Abstract
The effect of gamma interferon (IFN-γ) on elevation of reactive oxygen species and the viability of virulent wild-type and avirulent mutants of Salmonella enterica serovar Typhimurium and S. enterica serovar Infantis was studied in a murine macrophage cell line (J774.2 cells). S. enterica serovar Typhimurium 14028 phoP and a rough lipopolysaccharide mutant of S. enterica serovar Infantis 1326/28 (φr) (avirulent mutants) induced NADPH phagocytic oxidase gp91 (gp91phox) activity and a significant (P < 0.05) elevation of reactive oxygen species within 12 h without coculture with IFN-γ. This coincided with reduced survival of S. enterica serovar Typhimurium14028 phoP or stasis of S. enterica serovar Infantis φr. Fluorometric studies indicated that expression of IFN-γ on infected J774.2 cells was not significantly (P > 0.05) elevated. However, studies with the virulent S. enterica serovar Typhimurium strains showed that a comparable level of control of bacterial numbers could only be achieved by coculture with IFN-γ. This coincided with significant upregulation of IFN-γ receptor alpha expression on the surface of J774.2 cells and was completely abolished by N-acetyl-l-cysteine captopril (an inhibitor of reactive oxygen species). Delay in reactive oxygen species induction due to a requirement for IFN-γ and upregulation of IFN-γ receptor alpha in macrophages infected with virulent salmonellae may result in greater dissemination of virulent salmonellae in host tissue.
Over a number of years, Salmonella enterica serovar Typhimurium infection of mice has been used as a model of human typhoid, which remains a disease of global importance. Mutants of S. enterica serovar Typhimurium that are unable to survive in murine macrophages are avirulent (10), and this has stimulated a great deal of interest in the nature of the killing pathways employed by murine macrophages, including the generation of reactive nitrogen species via the inducible nitric oxide synthase pathway and reactive oxygen species (ROS) via the NADPH phagocytic oxidase system.
Previous studies have suggested that the oxidative burst is an important killing pathway employed by murine macrophages infected with salmonellae. Mutations in the S. enterica serovar Typhimurium recombination genes induce avirulence due to an inability of the salmonellae to repair DNA following exposure to the oxidative burst in J774 cells (2). Other studies have suggested that S. enterica serovar Typhimurium utilizes superoxide dismutase to protect periplasmic membranes against the effect of peroxynitrite formation and that sodC mutants are susceptible to peroxynitrite killing and are attenuated (5). Recent reports have indicated that reactive oxygen species (ROS) precede inducible nitric oxide synthase when knockout mice are challenged with S. enterica serovar Typhimurium, and it has been suggested that the timing of these responses is augmented by gamma interferon (IFN-γ) (24, 32).
The generation of ROS occurs via a membrane-bound flavocytochrome b558, consisting of two phagocytic oxidase components (gp91phox and p22phox) and four cytosolic components, p40phox, 47phox, p67phox, and a GTP-binding Rac protein. During activation, the cytosolic components translocate to the site of gp91phox/p22phox on the phagosomal membrane, forming a functional enzyme complex which generates ROS by catalyzing electron transfer from NADPH to molecular oxygen (21, 31). NADPH phagocytic oxidase (NADPHphox) is active in murine macrophages infected with S. enterica serovar Typhimurium (5), and greater numbers of S. enterica serovar Typhimurium are recovered from the livers and spleens of gp91phox−/− C57BL/6 mice than from wild-type mice (24). Furthermore, a more recent study suggested that Salmonella pathogenicity island 2 genes are required for evasion of the murine macrophage oxidative burst by preventing oxidase trafficking (32). Therefore, wild-type salmonellae may survive longer in macrophages due to their ability to evade ROS via Salmonella pathogenicity island 2 genes, but the NADPHphox system ultimately controls the early phase of infection and possibly prevents it from overwhelming the host immune response.
In the animal studies mentioned above, murine macrophages would have been stimulated by cytokines produced by infected cells at the site of invasion while ex vivo macrophages were stimulated with 20 U of IFN-γ per ml (24). Lower levels of IFN-γ are produced by natural killer cells early in infection, while the most productive source of IFN-γ comes from T lymphocytes active later in infection (19). Lipopolysaccharide and IFN-γ synergistically stimulate the signal-transducing activator of transcription 1 (STAT1) signaling pathway in murine macrophage-like RAW 264.7 cells (14). In vivo studies have also shown the importance of IFN-γ in mediating responses to lipopolysaccharide in mice, especially in the lethal shock reaction (Schwartzman reaction) (15). However, none of these studies elucidated the full effect of IFN-γ in terms of the generation of ROS during the early phase of infection with virulent or avirulent salmonellae.
The aim of the work described here was to investigate the oxidative burst in Salmonella-infected J774.2 cells and to determine the effect of IFN-γ on this killing pathway in the absence of an effective inducible nitric oxide synthase pathway during infection of J774.2 cells with virulent and avirulent S. enterica serovar Typhimurium. Furthermore, the study was designed to ascertain why ROS are important in Salmonella control early in infection compared to later control by inducible nitric oxide synthase.
MATERIALS AND METHODS
Bacterial culture and strains.
