Abstract
Recent publications have demonstrated that the protease caspase-1 is responsible for the processing of pro-interleukin 18 (IL-18) into the active form. Studies on cell lines and murine macrophages have shown that the bacterial invasion factor SipB activates caspase-1, triggering cell death. Thus, we investigated the role of SipB in the activation and release of IL-18 in human alveolar macrophages (AM), which are the first line of defense against inhaled pathogens. Under steady-state conditions, AM are a more important source of IL-18 than are dendritic cells (DC) and monocytes. Cytokine production by AM and DC was compared after both types of cells had been infected with a virulent strain of Salmonella enterica serovar Typhimurium and an isogenic sipB mutant, which were used as an infection model. Infection with virulent Salmonella led to marked cell death with features of apoptosis while both intracellular activation and release of IL-18 were demonstrated. In contrast, the sipB mutant did not induce such cell death or the release of active IL-18. The specific caspase-1 inhibitor Ac-YVAD-CMK blocked the early IL-18 release in AM infected with the virulent strain. However, the type of Salmonella infection did not differentially regulate IL-18 gene expression. We concluded that the bacterial virulence factor SipB plays an essential posttranslational role in the intracellular activation of IL-18 and the release of the cytokine in human AM.
An efficient immune response against pathogenic microorganisms is a key to survival. Gamma interferon (IFN-γ) is a cytokine known to mediate cellular immune responses that are essential for protection against many intracellular pathogens (24). In 1995, Okamura and coworkers (27) discovered a new cytokine called IFN-γ-inducing factor, which induces T-cell activation. Although this cytokine, now called interleukin 18 (IL-18), has significant structural similarities to IL-1β (10) and binds to receptors from the IL-1 family (30), it displays a different activity. Like IL-12, IL-18 strongly stimulates T cells to produce IFN-γ. IL-18 gene expression seems to be controlled by two distinct promoters, one upstream of exon 1 that is activated by lipopolysaccharide (LPS), and one upstream of exon 2 that appears to be constitutive (32). Like IL-1 β, IL-18 is synthesized as an inactive precursor lacking the typical signal peptide required for its secretion. The 24-kDa precursor protein is proteolytically processed into the 18-kDa mature active form by the IL-1β-converting enzyme, also called caspase-1 (12, 13, 27).
IL-18 has been shown to have a protective role in pulmonary infections, especially against intracellular pathogens such as Mycobacterium tuberculosis (33). In the lung, the main cells involved in initiation of immunity are alveolar macrophages (AM) and dendritic cells (DC). AM are the predominant cells in the alveoli (25), and they are able to trigger inflammatory or antiinflammatory cascades through their production of a wide array of cytokines. Immature DC (iDC) are found just below the epithelial cells of the airways, in the loose connective tissue surrounding the vessels and in the pleura. Although DC are considered to be the most efficient antigen-presenting cells (APC), the role of abundant AM in the lung in the induction of innate and specific immunity should not be underestimated. Although Salmonella enterica serovar Typhimurium is not known to infect the lung, it has been proposed as an intracellular pathogen model with which to study mucosal immunity in both the digestive and respiratory tracts and also as a vaccine vector against tuberculosis (7, 16). During infection, Salmonella genes are expressed, leading to the secretion of virulence factors such as SipB and SipC that activate the infection of phagocytic and nonphagocytic cells (14). The sipB gene is located in Salmonella pathogenicity island 1, a genome segment that encodes a type III secretion system. This protein was shown to bind to and activate caspase-1, thereby inducing cell death (15). These experiments were done with murine and cattle macrophages, monocyte (Mo)-derived macrophages, and macrophage cell lines (15, 21, 29) but not with human AM. In the present study, we investigated the role played by Salmonella virulence factor SipB in IL-18 activation and release by human AM. We also examined the role of caspase-1 in this process. Our results showed that SipB plays a posttranslational key role in both the activation and the release of IL-18 by AM. The involvement of caspase-1 in this process occurs early, while other mechanisms may be involved in later events leading to cell death.
MATERIALS AND METHODS
Cells, bacterial strains, and reagents.
AM were obtained by bronchoalveolar lavage (BAL) of lung specimens from patients with pulmonary cell carcinoma. BAL with sterile 0.9% NaCl was performed immediately after surgery in segments that were tumor free. At least 150 ml was injected into the major bronchial airways, and the cell-rich fluid was collected by aspiration. The cells obtained were >85% macrophages, as determined by differential counting of Wright-stained cytocentrifuge preparations. Cells were centrifuged and resuspended in RPMI 1640 medium (Life Technologies, Inc., Rockville, Md.) supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 U of streptomycin per ml, referred to as complete culture medium (CCM). The red blood cells were removed by centrifugation in Ficoll-Paque (research grade; AP Biotech, Uppsala, Sweden). Macrophages were washed three times with Hanks' balanced salt solution without Ca2+ and Mg2+ (HBSS) and plated in 24-well culture plates at a concentration of 5 × 105 cells/ml in CCM. The adherent AM were obtained after 24 h of incubation at 37°C in 5% CO2. Before the experiments, the cells were washed three times with HBSS and the adherent cells were cultured with 1 ml of CCM. In some experiments, AM were treated with a caspase-1-specific inhibitor (Ac-YVAD-CMK; Calbiochem, Nottingham, United Kingdom) at a concentration of 50 μM for 1 h before bacterial infection.
