Abstract
Apical membrane antigen 1 (AMA-1) of Plasmodium falciparum is a leading candidate antigen for inclusion in a malaria subunit vaccine. Its ectodomain can be divided into three subdomains, each with disulfide bond-stabilized structures. Since the majority of antibodies raised against the ectodomain appear to recognize strain-specific epitopes in domain I, we attempted to develop a vaccine formulation which directs the immune response to a region that contains more conserved epitopes. Here we demonstrate that a virosomal formulation of a peptide that mimics the semiconserved loop I of domain III elicits parasite growth-inhibitory antibodies. A synthetic peptide comprising residues 446 to 490 of AMA-1 (AMA-1446-490) was conjugated through the N terminus to a derivative of phosphatidylethanolamine and the phosphatidylethanolamine-peptide conjugate was incorporated into immunopotentiating reconstituted influenza virosomes as a human-compatible antigen delivery system. Both cyclized and linear versions of the peptide antigen elicited antibodies which specifically bound to parasite-expressed AMA-1 in Western blotting with parasite lysates as well as in immunofluorescence assays with blood stage parasites. All 11 peptidomimetic-specific monoclonal antibodies generated were cross-reactive with parasite-expressed AMA-1. Antigen binding assays with a library of overlapping cyclic peptides covering the target sequence revealed differences in the fine specificity of these monoclonal antibodies and provided evidence that at least some of them recognized discontinuous epitopes. The two immunodominant epitopes comprised the conserved linear sequences K459RIKLN464 and D467DEGNKKII475. A key feature of the synthetic vaccine formulation proposed here is the display of the peptide antigen in a native-like state on the surface of the virosome.
With up to 300 million people currently affected, malaria continues to be one of the major burdens on public health in many tropical countries (44). Because of the spread of drug-resistant parasites and the appearance of insecticide-resistant mosquitoes, the development of an effective vaccine against the most severe form of malaria caused by Plasmodium falciparum is an urgent priority. Vaccine candidates are being targeted against antigens expressed at various stages of the parasite's complex life cycle (35). The effector mechanisms that may confer immunity to malaria are incompletely understood, but several lines of evidence indicate that antibodies to antigens of asexual blood stage parasites can reduce the morbidity and mortality associated with malaria infection. Infected erythrocytes release merozoites, which are directly accessible to antibodies before reinvasion. In parasitemic children, passive transfer of antibodies from adults with naturally acquired immunity to malaria leads to markedly depressed parasite levels (5, 36).
Several merozoite antigens are thought to be able to induce protective antibodies and are currently considered candidate vaccine antigens (22). One of the leading candidates is apical membrane antigen 1 (AMA-1), an 83-kDa protein that is synthesized in mature stages of the parasite and is first localized in the neck of the rhoptries (8, 32). During the course of merozoite release, an additional N-terminally processed 66-kDa form of AMA-1 seems to spread around the merozoite surface (30, 32). Homologues of AMA-1 have been found in all Plasmodium species examined so far, and the protein seems to play an important role during the invasion of erythrocytes and in blood-stage growth (39, 40). Several passive and active immunization studies have indicated that AMA-1 is involved in eliciting protective immune responses (2, 7, 9, 10, 11, 20, 31, 45) and serves as a target for invasion-blocking antibodies (10, 11, 17, 19, 20).
AMA-1 is a type I integral membrane protein of low abundance. The overall structure of its ectodomain can be divided into subdomains I, II, and III. The structure of the protein is stabilized by eight intramolecular disulfide bonds (16) formed between 16 conserved (4, 24, 43) cysteine residues. The epitopes recognized by protective antibodies are not well characterized, but they seem to be primarily directed against conformational epitopes, since reduced and alkylated AMA-1 gives poor protection (2) and is poorly recognized by hyperimmune serum from individuals living in regions where malaria is endemic (17). The epitope recognized by a single merozoite invasion-blocking anti-P. falciparum AMA-1 monoclonal antibody (MAb) (20) has not been characterized. Other AMA-1 binding MAbs did not inhibit invasion (6), indicating that the fine specificity of an anti-AMA-1 antibody determines its ability to impair the function of the target protein. Similarly, it has been found that antibodies specific for P. falciparum merozoite surface protein 1 (MSP-1) can have invasion-inhibitory activity, be ineffective, or block the activity of inhibitory antibodies (41). In the present report, we provide evidence that it is possible to elicit parasite growth-inhibitory antibodies with a virosomal formulation of a synthetic peptidomimetic derived from loop I in domain III of AMA-1.
MATERIALS AND METHODS
Peptide synthesis. (i) AMA49-L1.
A 49-residue peptide comprising AMA-1 residues 446 to 490, with three additional amino acids (GGC) at the N terminus and one additional G residue at the C terminus (AMA-1446-490; Fig. 1) was prepared by solid-phase peptide synthesis on Sieber amide resin (0.5 mmol/g) with 9-fluorenylmethoxycarbonyl (Fmoc) chemistry and an ABI433A peptide synthesizer. The pseudoproline unit Fmoc-isoleucine-serine (2,2-dimethyl pseudo-proline) was used in place of Ile39 and Ser40. After assembly, the peptide was cleaved from a portion of the resin with trifluoroacetic acid (TFA)-H2O-1,2-ethanedithiol (EDT)-tri-isopropylsilane (TIS) (92.5:2.5:2.5:2.5) for 3.5 h at room temperature. After concentration in vacuo, the peptide was precipitated with diethyl ether and purified by reverse-phase high-pressure liquid chromatography (HPLC) (C18 column) with a gradient of acetonitrile (MeCN) in water (25% to 50%) with 0.1% TFA over 15 min. The purity was >95% by analytical HPLC. The constitution was confirmed by amino acid analysis and electrospray mass spectrometry (Table 1).
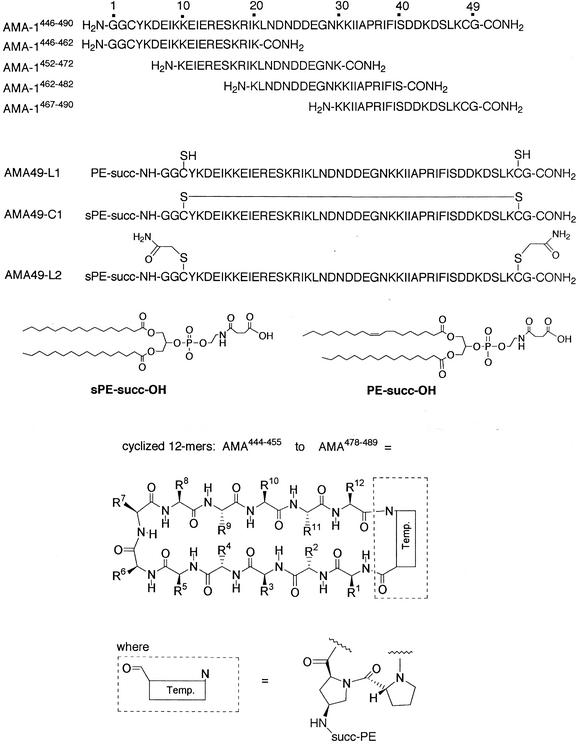
FIG. 1.