Bacteria were grown in Luria-Bertani (LB) broth (Life Technologies Ltd., Paisley, United Kingdom) for 18 h at 37°C under agitation. The bacteria were then subcultured in fresh LB broth for 4 h to late log phase (established by conventional counts of CFU). Prior to incubation with J774.2 cells, bacteria were adjusted to a multiplicity of infection (MOI) of 10. During this study, the following strains and mutants were used: S. enterica serovar Typhimurium 14028 (American Type Culture Collection strain); S. enterica serovar Typhimurium CS022 (phoP mutant of 14028, a gift from S. I. Miller, Harvard University), which does not survive in macrophages (25); S. enterica serovar Typhimurium F98 (virulent in mice); and a rough mutant of S. enterica serovar Infantis, 1326/28 φr, which is avirulent in mice (1).
In a separate study, the effect of other murine typhoid-inducing (S. enterica serovar Typhimurium 4/74, S. enterica serovar Enteritidis KMS1977, S. enterica serovar Dublin 2229, and S. enterica serovar Choleraesuis A50) and non-typhoid-inducing (S. enterica serovar Gallinarum 9, S. enterica serovar Kedougou GP, and S. enterica serovar Montevideo KMS) strains were analyzed. Growth curves for each serovar were obtained as previously stated.
Cell culture.
J774.2 cells were grown to confluence in 96-well plates (Nunc, Naperville, Ill.) containing RPMI 1640 medium at 37°C in CO2 (5%, vol/vol). The cells were then washed three times in phosphate-buffered saline (PBS) to remove medium and nonadherent cells and incubated in PBS at 22°C for 15 min prior to infection. Cells at passages of between 4 and 16 were used throughout this study.
Invasion and survival of salmonellae in J774.2 cells.
Survival of salmonellae in medium not supplemented with IFN-γ or supplemented with 10, 100, or 1,000 U of IFN-γ per ml was assessed by the method described previously (10). In separate experiments, Salmonella survival was also investigated in J774.2 cells incubated in medium supplemented with 1,000 U of IFN-γ per ml and N-acetyl-l-cysteine captopril (ACC) (10 mM) (Sigma, Poole, United Kingdom), which is an inhibitor of the oxidative burst.
Measurement of oxidative burst.
A nitroblue tetrazolium reduction assay (30) was used to detect oxygen metabolite activity in J774.2 cells. Briefly, cells were incubated with salmonellae (MOI, 10) in 96-well plates (Nunc, Naperville, Ill.) for 60 min at 37°C in CO2 (5%, vol/vol). The wells were washed with PBS and then incubated with 100 μg of gentamicin per ml for a further 60 min at 37°C. At various time points between 2 and 24 h postinfection, cells were incubated at 37°C with 50 μl of nitroblue tetrazolium (Sigma) (1 mg/ml in PBS) for 45 min. To stop the reaction, 100 μl of 1 N hydrochloric acid was added, and the plate was shaken gently at room temperature for 10 min. The wells were then washed with PBS and shaken again for 10 min at ambient temperature prior to resuspending the cells in 150 μl of dimethyl sulfoxide and adding 10 μl of 1 N sodium hydroxide to develop the color. The optical density of the reaction was read in a plate reader (Anthos Labtech Instruments, Hamburg, Germany) at 620 nm. Test samples were compared with induction of oxidative burst in J774 monolayers by zymosan (15) and cells that were not incubated with zymosan or bacteria (negative controls). In further experiments, oxidative burst was assessed in infected cells which had been cocultured in IFN-γ.
Construction of IFN-γRα-coated fluorescent microspheres.
Early experiments indicated that IFN-γ did not increase ROS activity in J774 cells infected with virulent salmonellae to the same degree as when cells were infected with avirulent mutants over a 24-h period. We therefore wanted to ascertain the effect of IFN-γ on anti-IFN-γ receptor alpha (IFN-γRα) expression on the surface of J774.2 cells following IFN-γ stimulation or following S. enterica serovar Typhimurium invasion prior to IFN-γ stimulation. A quantitative fluorescent microsphere assay for IFN-γRα (3) was used in which rabbit anti-mouse IFN-γRα (Autogenbioclear, Calne, Wiltshire, United Kingdom) was attached to Fluoresbrite carboxylated microspheres (0.5-μm diameter) (Polyscience, Warrington, Pa.) by a previously reported method (11). Briefly, 0.25 ml of microsphere suspension was centrifuged in a series of buffers (phosphate, carbonate, and borate) for 10 min at 13,000 × g in a bench top microcentrifuge. The microspheres were then resuspended in PBS containing carbodiimide (2%, wt/vol) (Sigma) and mixed at room temperature on an end-to-end shaker for 4 h. After 4 h, the microspheres were washed three times in PBS and resuspended in 200 μg of rabbit anti-mouse IFN-γRα in 1 ml of PBS. The microspheres were incubated overnight at 4°C on an end-to-end shaker, washed three times in PBS, and resuspended in bovine serum albumin (10 mg/ml; Sigma) for 60 min to block any remaining free binding sites. After 60 min, the microspheres were washed three times in PBS and stored in 1 ml of glycerol-sodium azide (Sigma) or resuspended in PBS and used within 48 h. To control for nonspecific microsphere binding via bovine serum albumin, microspheres which were coated only with bovine serum albumin were also constructed. To test whether proteins had successfully coated the microspheres, the microspheres were reacted with appropriate antibodies to analyze agglutination. Autoagglutination was also tested (11).
Quantitative determination of IFN-γRα expression on J774.2 cells.