DC were generated from peripheral blood mononuclear cells of healthy human donors by the method originally described by Sallusto and Lanzavecchia (28). These peripheral blood mononuclear cells were isolated by Ficoll-Paque density gradient centrifugation of buffy coats as described previously (5). After 1 h of adherence, the culture dishes were rinsed with HBSS and adherent cells were incubated overnight in CCM. Loosely adherent Mo, which were characterized by high expression of CD14 (more than 80%) and low expression of CD83 and CD86 (less than 5%) (5), were recovered after three rinses in HBSS. iDC were obtained from Mo after the cells had been cultured for 7 days in CCM with granulocyte-macrophage colony-stimulating factor (10 ng/ml; Immugenex Corp., Los Angeles, Calif.) and IL-4 (10 ng/ml; R&D Systems, Minneapolis, Minn.). Maturation of iDC was induced by addition of LPS purified from Salmonella (1 μg/ml). iDC and mature DC (mDC) were analyzed for expression of CD83 and CD86, which were upregulated in mDC, and CD14, which was downregulated in both cell types (5). Before the experiments, Mo and DC were washed three times, resuspended in CCM, seeded in wells at 2 × 105 cells per well in 1 ml of medium, and incubated in a 5% CO2 atmosphere.
S. enterica serovar Typhimurium strains and infection.
The strains of bacteria used in this study were the virulent strain of S. enterica serovar Typhimurium C53 and a mutant that lacks the sipB gene and therefore does not express a functional SipB protein. For infection, Salmonella strains were grown overnight without agitation in Luria broth (LB) medium supplemented with 300 mM KCl and 0.5% KNO3. On the day of the assay, bacteria were subcultured into fresh LB plus KCl and KNO3 and grown for 2 to 3 h at 37°C. Before being infected with bacteria, AM and DC were washed and cultured in RPMI medium free of antibiotic and supplemented with 0.1% fetal calf serum. At this stage, 100 μl of a bacterial suspension in prewarmed CCM, with a multiplicity of infection (MOI) of 25 bacteria/cell, was added to the cell culture. After 30 min of infection, gentamicin was added at a final concentration of 60 μg/ml to kill extracellular bacteria.
For evaluation of the number of infected cells, the AM were recovered in trypsin-EDTA 3 or 24 h after infection and the cell suspension was diluted in RPMI medium before plating on LB agar. One CFU was considered to correspond to one infected cell. Intracellular survival of the different strains was evaluated by the bacterial growth index, defined as the number of cells still containing live bacteria at 24 h after exposure, divided by the number of cells containing live bacteria at 3 h.
Cytotoxicity assays.
Supernatants of infected macrophages were evaluated for the presence of the cytoplasmic enzyme lactate dehydrogenase (LDH) with the Cytotox96 kit (Promega). The percentage of cytotoxicity was calculated with the formula 100 × [(experimental release − spontaneous release)/(total release − spontaneous release)].
Reverse transcription-PCR (RT-PCR).
Total cellular RNA was isolated by homogenizing the AM in Trizol (Life Technologies, Basel, Switzerland). Total RNA was reverse transcribed and amplified in one step, in accordance with the manufacturer's recommended procedure (Access RT-PCR; Promega, Madison, Wis.). Briefly, 100 ng of RNA was added to a reaction mixture containing 0.1 U of avian myeloblastosis virus reverse transcriptase per μl, 10 mM deoxynucleoside triphosphate, 0.1 U of Tfl DNA polymerase per μl, and 1.5 mM MgSO4. We also added the following oligonucleotides: IL-18 sense primer (5′-GCTTGAATCTAAATTATCAGTC-3′), IL-18 antisense primer (5′-TATTCTACGTTAAACTTAGAAC-3′), GAPDH (glyceraldehyde-3-phosphate dehydrogenase) sense primer (5′-GGACCTGACCTGCCGTCTAG-3′), and GAPDH antisense primer (5′GATGTCGTTFTCCCACCACC-3′). The reaction mixture was subjected to RT at 48°C for 45 min, and the products were amplified by PCR under the following conditions: 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min for 25 cycles (IL-18) and 94°C for 1 min, 59°C for 1 min, and 72°C for 1 min for 30 cycles (GAPDH). The resulting PCR products were separated by electrophoresis on 2% agarose gel, visualized by ethidium bromide staining, and analyzed by photography. To standardize the culture conditions of the various cell types, 1 h after LPS or bacterial infection was considered to be the earliest reasonable time point at which to compare baseline IL-18 levels.
IL-18 measurements.
For measurement of IL-18 contents, cell suspensions were collected 24 h after Salmonella infection. The cells and supernatants were separated by centrifugation and stored at −70°C. The cells were lysed by incubation for 30 min at 37°C in Triton X-100 (0.06%), and the volume was adjusted to 1 ml with HBSS. The IL-18 contents in the lysed cells and supernatant were measured with an ELISA kit with a polyclonal antibody (Diaclone, Besançon, France).
Immunoprecipitation.
Supernatant IL-18 was immunoprecipitated with 0.5 μg of anti-mIL-18 per ml by incubation for 2 h at 4°C and subsequent binding to protein G-Sepharose (Sigma, St. Louis, Mo.) for 2 h at 4°C. Before being subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the samples were boiled in 10 μl of sample buffer for 5 min.
Western analysis.