Structures of synthetic peptides used in this study, including AMA49-L1, AMA49-C1, and AMA49-L2, as well as the library of cyclic template-bound peptides each containing 12 residues from AMA-1. The sequences contained within each cyclic peptide are indicated in Table 2. The preferred conformations of each cyclic peptide are so far unknown. In AMA-1446-490, the amino acid residues GGC at the N terminus and G at the C terminus were added for reasons of synthesis and do not correspond to the numbering of the AMA-1 amino acid sequence. Temp., template (formula as shown in the figure).
TABLE 1.
Mass spectrometric characterization of peptides synthesized in this worka
| Peptide sequence | Predicted calculated mass (Da) | Measured mass (m/z) |
|---|---|---|
| AMA-1446-490 | 5,625.4 | 1125.9 [M+5H]5+, 938.9 [M+6H]6+, 804.6 [M+7H]7+, 704.4 [M+8H]8+. |
| AMA-1446-462 | 2,408.8 | 1204.7 [M+2H]2+, 803.8 [M+3H]3+, 603.0 [M+4H]4+ |
| AMA-1452-472 | 2,529.8 | 1265.5 [M+2H]2+, 844.5 [M+3H]3+, 633.4 [M+4H]4+ |
| AMA-1462-482 | 2,400.7 | 1200.7 [M+2H]2+, 800.8 [M+3H]3+ |
| AMA-1467-490 | 2,246.7 | 1124.1 [M+2H]2+, 749.8 [M+3H]3+ |
| AMA49-L1 | 6,425.4 | 1607.5 [M+4H]4+, 1285.8 [M+5H]5+, 1071.9 [M+6H]6+, 918.9 [M+7H]7+ |
| AMA49-C1 | 6,396.4 | 1280.2 [M+5H]5+, 1067.0 [M+6H]6+, 914.8 [M+7H]7+ |
| AMA49-L2 | 6,512.3 | 1303.4 [M+5H]5+, 1086.4 [M+6H]6+, 931.4 [M+7H]7+, 815.0 [M+8H]8+, 724.6 [M+9H]9+ |
Electrospray mass spectra were recorded on a Bruker Esquire-LC-MS or with a Finnegan-TSQ-700 spectrometer.
Another portion of the resin carrying the intact peptide chain (ca. 60 μmol) was treated with succinyl-1-oleyl-3-palmitoyl-2-glycerophosphatidylethanolamine (130 mg, 160 μmol) (Fig. 1), 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU; 61 mg, 160 μmol), 1-hydroxy-7-azabenzotriazole (HOAt; 22 mg, 160 μmol), and N,N-diisopropylethylamine iPr2EtN; 82 μl) in dimethylformamide (DMF)-CH2Cl2 (4 ml, 1:2) for 18 h at room temperature. After washing the resin with DMF and CH2Cl2 four times, the peptide conjugate was cleaved from the resin with 1% TFA in CH2Cl2 four times (4 ml). The combined organic fractions were concentrated in vacuo, and the conjugate was redissolved in TFA-H2O-EDT-TIS (92.5:2.5:2.5:2.5) for 4 h at room temperature. The TFA was then removed in vacuo, and AMA49-L1 was precipitated with di-isopropylether (iPr2O) and then purified by HPLC on a C4 column with a gradient of 10 to 100% MeCN-water with 0.1% TFA. The purity was >95% by analytical HPLC. The constitution was confirmed by amino acid analysis and electrospray mass spectrometry (Table 1). The presence of two free thiols was proven by an Ellman test and by reaction with excess N-ethylmaleimide (NEM) and detection of the bis-NEM derivative by HPLC-mass spectroscopy.
(ii) AMA49-C1.
AMA49-C1 was prepared in the same way except that succinyl-1,3-dipalmitoyl-2-glycerophosphatidylethanolamine (sPE-succ-OH) was used (Fig. 1), and in the final step the conjugate (4 mg) was oxidized in ammonium acetate buffer (50 mM, pH 8) and 2,2,2-trifluoroethanol (1:1,100 ml) for 4 days at room temperature. After addition of AcOH (100 μl) and lyophilization, AMA49-C1 was purified by HPLC as above for AMA49-L1. The formation of the disulfide bridge was confirmed by a negative Ellman test and by attempted derivatization with NEM, which gave no bis-NEM derivative by HPLC-mass spectroscopy. From the mass spectrum, it was clear that only the monomeric form of AMA49-C1 was obtained.
(iii) AMA49-L2.
For the synthesis of AMA49-L2, the dithiol (6 mg) was alkylated with iodoacetamide (43 μmol) in a mixture (5:4) of phosphate buffer (0.1 M, pH 7.5) and trifluoroethanol. AMA49-L2 was purified by HPLC on a C4 column with a gradient of 10 to 100% MeCN-water with 0.1% TFA. The purity was >95% by analytical HPLC. Electrospray mass spectrometry showed the expected mass (Table 1).
(iv) AMA1446-462, AMA1452-472, AMA1462-482, and AMA1467-490.
The linear peptides AMA1446-462, AMA1452-472, AMA1462-482, and AMA1467-490 were prepared by standard Fmoc solid-phase peptide synthesis (see above), purified by reverse-phase HPLC on a C18 column and a gradient of MeCN in water with 0.1% TFA, and characterized by amino acid analysis and electrospray mass spectrometry (Table 1).
(v) Library of 12-mers.
A library of 35 12-mer cyclic peptides which scan the AMA444-489 sequence (Fig. 1 and Table 2) was prepared by methods that will be described in detail elsewhere. Each mimetic was ≥95% pure by HPLC, and the structure was confirmed by electrospray mass spectrometry. The primary structure of all synthesized peptides was based on the AMA-1 domain III sequence of P. falciparum strain K1 (accession number U33279).
TABLE 2.