J774.2 cells were grown to confluency in 96-well plates containing RPMI 1640 medium. We ascertained that the peak of IFN-γ-induced oxidative burst over the 24-h postinfection period occurred at 12 h. We therefore measured IFN-γRα expression from 2 to 12 h postinfection. Salmonellae were grown and J774.2 cells were infected in the presence or absence of IFN-γ (1,000 U/ml). After 2, 7, and 12 h, cells were washed in PBS and fixed for 45 min in 3% para-formaldehyde at room temperature. After being washed in PBS, monolayers were incubated for 60 min at room temperature in 100 μl of PBS containing 5 μl of anti-IFN-γRα-coated microspheres (equivalent to 1.4 × 104 microspheres per cell) (Polyscience Inc. Biotechnology Product Handbook 1992-1993). After 60 min, the monolayers were washed three times in PBS, and fluorescent emission was measured at 485 and 535 nm in a Victor 1420 multichannel counter (PerkinElmer, Zaventem, Belgium). Fixed cells incubated with bovine serum albumin-coated microspheres were used as a control for bovine serum albumin used to block uncoupled binding sites on the surface of IFN-γRα-positive microspheres. Other controls included incubating IFN-γRα-positive microspheres with fixed cells which had not been exposed to salmonellae or IFN-γ. Autofluorescence was also measured in uninfected J774.2 cells which had not been exposed to IFN-γ or fluorescent microspheres. Each test was repeated three times on three occasions, and statistical comparisons were made between the mean fluorescent counts per second at each time point.
Immunocytochemical analysis of gp91phox activity.
The activity of gp91phox in infected and uninfected J774.2 cells was determined by standard immunocytochemical methods. Salmonella-infected cells which had or had not been incubated with IFN-γ (1,000 U/ml) were washed free of medium and permeabilized for 10 min at ambient temperature in Triton X-100 (0.05%) at 2, 7, 12, and 24 h postinfection. The cells were then fixed for 15 min in paraformaldehyde (5%) at room temperature and washed in PBS. Fixed cells were then incubated on an end-to-end shaker for 60 min at 4°C in the dark with goat anti-mouse gp91phox immunoglobulin G (IgG) (Autogenbioclear, London, United Kingdom) diluted 1:100 in PBS-Tween. The cells were then washed three times in PBS and incubated in identical conditions but with rabbit anti-goat IgG conjugated to fluorescein isothiocyanate (Sigma). Propidium iodide was used to stain bacteria. Samples were viewed on a TCS-NT confocal laser scanning microscope equipped with an argon laser (Leica, Hamburg, Germany). Controls included J774.2 cells which had not been infected or incubated with IFN-γ and also uninfected cells which had been incubated with IFN-γ (1,000 U/ml).
Effect of hydrogen peroxide on survival of salmonellae in LB broth.
To investigate whether there was a differential reaction to ROS by virulent wild-type and avirulent mutant salmonellae, Salmonella cultures were added to fresh LB containing H2O2 at 0.02, 0.04, and 0.06%. Salmonella numbers were estimated as stated earlier at time zero and every hour for 4 h. All treatments were compared with an H2O2-free control.
Statistical analysis.
Mann-Whitney analysis (Minitab) was used to measure significant difference at the 95% confidence limit between different test groups and between the same test groups at different time points.
RESULTS
Survival of salmonellae in J774.2 monolayers.
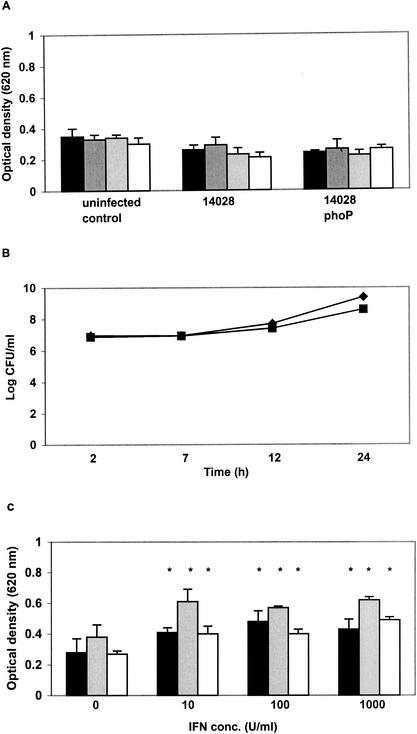
The numbers of both wild-type S. enterica serovar Typhimurium strains increased in J774.2 monolayers over the 24-h period measured, compared with a steady decline or stasis in the number of viable S. enterica serovar Typhimurium phoP and S. enterica serovar Infantis 1326/28 φr, respectively (Fig. 1A).
FIG. 1.
