Abstract
Ehrlichia chaffeensis, an obligate intracellular, tick-transmitted bacterium, is susceptible to antibody-mediated host defense, but the mechanism by which this occurs is not understood. One possible explanation is that antibodies directly access the bacteria in the extracellular environment of the host, perhaps during bacterial intercellular transfer. Accordingly, we investigated whether bacteria could be found outside of host cells during infection. Host cell-free plasma obtained from infected mice was found to contain ehrlichiae, and the host cell-free ehrlichiae readily transferred disease to susceptible SCID recipients. The host cell-free ehrlichiae were found during infection of both immunocompetent BALB/c and immunocompromised BALB/c-scid mice and reached levels as high as 108/ml in plasma during persistent infection in SCID mice. Approximately 10% of the blood-borne bacteria were found outside of host cells. Although it is generally accepted that replication of ehrlichiae occurs only within host cells, the cell-free bacteria were shown to undergo DNA replication and cell division in vitro for 3 to 5 days when incubated at 37°C in plasma. Paradoxically, both infectivity and virulence were lost after 24 h of ex vivo culture. The data indicate that E. chaffeensis is exposed to the extracellular milieu during infection, presumably during intercellular transfer, and reveal that these intracellular bacteria do not require the environment of the host cell for replication. Our findings reveal a possible mechanism by which antibodies can access the intracellular bacteria upon their release into the extracellular milieu and mediate host defense and also have implications for understanding the replication and transmission of this vector-borne pathogen.
Ehrlichia chaffeensis is an obligate intracellular monocytotropic bacterium that is the etiologic agent of human monocytic ehrlichiosis, a toxic shock-like illness (4, 10). The bacterium is transmitted by the tick vector Amblyomma americanum. How host defense is mediated towards the ehrlichiae is not well understood, but T cells appear to play an essential role in the immune response (9, 14, 39), and immunodeficient individuals are particularly susceptible to serious infections (28). However, we and others have also demonstrated that antibodies can be effective during this intracellular infection (27, 39). Our previous studies demonstrated that passive transfer of antibodies could provide long-term protection to susceptible SCID mice (22, 39). Although not a model for humoral immunity in immunocompetent mice or humans, SCID mice provide a means to investigate the function of antibodies in the absence of cellular responses, which can obscure the activity of antibodies.
Antibodies were found to be highly effective in SCID mice even when the antibodies were administered after infection had been well established, and the effects of the antibodies were often evident as early as 24 to 48 h after administration (39). Highly effective antibodies were found to recognize immunodominant outer membrane proteins. The outer membrane proteins in E. chaffeensis and related ehrlichiae comprise families of related proteins that exhibit antigenic variation (13, 26, 27, 31, 41). During infection of cattle by the ehrlichial pathogen Anaplasma marginale, antigenic phase variants are generated and selected, presumably under selection by antibodies (12). These data together provide support for the importance of the humoral immune response during ehrlichial infection in immunocompetent hosts and for the possible use of antibodies for therapeutic or prophylactic use in immunocompromised individuals.
Effective humoral immunity has also been demonstrated to occur during infection by other intracellular bacteria. Robust antibody responses are typically generated against intracellular bacterial pathogens, and in several cases humoral responses have been protective (5, 7, 19, 24). Humoral immunity during intracellular bacterial infections has often been considered paradoxical, however, because the infected host cells are thought to provide a protective niche for the pathogens. When antibodies have been demonstrated to be important, they have been proposed to function by various mechanisms, and often the mechanism is pathogen specific (3, 6). It is not known how antibodies mediate immunity during ehrlichial infection. In particular, it is not known how and where antibodies might access the ehrlichiae.
Because there is no evidence that antibodies have direct access to the ehrlichiae in the host macrophage, it was reasoned that the particular efficacy of antibodies during E. chaffeensis infection might reveal unexpected features of the bacterium's life cycle in the host. In the current study, we speculated that ehrlichiae were exposed to antibodies in the host extracellular milieu, perhaps during intercellular spreading, and so we investigated whether bacteria could be found outside of host cells during infection in vivo. Our findings reveal that significant numbers of infectious bacteria can be found outside of host macrophages, providing a possible mechanism to explain the susceptibility of these bacteria to antibodies. Our studies also led to the unexpected observation that E. chaffeensis retains a limited capacity to persist and replicate outside of the environment of the host cell, a finding that may have relevance for our understanding of ehrlichial microbiology and host-to-vector transmission.
MATERIALS AND METHODS
Mice and infections.
BALB/c-scid and BALB mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) or bred in the Wadsworth Center Animal Care Facility under institutional guidelines for animal care and use. BALB/c-scid mice were infected with 1 × 106 to 2 × 106 infected SCID splenocytes or similar numbers of infected DH82 cells via the peritoneum, as described previously (38). Quantitative PCR analyses revealed that this inoculum contained approximately 108 bacterial genomes. The mice were sacrificed when moribund, typically at day 21 postinfection. Morbidity was characterized by a lack of mobility, hunched posture, ruffled fur, and a pronounced loss of >30% of initial body weight (21). Blood samples were taken by cardiac puncture and collected in tubes containing EDTA as an anticoagulant. Tissue samples were harvested and stored at −80°C for further analyses.
For antibody treatment, mice were administered three doses of 200 μg of the outer membrane protein-specific monoclonal antibody (MAb) Ec56.5 (22) or an isotype-matched control antibody, KJ1-26 (16), via the peritoneum on days 6, 12, and 18 postinfection, and the mice were harvested on day 22 postinfection. Samples of liver, spleen, peritoneal exudate, peripheral blood mononuclear cells (PBMCs), and plasma were harvested and stored at −80°C.
Plasma and PBMC isolation.
Blood samples were mixed with anticoagulant during collection and immediately centrifuged at 230 × g for 10 min to separate the cellular fraction from the plasma. The collected plasma was subsequently passed through a 5-μm filter to ensure that any residual PBMCs were removed. The supernatant plasma fraction and the filtrate were analyzed histochemically (Diff-Quik; Dade Behring AG, Berne, Switzerland) to monitor the samples for the presence of any contaminating PBMCs. The PBMCs were purified from the cellular fraction after lysis of erythrocytes with an ammonium chloride lysing solution (150 mM NH4Cl, 10 mM NaHCO3, 1 mM disodium EDTA, pH 7.4). To monitor infectivity, plasma (200 μl) or PBMCs (1 × 106 cells) were transferred to BALB/c-scid recipients via the peritoneum.
Quantitation of bacteria in tissue and plasma.
