Abstract
The roles of interleukin-4 (IL-4) and IL-13 in the regulation of immunity to Leishmania donovani infection are still poorly understood. Here we show that the increased parasite load observed in IL-4−/− and IL-4 receptor α−/− mice correlates with retarded granuloma maturation and antileishmanial activity and that the increased parasite load observed in IL-4 receptor α−/− mice correlates with increased NOS2 expression and decreased serum gamma interferon levels. IL-4 and IL-13 appear to play little role in regulating collagen deposition in L. donovani-induced granulomas.
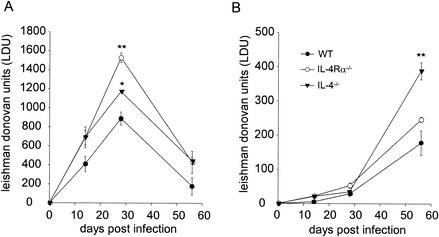
Although interleukin-4 (IL-4) is often associated with development of counterprotective type 2 immune responses in models of cutaneous leishmaniasis (10, 13, 20), previous studies have suggested that IL-4 does not exacerbate disease and may even be beneficial against Leishmania donovani infection (1, 20). The relative role of the related cytokine IL-13, which also has the capacity to signal through IL-4 receptor α (IL-4Rα) and can compensate for IL-4 in IL-4−/− BALB/c mice during cutaneous Leishmania major infection (17), has not been reported in murine models of visceral leishmaniasis. We therefore intravenously infected wild-type BALB/c mice, IL-4−/− BALB/c mice, and IL-4Rα−/− BALB/c mice with 2 × 107 L. donovani amastigotes prepared from the spleens of infected hamsters (22) and monitored the subsequent disease progression in the livers and the spleens by direct enumeration of amastigotes in Giemsa-stained impression smears. Although all three strains of mice were able to resolve the hepatic infection (Fig. 1), both IL-4−/− and IL-4Rα−/− mice had significantly increased parasite burdens at each time point studied. Significantly, IL-4Rα−/− mice had a higher peak parasite burden than IL-4−/− mice, indicating that IL-13 plays an additional or compensatory role in this infection. In both IL-4−/− and IL-4Rα−/− mice, the liver weight at the peak of infection was lower than the weight in wild-type mice, indicating that the increased number of leishman donovan units was not merely a reflection of greater inflammation (data not shown). Although IL-4−/− mice had higher splenic parasite burdens than wild-type mice, this was not the case for IL-4Rα−/− mice, suggesting that there is a complex and tissue-specific interplay between IL-4 and IL-13 in this model. The spleen weights in the groups of mice did not vary (data not shown).
FIG. 1.
Course of infection in L. donovani-infected BALB/c mice (•), IL-4−/− mice (▾), and IL-4Rα−/− mice (○). The parasite burden was determined in the liver (A) and spleen (B). The data are means ± standard errors for individual mice (n = 5), as determined from Giemsa-stained impression smears. One asterisk indicates that the P value is <0.05 compared to the value for wild-type (WT) mice, and two asterisks indicate that the P value is <0.01 compared to the value for wild-type mice. The results of one of two independent experiments are shown.
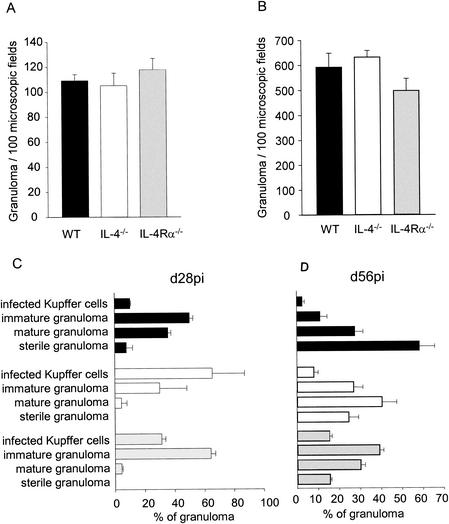
Resolution of hepatic parasite burden is associated with granuloma formation, in which various effector cell populations and the mediators that they produce are concentrated (reviewed in references 3 and 15). We therefore prepared paraffin-embedded sections of liver tissue, stained these sections with hematoxylin and eosin by conventional methods, and scored the progression of the granulomatous response. Each infected focus was classified as (i) an infected Kupffer cell with no associated cellular infiltrate, (ii) an early granuloma comprising an infected Kupffer cell surrounded by a few inflammatory cells but without organization, (iii) a mature granuloma having an organized structure, or (iv) a sterile granuloma, in which amastigotes had been killed as a result of effective antileishmanial immunity (14, 16). Although the three mouse strains had similar total numbers of hepatic granulomas on both day 28 and day 56 postinfection (Fig. 2A and B), differences in the kinetics of granuloma maturation were observed in IL-4−/− and IL-4Rα−/− mice (Fig. 2C and D). At day 28 postinfection this was reflected mostly by a significant decline in the frequency of mature granulomas (Fig. 2C) (P < 0.01 for wild-type mice compared with both IL-4−/− and IL-4Rα−/− mice), and at day 56 postinfection it was reflected by a decline in the frequency of sterile granulomas (Fig. 2D) (P < 0.01 for wild-type mice compared with both IL-4−/− and IL-4Rα−/− mice). As the rate of granuloma formation is believed to be related to the precursor frequency of Leishmania-specific T cells and/or their rate of clonal expansion (8, 12, 14), these data suggest that IL-4 and IL-13 may play a positive role in these processes. Furthermore, histologically mature granulomas fail to progress and acquire optimal antileishmanial activity.