Reactivity of anti-AMA49-C1 MAbsa
| Peptide | Reactivity with MAb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DV2 | DV6 | DV7 | DV5 | DV8 | DV1 | DV3 | DV4 | DV9 | DV10 | DV11 | |
| Full length | |||||||||||
| AMA446-490 | + | + | + | + | + | + | + | + | + | + | + |
| Linear | |||||||||||
| AMA446-462 | + | + | + | − | − | − | − | − | − | − | − |
| AMA452-472 | − | − | + | + | + | − | − | − | − | − | − |
| AMA462-482 | − | − | − | − | − | + | + | + | + | + | + |
| AMA472-490 | − | − | − | − | − | − | − | − | − | − | − |
| 12-mer cyclic | |||||||||||
| AMA444-455 | − | − | − | − | − | − | − | − | − | − | − |
| AMA445-456 | − | − | − | − | − | − | − | − | − | − | − |
| AMA446-457 | − | − | − | − | − | − | − | − | − | − | − |
| AMA447-458 | − | − | − | − | − | − | − | − | − | − | − |
| AMA448-459 | − | − | − | − | − | − | − | − | − | − | − |
| AMA449-460 | − | − | − | − | − | − | − | − | − | − | − |
| AMA450-461 | − | − | + | − | − | − | − | − | − | − | − |
| AMA451-462 | − | − | + | − | − | − | − | − | − | − | − |
| AMA452-463 | − | − | + | − | − | − | − | − | − | − | − |
| AMA453-464 | − | − | + | + | + | − | − | − | − | − | − |
| AMA454-465 | − | − | + | + | + | − | − | (+) | − | − | − |
| AMA455-466 | − | − | + | + | + | − | − | − | − | − | − |
| AMA456-467 | − | − | (+) | + | + | (+) | − | + | + | + | + |
| AMA457-468 | − | − | − | + | + | (+) | − | − | − | − | − |
| AMA458-469 | − | − | − | + | + | − | − | (+) | (+) | − | − |
| AMA459-470 | − | − | − | + | + | − | − | − | − | − | − |
| AMA460-471 | − | − | − | − | − | − | − | − | − | − | − |
| AMA461-472 | − | − | − | − | − | − | − | − | − | − | − |
| AMA462-473 | − | − | − | − | − | + | (+) | + | + | − | − |
| AMA463-474 | − | − | − | − | − | − | − | + | + | − | − |
| AMA464-475 | − | − | − | − | − | + | (+) | + | + | + | + |
| AMA465-476 | − | − | − | − | − | + | (+) | + | + | + | + |
| AMA466-477 | − | − | − | (+) | (+) | + | + | + | + | + | + |
| AMA467-478 | − | − | − | − | − | + | (+) | + | + | + | + |
| AMA468-479 | − | − | − | − | − | − | − | − | − | − | − |
| AMA469-480 | − | − | − | − | − | − | − | − | − | − | − |
| AMA470-481 | − | − | − | − | − | − | − | − | − | − | − |
| AMA471-482 | − | − | − | − | − | − | − | − | − | − | − |
| AMA472-483 | − | − | − | − | − | − | − | − | − | − | − |
| AMA473-484 | − | − | − | − | − | − | − | − | − | − | − |
| AMA474-485 | − | − | − | + | − | − | − | + | + | − | − |
| AMA475-486 | − | − | − | − | − | − | − | − | − | − | − |
| AMA476-487 | − | − | − | − | − | − | − | − | − | − | − |
| AMA477-488 | − | − | − | + | + | − | − | − | − | − | − |
| AMA478-489 | − | − | − | − | − | − | − | + | + | − | − |
Anti-AMA49-C1 MAbs were tested for their ELISA reactivity with AMA446-490 or 12-mer cyclic AMA-1 peptides (AMA444-455 to AMA478-489). Linear peptides AMA446-462, AMA452-472, AMA462- 482, and AMA472-490 were used in a competition ELISA to compete with the binding of anti-AMA49-C1 MAbs to coated AMA446-490. For ELISA experiments, antibody reactivities after 1 h of substrate incubation were classified as follows: +, OD405 ≥ 0.5; (+), 0.5 > OD405 ≥ 0.2; −, OD405 < 0.2. In competition ELISA experiments, linear peptides AMA446-462, AMA452-472, AMA462-482, and AMA472-490 were considered to react (+) or not (−) with anti-AMA49-C1 MAbs when they still inhibited binding of anti-AMA49-C1 MAbs to coated AMA49-C1 in a concentration of 12 μg/ml or less. In AMA444-455 and AMA445-456, amino acids 444 and 445 correspond to S444 and L445 from the AMA-1 sequence, respectively.
Preparation of peptide-loaded virosomes.
For the preparation of phosphatidylethanolamine-mimetic immunopotentiating reconstituted influenza virosomes (IRIVs), a solution of purified influenza A/Singapore virus hemagglutinin (4 mg) in phosphate-buffered saline (PBS) was centrifuged for 30 min at 100,000 × g, and the pellet was dissolved in PBS (1.33 ml) containing 100 mM octaethyleneglycolmonodecylether (PBS-OEG). Peptide-phosphatidylethanolamine conjugates (4 mg), phosphatidylcholine (32 mg; Lipoid, Ludwigshafen, Germany), and phosphatidylethanolamine (6 mg) were dissolved in a total volume of 2.66 ml of PBS-OEG. The phospholipid and the hemagglutinin solutions were mixed and sonicated for 1 min. This solution was then centrifuged for 1 h at 100,000 × g, and the supernatant was filtered (0.22 μm) under sterile conditions. Virosomes were then formed by detergent removal with SM BioBeads (Bio-Rad, Glattbrugg, Switzerland).
Mouse immunogenicity studies.
BALB/c mice were immunized intramuscularly with 0.1 ml of the commercial whole-virus influenza vaccine Inflexal Berna (Berna Biotech, Bern, Switzerland). At least 3 weeks later, they were immunized with peptide-loaded IRIVs at intervals of at least 2 weeks. Blood was collected before each immunization and 2 weeks after the final injection.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) analyses with peptide-phosphatidylethanolamine conjugates were performed essentially as described previously (28). Briefly, Polysorp plates (Nunc, Fisher Scientific, Wohlen, Switzerland) were coated overnight at 4°C with 100 μl of a 10-μg/ml solution of AMA-1 peptide-phosphatidylethanolamine conjugate in PBS (pH 7.2). Wells were then blocked with 5% milk powder in PBS for 30 min at 37°C, followed by three washes with PBS containing 0.05% Tween 20. Plates were then incubated with serial dilutions of antimimetic mouse serum or anti-AMA49-L1 monoclonal antibodies (MAbs) in PBS containing 0.05% Tween 20 and 0.5% milk powder for 2 h at 37°C. After being washed, plates were incubated with alkaline phosphatase-conjugated goat anti-mouse gamma heavy-chain antibodies (Sigma, St. Louis, Mo.) for 1 h at 37°C. After being washed again, phosphatase substrate solution (1 mg of p-nitrophenyl phosphate [Sigma] per ml in a pH 9.8 buffer solution containing 10% [vol/vol] diethanolamine and 0.02% MgCl2) was added, and the plates were incubated in the dark at room temperature until the colorimetric reaction had progressed sufficiently. The optical density was measured at 405 nm on a Titertek Multiscan MCC/340 reader (Labsystems, Helsinki, Finland).
In competition assays, peptide-phosphatidylethanolamine-coated plates were incubated for 2 h with MAbs in the presence of increasing concentrations of competitor peptides. Isotypes of anti-AMA49 MAbs were determined by detecting MAbs bound to peptide-phosphatidylethanolamine-coated plates with alkaline phosphatase-conjugated goat antibodies specific for mouse immunoglobulin γ1, γ2a, γ2b, γ3, κ, or λ chains (Southern Biotechnology, Birmingham, Ala.).
Indirect immunofluorescence assay.
Multiwell immunofluorescence microscopy slides (Flow Laboratories, Baar, Switzerland) were treated with 0.01% poly-l-lysine (Sigma) at room temperature for 30 min and washed five times with RPMI basal salts medium (Gibco-BRL, Basel, Switzerland). Erythrocytes from in vitro cultures (26) of P. falciparum strain K1 with a parasitemia of between 5 and 10% were washed twice in RPMI and resuspended in RPMI and 2 volumes of a solution containing 4% formaldehyde and 0.1% Triton X-100. From this cell suspension, 30 μl was added to each well, incubated at room temperature for 30 min, and washed five times with PBS. Wells were incubated for 30 min at room temperature with blocking solution containing 1% fatty acid-free bovine serum albumin in PBS.