Bacteria were quantitated in tissue samples by quantitative PCR analyses of the bacterial 16S ribosomal DNA (rDNA) gene, as described previously (21). To facilitate quantitation of small quantities of cell-free bacterial DNA, uninfected PBMCs from BALB/c-scid mice were added to the infected plasma samples prior to DNA extraction to provide a source of carrier DNA. The copy number of the bacterial 16S rDNA gene was determined by quantitative PCR, as described previously (21).
The bacteria were also enumerated by fluorescence assay with the Live/Dead BacLight bacterial viability kit (Molecular Probes, Eugene, Oreg.). The method used the SYTO9 green-fluorescent nucleic acid stain, which labels both viable and nonviable bacteria, and propidium iodide red-fluorescent nucleic acid stain, which labels nonviable bacteria and bacteria with damaged membranes. One hundred microliters of plasma was microcentrifuged at 10,000 × g for 10 min, and the bacteria were resuspended and washed twice in Hanks' balanced salt solution, followed by incubation in a 100-μl mixture of SYTO9 and propidium iodide, as described in the manufacturer's protocol. Ten microliters of the bacterial suspension was spotted on a microscope slide, and the number and percentage of viable bacteria within a series of three fields at 400× magnification were enumerated by epifluorescence microscopy.
Immunofluorescence assays.
Plasma samples were adsorbed onto microscope slides by centrifugation (Cytospin 3; Shandon Southern Instruments, Camberly, England), and the slides were air dried. In the absence of fixation, the slides were blocked with 10% normal goat serum in phosphate-buffered saline (PBS) at 4°C for 30 min, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated MAb Ec56.5 or an irrelevant isotype-matched antibody (FITC-conjugated KJ1-26) (16), at a concentration of 10 μg/ml at 4°C overnight. The slides were washed three times in PBS and analyzed with a Zeiss Axioskop fluorescence microscope, an AxioCam digital camera, and AxioVision 3.0 digital imaging software (Thornwood, N.Y.).
[3H]thymidine incorporation assays.
Freshly isolated plasma samples from infected BALB/c-scid mice were untreated or heat treated at 65°C for 30 min. Plasma samples were aliquoted into the wells of a 96-well flat-bottomed tissue culture plate (200 μl/well; Costar, Corning Incorporated, Corning, N.Y.), in triplicate. Plasma from uninfected BALB/c-scid mice was included as a control. [3H]thymidine (Amersham, Piscataway, N.J.) was added at a final concentration of 10 nCi/ml, and the microtiter plate was incubated in 5% CO2 at 37°C for 5 days. For measurement of [3H]thymidine incorporation, the cultures were collected on a glass fiber filter mat (Wallac, Gaithersburg, Md.) with a microtiter plate harvester (Tomtec, Hamden, Conn.). The filter was washed five times to remove unincorporated thymidine, the filter mat was air dried, and the [3H]thymidine remaining on the filter was enumerated with a beta plate liquid scintillation counter (Wallac).
Infectivity and virulence of cell-free ehrlichiae.
The infectivity of the cell-free ehrlichiae was determined by assay in the DH82 canine macrophage cell line (8). The DH82 cells (2 × 104 cells in 100 μl) were seeded in a 96-well flat-bottomed tissue culture plate (Costar, Corning Incorporated), and the cell monolayers were inoculated with 10-fold serial dilutions of plasma (100 μl, diluted in culture medium). The plates were incubated in 5% CO2 at 37°C for 7 to 8 days. Culture medium was removed from the wells, and the cell monolayers were washed twice with PBS and fixed with 2% paraformaldehyde in PBS for 20 min. The fixed cells were washed and blocked with 1% nonfat milk in PBS containing 0.1% saponin (Sigma, Saint Louis, Mo.). The cell monolayers were incubated with FITC-labeled MAb Ec56.5 (1:300; diluted in PBS containing 0.1% saponin) at room temperature for 2 h or at 4°C overnight. After incubation, the cell monolayers were washed twice with PBS containing 0.1% saponin and then twice with PBS, followed by addition of 100 μl of PBS per well. Each well was monitored by immunofluorescence assay for bacterial infection with an Olympus IX70 fluorescence microscope. The number of infected wells per plate at limiting dilution was used to calculate the concentration of infectious bacteria per milliliter in the test sample.
To measure bacterial virulence, serial 10-fold dilutions of infected plasma were transferred into SCID mice, and body weight change was monitored weekly, as described previously (21). The absolute number of bacteria in the inoculum was first measured by quantitative PCR, and the minimal infectious dose required to cause weight loss and morbidity in SCID mice was determined.
Ex vivo culture of infected peritoneal cells.
Peritoneal exudate cells (PECs) from infected and uninfected mice were collected after intraperitoneal injection of 6 ml of cold Eagle's minimal essential medium (MEM) containing 10% heat-inactivated fetal bovine serum and 2 mM l-glutamine. Peritoneal fluid was drawn through the abdominal wall with a 23-gauge needle. The fluid was centrifuged at 700 × g for 10 min, and the cells were washed twice with HBSS. Washed PEC suspensions were adjusted to 2 × 106 cell/ml in Eagle's MEM with 10% fetal bovine serum and 2 mM l-glutamine. An aliquot was also taken from the PEC suspension and treated with 10 μg of outer membrane protein-specific MAb Ec56.5 per ml. The cells were immediately seeded into a 96-well tissue culture plate (Costar, Corning Incorporated) and cultured in 5% CO2 at 37°C for 48 h. Samples of PEC-free culture supernatant were harvested in triplicate for PCR analysis. The viability of the PECs was assayed with the trypan blue dye exclusion method.
Statistical analyses.
The statistical significance of the data obtained from experimental groups was determined with the Mann-Whitney test (34). Calculated P values of 0.05 or less were considered significant.
RESULTS
Cell-free bacteria detected in the plasma of E. chaffeensis-infected mice.