Total cell-bound IL-18 and IL-18 immunoprecipitated from the supernatant of 5 × 105 cells/ml were analyzed by Western blotting. Samples were diluted in a reducing sample buffer and resolved by SDS-12% PAGE. After transfer to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany), IL-18 protein was detected with a monoclonal antibody raised against human IL-18 (R&D Systems Europe Ltd., Abingdon, United Kingdom). A secondary polyclonal antibody coupled to horseradish peroxidase (PharMingen, Hamburg, Germany) and the ECL-plus detection system (Amersham-Pharmacia, Freiburg, Germany) were used to visualize the IL-18.
Processing of cells for light and electron microscopy.
The infected human AM were washed in phosphate-buffered saline (Boehringer, Mannheim, Germany) at 4°C, fixed in a 2.5% phosphate-buffered glutaraldehyde solution, postfixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer, and contrasted in 0.5% uranyl acetate in 0.05 M maleate buffer. This was followed by dehydration in a graded ethanol series (70, 80, 96, and 100%) and gradual replacement of ethanol with propylene oxide before infiltration and embedding in epoxy resin.
Ultrathin and semithin sections were cut with a Reichert Austria ultramicrotome. The semithin sections were mounted on glass slides and stained with toluidine blue. The ultrathin sections were picked on 200-mesh carbon-coated copper grids, stained with uranyl acetate, counterstained with lead citrate, and observed with a Philips 300 transmission electron microscope at an accelerating voltage of 60 kV.
Statistical analysis.
Data were compared by using Student's t test. Differences were considered significant when P was <0.05. Results are expressed as the mean ± the standard error of the mean (SEM).
RESULTS
IL-18 content is greater in AM than in mDC, iDC, or Mo.
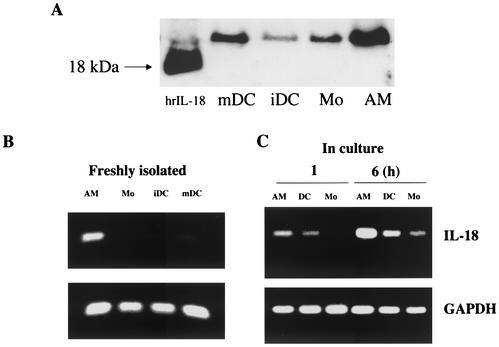
To analyze the extent of IL-18 production in nonactivated APC, the amounts of cell-bound IL-18 in freshly isolated AM obtained by BAL were compared to those found in Mo-derived iDC and in freshly isolated Mo by Western blotting (Fig. 1A). Compared to the other APC, AM contained considerably more of the inactive precursor form of IL-18 (which migrates as a 24-kDa band). The steady state of IL-18 mRNA was then compared in freshly isolated AM, Mo, iDC, and mDC (Fig. 1B), and IL-18 expression was then also analyzed in AM, Mo, and iDC at 1 and 6 h by RT-PCR (Fig. 1C). Little expression was observed after 1 h; however, the expression of IL-18 mRNA peaked after 6 h in all of the cells studied. Furthermore, IL-18 mRNA expression was higher in AM than in the other APC. These results suggest that IL-18 mRNA expression is high in AM. However, culture conditions such as plastic adhesion or the serum medium used seem to be sufficient to trigger IL-18 expression.
FIG. 1.
IL-18 content is greater in AM than in other APC. (A) Constitutive IL-18 contents of lysate from 105 freshly isolated AM, iDC, mDC, and Mo were analyzed by Western blotting. The proteins were resolved by SDS-PAGE under reducing conditions, electrotransferred onto a nitrocellulose membrane, and hybridized with an anti-IL-18 monoclonal antibody. hrIL-1, human recombinant IL-18. (B and C) One hundred nanograms of total RNA was reverse transcribed and subjected to PCR to quantify the expression of IL-18 mRNA in freshly isolated AM, Mo, iDC, and mDC (B) and in AM, iDC, and Mo cultured for 1 and 6 h (C). The results shown are from one representative of three independent experiments with similar profiles.
IL-18 production by AM and DC appears to be dependent on S. enterica serovar Typhimurium virulence factors.
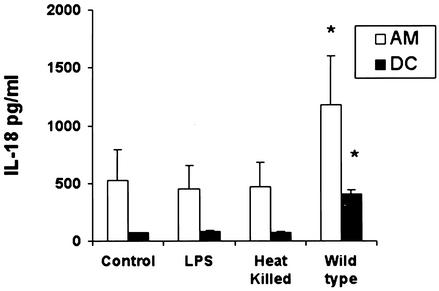
To gain insight into the interaction between a facultatively intracellular pathogen and human APC, AM and iDC were infected with virulent S. enterica serovar Typhimurium, heat-killed bacteria, or LPS. Exposure of the cells to LPS or heat-killed bacteria did not increase IL-18 secretion significantly at 24 h, whereas infection with virulent S. enterica serovar Typhimurium led to a threefold increase in IL-18 secretion by AM compared to that by DC (Fig. 2). This suggests that bacterial virulence factors are involved in IL-18 secretion. In view of its stimulatory role in caspase-1 activation, we focused our attention on the Salmonella invasion protein SipB.
FIG. 2.
S. enterica serovar Typhimurium virulence factors stimulate IL-18 release. Secretion of IL-18 by AM and iDC during the 24-h period after infection with wild-type S. enterica serovar Typhimurium (MOI, 25 bacteria per cell), treatment with heat-killed (65°C for 15 min) wild-type bacteria, or stimulation with LPS purified from Salmonella (1 μg/ml) was measured by ELISA. Results are given for an MOI of 5 × 105 cells/ml. Results are expressed as the mean of five independent experiments ± SEM. *, P < 0.05 compared to the control condition (cells alone).