Immunostaining was performed by incubating the wells with 25 μl of an appropriate antibody or serum dilution in blocking solution in a humid chamber for 1 h at room temperature. After five washes with blocking solution, 25 μl of 5-μg/ml indocarbocyanine dye-conjugated affinity-pure F(ab′)2 fragment goat anti-mouse IgG heavy-chain antibodies (Jackson ImmunoResearch Laboratories, West Grove, Pa.), diluted in blocking solution containing 0.01 mg of Hoechst dye no. 33256 (Sigma) per ml, were added to the wells and incubated for 1 h at room temperature. Finally, the wells were washed five times, mounted with mounting solution (90% [vol/vol] glycerol containing 0.1 M Tris-Cl [pH 8.0] and 2 mg of o-phenylenediamine per ml) and covered with a coverslip. Antibody binding and DNA staining were assessed by fluorescence microscopy on a Leitz Dialux 20 fluorescence microscope and documented with a Leica DC200 digital camera system.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Parasite lysates were prepared essentially as described previously (26) by saponin lysis of P. falciparum K1-infected erythrocytes. In brief, cultured parasites were washed three times with serum-free RPMI medium. Pelleted infected red blood cells were lysed by mixing with a large volume (adjusted to 5% hematocrit) of 0.015% (wt/vol) saponin in 150 mM NaCl and 15 mM sodium citrate (pH 7.0) and incubated on ice for 20 min. Finally, the pelleted parasites were resuspended in 3 volumes of SSC buffer (0.15 M NaCl plus 0.015 M sodium citrate) and stored at −80°C until further use.
A total of 50 μl of parasite lysate was solubilized in an equal volume of 2× loading buffer (1.7 ml of 0.5 M Tris-HCl [pH 6.8], 2 ml of glycerol, 4.5 ml of 10% sodium dodecyl sulfate, 1 ml of β-mercaptoethanol, 0.8 ml of bromophenol blue [0.3%, wt/vol]) and heated to 95°C for 10 min. Proteins were separated on an SDS-10% PAGE minigel. Separated proteins were electrophoretically transferred to a nitrocellulose filter (Protran Nitrocellulose, BA85; Schleicher & Schuell) by semidry blotting. Blots were blocked with PBS containing 5% milk powder and 0.1% Tween 20 overnight at 4°C. The filter was cut into strips and incubated with appropriate dilutions of immune serum in blocking buffer for 2 h at room temperature. Filter strips were then washed three times for 10 min in blocking buffer and incubated at room temperature for 1 h with alkaline phosphatase-conjugated goat anti-mouse γ heavy-chain antibodies diluted 1:30,000 in blocking buffer (Sigma, St. Louis, Mo.). After being washed again, blots were finally developed with 5-bromo-4-chloro-3-indolylphosphate (Bio-Rad, Reinach, Switzerland) and nitroblue tetrazolium (Bio-Rad) to visualize bands.
Generation of hybridomas and production of MAbs.
Hybridomas were generated from spleen cells of mice 3 days after a booster immunization with AMA49-C1-loaded IRIVs with PAI mouse myeloma cells as a fusion partner. Hybrids were selected in hypoxanthine-aminopterin-thymidine medium, and cells that secreted anti-AMA49-L1 MAbs were identified by ELISA. For large-scale MAb production, hybridoma cell lines were cultured in 1-liter spinner bottles, and MAbs were purified by protein G affinity chromatography. Purified MAbs were dialyzed against PBS, aliquoted, and stored at −80°C.
Parasite culture and in vitro growth inhibition assay.
P. falciparum strain K1 was cultured essentially as described previously (26). The culture medium was supplemented with 0.5% AlbuMAX (Gibco, Paisley, Scotland) as a substitute for human serum (12). Synchronization of cultures was achieved by sorbitol treatment as described previously (23). Serogroup A+ erythrocytes for passages were obtained from the Swiss Red Cross (Basel, Switzerland).
For in vitro growth inhibition assays, synchronous late trophozoites or schizonts were diluted with fresh red blood cells to give a parasitemia of 0.5% and mixed with purified MAb. The final hematocrit in cultures was adjusted to 0.5%. Each culture was set up in sextuplicate in 96-well flat-bottomed culture plates. After 96 h, the plates were centrifuged at 180 × g for 5 min, and the culture supernatants were discarded. Pelleted erythrocytes were resuspended in 200 μl of PBS supplemented with 15 μg of hydroethidine fluorescent vital stain (Polysciences Inc., Warrington, Pa.) per ml and incubated at 37°C for 30 min. The erythrocytes were washed twice with PBS, resuspended in 400 μl of PBS, and analyzed in a FACSscan flow cytometer (Becton Dickinson, San Jose, Calif.) with CellQuest 3.2.1fl software. The hydroethidine emission was detected in the FL2 channel by logarithmic amplification, and the erythrocytes were gated on the basis of their forward and side scatters. A total of 30,000 cells per sample were analyzed. Percent inhibition was calculated from the geometric mean parasitemias of sextuplicate test and control wells as 100 × [(control − test)/control]. Statistical significance was calculated by a two-sided t test. Confidence intervals (P < 95%) were calculated by antilogging the confidence limits calculated on the log scale.
RESULTS
A sequence comprising 45 amino acid residues (Y446KDEIKKEIERESKRIKLNDNDDEGNKKIIAPRIFISDDKDSLKC490) from loop I in domain III of AMA-1 (Fig. 2) with a GGC sequence added to the N terminus and an additional glycine residue at the C terminus was synthesized by standard solid-phase peptide chemistry. In order to load the peptide onto virosomes, it was conjugated through a succinate linker at the N terminus to a regioisomer of phosphatidylethanolamine, and the phosphatidylethanolamine-peptide conjugate was designated AMA49-L1 (Fig. 1) and incorporated into IRIV as the antigen delivery system. After one immunization with the influenza vaccine Inflexal, mice were immunized with AMA49-L1-loaded IRIV. All mice immunized produced antibodies that reacted with AMA49-L1 in an ELISA (Fig. 3A) and were cross-reactive with blood stage parasites in the immunofluorescence assay (Fig. 4), yielding a punctuate staining pattern characteristic of AMA-1 (17, 27).
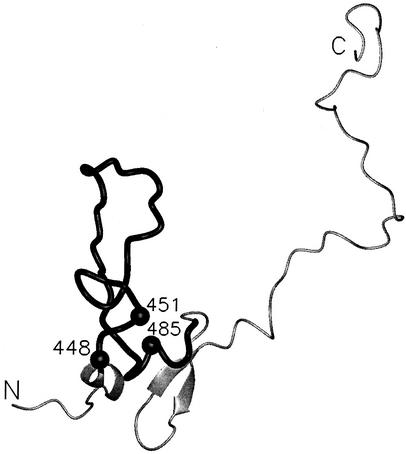
FIG. 2.
Representation of the structure of loop 1 from domain III of AMA-1. The image was made with the PDB file 1HN6, which includes residues 436 to 545 of domain III. The sequence stretch incorporated into the synthetic AMA49 molecules (AMA446-490) is shown as a black cylinder. The residues showing dimorphism (D/N448, K/M451, and K/I485) are each depicted by a black ball.
