only 4 of 10 mice survived challenge with TIGR4 (Fig. 3B). The difference in the protection elicited by HR108 against BR93.1 and TIGR4 in BALB/c mice was statistically significant (P = 0.04) (Fig. 3).
The biggest difference between the protection elicited by SW111 and that by HR108 was that HR108, unlike SW111, appeared to be at least partially protective against fatal infection with WU2 in BALB/c mice. This difference in protection elicited by HR108 and SW111 was significant at P = 0.029. This result was unanticipated because the amino acid sequence of HR108 is included within SW111. One possible explanation is that SW111 contains PspA epitopes that are more immunogenic than those in HR108 but which elicit highly cross-protective antibodies. The fact that HR108 was observed previously not to elicit protection against A66.1 (another capsular type 3, family 1 PspA strain) in BALB/c mice (29) might be explained by the higher virulence of A66.1 than of WU2 (5, 8) or the small differences in their PspAs.
Relative effects of the genetic backgrounds and PspA family of challenge strains on the ability of recombinant family 1 PspA fragments to elicit protection.
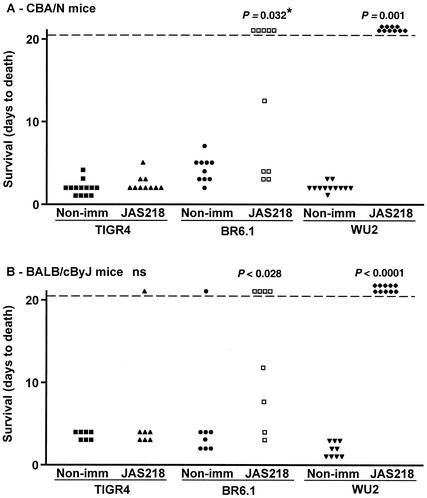
CBA/N and BALB/c mice were immunized with JAS218 (aa 170 to 288 of PspA/Rx1). This recombinant 119-aa fragment includes the region of PspA/Rx1, which has previously been shown to be important in elicitation of cross-protection (22) and is homologous to HR108 for PspA/TIGR4. The mice were then infected with WU2, BR6.1, or TIGR4. BR6.1 is a variant of TIGR4 that expresses PspA/Rx1, which is a family 1 PspA very similar to family 1 PspA/WU2 (18).
Immunization of CBA/N mice with JAS218 gave significant protection against both WU2 and BR6.1 (Fig. 4A). JAS218 immunization of BALB/c mice resulted in complete protection against WU2 (P < 0.0001), and four of eight mice survived challenge with BR6.1 (P = 0.028) (Fig. 4B). For both mouse strains, immunization with JAS218 appeared to elicit better protection against WU2 than against BR96.1. Although these strains both express family 1 PspA, they differ in their genetic background and capsular type. The greater protection against WU2 than against BR96.1 was statistically significant in comparison of live with dead mice for the experiments in both CBA/N (P = 0.03) and BALB/c (P = 0.02) mice.
FIG. 4.
Protection against pneumococcal infection after immunization with PspA/Rx1 fragment JAS218. Each point represents the results of a single mouse. P values were calculated from comparison of days to death between immunized and nonimmunized groups by the Mann-Whitney U test. No P values were given if the value was ≥0.05. The only exception is the value marked by an asterisk, which was 0.032 when comparison of live and dead mice was conducted with the Fisher exact test. This difference was not significant by the Mann-Whitney U test. The value provided was calculated by comparison of live with dead mice. (A) CBA/N mice were immunized with JAS218. After a booster immunization, the mice were infected with 270 CFU of TIGR4, 320 CFU of WU2, or 270 CFU of BR6.1. (B) BALB/c mice were immunized with JAS218. After a booster immunization, the mice were infected with 2.5 × 106 CFU of TIGR4, 1.0 × 106 CFU of WU2, or 1.1 × 106 CFU of BR6.1.
Immunization with JAS218 did not confer protection against TIGR4 in CBA/N or BALB/c mice, even though it had provided protection against strain BR6.1, which is also capsular type 4 but expresses the immunizing family 1 PspA. This finding indicated that insufficient cross-protective antibodies reactive with family 2 PspA were elicited by the family 1 PspA to be protective against a strain on the TIGR4 background expressing a family 2 PspA (Fig. 4).
As was the case with the immunizations with the family 2 PspA fragments, JAS218 provided better protection against pneumococci expressing a PspA homologous to the cognate immunogen than against pneumococci expressing noncognate PspA. We also observed that, when the challenge strains expressed the same PspA that was used for immunization, somewhat better protection was observed if the challenge strain had the capsular type 3 WU2 genetic background rather than the capsular type 4 TIGR4 genetic background.