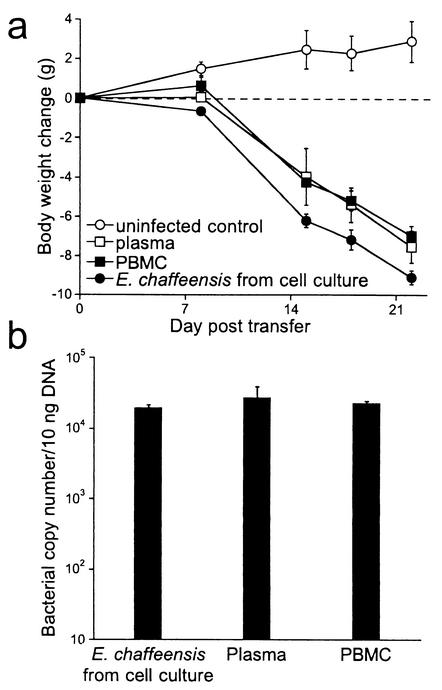
To determine whether bacteria could be found outside the confines of host cells during infection, peripheral blood was collected from day 21 infected BALB/c-scid mice and separated into PBMCs and plasma fractions by centrifugation. No mononuclear cells were detected in the plasma supernatant with histochemical staining techniques. The plasma was typically passed through a 5-μm filter to eliminate any possibility that nucleated blood cells may have sedimented, although the filtration step was not required. The cell-free plasma (0.2 ml) and the isolated PBMCs (106 cells) both induced disease after transfer to susceptible SCID mice (Fig. 1). The recipient mice exhibited pronounced body weight loss and levels of liver infection characteristic of animals infected with E. chaffeensis obtained from cell culture. These findings indicated that the cell-free plasma was a source of infectious ehrlichiae.
FIG. 1.
Transfer of plasma from E. chaffeensis-infected mice caused disease in recipient SCID mice. (a) Groups of three BALB/c-scid mice were uninfected or injected with either 200 μl of plasma, 106 PBMCs isolated from 21-day-infected SCID mice, or 106 E. chaffeensis-infected DH82 cells via the peritoneum. Body weight changes were monitored at weekly intervals. The average body weight and standard deviation of groups of three mice are shown. Statistical comparison of each group of infected mice with the uninfected control group revealed P values of 0.05, as determined with the Mann-Whitney test. (b) Quantitation of bacterial colonization in the livers of mice from panel a on day 22 posttransfer. The data are representative of three independent experiments.
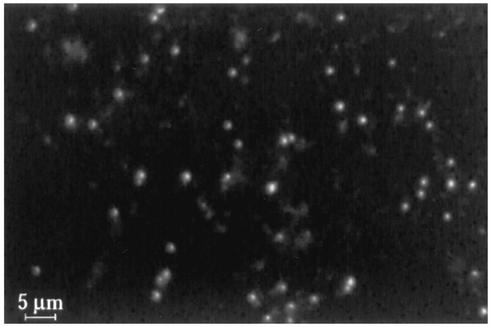
To visualize the bacteria in the host cell-free fraction, the plasma was centrifuged onto microscope slides with a Cytospin apparatus, and the bacteria were detected with the previously described outer membrane protein-specific antibody Ec56.5 (22). The analysis was performed in the absence of fixation and detergent permeabilization in order to reduce the possibility that bacteria were detected within host membranes. Control experiments were performed to confirm that bacteria were not detected within host cells in the absence of permeabilization. Cell-free bacteria were readily detected with this approach (Fig. 2). The bacteria were pleomorphic and ranged in size from 0.5 to 1 μm, consistent with measurements obtained from ultrastructural studies of intracellular ehrlichiae (30, 32).
FIG. 2.
Detection of cell-free ehrlichiae by direct immunofluorescence assay. Freshly isolated plasma from infected mice was centrifuged onto microscope slides with a Cytospin apparatus and air dried. The slides were blocked and incubated with the E. chaffeensis-specific FITC-conjugated MAb Ec56.5 in the absence of permeabilization or fixation. The cell-free ehrlichiae ranged in size from 0.5 to 1 μm (scale bar, 5 μm). No staining was observed with an isotype-matched control antibody (not shown).
Detection of extracellular ehrlichiae during infection.
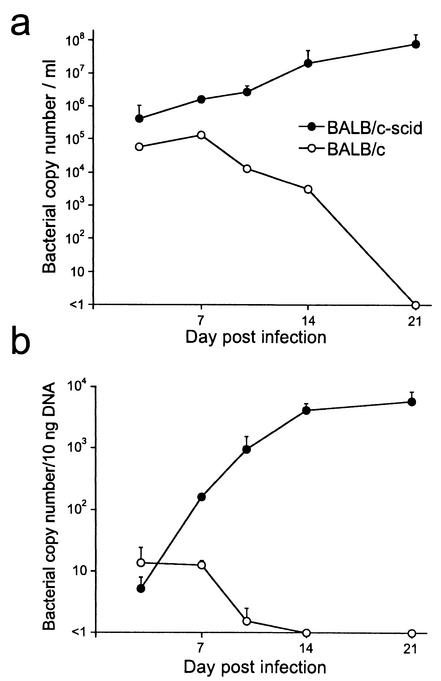
To determine when the bacteria were present in the cell-free plasma, the extracellular ehrlichiae were enumerated at various times after infection of both immunocompromised BALB/c-scid and immunocompetent BALB/c mice. The latter mice are known from previous studies to exhibit transient E. chaffeensis infection that is resolved within 14 to 21 days (38). Extracellular bacteria were detected in the plasma within 3 days postinfection in both BALB/c and BALB/c-scid mice (Fig. 3a). The number of cell-free ehrlichiae in the BALB/c mice reached a maximum of 105/ml at 7 days postinfection and declined to undetectable levels within 21 days postinfection. In the SCID mice, the cell-free bacteria increased exponentially for 21 days, at which time the mice were moribund and were sacrificed.
FIG. 3.
Detection of cell-free ehrlichiae in plasma during infection. BALB/c and BALB/c-scid mice were inoculated with 106 E. chaffeensis-infected, sex-matched BALB/c-scid splenocytes (approximately 108 genome units) via the peritoneum. Groups of three mice were harvested at various times postinfection, and the bacterial numbers in plasma and liver tissues were measured by quantitative PCR. The kinetics of the appearance of cell-free ehrlichiae in plasma are shown in a, and colonization of the liver is shown in b. The BALB/c-scid mice became moribund and succumbed to infection within 21 days postinfection. The experiments were repeated at least two times with similar results. Mean and standard deviation are indicated.
Bacterial numbers in the plasma increased approximately 10-fold between days 7 and 14 postinfection, but colonization of the liver increased by nearly 100-fold during the same period (Fig. 3a and 3b). The numbers of cell-free ehrlichiae were approximately 10-fold higher in the SCID mice relative to the peak level in BALB/c mice on day 7 postinfection. Bacterial numbers in the plasma of the SCID mice reached levels as high as 108/ml by day 21 postinfection. The rate of decline of infection in the BALB/c mice was similar in plasma and liver. The apparent prolonged infection in the plasma versus the liver in the BALB/c mice on day 14 postinfection was probably because the cell-free bacteria were more readily detected than those in liver tissue. These findings demonstrate that extracellular ehrlichiae are found throughout infection in both immunocompetent and immunocompromised mice.