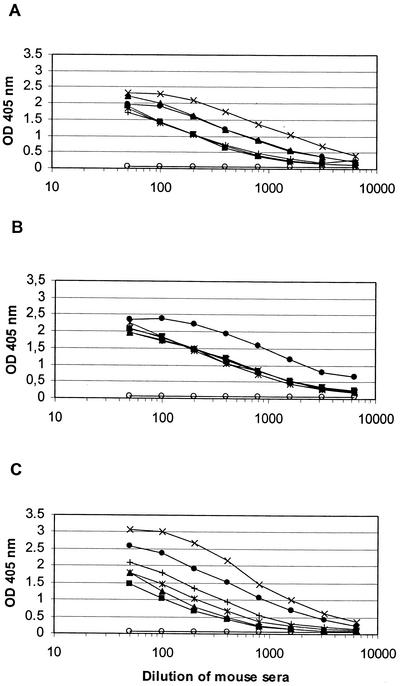
FIG. 3.
AMA49-L1- (A), AMA49-C1- (B), and AMA49-L2- (C) specific IgG ELISA titers after three immunizations of mice with AMA49-L1-, AMA49-C1-, and AMA49-L2-loaded IRIV, respectively. Responses of individual mice are shown. Preimmune serum samples showed no significant reactivity with the corresponding immunogen, and for each group one typical preimmune serum (open circles) is shown.
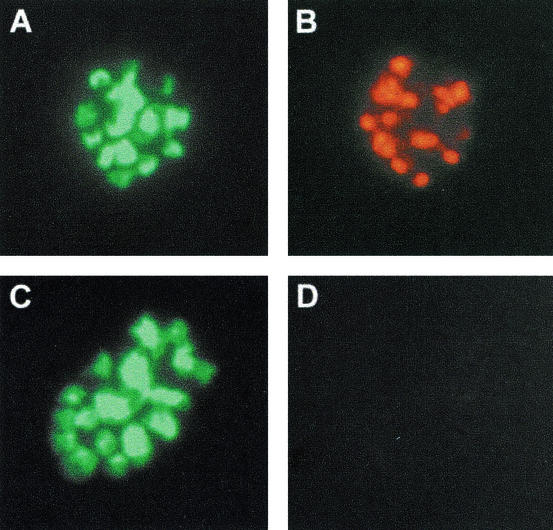
FIG. 4.
Immunofluorescence staining of mature schizonts of P. falciparum strain K1. (A and C) Parasite DNA control staining with Hoechst dye 33258. (B) Characteristic AMA-1 immunostaining with anti-AMA49-L1 immune serum (diluted 1:100). (D) Lack of staining with preimmune serum (diluted 1:100). Representative results with serum samples derived from one of the immunized mice are shown. Sera from all other AMA49-L1-, AMA49-C1-, and AMA49-L2-immunized mice yielded comparable immunofluorescence assay staining patterns.
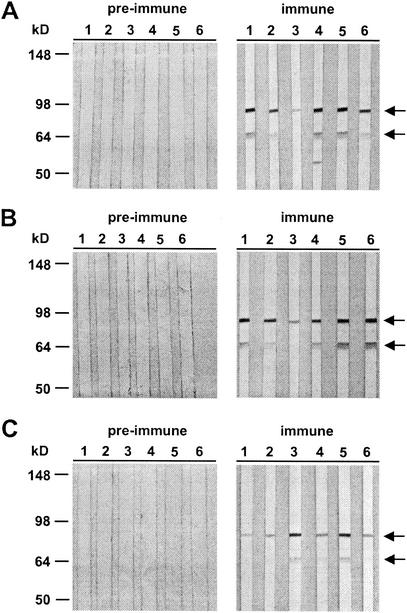
Most of the serum samples (five of six) had an immunofluorescence assay titer greater than 1:1,000. The interaction of mouse anti-AMA49-L1 serum samples with parasite-expressed AMA-1 was reconfirmed by Western blot analysis with total protein lysates of P. falciparum blood stage parasites (Fig. 5A). The anti-AMA49-L1 antisera primarily stained proteins with apparent molecular masses of approximately 83 and 66 kDa, which are the sizes of the full-length AMA-1 and its major processing product, respectively (17, 30). Some of the anti-AMA49-L1 antisera stained an additional protein band, which may correspond to a previously described processing product of AMA-1 (18) that possesses the same N terminus as the 66-kDa fragment.
FIG. 5.
Western blot analysis of the reactivity of anti-AMA49-L1 (A), anti-AMA49-C1 (B), and anti-AMA49-L2 (C) antisera with P. falciparum K1 blood stage lysates as the antigen. Sera yielded a double band pattern (indicated by arrows) characteristic of full-length AMA-1 and its major processing product (83 and 66 kDa, respectively). Each lane represents serum from one individual mouse. No staining was observed with the corresponding preimmune serum samples. All serum samples were used at a dilution of 1:100.
In order to investigate whether cyclization of the peptide via the cysteine residues close to the C and N termini would improve the yield of parasite-binding antibodies, mice were immunized with IRIV loaded with a cyclized (AMA49-C1) and a linear (AMA49-L2) version of AMA49-L1 (Fig. 1). While AMA49-L1 contained free thiols, both thiol groups of the terminal cysteine residues were blocked by alkylation in the case of AMA49-L2. ELISA (Fig. 3B and C) and immunofluorescence assay titers obtained with the two constructs were comparable to those obtained with AMA49-L1. All anti-AMA49 serum samples were cross-reactive with all three structural variants of AMA49 in the ELISA (data not shown). In Western blot analyses with P. falciparum lysates, all anti-AMA49-C1 and -L2 serum samples yielded staining patterns (Fig. 5B and C) comparable to those obtained with anti-AMA49-L1 antibodies (Fig. 5A).
For a detailed analysis of the humoral immune response, AMA49-C1-specific mouse B-cell hybridomas were generated. All 11 hybridoma clones obtained secreted IgG:κ (10 MAbs were IgG1; MAb DV3 was IgG2a) that reacted in the ELISA with both the cyclic and the linear peptides with comparable efficacy. These 11 MAbs all stained blood stage parasites of strain K1 in the immunofluorescence assay (data not shown).
Competition ELISA experiments with a set of four overlapping linear peptides, AMA446-462, AMA452-472, AMA462-482, and AMA472-490 (Fig. 1), were used for epitope mapping and differentiated four groups of antibodies (Table 2). Antigen binding of MAbs DV2 and DV6 was blocked by AMA446-462, MAb DV7 was blocked by both AMA446-462 and AMA452-472, MAbs DV5 and DV8 were blocked by AMA452-472, and MAbs DV1, DV3, DV4, DV9, DV10, and DV11 were blocked by AMA462-482. None of the antibodies was blocked by the C-terminal sequence AMA472-490.
A library of 35 cyclic peptides, each containing 12 residues scanning the AMA444-489 sequence and each with an offset of one amino acid, were used for more detailed epitope mapping (Fig. 1 and Table 2). These peptides were conformationally restrained by cyclization through linkage to a dipeptide template comprising a d-proline and an l-4-aminoproline conjugated to succinyl-l-oleyl-3-palmitoyl-2-glycerophosphatidylethanolamine (succPE). In the ELISA, both MAbs DV2 and DV6 inhibited by AMA446-462 bound to none of the short cyclic peptides. MAb DV7 bound to the consecutive cyclic peptides comprising residues 450 to 461 through 455 to 466, which share the sequence E455RESKRI461 that is also present in the overlap of the two long inhibitory peptides AMA446-462 and AMA452-472. MAbs DV5 and DV8 bound to cyclic peptides 453 to 464 through 459 to 470, which share the sequence K459RIKLN464 located in the center of the inhibitory peptide AMA452-472. Additional binding of both MAbs to the nonconsecutive cyclic peptide 477 to 488 and of MAb DV5 to cyclic peptide 474 to 485 is indicative of a discontinuous epitope.