DISCUSSION
This study found that both the pneumococcal genetic background and the similarity between the immunizing PspA and the PspA of the challenge strain affected the degree of protection elicited by immunization with PspA. There was no evidence, however, that PspA/TIGR4 was a poorer protective target for antibodies to PspA than was PspA/WU2 or PspA/Rx1. Moreover, the family 1 and family 2 PspAs showed essentially the same abilities to support virulence in a sepsis model.
In the past, the general observation was that, even though PspA families can differ in their clade-defining region sequences by 60% or more (18), cross-protection between PspA families 1 and 2 had been the outcome of virtually all studies. These demonstrations were primarily from studies in which mice immunized with family 1 PspA/Rx1 or with family 2 PspA/EF5668 were challenged with a variety of strains (6, 9, 21). Although the present study has provided some evidence for this type of broad cross-protection with certain recombinant fragments in one of the mouse strains used, the important observation here was that the family of the immunizing PspA could affect the protection achieved. This observation is also supported in part by a recent paper showing that immunization with family 2 PspA/EF3296 (TIGR4) fragments elicited no protection against strain A66.1 (a capsular type 3 strain expressing a family 1 PspA). However, that same study also contained data indicating that cross-protection between family 1 and 2 PspAs could sometimes be observed (29).
In the present study, we focused on this question in detail and sought to determine if the reason that the capsular type 4 strains had been observed to be more difficult to protect against might be their genetic background or the particular PspAs that they carried or possibly because in the previous studies immunization was generally not with PspAs of the same family as the type 4 strains. These questions were investigated with the aid of some customized strains developed by genetic means.
Although family-specific protection was common in this study, we observed that one fragment of family 2 PspA/TIGR4 (HR108) could elicit protection against WU2 (family 1 PspA), but only in BALB/c mice and not in CBA/N mice. No protection was seen in either strain of mice when the family 1 PspA/Rx1 (JAS218) was used to immunize mice later challenged with TIGR4. This finding suggests that the family 1 PspA and the family 2 PspA that we examined in the present study may share too few immunogenic protection-eliciting epitopes to be universally cross-protective.
In these studies we also observed that, if the PspA expressed was held constant, the pneumococci on the TIGR4 background were more difficult to protect against than were strains with the WU2 background. The way in which the studies were conducted, however, did not allow us to tell whether the differences in resistance to PspA immunity were related to capsular type or to other genetic factors. The possibility that PspA is less well expressed on TIGR4 seems unlikely in light of our flow cytometry assays, which showed similar degrees of antibody binding to the two genetic backgrounds expressing the same PspA. The virulence strategy of the strains may differ, however. For example, if TIGR4's virulence strategy minimizes the importance of PspA's ability to inhibit complement fixation, it could explain why antibody to PspA has more difficulty protecting against the strains of the TIGR4 than the WU2 background.
The robustness of the results of these studies is supported by the fact that the same general results were obtained using CBA/N and BALB/c mice, even though the former are much more susceptible and required a challenge dose for these experiments of about 1/1,000 of that of BALB/c mice. One significant difference between the results obtained with the two mouse strains was that immunization with HR108 elicited protection against WU2 in BALB/c but not in CBA/N mice. The reason for this difference is not known but could be related to the fact that the normal serum of BALB/c mice contains antibody to phosphocholine, which can be protective against low challenge inocula of S. pneumoniae (7, 10).
Taken together, the present and past data regarding immunity to PspA demonstrate that the best way to use a PspA-containing vaccine to protect against diverse pneumococci with different PspAs and different genetic backgrounds is probably to immunize with at least one PspA from each of the two major PspA families. Our past observations of broad cross-protection among diverse PspAs (9, 21, 29) and studies of PspA diversity (18, 34) lend confidence to our conclusion that the number of different PspAs required in such a vaccine will not be large.
Acknowledgments
We acknowledge Susan Hollingshead for her advice with genetic and molecular manipulations and Flora Gathof, whose handling of the administrative details greatly facilitated this study. We are also grateful to our collaborators at Aventis Inc. for providing monoclonal antibody PC3.1 and PspA/EF3296 construct SW111.
This study was supported by the Swedish Cancer Society (A.H.); National Institute of Health grants AI21548 and HL54818 and the Carsten Cole Buckley Memorial Pediatric Meningitis Research Fund (D.E.B.); and NIH grant AI143653 (L.S.M.).