IL-18 release is reduced by a Salmonella sipB mutant.
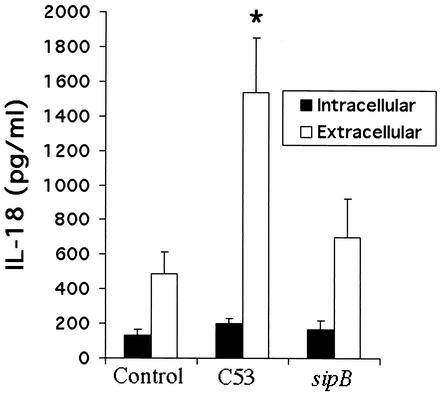
The results described above suggest that bacterial virulence factors contribute to the release of IL-18 and that AM are a major source of this cytokine in the lung. Previous work has shown that the expression and secretion of invasion proteins encoded by Salmonella pathogenicity island 1, such as SipB, are essential for Salmonella infection (15). SipB has been shown in murine macrophages to activate caspase-1 (15), which cleaves IL-18 into its mature form. To demonstrate that SipB is involved in the release of active IL-18 by AM, cells were infected with two Salmonella strains: the virulent C53 strain and the isogenic sipB mutant. Figure 3 shows intracellular and extracellular production of IL-18 measured by ELISA. Intracellular IL-18 content did not seem to be enhanced by the expression of sipB. In contrast, cytokine release was considerably higher with virulent strain C53 than with the isogenic sipB mutant (P < 0.05) at 24 h after infection. This suggests that the SipB protein markedly stimulates the production and immediate release of IL-18 in AM.
FIG. 3.
IL-18 release by AM is SipB dependent. Intracellular and extracellular IL-18 production by AM during the 24 h after infection with two strains of S. enterica serovar Typhimurium, virulent strain C53 and the isogenic sipB mutant, was measured by ELISA. Results are expressed as the mean of three independent experiments ± SEM. *, P < 0.05 compared to the control condition (cells alone).
S. enterica serovar Typhimurium C53 and the sipB mutant efficiently survive in AM.
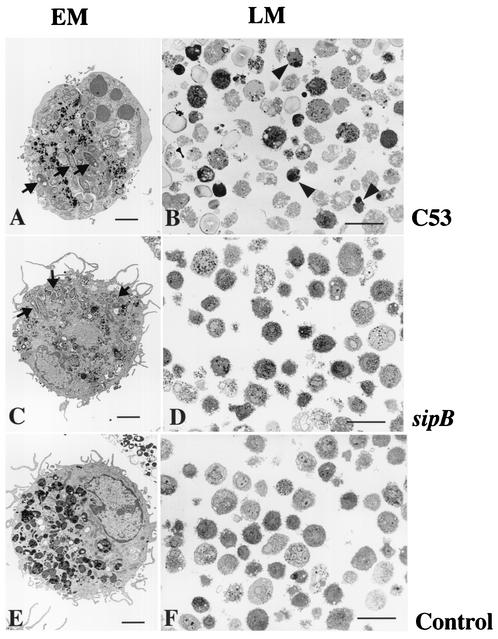
To evaluate the level of bacterial infection, we used morphological characterization of human AM infected with S. enterica serovar Typhimurium C53 and the sipB mutant at 24 h (Fig. 4). These observations revealed that AM were efficiently infected by both the virulent and mutant Salmonella strains. A gentamicin protection assay at 3 h showed that 57.87% ± 27.8% (n = 3) of the macrophages were infected by S. enterica serovar Typhimurium C53, whereas 24.4% ± 12.10% (n = 3) were infected by the Salmonella sipB mutant. However, in both cases, salmonellae survived within the cells after 24 h (bacterial growth indexes, 2 ± 0.3 and 0.76 ± 0.08, respectively), demonstrating the greater virulence of C53 and its ability to multiply in infected AM. Infection by strain C53 resulted in cell death with features of apoptosis (Fig. 4A and B), as demonstrated by chromatin condensation and karyohexis (6, 34). We also observed higher LDH concentrations in the supernatants of C53-infected cells after 24 h, with a cytotoxicity index of 86.92% ± 0.14%, than in those of uninfected cells (cytotoxicity index, 24.4% ± 4.7%). However, the strain carrying a mutation in the sipB gene (Fig. 4C and D) infected cells without inducing cell death, as the LDH concentrations were lower than that of the control (−15% ± 7.3%). This was confirmed by electron microscopy which showed that AM morphology was similar to that of the control cells (Fig. 4E and F). These results corroborate previous studies in which virulence-defective mutants were found to be noncytotoxic (4, 19) and provide evidence for the role of SipB in the induction of cell death in human AM.
FIG. 4.
Salmonella strain C53 and the sipB mutant efficiently infect AM. Light microscopy (LM) and electron microscopy (EM) of AM 24 h after Salmonella infection. (A and B) AM infected with strain C53 displaying chromatin condensation. (C and D) AM infected with the sipB mutant strain displaying intracellular localization of Salmonella and intact nuclear chromatin. (E and F) Control cells incubated without infection. N, nucleus; arrows, intracellular salmonellae; arrowheads, condensation of nuclear chromatin. Scale bars: panels B, D, and F, 40 μm; panels A, C, and E, 3 μm.