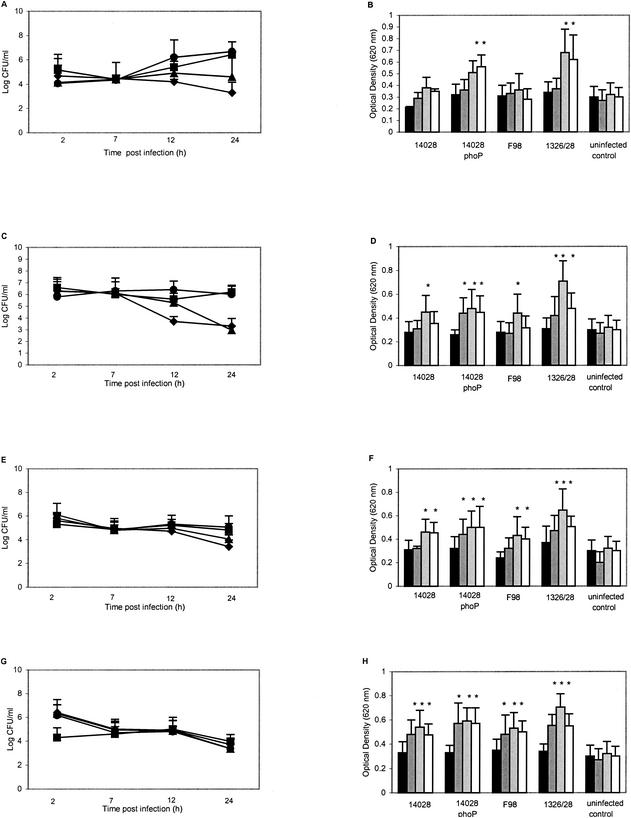
Survival of Salmonella spp. and induction of respiratory burst (ROS) in J774.2 monolayers cocultured with IFN-γ from 2 to 24 h postinfection. ▪, wild-type S. enterica serovar Typhimurium 14028; ♦, S. enterica serovar Typhimurium 14028 phoP; •, S. enterica serovar Typhimurium F98; ▪, S. enterica serovar Infantis 1326/28 φr. (A, C, E, and G) Salmonella survival; (B, D, F, and H) oxidative burst. (A and B) Zero IFN-γ; (C and D) cocultured with IFN-γ (10 U/ml), (E and F) cocultured with IFN-γ (100 U/ml); (G and H) cocultured with IFN-γ (1,000 U/ml). Black bars, 2 h postinfection; dark gray bars, 7 h postinfection; light gray bars, 12 h postinfection; open bars, 24 h postinfection. *, significant (P = 0.05) increase from control values at the same time point. All points represent a mean of three replicate experiments performed on at least five different occasions. MOI was 10 in all experiments.
When 10 U of IFN-γ per ml was added to the culture medium, the numbers of both virulent S. enterica serovar Typhimurium 14028 and F98 exhibited stasis, while a bactericidal effect was observed for both mutants (Fig. 1C). Further increasing the IFN-γ concentration (100 or 1,000 U/ml) did not increase the bactericidal effect on the Salmonella mutants but did so in both virulent strains (Fig. 1E and 1G). Thus, the overall effect of increasing the IFN-γ concentration on virulent Salmonella populations in J774.2 cells 24 h postinfection was to stimulate survival trends similar to those observed for avirulent mutants, and this was most apparent at high IFN-γ concentrations.
Induction of ROS in Salmonella-infected J774.2 cells.
Infected J774.2 monolayers were analyzed for oxidative burst with a nitroblue tetrazolium reduction assay. Significantly higher (P < 0.05) levels of ROS activity were measured in J774.2 cells infected with S. enterica serovar Typhimurium 14028 phoP or S. enterica serovar Infantis 1328/28 φr from 12 h postinfection compared with uninfected control monolayers (Fig. 1B). Small but nonsignificant increases in ROS activity were detected after infection with virulent S. enterica serovar Typhimurium 14028 or F98 throughout the 24-h postinfection period studied.
When IFN-γ (10 U/ml) was added to the culture medium, the mutant strains significantly (P < 0.05) elevated oxidative burst from 7 h postinfection, and wild-type strains significantly (P < 0.05) increased oxidative burst from 12 h postinfection (Fig. 1D). This trend continued when the infected cells were cocultured with 100 U of IFN-γ per ml (Fig. 1F) and 1,000 U of IFN-γ per ml (Fig. 1H), but at these higher IFN-γ concentrations, a significant level of oxidative burst was shifted to an earlier time point (Fig. 2F and H). Further experiments showed that significantly higher levels of oxidative burst could be achieved at earlier time points by increasing the MOI to 100, without IFN-γ, and when both an MOI of 100 and an IFN-γ concentration of 1,000 U/ml were investigated, significant induction of oxidative burst could be achieved at 2 h postinfection (data not shown).
FIG. 2.
Effect of coculture with N-acetyl-l-cysteine captopril (ACC) and IFN-γ on Salmonella survival and ROS induction in J774.2 cells. (A) J774 cells infected with salmonellae (MOI = 10) and cocultured with ACC (10 mM) and IFN-γ (1,000 U/ml) from 2 to 24 h postinfection. Black bars, 2 h postinfection; dark gray bars, 7 h postinfection; light gray bars, 12 h postinfection; open bars, 24 h postinfection. (B) Survival of salmonellae in J774 cells cocultured with ACC (10 mM) and IFN-γ (1,000 U/ml). ⋄, wild-type 14028 with ACC; □, 14028 phoP mutant with ACC; ♦, wild-type 14028 without ACC; ▪, 14028 phoP mutant without ACC. (C) Effect of increasing IFN-γ concentration on the induction of ROS in J774 cells. Black bars, 2 h postinfection; dark gray bars, 7 h postinfection; light gray bars, 12 h postinfection; open bars, 24 h postinfection. *, significant (P = 0.05) increase above the control (zero IFN-γ) at the same time point. Each point represents a mean of three replicate experiments repeated on at least five separate occasions at an MOI of 10.
Effect of ACC on ROS induction and Salmonella survival in J774.2 cells.
Throughout the infection period, addition of N-acetyl-l-cysteine captopril (ACC) to the culture medium inhibited ROS activity to levels recorded in uninfected controls, even in the presence of IFN-γ (1,000 U/ml) (Fig. 2A). Inhibition of ROS in these cells coincided with recovery of significantly (P < 0.05) higher numbers of S. enterica serovar Typhimurium 14028 and its phoP mutant (Fig. 2B).