Editor: V. J. DiRita
REFERENCES
- 1.Aaberge, I. S., J. Eng, G. Lermark, and M. Lovik. 1995. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb. Pathog. 18:141-152. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, J. E., R. A. Lock, C. C. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, B., J. Dahmén, T. Freijd, H. Leffler, G. Magnusson, G. Noori, and C. Svanborg-Edén. 1983. Identification of a disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. A strong association between capsular type and mouse virulence among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., J. Horowitz, L. S. McDaniel, W. H. Benjamin, Jr., J. L. Claflin, C. L. Booker, G. Scott, and C. Forman. 1986. Genetic control of susceptibility to pneumococcal infection. Curr. Top. Microbiol. Immunol. 124:103-120. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., J. D. King, M. A. Gray, L. S. McDaniel, E. Swiatlo, and K. A. Benton. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14:858-867. [DOI] [PubMed] [Google Scholar]
- 9.Briles, D. E., G. S. Nabors, A. Brooks-Walter, J. C. Paton, and S. Hollingshead. 2001. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine 19:S87-S95. [DOI] [PubMed] [Google Scholar]
- 10.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles, D. E., R. C. Tart, H.-Y. Wu, B. A. Ralph, M. W. Russell, and L. S. McDaniel. 1996. Systemic and mucosal protective immunity to pneumococcal surface protein A. Ann. N. Y. Acad. Sci. 797:118-126. [DOI] [PubMed] [Google Scholar]
- 12.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaneda, E., A. L. Leal, O. Castillo, F. De La Hoz, M. C. Vela, M. Arango, H. Trujillo, A. Levy, M. E. Gama, M. Calle, M. L. Valencia, W. Parra, N. Agudelo, G. I. Mejia, S. Jaramillo, F. Montoya, H. Porras, A. Sanchez, D. Saa, J. L. Di Fabio, A. Homma, et al. 1997. Distribution of capsular types and antimicrobial susceptibility of invasive isolates of Streptococcus pneumoniae in Colombian children. Microb. Drug Resist. 3:147-152. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 46:1-24. [Google Scholar]
- 15.Cowan, M. J., A. J. Ammann, D. W. Wara, V. M. Howie, L. Schultz, N. Doyle, and M. Kaplan. 1978. Pneumococcal polysaccharide immunization in infants and children. Pediatrics 62:721-727. [PubMed] [Google Scholar]
- 16.Crain, M. J., W. D. Waltman II, J. S. Turner, J. Yother, D. F. Talkington, L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakansson, A., H. Roche, S. Mirza, L. S. McDaniel, A. Brooks-Walter, and D. E. Briles. 2001. Characterization of binding of human lactoferrin to pneumococcal surface protein A. Infect. Immun. 69:3372-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kertesz, D. A., J. L. Di Fabio, M. C. de Cunto Brandileone, E. Castaneda, G. Echaniz-Aviles, I. Heitmann, A. Homma, M. Hortal, M. Lovgren, R. O. Ruvinsky, J. A. Talbot, J. Weekes, and J. S. Spika. 1998. Invasive Streptococcus pneumoniae infection in Latin American children: results of the Pan American Health Organization Surveillance Study. Clin. Infect. Dis. 26:1355-1361. [DOI] [PubMed] [Google Scholar]
- 20.Koskela, M., M. Leinonen, V. M. Haiva, M. Timonen, and P. H. Makela. 1986. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr. Infect. Dis. J. 5:45-50. [DOI] [PubMed] [Google Scholar]
- 21.McDaniel, L. S., D. O. McDaniel, S. K. Hollingshead, and D. E. Briles. 1998. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect. Immun. 66:4748-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaniel, L. S., B. A. Ralph, D. O. McDaniel, and D. E. Briles. 1994. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17:323-337. [DOI] [PubMed] [Google Scholar]
- 23.McDaniel, L. S., G. Scott, J. F. Kearney, and D. E. Briles. 1984. Monoclonal antibodies against protease sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J. Exp. Med. 160:386-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel, L. S., J. S. Sheffield, P. Delucchi, and D. E. Briles. 1991. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect. Immun. 59:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogunniyi, A. D., M. C. Woodrow, J. T. Poolman, and J. C. Paton. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton, J. C., R. A. Lock, and D. J. Hansman. 1983. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 40:548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralph, B., D. E. Briles, and L. S. McDaniel. 1994. Cross-reactive protection eliciting epitopes of pneumococcal surface protein A. Ann. N. Y. Acad. Sci. 730:361-363. [DOI] [PubMed] [Google Scholar]
- 28.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsule serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche, H., A. Hakansson, S. K. Hollingshead, and D. E. Briles. 2003. Regions of PspA/EF3296 best able to elicit protection against Streptococcus pneumoniae in a murine infection model. Infect. Immun. 71:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro, E. D., A. T. Berg, R. Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 32.Tart, R. C., L. S. McDaniel, B. A. Ralph, and D. E. Briles. 1996. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J. Infect. Dis. 173:380-386. [DOI] [PubMed] [Google Scholar]
- 33.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 34.Vela Coral, M. C., N. Fonseca, E. Castaneda, J. L. Di Fabio, S. K. Hollingshead, and D. E. Briles. 2001. Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates recovered from Colombian children. Emerg. Infect. Dis. 7:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 36.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]