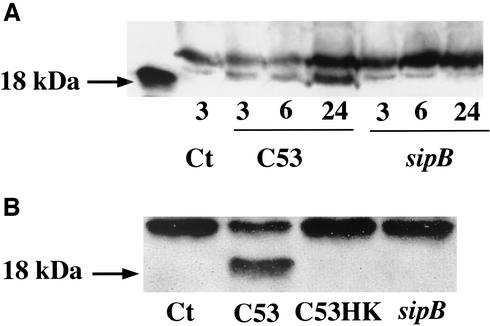
Intracellularly active IL-18 content (18 kDa) increases progressively over time following Salmonella strain C53 infection.
In the experiments described in the previous section, we showed that Salmonella strain C53 increased the production and immediate release of IL-18 in AM, but the ELISA method could not measure the amount of active IL-18. To determine the presence of the active form, we performed a time course analysis of intracellular IL-18 in AM infected with Salmonella strain C53 and the sipB mutant by Western blotting (Fig. 5A). The mature form of IL-18 appeared to increase gradually over time during the 24 h of infection, whereas IL-18 in AM infected with the sipB mutant appeared to decrease over time until it disappeared at 24 h. When we examined the IL-18 content in the AM supernatant collected 24 h after bacterial infection, active IL-18 was detected exclusively in the medium after Salmonella strain C53 infection (Fig. 5B). This suggests that the activation process occurs initially at the intracellular level and that mature IL-18 is then rapidly released into the extracellular medium.
FIG. 5.
SipB-induced intracellular activation and release of IL-18. (A) Western blot analysis of intracellular IL-18 in AM (2.5 × 106 cells) analyzed at 3, 6, and 24 h after infection with S. enterica serovar Typhimurium strain C53 or the sipB mutant. Samples were resolved by SDS-PAGE, transferred to nitrocellulose, and hybridized with an anti-IL-18 monoclonal antibody. (B) Extracellular IL-18 from AM 24 h after infection with S. enterica serovar Typhimurium strain C53 and the sipB mutant was analyzed by Western blotting. Supernatants from 2.5 × 106 AM cells were immunoprecipitated with 0.5 μg of monoclonal anti-IL-18 and protein G-Sepharose per ml (as described in Materials and Methods). Immunocomplexes were resolved by SDS-12% PAGE under reducing conditions and blotted with an anti IL-18 monoclonal antibody. Ct, cells alone; C53, wild-type sipB; sipB, mutant lacking the protein that activates caspase-1; C53HK, heat-killed bacteria.
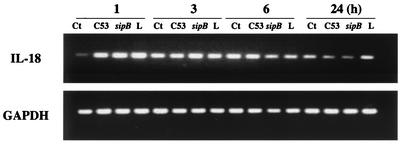
IL-18 gene expression is not specifically modulated by Salmonella infection.
IL-18 mRNA expression in AM was analyzed during the 24-h period after infection with S. enterica serovar Typhimurium virulent strain C53 or the sipB mutant and compared to LPS stimulation. Under the culture conditions used, little or no constitutive IL-18 expression was observed at 1 h but its expression was induced at 3 h (Fig. 6). Furthermore, the accumulation of IL-18 mRNA in cells stimulated by Salmonella or LPS was already high after 1 h, peaked at 3 h, and decreased slightly after 6 h to a barely detectable level at 24 h. The results were similar when fewer PCR cycles were used (data not shown). These results suggest that IL-18 mRNA expression is modulated at an early stage by bacterial infection or LPS stimulation; however, because the control conditions already induced accumulation after 3 h, Salmonella infection or LPS stimulation does not appear to be able to modulate further IL-18 mRNA expression beyond the first hour. This poor modulation of IL-18 expression highlights the important role played by posttranslational events, particularly SipB, which activates caspase-1 and facilitates the release of IL-18.
FIG. 6.
IL-18 gene expression in AM is not modulated by S. enterica serovar Typhimurium infection. AM were cultured at 5 × 105 cells/ml with LPS purified from Salmonella (1 μg/ml), with virulent Salmonella strain C53, or with the sipB mutant for 1, 3, 6, or 24 h, and IL-18 mRNA accumulation was analyzed by RT-PCR. Results are representative of three separate experiments. Ct, cells alone; C53, wild-type sipB; sipB, mutant lacking the protein that activates caspase-1; L, lipopolysaccharide.
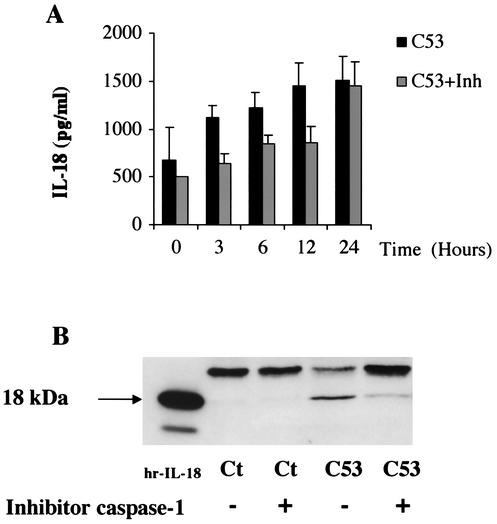
Caspase-1 inhibitor reduces the release of mature IL-18 induced by Salmonella strain C53.