FIG. 2.
Paraffin-embedded liver tissues of wild-type mice (WT) (solid bars), IL-4−/− mice (open bars), and IL-4Rα−/− mice (gray bars) infected for 28 days with L. donovani (d28pi) (A and C) or 56 days with L. donovani (d56pi) (B and D) were stained with hematoxylin and eosin. (A and B) Number of granulomas per 100 microscopic fields (magnification, ×400). (C and D) Percentages of granuloma formation stages for each mouse strain (n = 5). The data are means ± standard errors for one of two independent experiments.
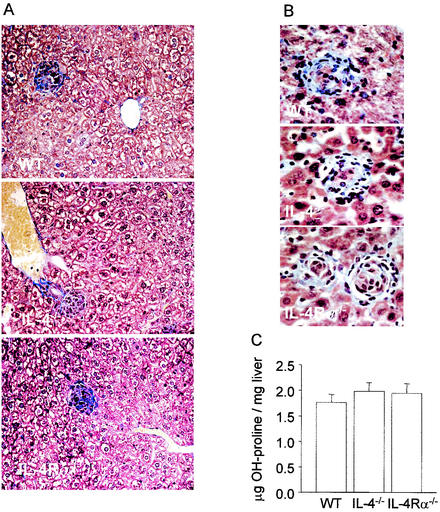
IL-13, in contrast to IL-4, has been shown to have a major role in the regulation of collagen synthesis in granulomas induced by Schistosoma mansoni eggs (6). We therefore stained sections from day-28 and -56 infected mice for collagen deposition by using conventional Martius, Scarlet, and Celestine Blue staining (21). As shown in Fig. 3A and B, mature amastigote-containing granulomas from BALB/c mice had clearly evident collagen deposition. No obvious differences in either staining intensity or pattern were found in IL-4−/− or IL-4Rα−/− mice. To quantify collagen deposition, we measured tissue hydroxyproline levels (4). As shown in Fig. 3C, no differences were observed among the three strains of mice. Thus, collagen deposition is not critically regulated by IL-13 in this model. It is important to note, however, that unlike patients with acute visceral leishmaniasis, BALB/c mice do not develop systemic fibrosis of the hepatic lobule (11). As IL-13 is readily detected in many patients with acute visceral leishmaniasis (2), a role for this cytokine in the more extreme fibrosis seen in humans cannot be discounted.
FIG. 3.
Liver sections from mice infected for 28 days (A) or 56 days (B) stained for collagen deposition. Collagen is blue. The images are representative low-power (A) and high-power (B) images of granulomas from IL-4+/+, IL-4−/−, and IL-4Rα−/− mice. (C) Hydroxyproline contents of the livers of day-56 infected IL-4+/+(WT), IL-4−/−, and IL-4Rα−/− mice.