Survival and replication of cell-free bacteria outside of host cells.
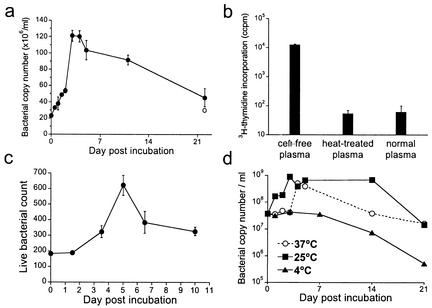
Obligate intracellular bacteria such as ehrlichiae are generally considered to be incapable of replication outside the environment of the host cell (18). Moreover, the ehrlichiae are not thought to survive outside of host cells for more than brief periods. We considered, however, that the environment of the plasma might promote survival or perhaps even replication of the ehrlichiae outside the host cell environment. To test these hypotheses, the cell-free bacteria were incubated in plasma at 37°C, and bacteria were quantitated at several intervals thereafter (Fig. 4). Bacterial copy number, detected by PCR, was found to increase for the first 3 to 5 days of ex vivo culture (Fig. 4a). Bacterial copy numbers increased by 6- to 14-fold within 3 to 5 days and declined gradually to starting levels within approximately 3 weeks. Although the reason for the decline in copy number is not known, it was probably not due to degradation of free DNA by plasma DNases, since experimental treatment of the cell-free bacteria with DNase I did not significantly reduce the copy number detected by PCR.
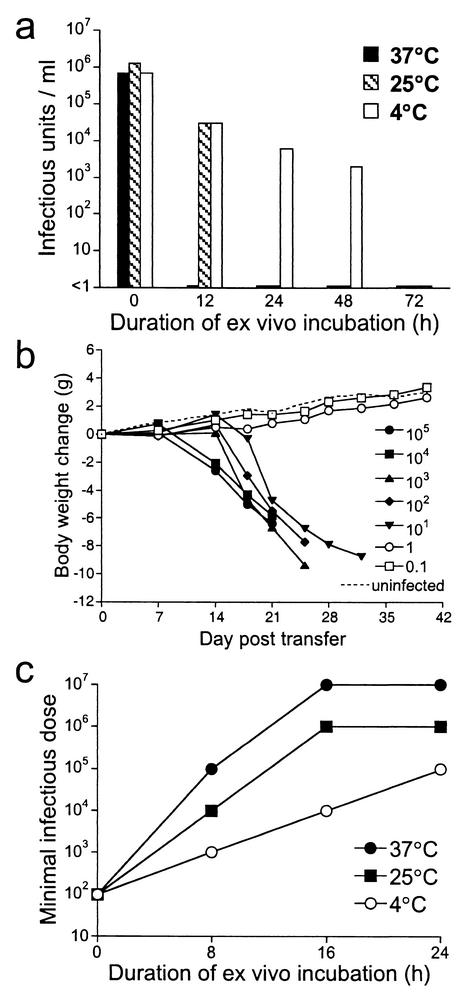
FIG. 4.
Replication of cell-free ehrlichiae. (a) Plasma was isolated from infected BALB/c-scid mice and incubated at 37°C, and the copy number of the cell-free ehrlichiae was determined by quantitative PCR at various intervals. On day 22 postincubation, an aliquot of the plasma was treated with DNase I prior to DNA extraction (open circle). (b) Freshly isolated cell-free ehrlichiae were incubated in plasma at 37°C in the presence of [3H]thymidine for 5 days, and incorporation of [3H]thymidine was measured. Cell-free ehrlichiae that had been heat treated at 65°C for 30 min and normal mouse plasma incubated under the same conditions were used as controls. The experiment shown is representative of two separate experiments. The error bars indicate the standard deviation observed from triplicate samples. (c) Freshly isolated cell-free ehrlichiae were isolated and stained with SYTO9 and propidium iodide, and the relative numbers of viable bacteria were enumerated by epifluorescence at various intervals during ex vivo incubation at 37°C. The data represent the mean and standard deviation of counts obtained from three randomly chosen fields at 400× magnification. (d) Plasma samples isolated from infected BALB/c-scid mice were incubated at the indicated temperatures, and the ehrlichial copy number was measured at various intervals. Data are representative of four independent experiments.
The increase in copy number suggested that the bacteria had undergone three to four cell divisions during the incubation period. To confirm that the increase in copy number was due to DNA replication, ex vivo culture of the cell-free ehrlichiae was performed in the presence of [3H]thymidine, and incorporation of the radiolabel in DNA was measured 5 days later (Fig. 4b). Thymidine incorporation in the cell-free bacteria was over 100-fold higher than background incorporation obtained with nonviable, heat-treated bacteria or plasma from uninfected mice, which corroborated the PCR data.
To address whether DNA replication and thymidine incorporation were accompanied by cell division during the ex vivo incubation, the cell-free bacteria were enumerated directly by microscopy. The cell-free bacteria were stained with the membrane-permeating green-fluorescent nucleic acid stain SYTO9, which detects both viable and nonviable bacteria, and the membrane-nonpermeating red-fluorescent nucleic acid stain propidium iodide, which is excluded by viable bacteria with intact membranes. The number and viability of the cell-free bacteria were enumerated by epifluorescence microscopy. The number of ehrlichiae that excluded propidium iodide increased by at least threefold during the first 5 days of ex vivo incubation at 37°C (Fig. 4c), and the apparent viability remained at approximately 90% during this period (data not shown). As was observed in the PCR assays, the onset of bacterial replication was delayed for 2 to 3 days after ex vivo culture. It is possible that data obtained by direct measurement represent an underestimate of the total bacterial numbers, because the assay may have scored doublets as single bacteria. Alternatively, it is possible that the cell-free bacteria experience physiological stress when released into the extracellular milieu, which may cause defective cell division despite normal DNA replication (29). These interpretations may explain the apparent lower replication observed with the direct assay relative to the PCR assay.
Because E. chaffeensis is transmitted to mammalian hosts by the tick Amblyomma americanum, the bacteria typically encounter diverse physiological environments during their transmission cycle. Therefore, we investigated whether temperature affected the capacity for replication of the extracellular bacteria in vitro. A similar increase in bacterial copy number was observed when cell-free ehrlichiae were incubated at either 25°C or 37°C. In contrast, incubation of cell-free bacteria at 4°C abrogated the increase in copy number (Fig. 4d). Thus, replication could occur at either physiological or ambient temperature, but it did not occur in the cold.