All six MAbs that were inhibited by AMA462-482 exhibited binding to the consecutive peptides 464 to 475 through 467 to 478, which share the sequence D467DEGNKKII475 located in the center of the long inhibitory peptide AMA462-482. MAb DV1 reacted in addition with the overlapping peptide 462 to 473. In the case of MAb DV3, the reactivity with peptide N466DDEGNKKIIAP477 was outstanding. With the other four MAbs, DV4, DV9, DV10, and DV11 reactivity patterns with nonoverlapping peptides indicated recognition of a discontinuous epitope. All four MAbs showed reactivity with peptide 456 to 467, which only overlaps at position D467 with the putative central D467DEGNKKII475 recognition sequence. In addition, MAbs DV4 and DV9 also bound to the nonconsecutive peptides 474 to 485 and 478 to 489 and to the peptides 462 to 473 and 463 to 474, which share the sequence D467DEGNKK473 with the central recognition sequence.
While immunofluorescence assay cross-reactivities with P. falciparum strains expressing natural sequence variants of AMA446-490 (strain 3D7, D448M451K485; strain RPF2, D448K451I485; and strain FC27, N448M451K485) were observed, none of the MAbs reacted with Plasmodium berghei blood stage parasites expressing a shorter loop 1 sequence (YKNKINEEIKVLNKNISNGNNSIEFPRIFISTDKNSLNC) with 59% identity to the P. falciparum sequence (data not shown).
Mice were immunized with IRIV loaded with phosphatidylethanolamine conjugates of each of the cyclic 12-mer peptides in order to analyze whether some of them could act as mimotopes of AMA-1 surface loops and elicit parasite-binding antibodies. While all 35 structures elicited significant antibody titers against the respective immunizing peptide sequence itself, only serum samples raised against AMA458-469 (containing the central recognition unit K459RIKLN464 of MAbs DV5 and DV8) and AMA464-475 (containing the central recognition unit D467DEGNKKII475 of MAbs DV1, DV3, DV4, DV9, DV10, and DV11) were weakly cross-reactive with blood stage parasites in the immunofluorescence assay (data not shown). Hyperimmune serum samples from individuals living in regions where malaria is endemic were reactive with all forms of AMA446-490 in the ELISA but showed either no or only very weak binding to the 35 cyclic 12-mer peptides (data not shown).
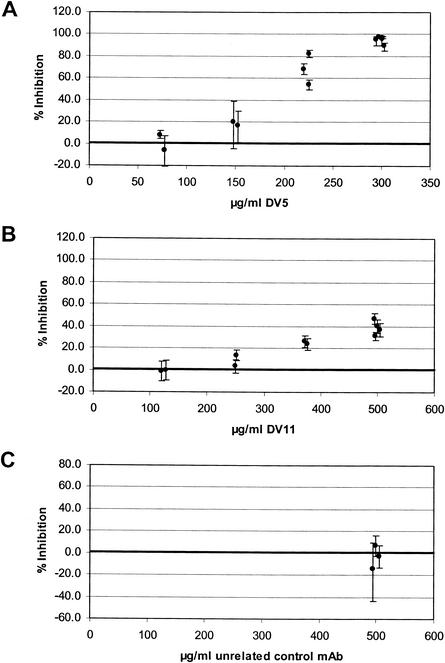
When MAbs DV5 and DV11, representing the two major fine specificities, were tested in a P. falciparum in vitro growth inhibition assay, both exhibited substantial inhibitory activity (Fig. 6). A 95.3% reduction of parasite growth was observed after addition of MAb DV5 at a final concentration of 300 μg/ml (average of five independent sextuplicate experiments). MAb DV11 also exhibited a significant but lower growth-inhibitory activity, while an isotype-matched control MAb had no effect. Taken together, these data clearly demonstrate that it is possible to elicit parasite binding and in vitro growth-inhibitory antibodies by immunization with AMA-1446-490 in combination with IRIV as a human-compatible antigen delivery system.
FIG. 6.
In vitro parasite growth-inhibitory activity of anti-AMA49 MAbs. (A) MAb DV5; (B) MAb DV11; (C) isotype-matched control antibodies. Each symbol represents the mean of an independent sextuplicate experiment, and error bars indicate the 95% confidence intervals (P < 0.05, two-sided t test).
DISCUSSION
AMA-1 is a leading malaria blood stage vaccine candidate which has been shown to elicit protective immune responses in malaria infection models. The parasite invasion-inhibitory activities of anti-AMA-1 antibodies indicate that the humoral arm of the immune response plays an important role in AMA-1-mediated immune protection. The ectodomain of AMA-1 is divided into three subdomains, defined by disulfide bonds (16). There is evidence that the majority of inhibitory antibodies in serum samples from malaria-exposed humans and in rabbit antisera raised against the refolded AMA-1 ectodomain are directed against strain-specific epitopes in domain I (17).
An analysis of tryptic fragments of P. chabaudi adami AMA-1 recently identified a loop-like structure within the putative domain I as a target of antibodies from hyperimmune mouse serum samples (37). A synthetic 45-mer loop mimetic incorporating this element was found to elicit AMA-1 binding antibodies. However, these did not protect P. chabaudi adami-challenged mice in passive immunization experiments. Since domain I is the most diverse region of AMA-1 (25, 46), an AMA-1 vaccine component which lacks this domain may be preferable in order to direct the immune response to a region(s) that contain more conserved epitopes (17). Since the production of large batches of clinical-grade recombinantly expressed AMA-1 has been notoriously difficult, we are investigating the possibility of developing a synthetic peptide-based AMA-1 vaccine formulation. We demonstrate in this report that it is possible to elicit parasite growth-inhibitory antibodies with a virosomal formulation of a peptide comprising the sequence of loop I from domain III of P. falciparum AMA-1. In agreement with these findings, it has recently been shown that epitopes in domain III are targets of inhibitory human antibodies (29).
The development of peptide-based vaccines is hampered both by the poor immunogenicity of many peptides and by a lack of conformational similarity between small linear peptides and the corresponding sequence in the native target protein. In an attempt to overcome both problems, we are evaluating the use of a human-compatible delivery system comprising IRIVs in synthetic peptide vaccine design (28, 34). IRIVs are spherical, unilammelar vesicles prepared by detergent removal from a mixture of natural and synthetic phospholipids and influenza virus surface glycoproteins. They have been shown to act as an efficient and highly effective means of enhancing the immune response to a variety of antigens, illustrating their broad suitability as a vaccine delivery system (14).