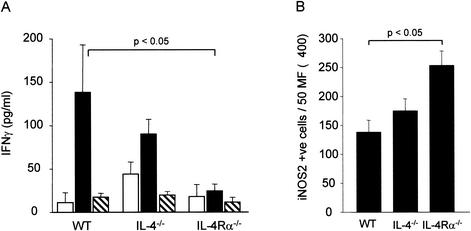
To determine the reason behind the failure of L. donovani-infected IL-4−/− and IL-4Rα−/− mice to produce functionally optimal mature granulomas, the levels of gamma interferon (IFN-γ) in the sera of these mice were determined by using a previously described enzyme-linked immunosorbent assay (7) since IFN-γ drives the development of mature granulomas (Fig. 4A) (23). The level of serum IFN-γ in BALB/c mice was maximal at 14 days postinfection and decreased by day 28. Similar kinetics for serum IFN-γ have previously been reported by Wilson and colleagues following infection with Leishmania chagasi (24). Although IL-4−/− mice did not have significantly different levels of serum IFN-γ than wild-type mice, strikingly, IL-4Rα−/− mice exhibited no detectable serum IFN-γ response to L. donovani infection. These findings concerning serum IFN-γ levels correlated well with the course of infection (Fig. 1A) and the effectiveness of mature granulomas for killing amastigotes (Fig. 2C and D) and suggest that together these type 2 cytokines are in fact essential for optimal development of IFN-γ responses during L. donovani infection. We next used an immunohistochemical approach to examine NOS2 protein expression in granulomas from the mice. Although IL-4 has been shown to be involved in the downregulation of NO production (9, 19), our data show that the absence of IL-4 did not influence the frequency of NOS2+ cells found in granulomas at day 14 postinfection (Fig. 4B). In contrast, significantly more NOS2+ cells were observed in IL-4Rα−/− mice (P < 0.05), suggesting that IL-13 has a strong negative regulatory role in NOS2 expression, as previously observed in in vitro experiments (5, 19). In IL-4Rα−/− mice, NOS2+ cells were mainly localized in the outer edges of the granuloma, and most infected cells were not expressing NOS2 at this time (Fig. 4C). This increase in the frequency of inflammatory NOS2+ cells in IL-4Rα−/− mice correlated with the absence of serum IFN-γ at day 14 postinfection, suggesting that high NO levels might impair local T-cell functions and consequently IFN-γ production. A similar conclusion was reached for the role of IL-4−/− mice during S. mansoni infection (18).
FIG. 4.
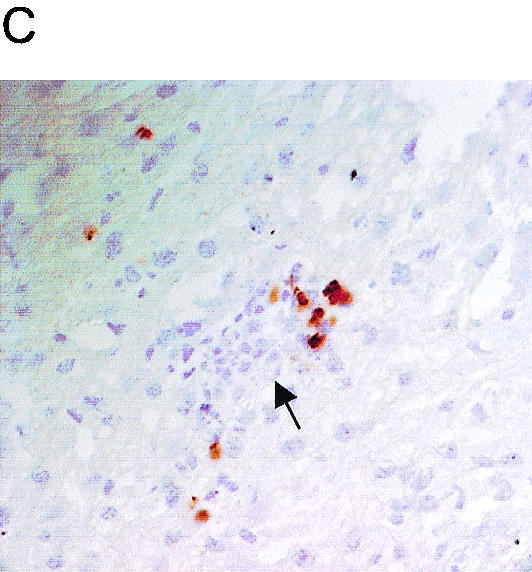
(A) Serum IFN-γ levels for naive mice (open bars), mice at 14 days after infection with L. donovani (solid bars), and mice at 28 days after infection with L. donovani (cross-hatched bars). The data are the means ± standard errors for each mouse strain (n = 5). WT, wild type. (B) Cryosections from livers at 14 days after infection were stained for NOS2. The data are means ± standard errors for NOS2-positive cells within liver granulomas, as determined from approximately 250 granulomas per mouse (n = 5). (C) Representative immature granuloma from an IL-R4α−/− mouse infected for 14 days. The cryosection was stained for NOS2 (brown), and an infected macrophage is indicated by the arrow.
In conclusion, in this study we demonstrated that both IL-4 and IL-13 play important positive roles as regulators of immunity to L. donovani and that the absence of a functional IL-4Rα results in an increase in the frequency of cells producing NOS2, a decrease in IFN-γ serum levels, and retardation of granuloma maturation and effector function. Production of mice with spatial and temporal regulation of IL-4Rα gene expression should in the future facilitate dissection of the IL-4 and IL-13 effects at both the onset and later stages of the granulomatous response.