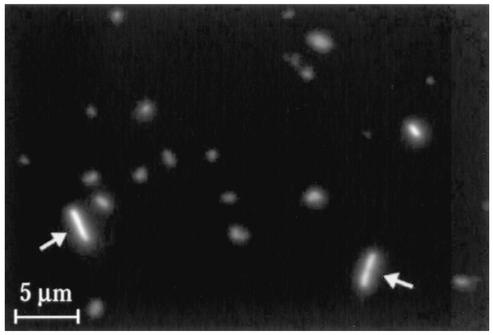
Finally, direct immunofluorescence assay of the cell-free ehrlichiae was performed after the infected plasma was incubated for 5 days at 37°C. At this time, some bacteria were elongated and appeared to have undergone binary fission (Fig. 5). These data, from four independent assays, provide strong evidence that, under these conditions, the ehrlichiae replicate outside of host cells.
FIG. 5.
Immunofluorescence analysis of cell-free ehrlichiae after ex vivo incubation. Cell-free plasma obtained from infected SCID mice was incubated at 37° for 5 days, and the immunofluorescence assay for E. chaffeensis was performed with FITC-conjugated MAb Ec56.5. Some bacteria appeared to have undergone cell division by binary fission (arrows). A scale bar is indicated.
Cell-free ehrlichiae rapidly lose both infectivity and virulence.
Although the cell-free ehrlichiae replicated outside of host cells, it was not known whether the bacteria remained infectious within this environment. Infectivity was tested in vitro after incubation of the cell-free bacteria in plasma at various temperatures for as long as 72 h. Serial dilutions of cell-free bacteria that had been incubated for various periods were cultured on monolayers of DH82 cells in 96-well tissue culture plates for 7 to 8 days to allow propagation of infectious ehrlichiae. The cell monolayers were fixed and permeabilized, and wells containing bacteria were detected by staining with FITC-labeled ehrlichia-specific MAb Ec56.5. The concentration of infectious units in the plasma was determined by enumerating the number of positive wells per plate at limiting dilution. In vitro infectivity of the cell-free bacteria was lost within 12 to 24 h after incubation at either 25°C or 37°C (Fig. 6a). Loss of infectivity occurred more slowly when the cell-free ehrlichiae were incubated at 4°C. Infectious ehrlichiae were detectable for as long as 2 days after incubation, and the infectivity was lost after 3 days of incubation at 4°C (Fig. 6a).
FIG. 6.
Infectivity and virulence of cell-free ehrlichiae. (a) Cell-free ehrlichiae in plasma were incubated in vitro at the indicated temperatures for 12 to 72 h. At various times postincubation, infectious bacteria were enumerated in vitro at limiting dilution with an in vitro infection assay. (b) Virulence of freshly isolated cell-free ehrlichiae was demonstrated by their ability to cause disease in the susceptible SCID mice. The minimal infectious dose, which is inversely correlated with virulence, was determined in vivo by injection of serial dilutions of cell-free ehrlichiae to BALB/c-scid mice via the peritoneum, and weight change was monitored in the recipient mice. The legend indicates the approximate copy number of ehrlichiae used for inoculation. (c) Infected cell-free plasma was incubated for 0 to 24 h at the indicated temperatures, and the minimal infectious dose required to cause disease and morbidity in SCID recipients was determined. The experiments were repeated two times with similar results.
To assay virulence, serial dilutions of freshly isolated cell-free ehrlichiae were transferred to SCID recipients, and the recipient mice were monitored for disease and morbidity, as indicated by weight loss. SCID mice appear to lack any ability to control the infection, because a sharp titration endpoint was observed when as few as 1 to 10 bacteria were administered (Fig. 6b). The virulence assay, which was more sensitive than the infectivity assay, provided a means of quantifying the number of virulent bacteria in culture and suggested that most, if not all, of the freshly isolated cell-free bacteria were viable and virulent.
To determine whether virulence was lost during in vitro culture, dilutions of cell-free bacteria that had been cultured in vitro for various periods were transferred to SCID recipient mice, and the dilutions that were required to induced disease in SCID recipients were determined, as for Fig. 6b. The bacterial copy number in each of the samples was determined by PCR and used to calculate the minimum infectious dose. A loss of virulence was correlated with the observation that larger doses of bacteria were required to induce disease. The number of virulent bacteria remaining after in vitro culture declined rapidly, and after 16 h of incubation at either 25°C or 37°C very few virulent bacteria were detected in the cultures (Fig. 6c). As was observed in the infectivity assays, the loss of virulence was delayed when the ehrlichiae were incubated at 4°C. These data revealed that although the ehrlichiae were capable of replication for as long as 5 days in the cell-free plasma, the bacteria were no longer either infectious or virulent after incubation at 25° or 37°C for 24 h outside the environment of the host cell.
Effect of antibody treatment in vivo.
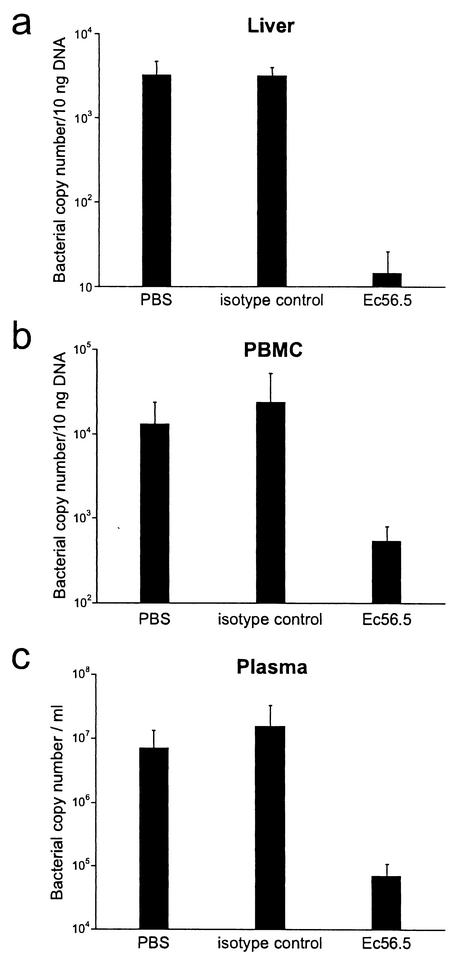
Our previous studies have shown that antibodies can effectively mediate clearance of intracellular ehrlichiae from tissues of infected mice (21, 22). To determine whether the cell-free ehrlichiae were also susceptible to antibody-mediated immunity, the levels of cell-free ehrlichiae in the plasma of untreated and antibody-treated infected SCID mice were compared. Infected BALB/c-scid mice were either untreated or treated with three doses of MAb Ec56.5 (200 μg) or an isotype control antibody (KJ1-26) on days 6, 12, and 18 postinfection. Bacterial numbers in liver, PBMCs, and plasma fractions were analyzed by quantitative PCR on day 22 postinfection, at which time the untreated mice and mice treated with the isotype control antibody were moribund, while mice treated with MAb Ec56.5 exhibited little or no sign of disease. The E. chaffeensis-specific antibody caused a 20- to 200-fold decrease in bacterial numbers in liver, PBMCs, and plasma (Fig. 7), indicating that the cell-free ehrlichiae were a target of antibody-mediated host defenses.