The hemagglutinin membrane glycoprotein of influenza virus plays a key role in the mode of action of IRIVs. This major antigen of influenza virus is a fusion-inducing component which facilitates antigen delivery to immunocompetent cells. In the case of the IRIV-based hepatitis A vaccine Epaxal-Berna, which is the first licensed vaccine in which IRIVs are used as a delivery system for a non-influenza virus antigen, the hepatitis A virus antigen spontaneously binds to the IRIVs. For smaller synthetic antigens, we have developed and evaluated a method to link the antigenic molecule to a phospholipid (phosphatidylethanolamine) and to integrate the phosphatidylethanolamine-antigen conjugates into the virosomal membrane during the virosome reconstitution process (28, 34). When we compared a virosome formulation loaded with a phosphatidylethanolamine conjugate of a cyclic peptide mimotope of the repeat region of the P. falciparum circumsporozoite protein with an alum-adjuvanted mimotope-multiple antigenic peptide construct, we found that both formulations elicited comparable levels of antimimotope antibody responses in mice. However, only the antibodies against the virosomal formulation bind effectively to the parasites, indicating that phosphatidylethanolamine-coupled antigens are located in a more native-like state on the surface of the virosomes. Apparently, adsorption to alum dramatically disturbs the conformation of the mimetic (28).
Several lines of evidence indicate that for an AMA-1 vaccine, the correct conformation is critical. In view of this, our finding that a linear and a cyclized version of the AMA-1446-490 sequence had comparable properties is noteworthy. It remains to be seen whether different cyclic forms of this peptide may generate improved immune responses. In any case, the results may indicate that intramolecular interactions lead to a correct folding of the linear peptide and that the anchoring to the surface of IRIVs has no deleterious effects on this process. Apparently, the solution structure of the AMA-1 domain III, determined by nuclear magnetic resonance spectroscopy, consists of a disulfide-stabilized core region including all three disulfide bonds, but also contains significant regions of disorder (29). Our epitope analyses of growth-inhibitory anti-AMA49 MAbs with a library of 12-residue cyclic peptides covering the AMA444-489 sequence provided evidence that at least some of the MAbs may recognize discontinuous epitopes. Since discontinuous epitopes may contain short stretches of continuous sequences (1, 3, 38), analyses with sets of overlapping peptides are suitable to define both continuous linear epitopes and parts of discontinuous epitopes (15, 41). Our analyses indicate that K459RIKLN464 and D467DEGNKKII475 represent sequence stretches of discontinuous epitopes recognized by inhibitory anti-AMA-1 MAbs.
AMA-1 lacks tandem repeat sequences found in many other P. falciparum antigens. However, a significant degree of sequence diversity is observed, which may reflect diversifying selection pressure from naturally acquired immune responses (9, 13, 33, 42). Most of the polymorphic or dimorphic amino acid residues of the relatively conserved domain III are located far apart from each other in the primary sequence but may cluster in the region of the disulfide core in the three-dimensional structure of the molecule (29). This is also the case for the three variable residues (D448, K451, and K485) present in the AMA-1446-490 sequence analyzed here. The virosomal formulation of this peptide seems to focus the antibody response primarily to conserved loop structures away from this core region. Cross-protection obtained with antibodies raised against recombinantly expressed AMA-1 has provided evidence for the existence of such common protective epitopes (17, 21). Taken together, our results indicate that the loop I sequence from domain III of AMA-1 represents a suitable component of an IRIV-based multiantigen multistage synthetic peptide malaria vaccine candidate.
Acknowledgments
This work was supported by grant 4388.1 from the Swiss Commission for Technology and Innovation (KTI).
The authors in Zurich thank the World Health Organization for financial support through the UNDP/World Bank/WHO special program for research and training in tropical diseases.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Amit, A. G., R. A. Mariuzza, S. E. Phillips, and R. J. Poljak. 1986. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science 233:747-753. [DOI] [PubMed] [Google Scholar]
- 2.Anders, R. F., P. E. Crewther, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. [DOI] [PubMed] [Google Scholar]
- 3.Barlow, D. J., M. S. Edwards, and J. M. Thornton. 1986. Continuous and discontinuous protein antigenic determinants. Nature 322:747-748. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, Q., and A. Saul. 1994. Sequence analysis of the apical membrane antigen I (AMA-1) of Plasmodium vivax. Mol. Biochem. Parasitol. 65:183-187. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, S., I. A. McGregor, and S. Carrington. 1961. Gamma globulin and acquired immunity to human malaria. Nature 192:733-737. [DOI] [PubMed] [Google Scholar]
- 6.Coley, A. M., N. V. Campanale, J. L. Casey, A. N. Hodder, P. E. Crewther, R. F. Anders, L. M. Tilley, and M. Foley. 2001. Rapid and precise epitope mapping of monoclonal antibodies against Plasmodium falciparum AMA1 by combined phage display of fragments and random peptides. Protein Eng. 14:691-698. [DOI] [PubMed] [Google Scholar]
- 7.Collins, W. E., D. Pye, P. E. Crewther, K. L. Vandenberg, G. G. Galland, A. J. Sulzer, D. J. Kemp, S. J. Edwards, R. L. Coppel, J. S. Sullivan, C. L. Morris, and R. F. Anders. 1994. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51:711-719. [DOI] [PubMed] [Google Scholar]
- 8.Crewther, P. E., J. G. Culvenor, A. Silva, J. A. Cooper, and R. F. Anders. 1990. Plasmodium falciparum: two antigens of similar size are located in different compartments of the rhoptry. Exp. Parasitol. 70:193-206. [DOI] [PubMed] [Google Scholar]
- 9.Crewther, P. E., M. L. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deans, J. A., T. Alderson, A. W. Thomas, G. H. Mitchell, E. S. Lennox, and S. Cohen. 1982. Rat monoclonal antibodies which inhibit the in vitro multiplication of Plasmodium knowlesi. Clin. Exp. Immunol. 49:297-309. [PMC free article] [PubMed] [Google Scholar]
- 11.Deans, J. A., A. M. Knight, W. C. Jean, A. P. Waters, S. Cohen, and G. H. Mitchell. 1988. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 10:535-552. [DOI] [PubMed] [Google Scholar]
- 12.Dorn, A., R. Stoffel, H. Matile, A. Bubendorf, and R. G. Ridley. 1995. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 374:269-271. [DOI] [PubMed] [Google Scholar]
- 13.Escalante, A. A., H. M. Grebert, S. C. Chaiyaroj, M. Magris, S. Biswas, B. L. Nahlen, and A. A. Lal. 2001. Polymorphism in the gene encoding the apical membrane antigen-1 (AMA-1) of Plasmodium falciparum. X. Asembo Bay Cohort Project. Mol. Biochem. Parasitol. 113:279-287. [DOI] [PubMed] [Google Scholar]
- 14.Gluck, R. 1999. Adjuvant activity of immunopotentiating reconstituted influenza virosomes (IRIVs). Vaccine 17:1782-1787. [DOI] [PubMed] [Google Scholar]