Effect of IFN-γ on oxidative burst in uninfected J774.2 cells.
When uninfected J774.2 cells were cultured with IFN-γ, ROS activity increased significantly (P < 0.05) in comparison to ROS activity measured in uninfected control cells which had not been incubated with IFN-γ (Fig. 2C). However, the effect of IFN-γ on ROS was not dose dependent, since concentrations of IFN-γ ranging from 10 to 1,000 U/ml did not have an increased effect (Fig. 2C). The length of time that cells were exposed to IFN-γ, however, did have an effect, with 12 h being the optimal time of exposure for greatest ROS activity (Fig. 2C).
Immunocytochemical detection of gp91phox in Salmonella-infected J774.2 cells.
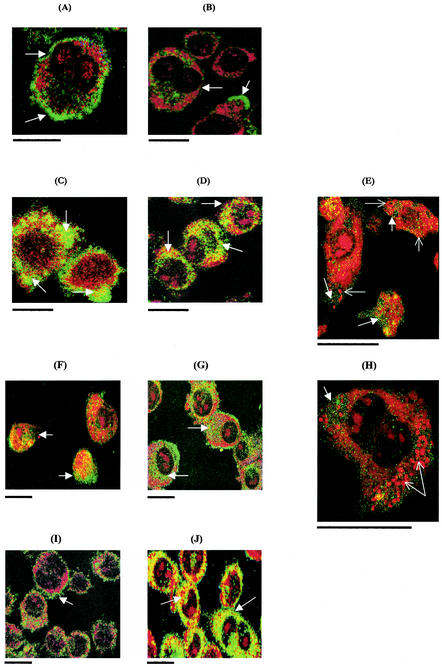
Confocal laser scanning microscopy was used to compare gp91phox activity in J774.2 cells infected with virulent S. enterica serovar Typhimurium 14028 or avirulent S. enterica serovar Typhimurium phoP. Cells were infected with salmonellae without the addition of IFN-γ to the culture medium or in the presence of IFN-γ (1,000 U/ml). Uninfected cells exhibited cell membrane localization of gp91phox after 2 and 12 h in culture, although slight cytoplasmic activity was noted after 12 h (Fig. 3A and B). Cells infected with S. enterica serovar Typhimurium 14028 exhibited little cytoplasmic localization of gp91phox even after 12 h of infection (Fig. 3C), but gp91phox cytoplasmic activity during infection was greatly enhanced by the addition of IFN-γ to the culture medium for 12 h (Fig. 3D). Cells infected with S. enterica serovar Typhimurium 14028 phoP, however, began to show strong cytoplasmic gp91phox activity at 12 h postinfection without addition of IFN-γ to the culture medium (Fig. 3F), while the addition of IFN-γ to the medium increased the intensity of the response even further (Fig. 3G). Further analysis, below the cell surface and at higher power, indicated that gp91phox activity was present in cytoplasmic compartments but was not strictly localized to areas which were infected by the salmonellae (Fig. 3E and H). The effect of IFN-γ (1,000 U/ml) alone on gp91phox activity in J774.2 cells was also investigated and showed that cytoplasmic localization was present after 2 h in culture with IFN-γ (Fig. 3I), and this was further enhanced by 12 h (Fig. 3J).
FIG.3.
Confocal laser scanning microscope images of gp91phox activity in J774.2 macrophages infected with salmonellae and cocultured with or without IFN-γ (1,000 U/ml). (A) Uninfected control, 2 h in culture; (B) uninfected control, 12 h in culture; (C) 14028 phoP mutant, 12 h postinfection, without IFN-γ; (D) 14028 phoP, 12 h postinfection, with IFN-γ; (E) high-power image of 14028 phoP mutant-infected cell, 12 h postinfection, without IFN-γ (image taken 4 μm below cell surface); (F) wild-type 14028, 12 h postinfection, without IFN-γ; (G) wild-type 14028, 12 h postinfection, with IFN-γ; (H) high-power image of wild-type 14028, 12 h postinfection, without IFN-γ (4 μm below cell surface); (I) IFN-γ only, 2 h in culture; (J) IFN-γ only, 12 h in culture. Yellow-green shows gp91phox activity. Open arrow shows gp91phox localization on cell membrane; solid arrow shows gp91phox cytoplasmic localization. Solid arrows in E and H show bacteria in cells. Scale bars, 10 μm.
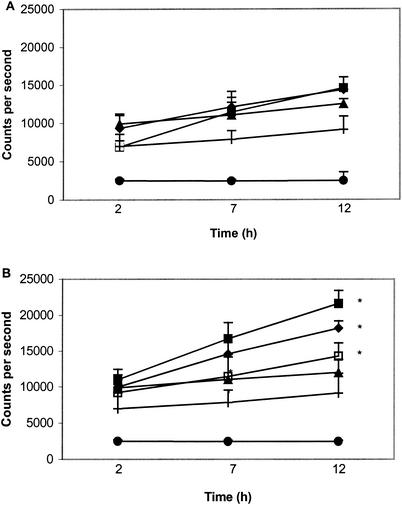
IFN-γRα expression on Salmonella-infected J774.2 cells.