To establish a correlation between IL-18 secretion and its caspase-1-mediated activation, we examined the effect of the caspase-1 inhibitor Ac-YVAD-CMK on the secretion of IL-18 by Salmonella-infected AM. The caspase-1 inhibitor was added to cell cultures 1 h before Salmonella strain C53 infection, and IL-18 production was measured 0, 3, 6, 12, and 24 h after infection. Inhibition of IL-18 secretion by the caspase-1 inhibitor was observed in the cell supernatants (Fig. 7A), with maximum inhibition at 3 h (i.e., 44% of the inhibition reached at the same time point in infected cells without the caspase-1 inhibitor). No inhibitory effect was observed at 24 h. Western blot analysis of the IL-18 released from AM infected with the virulent Salmonella strains, with or without the caspase-1 inhibitor (Fig. 7B), showed that 24 h after bacterial infection, the caspase-1 inhibitor prevented the processing of IL-18, thereby leading to accumulation of pro-IL-18. At this point, we consider it relevant to mention that cell death, analyzed by LDH release in the supernatants or enzyme immunoassay determination of cytoplasmic histone-associated DNA fragments during the 24-h period after Salmonella strain C53 infection, was not inhibited by caspase-1 (data not shown). These results suggest that the protein SipB act as a posttranslational regulator, allowing pro-IL-18 to accumulate when the processing of IL-18 is blocked. Therefore, caspase-1 appears to be largely involved in the processing and release of IL-18 in the early stages of Salmonella infection, without being the only biological cascade likely implicated in the AM cell death process.
FIG. 7.
Caspase-1-mediated IL-18 activation and early release. (A) Cells (5 × 105) were incubated with the caspase-1 inhibitor (Inh) Ac-YVAD-CMK (50 μM) for 1 h before infection with virulent Salmonella strain C53. Cells were harvested at different times following infection (3, 6, 12, and 24 h), and IL-18 was measured by ELISA. Results are expressed as the mean of three separates experiments ± SEM. (B) Western blot analysis of released IL-18 at 24 h after bacterial infection with or without Ac-YVAD-CMK. hr-IL-18, human recombinant IL-18; Ct, cells alone; C53, wild-type sipB.
DISCUSSION
In the distal airways of the lung, AM represent the first line of defense (26). Their ability to enhance acquired immunity by the release of active IL-18 is still poorly understood. In the present study, we decided to use S. enterica serovar Typhimurium as an intracellular pathogen model with which to examine the induction of innate and acquired immunity in human AM. This bacterium offers several advantages in the study of lung immunity, including genetic flexibility, a well-characterized virulence pathway, the availability of attenuated mutants, and absence from AM obtained from human subjects. In addition, Salmonella is widely considered as a potential vector for vaccines to be administered via the nasal route. Although salmonellae are not respiratory pathogens, homologous virulence factors have been recovered from a wide variety of human pathogens, such as chlamydiae (11, 20).
In 1996, Chen and coworkers (4) demonstrated that Salmonella spp. exert a cytotoxic effect on cultured murine macrophages via a mechanism induced by the bacteria. It has been shown that macrophage cell death is induced by at least two pathways (15, 18), one of which involves the activation of caspase-1. As caspase-1 is also essential for the intracellular activation of the proinflammatory cytokine IL-18, we decided to investigate the role of the bacterial activating factor of caspase-1, SipB, in the production and release of IL-18 by human AM. Our results show that AM are a more important reservoir of IL-18 than are DC and Mo. Furthermore, AM can release mature IL-18 when exposed to virulent Salmonella. In contrast, heat-killed bacteria, despite membrane-bound LPS, and SipB-deficient Salmonella both lack the capacity to induce IL-18 activation and release.
In healthy persons, AM are efficient phagocytic cells and poor APC (31). This lack of antigen-presenting capacity has been related to the absence of the costimulatory molecule CD80 or CD86 on their surface (3). The ability of AM to release cytokines, compared to that of Mo or DC, is not yet fully understood, although it is well known that they have a greater ability to produce tumor necrosis factor alpha and a lesser ability to release IL-10 (1). Previous studies have also shown that AM produce markedly less IL-1 than do Mo (9). We show that AM have a much higher constitutive IL-18 content than do either Mo or DC. This could be related to enhanced expression of IL-18 mRNA in the cell under various stimuli, including the culture conditions used, but also to greater accumulation of the unprocessed form. This is of considerable importance, as it was later shown that AM are also a better source of IL-12 than are Mo (17). This strongly suggests that if AM are adequately activated, they may release IL-18 in the alveolar milieu, where the cytokine could play an important role in amplifying local immunity, favoring either Th1 immunity in the presence of IL-12 or a Th2 response (24, 35).
When we stimulated AM and DC with virulent Salmonella strains, the release of IL-18 by AM was increased severalfold. Furthermore, this release was greater when the cells were exposed to live strains instead of heat-killed bacteria or LPS, suggesting that virulence factors produced during infection are involved in the release of IL-18. By contrast, in a virulent defective strain, the attenuation resulting from deletion of the sipB gene led to a decrease in IL-18 secretion (Fig. 3). Therefore, in order to differentiate the role of Salmonella membrane-bound structures and the potential impact of sipB on IL-18 production and release, we compared virulent strain C53 and the defective sipB mutant, which lacks the activator of caspase-1. Although slight differences between the bacterial infections were observed at 3 h, the growth index demonstrated that the Salmonella sipB mutant was able to survive in macrophages, and the results observed were caused by the deletion of sipB. The results showed that the intracellular content of IL-18 was not affected by sipB expression even though the cells were efficiently infected, whereas the infection by virulent strain C53 stimulated the release of IL-18.