FIG. 7.
Antibody-mediated bacterial clearance. Groups of three to four BALB/c-scid mice were infected with 106 splenocytes from infected BALB/c-scid mice. On days 6, 12, and 18 postinfection, the infected mice were either left untreated or injected with 200 μg of Ec56.5 or the isotype-matched irrelevant antibody (KJ1-26) via the peritoneum. Bacterial copy number in the liver, PBMCs, and cell-free plasma was measured, as indicated. The experiment was repeated two times with similar results. Statistical comparisons were made between groups of three to four mice treated with either PBS,or the isotype control antibody and mice treated with the specific antibody Ec56.5. In all cases P values were less than 0.05, as determined with the Mann Whitney test.
In vitro recovery of cell-free bacteria.
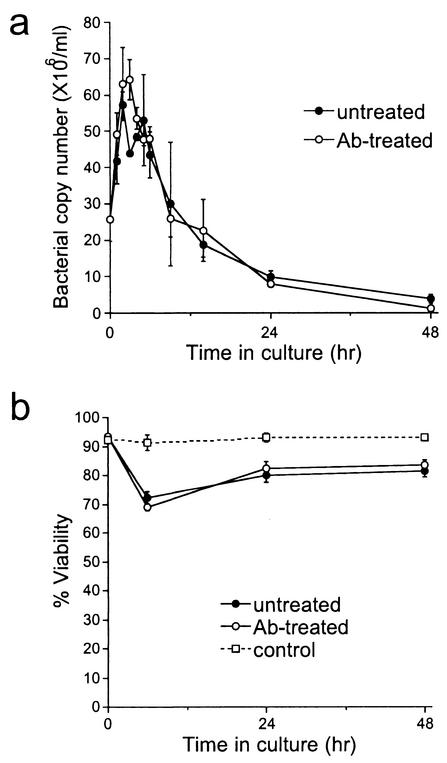
The data suggested that cell-free bacteria were released from infected cells in vivo, so experiments were performed to determine whether the findings could be reproduced in vitro. Peritoneal exudate cells (PECs; 2 × 106/ml), typically greater than 90% infected, were isolated from infected SCID mice on day 21 postinfection, washed, and cultured in medium at 37°C for 0 to 48 h. The PEC-free culture supernatants were analyzed for the presence of ehrlichiae by PCR, and the viability of the PECs was monitored throughout the culture period. Large numbers of cell-free ehrlichiae (6 × 107/ml) were recovered from the supernatants within 4 to 6 h (Fig. 8). Release was transient, however, as no increase in the number of cell-free bacteria was observed at later intervals. Treatment with the E. chaffeensis-specific MAb Ec56.5 did not affect the release or recovery of bacteria from the supernatants (Fig. 8a). Similar findings were observed with PECs from infected immunocompetent BALB/c mice on day 7 postinfection (data not shown). Thus, the release of cell-free bacteria from infected PECs was observed in vitro and was correlated with a transient loss of host cell viability (Fig. 8b) but was not affected by the presence of antibodies during the first 48 h of culture. The data suggest that bacteria are released from infected cells upon lysis but that the antibodies do not function by blocking the release of the ehrlichiae from host cells.
FIG. 8.
Cell-free ehrlichiae were detected after ex vivo culture of infected peritoneal cells. (a) Peritoneal exudate cells (PECs) isolated from 21-day-infected BALB/c-scid mice (>90% infected) were washed and cultured in Eagle's MEM containing 10% fetal bovine serum and 2 mM l-glutamine, with or without 10 μg of the E. chaffeensis-specific MAb Ec56.5 per ml at a concentration of 106 cell/ml at 37°C for 48 h. Bacterial copy number was measured in the supernatant. No significant differences were observed between the untreated and antibody-treated groups. (b) The viability of the infected PECs was monitored with the trypan blue exclusion method. Also shown is the viability of thioglycolate-elicited PECs from uninfected BALB/c-scid mice cultured under identical conditions (control). Data are representative of four independent experiments. The difference observed between the uninfected control group and the infected groups (untreated and antibody treated) at 6 h was statistically significant (P < 0.05).
DISCUSSION
In this study we have shown that E. chaffeensis can be found outside of host cells during infection in both immunocompromised SCID and normal BALB/c mice. These observations were not artifactual, because the gentle isolation conditions used in this study were unlikely to have caused cell lysis as a result of mechanical damage and there was no evidence of contaminating nucleated cells in the preparations. Approximately 10% of blood-borne ehrlichiae were found in the plasma, of which approximately 90% were viable, as determined with vital dyes. As few as 1 to 10 cell-free bacteria could readily transfer disease to susceptible SCID mice, supporting the idea that the majority of the freshly isolated cell-free bacteria were viable and infectious. The sizes of the cell-free ehrlichiae were consistent with previous characterizations of intracellular ehrlichiae (30, 32). Ultrastructural analyses that might reveal whether particular morphological changes accompany the release of the bacteria into the host extracellular milieu are not yet available. It is possible, for example, that exposure to a novel environment triggers bacterial differentiation, as has been observed in other intracellular bacterial infections (33, 40). Recent preliminary data suggested that cell-free bacteria were not detected during infection of SCID mice with the related granulocytic ehrlichia species Anaplasma phagocytophilum (J. Li and G.Winslow, unpublished data), suggesting that the phenomenon that we have described may not occur during other Ehrlichia infections.
Cell-free ehrlichiae were also found to be released from infected host cells in culture, and the release of the ehrlichiae was correlated with a transient decrease in host cell viability. The mechanism of bacterial release from host cells is not known, but it probably does not involve apoptosis, because this mechanism would likely result in the sequestering of the pathogens within apoptotic bodies (11). Necrotic host cell lysis could be involved, because necrosis would likely release cellular contents into the extracellular milieu. Host cell lysis could be induced by the bacteria in vivo, as E. chaffeensis is lytic in vitro; alternatively, macrophage cell death could be induced by host inflammatory factors. The latter possibility is supported by data that demonstrated only transient bacterial release from infected macrophages after ex vivo culture in medium. Alternatively, a mechanism similar to that used by some intracellular bacteria, such as Coxiella burnetii (17) and Chlamydia trachomatis biovar trachoma (25), might be involved, by which bacteria can be released from host cells by a process resembling exocytosis. Support for such a mechanism is suggested from early studies of E. chaffeensis, where it was observed that infection was noncytolytic in a human embryonic lung fibroblast cell line (2). Further studies will be necessary to resolve the relevant mechanism of bacterial release.