Fluorometry was used to measure changes in surface expression of IFN-γRα following infection of J774.2 cells with S. enterica serovar Typhimurium 14028 or its phoP mutant, with or without the addition of IFN-γ (1,000 U/ml) to the culture medium. In cells infected with the wild type or phoP mutant, IFN-γRα expression was not significantly (P > 0.05) raised above levels detected on the surface of uninfected cells, although expression of IFN-γRα on the surface of uninfected cells was actually raised in culture during the 12-h period investigated (Fig. 4A). The addition of IFN-γ (1,000 U/ml) to the culture medium significantly (P < 0.05) elevated IFN-γRα expression on the surface of cells infected with either wild-type 14028 or the phoP mutant after 7 h, with the greatest increase measured on the surface of cells infected with wild-type 14028 (Fig. 4B).
FIG. 4.
Fluorometric microsphere analysis of IFN-γRα surface expression on J774.2 cells following Salmonella infection (MOI, 10) and cocultured with or without IFN-γ (1,000 U/ml) from 2 to 12 h postinfection. (A) Cocultured without IFN-γ; (B) cocultured with IFN-γ. ▪, wild-type 14028; ▴, 14028 phoP mutant; ♦, uninfected control; □, IFN-γ only; +, microspheres coated with bovine serum albumin; •, cultured cells not exposed to any treatment (autofluorescence). *, significant (P = 0.05) increase above the uninfected control value. Each experiment represents a mean of three replicates repeated on at least five separate occasions.
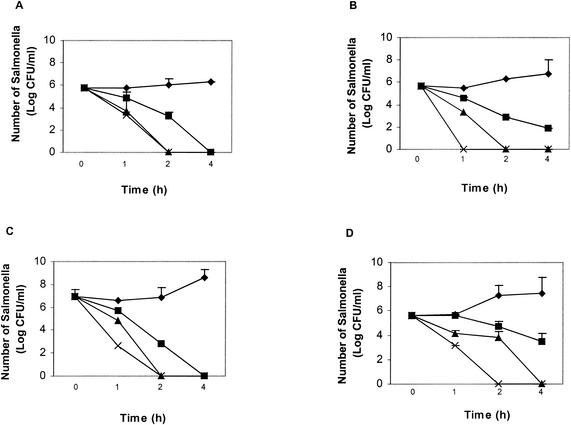
Effect of hydrogen peroxide on Salmonella survival in LB broth.
To examine whether or not some of the Salmonella strains used in this study have a natural resistance or susceptibility to ROS, we grew virulent S. enterica serovar Typhimurium 14028 and F98 and avirulent S. enterica serovar Typhimurium 14028 phoP and S. enterica serovar Infantis φr in LB broth supplemented with H2O2 (concentration range, 0.02 to 0.06%). The results indicated a comparable level of survival of all Salmonella strains tested, with a reduction in numbers within 4 h at the lowest H2O2 concentration (0.02%) and complete killing at the highest concentration (0.06%) (Fig. 5).
FIG. 5.
Survival of salmonellae in hydrogen peroxide. (A) Wild-type 14028; (B) S. enterica serovar Typhimurium 14028 phoP; (C) S. enterica serovar Typhimurium F98; (D) S. enterica serovar Infantis 1326/28 φr. Each experiment was repeated three times on three separate occasions.
DISCUSSION
The results from this study indicate that in the absence of IFN-γ, a significant elevation of ROS is induced over the first 24 h of infection in J774.2 cells infected with avirulent Salmonella strains (S. enterica serovar Typhimurium 14028 phoP and S. enterica serovar Infantis 1326/28 φr) compared with virulent wild-type strains (S. enterica serovar Typhimurium 14028 and S. enterica serovar Typhimurium F98). Cytoplasmic localization of gp91phox was also evident in cells infected with S. enterica serovar Typhimurium 14028 phoP after 2 h but was not seen in cells infected with the wild-type strain. At 12 h postinfection, the levels of cytoplasmic gp91phox activity also appeared to be much greater in cells infected with the phoP mutant. This coincided with increased growth of virulent strains compared with decreased growth of the 14028 phoP mutant or stasis in the case of S. enterica serovar Infantis 1326/28 φr and is consistent with studies which have shown that phoP mutants are avirulent in mice (12, 25) and our own studies which have shown that S. enterica serovar Infantis φr is also avirulent for mice (1; Foster et al., unpublished data).
When IFN-γ was added to the cells, low levels (10 U/ml) induced a significant fall in the number of avirulent mutants surviving over the 24-h postinfection period and to stasis of virulent Salmonella strains in J774.2 cells. Higher concentrations of IFN-γ (100 or 1,000 U/ml) increased gp91phox mRNA expression, cytoplasmic localization of gp91phox, and increased ROS activity. However, the greatest effect of IFN-γ on infected J774.2 cells was that it decreased the time between invasion and the induction of significant oxidative burst. This occurred in cells infected with wild-type and mutant strains and coincided with a reduction in the number of viable salmonellae recovered from these cells over the 24-h postinfection period.