Editor: J. M. Mansfield
REFERENCES
- 1.Alexander, J., K. C. Carter, N. Al-Fasi, A. Satoskar, and F. Brombacher. 2000. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur. J. Immunol. 30:2935-2943. [DOI] [PubMed] [Google Scholar]
- 2.Babaloo, Z., P. M. Kaye, and M. B. Eslami. 2001. Interleukin-13 in Iranian patients with visceral leishmaniasis: relationship to other Th2 and Th1 cytokines. Trans. R. Soc. Trop. Med. Hyg. 95:85-88. [DOI] [PubMed] [Google Scholar]
- 3.Baker, R. C. P., and P. M. Kaye. 1999. Leishmaniasis, p. 212-234. In D. G. J. A. Zumla (ed.), The granulomatous disorders. Cambridge University Press, Cambridge, United Kingdom.
- 4.Bergman, I., and R. Loxley. 1963. Two improved and simplified methods for the spectophotometric determination of hydroxyproline. Anal. Chem. 35:1961-1965.
- 5.Bogdan, C., H. Thuring, M. Dlaska, M. Rollinghoff, and G. Weiss. 1997. Mechanism of suppression of macrophage nitric oxide release by IL-13: influence of the macrophage population. J. Immunol. 159:4506-4513. [PubMed] [Google Scholar]
- 6.Chiaramonte, M. G., D. D. Donaldson, A. W. Cheever, and T. A. Wynn. 1999. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Investig. 104:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye, P. M., A. J. Curry, and J. M. Blackwell. 1991. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J. Immunol. 146:2763-2770. [PubMed] [Google Scholar]
- 8.Kaye, P. M., and C. R. Engwerda. 2003. Murine leishmaniasis, p. 117-146. In D. Boros (ed.), Granulomatous infections and inflammations. ASM Press Inc., Washington, D.C.
- 9.La Flamme, A. C., E. A. Patton, B. Bauman, and E. J. Pearce. 2001. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J. Immunol. 166:1903-1911. [DOI] [PubMed] [Google Scholar]
- 10.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 11.Leite, V. H., and S. L. Croft. 1996. Hepatic extracellular matrix in BALB/c mice infected with Leishmania donovani. Int. J. Exp. Pathol. 77:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElrath, M. J., H. W. Murray, and Z. A. Cohn. 1988. The dynamics of granuloma formation in experimental visceral leishmaniasis. J. Exp. Med. 167:1927-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohrs, M., B. Ledermann, G. Kohler, A. Dorfmuller, A. Gessner, and F. Brombacher. 1999. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 162:7302-7308. [PubMed] [Google Scholar]
- 14.Murphy, M. L., S. E. Cotterell, P. M. Gorak, C. R. Engwerda, and P. M. Kaye. 1998. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen, Leishmania donovani. J. Immunol. 161:4153-4160. [PubMed] [Google Scholar]
- 15.Murray, H. W. 1999. Granulomatous inflammation: host antimicrobial defense in the tissue in visceral leishmaniasis, p. 977-994. In J. Gallin, R. Snyderman, D. Fearon, B. Haynes, and C. Nathan (ed.), Inflammation: basic principles and clinical correlates, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 16.Murray, H. W. 2001. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 82:249-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noben-Trauth, N., W. E. Paul, and D. L. Sacks. 1999. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 162:6132-6140. [PubMed] [Google Scholar]
- 18.Patton, E. A., A. C. La Flamme, J. A. Pedras-Vasoncelos, and E. J. Pearce. 2002. Central role for interleukin-4 in regulating nitric oxide-mediated inhibition of T-cell proliferation and gamma interferon production in schistosomiasis. Infect. Immun. 70:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutschman, R., R. Lang, M. Hesse, J. N. Ihle, T. A. Wynn, and P. J. Murray. 2001. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 166:2173-2177. [DOI] [PubMed] [Google Scholar]
- 20.Satoskar, A., H. Bluethmann, and J. Alexander. 1995. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect. Immun. 63:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, D., H. Hansch, G. Bancroft, and S. Ehlers. 1997. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-alpha and interferon-gamma. Immunology 92:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stager, S., D. F. Smith, and P. M. Kaye. 2000. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J. Immunol. 165:7064-7071. [DOI] [PubMed] [Google Scholar]
- 23.Taylor, A. P., and H. W. Murray. 1997. Intracellular antimicrobial activity in the absence of interferon-gamma: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-gamma gene-disrupted mice. J. Exp. Med. 185:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson, M. E., B. M. Young, B. L. Davidson, K. A. Mente, and S. E. McGowan. 1998. The importance of TGF-beta in murine visceral leishmaniasis. J. Immunol. 161:6148-6155. [PubMed] [Google Scholar]