Our observations that significant numbers of bacteria can reside outside of host cells suggest a mechanism by which antibodies access these obligate intracellular bacteria and mediate host defense. Antibodies likely bind the bacteria released from host cells and thereby passively prevent reinfection (19) and/or act as opsonins and target the bacteria for uptake and killing by host phagocytes by as yet unknown mechanisms. However, these models do not satisfactorily explain our observations that bacterial clearance can be observed within 1 to 2 days of a single antibody administration. Reconciliation of these data with the proposed models may require that host cell lysis and/or intercellular spreading be rapid and extensive, so that most intracellular ehrlichiae become exposed to the extracellular milieu during the relatively short period within which antibodies have been shown to be effective. Perhaps this explanation is correct, and rapid intercellular spreading is a unique characteristic of the ehrlichiae.
A related ehrlichia (Anaplasma phagocytophilum) infects short-lived granulocytes (35), so it is possible that rapid intercellular spreading is a feature of the ehrlichiae in general. Such a characteristic may render them particularly susceptible to antibody-mediated host defenses. It is also possible that antibodies mediate generalized macrophage activation in vivo that enhances the release of bacteria into the extracellular milieu, even though our in vitro studies did not provide support for such a mechanism. An alternative explanation is that antibodies act upon bacteria that are residing within host cells, perhaps utilizing novel mechanisms to activate host cells. Such an indirect mechanism has been suggested by studies that have demonstrated that Ehrlichia immune complexes were capable of eliciting inflammatory cytokine production from host macrophages, which may in turn enhance microbicidal activities that affect the survival of resident intracellular bacteria (20).
In addition to providing insight into the mechanism of humoral immunity, our data may be significant for the understanding of ehrlichial microbiology in a mammalian host. Indeed, the classification of the ehrlichiae as obligate intracellular bacteria may be somewhat misleading, since our data suggest that E. chaffeensis may not always reside in host cells. Obligate intracellular bacteria are conventionally classified on the basis of their dependence on the host cell for survival and replication (3, 18). Our data demonstrate that E. chaffeensis can survive outside of host cells, even if only transiently. These findings are supported by studies of McKechnie and colleagues, which reported that viable E. chaffeensis could survive in white blood cell-depleted human blood stored at 4°C for up to 5 days (23). Although their findings and ours reveal that E. chaffeensis can survive outside of host cells, they do not challenge current dogma, because survival is likely transient. However, our data do challenge the dogma that replication of the ehrlichiae can only occur within the environment of the host cell. We detected DNA replication, by two independent methods, for as long as 5 days ex vivo. Quantitation revealed a 6- to 14-fold increase in the copy number of the 16S rDNA gene, suggesting a minimum of three to four rounds of replication within the population. DNA replication was accompanied by cell division, because the relative number of cell-free ehrlichiae was also found to increase during ex vivo incubation. To our knowledge, this is the first study to demonstrate that rickettsiae can replicate outside of the environment of a host cell.
It is not known how the ehrlichiae could retain the capacity to replicate, even transiently, in the extracellular milieu of the host, although some obligate intracellular bacteria, such as E. sennetsu, E. risticii, and Rickettsia prowazekii are known to exhibit transient metabolic activity outside of the host cell (15, 36). When isolated and purified from the host cells, these obligate intracellular bacteria retained the ability to synthesize ATP, DNA, RNA, and proteins under appropriate conditions in vitro (37), so it is possible that E. chaffeensis likewise retains some biosynthetic capacity to allow limited metabolism outside of host cells. Another possibility is that the bacteria utilize stored metabolites that they acquire from host cells prior to their escape and that replication ceases once the metabolites are depleted. E. chaffeensis may require an external source of ATP for its replication, because ehrlichiae have frequently been found adjacent to mitochondria inside host cells (29, 30). It is not known whether E. chaffeensis possesses an ATP transport system, but ATP transport proteins have been observed in Rickettsia and Chlamydia spp. (1). A requirement for host cell metabolites probably necessitates that the ehrlichiae either access a new host cell within a relatively short period or else adjust their metabolic requirements to accommodate a novel environment.
Once isolated from infected mice, the cell-free ehrlichiae rapidly lost virulence when incubated at 37°C and 25°C, even though they still underwent DNA replication under the same conditions. One resolution of this paradox is that replication is of no consequence once virulence has been lost and that this represents a dead end. An alternative possibility is that the cell-free ehrlichiae, after incubation in the absence of mammalian host cells, retain the capacity to infect their arthropod hosts. Vector-borne pathogens must adapt to physiological changes during the transmission cycle between mammalian hosts-carriers and arthropod vectors, so loss of virulence for the mammalian host may not necessarily be accompanied by a loss of the capacity to infect cells of the vector. This hypothesis can be tested experimentally. In either event, our findings suggest a mechanism by which ehrlichiae that colonize many different host tissues may contribute to the pool of bacteria available for vector transmission. Thus, our findings may have implications not only for possible mechanisms of antibody-mediated immunity, but also for natural transmission of the ehrlichiae.
Acknowledgments
We thank Marcia Blackman of the Trudeau Institute for critical reading of the manuscript and Jennifer Huntington for excellent technical assistance. We also thank the Wadsworth Center Immunology Core Facility and the Animal Care Facility.
This work was supported by U.S. Public Health Service grant R01AI47963-01 to G.M.W.