Synergy in the induction of expression of IFN-γRα was indicated by increased expression in cells infected with salmonellae and cocultured in IFN-γ (1,000 U/ml) compared with expression of IFN-γRα both in cells infected with salmonellae and in uninfected cells cocultured with IFN-γ. Impaired absorption of IFN-γ to both J774 and P388D murine macrophage cells in comparison with murine peritoneal macrophages has been shown previously. Decreased binding of IFN-γ-coated fluorescent microspheres has been shown, at least for P388D (3). However, our study suggests that IFN-γ, acting alone, still induces increased cytoplasmic accumulation of gp91phox and significant levels of ROS in J774.2 cells and significantly increases IFN-γRα expression, which indicates a more efficient utilization of IFN-γ in these cells than was previously suggested (3). Further evidence to support the hypothesis that ROS were responsible for decreased Salmonella viability within the first 24 h was obtained by the addition of ACC to the culture medium. ACC significantly increased the number of S. enterica serovar Typhimurium 14028 wild-type and phoP mutant cells recovered from J774 cells even in the presence of IFN-γ (1,000 U/ml) within the first 24 h postinfection, and this coincided with a significant decrease in levels of ROS.
The relative timing of the induction of killing pathways in our J774.2 model system agrees with previous studies on knockout mice which have shown that oxidative pathways are required to reduce Salmonella numbers and host survival early postinfection, whereas nitrosative pathways are important later in infection (24). The results of our study may explain this phenomenon in that they suggest that this difference may be due to a difference in the responsiveness of these pathways to IFN-γ and that this is due to the exposure period rather than concentration, ROS being elevated more readily in response to IFN-γ at low concentrations and over a shorter period of time. We therefore hypothesize that uninfected macrophages respond to an environment of low IFN-γ concentration, such as may occur early in infection, to produce ROS rather than reactive nitrogen species and that IFN-γ itself does not augment the response.
Previous studies have also shown the direct importance of IFN-γ in its capacity to enhance macrophage killing of salmonellae (18, 20, 22). Splenic T lymphocytes of BALB/c mice infected with S. enterica serovar Typhimurium SL3261 aroA produce increased levels of IFN-γ after 48 h, leading to immune protection (13), while the viability of S. enterica serovar Typhimurium in the livers and spleens of IFN-γR−/− mice is significantly increased within 5 days postinfection compared to normal mice (23). Investigations into the importance of IFN-γ in the early stages of infection have shown that the viability of S. enterica serovar Typhimurium C5 is increased in CBA mice if the mice are inoculated with antibodies to IFN-γ 24 h before infection (27) or that IFN-γ may have a bacteriostatic effect within the first week of infection with virulent strain S. enterica serovar Typhimurium SL1344 (26). IFN-γ is also the recommended therapeutic agent for patients suffering from chronic granulomatous disease (8, 9), in which defective phox genes (and consequently reduced induction of ROS) lead to recurrent bacterial infections (6, 28, 29).
IFN-γ therapy enhances phagocytic oxidative killing of bacteria, possibly at the level of the NADPH phagocytic oxidase-producing genes (4, 7). All of these studies are consistent with our current findings that IFN-γ affects the establishment of infection by virulent strains of Salmonella in macrophages by stimulating an enhanced phagocytic oxidase response leading to an increased and prolonged oxidative burst.
We have established that, unless IFN-γ is present, the induction of ROS in J774.2 macrophages infected with a virulent S. enterica serovar Typhimurium strain is impaired compared to that induced in cells infected with a phoP mutant. However, the virulent Salmonella strain used in our study did not have an innate resistance to ROS, as shown by equivalent growth of virulent and avirulent mutants in broth containing H2O2. Mycobacteria survive in macrophages for extended periods of time, and Mycobacterium tuberculosis is able to downregulate IFN-γ-stimulated HLA-DR expression (16), while Mycobacterium avium downregulates IFN-γRα on murine macrophages, leading to disruption of the IFN-γ signaling pathway (17). Although our investigation shows clearly that wild-type S. enterica serovar Typhimurium 14028 does not downregulate IFN-γRα, we do not know if subsequent Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways were affected, and this should be elucidated in future studies.
Our study has shown that IFN-γ and the subsequent upregulation of IFN-γRα are important in macrophage killing of more persistent strains of S. enterica serovar Typhimurium by stimulating an earlier and more prolonged increase in ROS. This occurs via its cytoplasmic localization, but regulation at the level of transcription may also be involved. However, this requirement for IFN-γ may allow dissemination of the infection in mice until adequate stimulation of macrophages by IFN-γ-producing cells can be achieved. In comparison, avirulent strains, which induce high levels of ROS via gp91phox in J774.2 cells without the associated IFN-γRα upregulation or the need for IFN-γ, may be killed in macrophages before they are allowed to disseminate.
Acknowledgments
We thank Elaine Bennett for assistance with cell culture.
This work was supported by a European Union grant (Fair 98-4006) and the Department of Food, the Environment and Rural Affairs, United Kingdom.