A Western blot analysis was performed to differentiate the production of mature and immature forms of IL-18. The active form of IL-18 was detected intracellularly and extracellularly after infection with virulent Salmonella, suggesting that the cleavage of immature into mature IL-18 occurs, at least in part, intracellularly before its release (Fig. 5A and B). In contrast, only the inactive form was observed after exposure of AM to the Salmonella sipB mutant and heat-killed bacteria. These results suggest that the release of active IL-18 is tightly controlled by SipB, while inactive IL-18 can be found outside the cell even in the absence of SipB-mediated caspase-1 activation or when a caspase-1 inhibitor is added. This observation corroborates the data of Mehta and coworkers (22), who showed that precursor cleavage is not required for release of the cytokine. To analyze the extent of the involvement of caspase-1 during Salmonella infection, we used a specific caspase-1 inhibitor and measured the extracellular IL-18. The effect of the inhibitor on the release of IL-18 decreased after 12 h. This confirms that caspase-1 is a key factor in IL-18 activation and early release by AM following their infection with Salmonella expressing sipB, but other biologic cascades are likely to be involved since the Ac-YVAD-CMK inhibitor did not prevent AM cell death or delayed IL-18 release after 24 h.
The experiments on the mouse model and cell lines demonstrated that the virulence factor SipB is required for Salmonella-mediated macrophage cell death through caspase-1 (15). Also, there is evidence of a caspase-1-independent mechanism that leads to cell death (2, 18, 23). Although it is difficult to dissociate caspase-1 activation and IL-18 release from the capacity of Salmonella to evoke cell death, it is clear that both mechanisms have enzymatic activities in common and a clear correlation was found recently between cell death induced by Salmonella and the capacity of DC to release IL-18 (8). In our experiments, even though IL-18 activation was inhibited by Ac-YVAD-CMK, macrophage cell death could not be prevented by use of the inhibitor after Salmonella strain C53 infection. This implies that AM cell death could be implicated, to some extent, in the release of IL-18, not necessarily as a unique consequence of caspase-1 activation.
A particularly important aspect of IL-18 regulation involves the release pathways. Mehta and coworkers (22) suggested that the release of IL-18 is stimulated by exogenous ATP and requires priming by LPS. This release was shown to occur through a purinoreceptor, P2X7, which upon the sustained action of stimulators such as ATP, leads to the formation of a nonselective pore that is permeable to low-molecular-weight solutes. We used oxidized ATP, an antagonist of ATP, to determine the extent of ATP's involvement in the release of IL-18 after Salmonella infection. We observed that, under such conditions, the activation and release of IL-18 were inhibited (data not shown). However, the extent of SipB's role as an activator like ATP, or in the stimulation of ATP following bacterial infection, is not understood. However, it should be investigated as an alternative pathway for the release of IL-18.
Our studies with live intracellular pathogens underline the importance of their genetic backgrounds. Our observations will help optimize live-vaccine vectors and show their superiority over the delivery of peptides combined with standard adjuvants. The adjuvant effect of live vectors thus appears to be dependent not only on the type of APC involved or on the surface interactions between the APC and the pathogen but also on the enzymatic activity triggered by intracellular pathogens, such as the virulence factor SipB secreted by Salmonella. These observations will have a major impact on our understanding of the modulation of mucosal or systemic immunity by intracellular pathogens and on the development of potential new vaccine strategies using S. enterica serovar Typhimurium.
Acknowledgments
We are grateful to Izui Shozo for critical review of the manuscript. We also express our sincere thanks to Silvia Joudier and Christine Devenoges for excellent technical assistance and to Ursula Gerber for expert technical assistance in electron microscopy.
This work was supported by the European Respiratory Society and by Swiss National Science Foundation grants 31-63.660.00 and 037-061164/1.