- 15.Gomme, P. T., P. G. Stanton, and M. T. Hearn. 1999. Evaluation of a pepscan approach to identify epitopes recognised by anti-hTSH monoclonal antibodies. J. Biochem. Biophys. Methods 38:53-70. [DOI] [PubMed] [Google Scholar]
- 16.Hodder, A. N., P. E. Crewther, M. L. Matthew, G. E. Reid, R. L. Moritz, R. J. Simpson, and R. F. Anders. 1996. The disulfide bond structure of Plasmodium apical membrane antigen-1. J. Biol. Chem. 271:29446-29452. [DOI] [PubMed] [Google Scholar]
- 17.Hodder, A. N., P. E. Crewther, and R. F. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69:3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell, S. A., C. Withers-Martinez, C. H. Kocken, A. W. Thomas, and M. J. Blackman. 2001. Proteolytic processing and primary structure of Plasmodium falciparum apical membrane antigen-1. J. Biol. Chem. 276:31311-31320. [DOI] [PubMed] [Google Scholar]
- 19.Kocken, C. H., A. M. van der Wel, M. A. Dubbeld, D. L. Narum, F. M. van de Rijke, G. J. van Gemert, d. L. van, X., L. H. Bannister, C. Janse, A. P. Waters, and A. W. Thomas. 1998. Precise timing of expression of a Plasmodium falciparum-derived transgene in Plasmodium berghei is a critical determinant of subsequent subcellular localization. J. Biol. Chem. 273:15119-15124. [DOI] [PubMed] [Google Scholar]
- 20.Kocken, C. H., Narum1 DL, A. Massougbodji, B. Ayivi, M. A. Dubbeld, W. A. van der, D. J. Conway, A. Sanni, and A. W. Thomas. 2000. Molecular characterisation of Plasmodium reichenowi apical membrane antigen-1 (AMA-1), comparison with P. falciparum AMA-1, and antibody-mediated inhibition of red cell invasion. Mol. Biochem. Parasitol. 109:147-156. [DOI] [PubMed] [Google Scholar]
- 21.Kocken, C. H., C. Withers-Martinez, M. A. Dubbeld, W. A. van der, F. Hackett, A. Valderrama, M. J. Blackman, and A. W. Thomas. 2002. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect. Immun. 70:4471-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, S., J. E. Epstein, and T. L. Richie. 2002. Vaccines against asexual stage malaria parasites. Chem. Immunol. 80:262-286. [DOI] [PubMed] [Google Scholar]
- 23.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 24.Marshall, V. M., M. G. Peterson, A. M. Lew, and D. J. Kemp. 1989. Structure of the apical membrane antigen I (AMA-1) of Plasmodium chabaudi. Mol. Biochem. Parasitol. 37:281-283. [DOI] [PubMed] [Google Scholar]
- 25.Marshall, V. M., L. Zhang, R. F. Anders, and R. L. Coppel. 1996. Diversity of the vaccine candidate AMA-1 of Plasmodium falciparum. Mol. Biochem. Parasitol. 77:109-113. [DOI] [PubMed] [Google Scholar]
- 26.Matile, H., and J. Pink. 1990. Plasmodium falciparum malaria parasite cultures and their use in immunology, p. 221-234. In I. Lefkovits and P. Benvenuto (ed.), Immunological methods. Academic Press, Inc., San Diego, Calif.
- 27.Miller, L. H., and S. L. Hoffman. 1998. Research toward vaccines against malaria. Nat. Med. 4:520-524. [DOI] [PubMed] [Google Scholar]
- 28.Moreno, R., L. Jiang, K. Moehle, R. Zurbriggen, R. Gluck, J. A. Robinson, and G. Pluschke. 2001. Exploiting conformationally constrained peptidomimetics and an efficient human-compatible delivery system in synthetic vaccine design. Chem. Biochem. 2:838-843. [DOI] [PubMed] [Google Scholar]
- 29.Nair, M., M. Hinds, A. Coley, A. Hodder, M. Foley, R. Anders, and R. Norton. 2002. Structure of domain III of the blood-stage malaria vaccine candidate, Plasmodium falciparum apical membrane antigen 1 (AMA1). J. Mol. Biol. 322:741-753. [DOI] [PubMed] [Google Scholar]
- 30.Narum, D. L., and A. W. Thomas. 1994. Differential localization of full-length and processed forms of PF83/AMA-1 an apical membrane antigen of Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 67:59-68. [DOI] [PubMed] [Google Scholar]
- 31.Narum, D. L., S. A. Ogun, A. W. Thomas, and A. A. Holder. 2000. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect. Immun. 68:2899-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson, M. G., V. M. Marshall, J. A. Smythe, P. E. Crewther, A. Lew, A. Silva, R. F. Anders, and D. J. Kemp. 1989. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol. Cell. Biol. 9:3151-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polley, S. D., and D. J. Conway. 2001. Strong diversifying selection on domains of the Plasmodium falciparum apical membrane antigen 1 gene. Genetics 158:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poltl-Frank, F., R. Zurbriggen, A. Helg, F. Stuart, J. Robinson, R. Gluck, and G. Pluschke. 1999. Use of reconstituted influenza virus virosomes as an immunopotentiating delivery system for a peptide-based vaccine. Clin. Exp. Immunol. 117:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richie, T. L., and A. Saul. 2002. Progress and challenges for malaria vaccines. Nature 415:694-701. [DOI] [PubMed] [Google Scholar]
- 36.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 37.Salvatore, D., A. Hodder, W. Zeng, L. Brown, R. Anders, and D. Jackson. 2002. Identification of antigenically active tryptic fragments of apical membrane antigen-1 (AMA1) of Plasmodium chabaudi malaria: strategies for assembly of immunologically active peptides. Vaccine 20:3477-3484. [DOI] [PubMed] [Google Scholar]
- 38.Sheriff, S., E. W. Silverton, E. A. Padlan, G. H. Cohen, S. J. Smith-Gill, B. C. Finzel, and D. R. Davies. 1987. Three-dimensional structure of an antibody-antigen complex. Proc. Natl. Acad. Sci. USA 84:8075-8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Triglia, T., J. Healer, S. R. Caruana, A. N. Hodder, R. F. Anders, B. S. Crabb, and A. F. Cowman. 2000. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 38:706-718. [DOI] [PubMed] [Google Scholar]
- 40.Urquiza, M., J. E. Suarez, C. Cardenas, R. Lopez, A. Puentes, F. Chavez, J. C. Calvo, and M. E. Patarroyo. 2000. Plasmodium falciparum AMA-1 erythrocyte binding peptides implicate AMA-1 as erythrocyte binding protein. Vaccine 19:508-513. [DOI] [PubMed] [Google Scholar]
- 41.Uthaipibull, C., B. Aufiero, S. E. Syed, B. Hansen, J. A. Guevara Patino, E. Angov, I. T. Ling, K. Fegeding, W. D. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. A. Lyon, and A. A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381-1394. [DOI] [PubMed] [Google Scholar]
- 42.Verra, F., and A. L. Hughes. 2000. Evidence for ancient balanced polymorphism at the apical membrane antigen-1 (AMA-1) locus of Plasmodium falciparum. Mol. Biochem. Parasitol. 105:149-153. [DOI] [PubMed] [Google Scholar]
- 43.Waters, A. P., A. W. Thomas, J. A. Deans, G. H. Mitchell, D. E. Hudson, L. H. Miller, T. F. McCutchan, and S. Cohen. 1990. A merozoite receptor protein from Plasmodium knowlesi is highly conserved and distributed throughout Plasmodium. J. Biol. Chem. 265:17974-17979. [PubMed] [Google Scholar]
- 44.World Health Organization. 1997. World malaria situation in 1994. Wkly. Epidemiol. Rec. 69:309-314. [Google Scholar]
- 45.Xu, H., A. N. Hodder, H. Yan, P. E. Crewther, R. F. Anders, and M. F. Good. 2000. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J. Immunol. 165:389-396. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, L., B. Zhan, J. Wang, and X. Feng. 1995. Sequence analysis of apical membrane antigen I from a Plasmodium falciparum isolate collected from Mengpeng Township, Yunnan Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 13:203-208. [PubMed] [Google Scholar]