Editor: B. B. Finlay
REFERENCES
- 1.Barrow, P. A., M. B. Huggins, and M. A. Lovell. 1994. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect. Immun. 62:4602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchmeier, N. A., C. J. Lipps, Y. Magdalene, Y. So, and F. Heffron. 1993. Recombinant deficient mutants of Salmonella are avirulent and sensitive to oxidative burst. Mol. Microbiol. 7:933-936. [DOI] [PubMed] [Google Scholar]
- 3.Cellada, A., P. W. Gray, E. Rinderknecht, and R. D. Shreiber. 1984. Evidence for a gamma-receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condino-Neto, A., and P. E. Newburger. 2000. Interferon-gamma improves splicing efficiency of CYBB gene transcripts in an interferon responsive variant of chronic granulomatous disease due to splice site consensus region mutation. Blood 95:3548-3554. [PubMed] [Google Scholar]
- 5.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vasquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94:13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinauer, M. C., L. L. Ling, H. Bjorgvinsdottir, C. Ding, and N. Pech. 1999. Long term correction of phagocyte NADPH oxidase activity by retroviral-mediated gene transfer in murine X-linked chronic granulomatous disease. Blood 94:914-922. [PubMed] [Google Scholar]
- 7.Eklund, E. L., W. Luo, and D. G. Skalnik. 1996. Characterization of three promoter elements and cognate DNA binding protein(s) necessary for IFN-γ induction of gp91-phox transcription. J. Immunol. 157:2418-2429. [PubMed] [Google Scholar]
- 8.Ezekowitz, R. A., M. C. Dinauer, H. S. Jaffe, H. Orkin, and P. E. Newburger. 1988. Partial correction of the phagocyte defect in patients with chronic granulomatous disease by subcutaneous interferon gamma. N. Engl. J. Med. 319:146-151. [DOI] [PubMed] [Google Scholar]
- 9.Ezekowitz, R. A., C. A. Sieff, M. C. Dinauer, C. Nathan, S. H. Orkin, and P. E. Newburger. 1990. Restoration of phagocyte function by interferon-gamma in X-linked chronic granulomatous disease occurs at the level of the progenitor cell. Blood 76:2443-2448. [PubMed] [Google Scholar]
- 10.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, N., M. A. Clark, M. A. Jepson, and B. H. Hirst. 1998. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M cells in vivo. Vaccine 16:536-541. [DOI] [PubMed] [Google Scholar]
- 12.Groisman, E. A., and M. H. Saier. 1990. Salmonella virulence: new clues to intramacrophage survival. Trends Biochem. Sci. 15:30-33. [DOI] [PubMed] [Google Scholar]
- 13.Harrison, J. A., B. Villarreal-Ramos, P. Mastroeni, R. Demarco De Hormaeche, and C. E. Hormaeche. 1997. Correlates of protection induced by live Aro− Salmonella typhimurium vaccines in the murine typhoid model. Immunology 90:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Held, T. K., W. Xiao W. Yuan, D. V. Kavakolanu, and A. S. Cross. 1999. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect. Immun. 67:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heremans, H., J. Van Damme, C. Dillon, R. Dijkmans, and A. Billau. 1990. Interferon gamma, a mediator of lethal Schwartzman-like shock reaction in mice. J. Exp. Med. 171:1853-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hmama, Z., R. Gabathuler, W. A. Jefferies, G. De Jong, and N. E. Reiner. 1998. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immiature class II heterodimers. J. Immunol. 161:4882-4893. [PubMed] [Google Scholar]
- 17.Hussain, S., B. S. Zwilling, and W. P. Lafuse. 1999. Mycobacterium avium infection of mouse macrophages inhibits IFN-γ Janus kinase-STAT signalling and gene induction by downregulation of the IFN-γ receptor. J. Immunol. 163:2041-2048. [PubMed] [Google Scholar]
- 18.Kagaya, K., K. Watnabe, and J. Fukazawa. 1989. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect. Immun. 57:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman, S. H. E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 20.Kincy-Cain, T., J. D. Clements, and L. Bost. 1996. Endogenous and exogenous interleukin-12 augment protective immune response in mice orally challenged with Salmonella dublin. Infect. Immun. 64:1437-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leusen, J. H. W., M. deBoer, B. G. J. M. Bolscher, P. M. Hilaries, R. S. Weening, H. J. Ochs, D. Roos, and A. J. Verhoeven. 1994. A point mutation in gp91phox of cytochrome b558 of human NADPH oxidase leading to defective translocation of the cytosolic proteins p47-phox and p67-phox. J. Clin. Investig. 93:2120-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastroeni, P., J. A. Harrison, J. A. Chabalgoity, and C. E. Hormaeche. 1996. Effect of interleukin-12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect. Immun. 64:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastroeni, P., S. Clare, S. Khan, J. E. Harrison, C. E. Hormaeche, H. Okamura, M. Kurimoto, and G. Dougan. 1999. Interleukin-18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastroeni, P., A. Vasquez-Torres, F. C. Fang, Y. Yisheng, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival. J. Exp. Med. 192:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, S. I., and J. J. Mekalanos. 1990. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 172:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muotiala, A., and P. H. Makela. 1993. Role of gamma interferon in late stages of murine salmonellosis. Infect. Immunol. 61:4248-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauciel, C., and F. Espinasse-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rae, J., P. E. Newburger, M. C. Dinauern, D. Noack, P. J. Hopkins, R. Kuruto, and J. T. Curnutte. 1998. X-linked chronic granulomatous disease: mutations in the CYBB gene encoding the gp91-phox component of the respiratory burst oxidase. Am. J. Hum. Genet. 62:1320-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos, D., M. de Boer, F. Kuribayashi, C. Meischel, R. Weening, A. Segal, A. Ahlin, K. Nemet, J. Hossle, E. Bernatowska-Matuszkiewicz, and H. Middleton-Price. 1996. Mutations in the x-linked and autosomal recessive forms of chronic granulomatous disease. Blood 87:1663.. [PubMed] [Google Scholar]
- 30.Segal, A. W. 1974. Nitroblue-tetrazolium tests. Lancet ii:1248-1252. [DOI] [PubMed]
- 31.Segal, A. W., and K. P. Shatwell. 1997. The NADPH oxidase of phagocytic leucocytes. Ann. N.Y. Acad. Sci. 832:215-222. [DOI] [PubMed] [Google Scholar]
- 32.Vasquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulous, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]