Editor: T. R. Kozel
REFERENCES
- 1.Andersson, S. G. 1998. Bioenergetics of the obligate intracellular parasite Rickettsia prowazekii. Biochim. Biophys. Acta 1365:105-111. [DOI] [PubMed] [Google Scholar]
- 2.Brouqui, P., M. L. Birg, and D. Raoult. 1994. Cytopathic effect, plaque formation, and lysis of Ehrlichia chaffeensis grown on continuous cell lines. Infect. Immun. 62:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall, A. 1998. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 6:102-107. [DOI] [PubMed] [Google Scholar]
- 4.Dumler, J. S., and D. H. Walker. 2001. Tick-borne ehrlichioses. Lancet Infect. Dis. i:21-28. [Google Scholar]
- 5.Edelson, B. T., P. Cossart, and E. R. Unanue. 1999. Paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 163:4087-4090. [PubMed] [Google Scholar]
- 6.Edelson, B. T., and E. R. Unanue. 2001. Intracellular antibody neutralizes listeria growth. Immunity 14:503-512. [DOI] [PubMed] [Google Scholar]
- 7.Eisenstein, T. K., L. M. Killar, and B. M. Sultzer. 1984. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J. Infect. Dis. 150:425-435. [DOI] [PubMed] [Google Scholar]
- 8.Felek, S., A. Unver, R. W. Stich, and Y. Rikihisa. 2001. Sensitive detection of Ehrlichia chaffeensis in cell culture, blood, and tick specimens by reverse transcription-PCR. J. Clin. Microbiol. 39:460-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, H.-M., and D. H. Walker. 1999. Mechanisms of immunity to Ehrlichia chaffeensis in mice, p. 145-150. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millennium. Elsevier, Paris, France.
- 10.Fishbein, D. B., J. E. Dawson, and L. E. Robinson. 1994. Human ehrlichiosis in the United States, 1985 to 1990. Ann. Intern. Med. 120:736-743. [DOI] [PubMed] [Google Scholar]
- 11.Fratazzi, C., R. D. Arbeit, C. Carini, M. K. Balcewicz-Sablinska, J. Keane, H. Kornfeld, and H. G. Remold. 1999. Macrophage apoptosis in mycobacterial infections. J. Leukoc. Biol. 66:763-764. [DOI] [PubMed] [Google Scholar]
- 12.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French, D. M., T. F. McElwain, T. C. McGuire, and G. H. Palmer. 1998. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 66:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganta, R. R., M. J. Wilkerson, C. Cheng, A. M. Rokey, and S. K. Chapes. 2002. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect. Immun. 70:380-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackstadt, T. 1996. The biology of rickettsiae. Infect. Agents Dis. 5:127-143. [PubMed] [Google Scholar]
- 16.Haskins, K., R. Kubo, J. White, M. Pigeon, J. Kappler, and P. Marrack. 1983. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J. Exp. Med. 157:1149-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe, D., and L. P. Mallavia. 2000. Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A0.1 cells. Infect. Immun. 68:3815-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail, N., J. P. Olano, H. M. Feng, and D. H. Walker. 2002. Current status of immune mechanisms of killing of intracellular microorganisms. FEMS Microbiol. Lett. 207:111-120. [DOI] [PubMed] [Google Scholar]
- 19.Koesling, J., T. Aebischer, C. Falch, R. Schulein, and C. Dehio. 2001. Cutting edge: antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen Bartonella grahamii. J. Immunol. 167:11-14. [DOI] [PubMed] [Google Scholar]
- 20.Lee, E., and Y. Rikihisa. 1997. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IκB-α and activation of NF-κB. Infect. Immun. 65:2890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, J. S., F. Chu, A. Reilly, and G. M. Winslow. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169:1419-1425. [DOI] [PubMed] [Google Scholar]
- 22.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, F. K. Chu, and G. Winslow. 2001. Outer membrane protein specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 23.McKechnie, D. B., K. S. Slater, J. E. Childs, R. F. Massung, and C. D. Paddock. 2000. Survival of Ehrlichia chaffeensis in refrigerated, ADSOL-treated RBCs. Transfusion 40:1041-1047. [DOI] [PubMed] [Google Scholar]
- 24.McSorley, S. J., and M. K. Jenkins. 2000. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect. Immun. 68:3344-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paddock, C. D., S. M. Folk, G. M. Shore, L. J. Machado, M. M. Huycke, L. N. Slater, A. M. Liddell, R. S. Buller, G. A. Storch, T. P. Monson, D. Rimland, J. W. Sumner, J. Singleton, K. C. Bloch, Y. W. Tang, S. M. Standaert, and J. E. Childs. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 33:1586-1594. [DOI] [PubMed] [Google Scholar]
- 29.Popov, V. L., S.-M. Chen, H.-M. Feng, and D. H. Walker. 1995. Ultrastructural variation of cultured Ehrlichia chaffeensis. J. Med. Microbiol. 43:411-421. [DOI] [PubMed] [Google Scholar]
- 30.Popov, V. L., V. C. Han, S. M. Chen, J. S. Dumler, H. M. Feng, T. G. Andreadis, R. B. Tesh, and D. H. Walker. 1998. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J. Med. Microbiol. 47:235-251. [DOI] [PubMed] [Google Scholar]
- 31.Reddy, G. R., C. R. Sulsona, A. F. Barbet, S. M. Mahan, M. J. Burridge, and A. R. Alleman. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe ehrlichiae. Biochem. Biophys. Res. Commun. 247:636-643. [DOI] [PubMed] [Google Scholar]
- 32.Rikihisa, Y. 1999. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1:367-376. [DOI] [PubMed] [Google Scholar]
- 33.Shaw, E. I., C. A. Dooley, E. R. Fischer, M. A. Scidmore, K. A. Fields, and T. Hackstadt. 2000. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol. 37:913-925. [DOI] [PubMed] [Google Scholar]
- 34.Siegel, S. 1956. Nonparametric statistics for the behavioral sciences. McGraw-Hill Book Company, Inc., York, Pa.
- 35.Walker, D. H., and J. S. Dumler. 1997. Human monocytic and granulocytic ehrlichioses. Arch. Pathol. Lab. Med. 121:785-791. [PubMed] [Google Scholar]
- 36.Weiss, E., G. A. Dasch, Y.-H. Kang, and H. N. Westfall. 1988. Substrate utilization by Ehrlichia sennetsu and Ehrlichia risticii separated from host constituents by renografin gradient centrifugation. J. Bacteriol. 170:5012-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler, H. H. 1987. Protein and RNA synthesis by isolated Rickettsia prowazekii. Infect. Immun. 55:2032-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winslow, G., E. Yager, K. Shilo, D. N. Collins, and F. K. Chu. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 66:3892-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winslow, G. M., E. Yager, K. Shilo, E. Volk, and F. K. Chu. 2000. Antibody mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyrick, P. B. 2000. Intracellular survival by Chlamydia. Cell. Microbiol. 2:275-282. [DOI] [PubMed] [Google Scholar]
- 41.Yu, X. J., J. McBride, and D. H. Walker. 1999. Genetic diversity of the 28-kilodalton outer membrane protein gene in human isolates of Ehrlichia chaffeensis. J. Clin. Microbiol. 37:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]