Editor: F. C. Fang
REFERENCES
- 1.Boehringer, N., G. Hagens, F. Songeon, P. Isler, and L. P. Nicod. 1999. Differential regulation of tumor necrosis factor-alpha (TNF-α) and interleukin-10 (IL-10) secretion by protein kinase and phosphatase inhibitors in human alveolar macrophages. Eur. Cytokine Netw. 10:211-218. [PubMed] [Google Scholar]
- 2.Brennan, M. A., and B. T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31-34. [DOI] [PubMed] [Google Scholar]
- 3.Chelen, C. J., Y. Fang, G. J. Freeman, H. Secrist, J. D. Marshall, P. T. Hwang, L. R. Frankel, R. H. DeKruyff, and D. T. Umetsu. 1995. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J. Clin. Investig. 95:1415-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L. M., K. Kaniga, and J. E. Galan. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101-1115. [DOI] [PubMed] [Google Scholar]
- 5.Cochand, L., P. Isler, F. Songeon, and L. P. Nicod. 1999. Human lung dendritic cells have an immature phenotype with efficient mannose receptors. Am. J. Respir. Cell Mol. Biol. 21:547-554. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, G. M., X. M. Sun, R. T. Snowden, D. Dinsdale, and D. N. Skilleter. 1992. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem. J. 286:331-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreher, D., M. Kok, L. Cochand, S. G. Kiama, P. Gehr, J. C. Pechere, and L. P. Nicod. 2001. Genetic background of attenuated Salmonella typhimurium has profound influence on infection and cytokine patterns in human dendritic cells. J. Leukoc. Biol. 69:583-589. [PubMed] [Google Scholar]
- 8.Dreher, D., M. Kok, C. Obregon, S. G. Kiama, P. Gehr, and L. P. Nicod. 2002. Salmonella virulence factor SipB induces activation and release of IL-18 in human dendritic cells. J. Leukoc. Biol. 72:743-751. [PubMed] [Google Scholar]
- 9.Elias, J. A., A. D. Schreiber, K. Gustilo, P. Chien, M. D. Rossman, P. J. Lammie, and R. P. Daniele. 1985. Differential interleukin 1 elaboration by unfractionated and density fractionated human alveolar macrophages and blood monocytes: relationship to cell maturity. J. Immunol. 135:3198-3204. [PubMed] [Google Scholar]
- 10.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1β: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 19:1-11. [DOI] [PubMed] [Google Scholar]
- 11.Fields, K. A., and T. Hackstadt. 2000. Evidence for the secretion of Chlamydia trachomatis CopN by a type III secretion mechanism. Mol. Microbiol. 38:1048-1060. [DOI] [PubMed] [Google Scholar]
- 12.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, W. Wong, R. Kamen, D. Tracey, and H. Allen. 1997. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386:619-623. [DOI] [PubMed] [Google Scholar]
- 13.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding, D. J. Livingston, and S. Su. 1997. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 14.Hayward, R. D., E. J. McGhie, and V. Koronakis. 2000. Membrane fusion activity of purified SipB, a Salmonella surface protein essential for mammalian cell invasion. Mol. Microbiol. 37:727-739. [DOI] [PubMed] [Google Scholar]
- 15.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins, S., J. P. Kraehenbuhl, F. Schodel, A. Potts, D. Peterson, P. de Grandi, and D. Nardelli-Haefliger. 1995. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 63:3279-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isler, P., B. G. de Rochemonteix, F. Songeon, N. Boehringer, and L. P. Nicod. 1999. Interleukin-12 production by human alveolar macrophages is controlled by the autocrine production of interleukin-10. Am. J. Respir. Cell Mol. Biol. 20:270-278. [DOI] [PubMed] [Google Scholar]
- 18.Jesenberger, V., K. J. Procyk, J. Yuan, S. Reipert, and M. Baccarini. 2000. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 192:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindgren, S. W., I. Stojiljkovic, and F. Heffron. 1996. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu, H., C. Shen, and R. C. Brunham. 2000. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J. Immunol. 165:1463-1469. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg, U., U. Vinatzer, D. Berdnik, A. von Gabain, and M. Baccarini. 1999. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J. Bacteriol. 181:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta, V. B., J. Hart, and M. D. Wewers. 2001. ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J. Biol. Chem. 276:3820-3826. [DOI] [PubMed] [Google Scholar]
- 23.Monack, D. M., W. W. Navarre, and S. Falkow. 2001. Salmonella-induced macrophage death: the role of caspase-1 in death and inflammation. Microbes Infect. 3:1201-1212. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both th1 and th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 25.Nicod, L. P. 1997. Function of human lung dendritic cells, p. 311-334. In M. F. Lipscomb and S. W. Russell (ed.), Lung macrophages and dendritic cells in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 26.Nicod, L. P., L. Cochand, and D. Dreher. 2000. Antigen presentation in the lung: dendritic cells and macrophages. Sarcoidosis Vasc. Diffuse Lung Dis. 17:246-255. [PubMed] [Google Scholar]
- 27.Okamura, H., H. Tsutsui, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin-4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos, R. L., R. M. Tsolis, A. J. Baumler, R. Smith III, and L. G. Adams. 2001. Salmonella enterica serovar Typhimurium induces cell death in bovine monocyte-derived macrophages by early SipB-dependent and delayed SipB-independent mechanisms. Infect. Immun. 69:2293-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomassen, E., T. A. Bird, B. R. Renshaw, M. K. Kennedy, and J. E. Sims. 1998. Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1. J. Interferon Cytokine Res. 18:1077-1088. [DOI] [PubMed] [Google Scholar]
- 31.Toews, G. B., W. C. Vial, M. M. Dunn, P. Guzzetta, G. Nunez, P. Stastny, and M. F. Lipscomb. 1984. The accessory cell function of human alveolar macrophages in specific T cell proliferation. J. Immunol. 132:181-186. [PubMed] [Google Scholar]
- 32.Tone, M, S. A. Thompson, Y. Tone, P. J. Fairchild, and H. Waldmann. 1997. Regulation of IL-18 (IFN-γ-inducing factor) gene expression. J. Immunol. 159:6156-6163. [PubMed] [Google Scholar]
- 33.Vankayalapati, R., B. Wizel, S. E. Weis, B. Samten, W. M. Girard, and P. F. Barnes. 2000. Production of interleukin-18 in human tuberculosis. J. Infect. Dis. 182:234-239. [DOI] [PubMed] [Google Scholar]
- 34.Wyllie, A. H., J. F. Kerr, and A. R. Currie. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251-306. [DOI] [PubMed] [Google Scholar]
- 35.Xu, D., V. Trajkovic, D. Hunter, B. P. Leung, K. Schulz, J. A. Gracie, I. B. McInnes, and F. Y. Liew. 2000. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur. J. Immunol. 30:3147-3156. [DOI] [PubMed] [Google Scholar]