Abstract
The gram-negative bacterium Actinobacillus pleuropneumoniae is the causative agent of porcine fibrinohemorrhagic necrotizing pleuropneumonia, a disease that causes important economic losses to the swine industry worldwide. In general, the initial step of bacterial colonization is attachment to host cells. The purpose of the present study was to evaluate the binding of A. pleuropneumoniae serotype 1 to phospholipids, which are the major constituents of biological membranes. Phospholipids serve as receptors for several bacteria, including respiratory pathogens. To study this effect, we used thin-layer chromatography overlay binding assays to test commercial phospholipids such as phosphatidic acid, phosphatidylcholine, phosphatidylserine, phosphatidylinositol, phosphatidylglycerol, and phosphatidylethanolamine (PE). Our results indicate that A. pleuropneumoniae serotype 1 binds to PE but not to the other phospholipids tested. Serotypes 5b and 7, which, along with serotype 1, are the most prevalent serotypes of A. pleuropneumoniae in North America, share the ability to bind PE. Inhibition of binding with a monoclonal antibody against A. pleuropneumoniae serotype 1 O antigen and the use of isogenic lipopolysaccharide (LPS) mutants of A. pleuropneumoniae serotype 1 showed that the O antigen seems to be implicated in the binding to PE, at least for A. pleuropneumoniae serotype 1. A. pleuropneumoniae was also shown to bind to a phospholipid extracted from swine lungs by using the method of Folch. Chemical staining with molybdenum blue and ninhydrin, migration with neutral, acidic, and basic solvent systems, and mass spectrometry analysis all indicated that this lipid is PE. This study is, to the best of our knowledge, the first description of A. pleuropneumoniae binding to phospholipids. Our data also suggest that LPS O antigens could be involved in binding to PE.
Actinobacillus pleuropneumoniae is the causative agent of porcine pleuropneumonia, which has been reported in most countries for which the pig industry is important (41). Twelve serotypes of NAD-dependent A. pleuropneumoniae have been recognized on the basis of capsular and lipopolysaccharide (LPS) antigens (29), and an additional serotype has recently been proposed (6). In North America, serotypes 1, 5b, and 7 are the most prevalent (11).
While the pathogenesis of porcine pleuropneumonia has been studied by many groups, the disease is still not completely understood. Several virulence factors have already been identified, such as capsule, LPS, outer membrane proteins (OMPs), and RTX (repeat in toxin) toxins (7, 13, 17, 40). Some of the factors that could potentially be involved in A. pleuropneumoniae colonization of the respiratory tract have also been reported. Overbeke et al. recently reported that the expression of a 55-kDa OMP and fimbriae play a role in the adherence of strains from serotypes 5a, 9, and 10 to alveolar epithelial cells in culture (31).
Our group has previously shown that the LPS molecule plays an important role in adherence of the bacterium to porcine respiratory tract cells and mucus (4, 5, 20, 21, 32). LPS molecules are major components of the outer membranes of gram-negative bacteria. They consist of a polysaccharide and a lipid moiety. The polysaccharide part is composed of a core region, which is an oligosaccharide that contains 3-deoxy-d-manno-octulosonic acid (Kdo), and the O antigen, a polysaccharide chain that consists of repeated units (18). The polysaccharide portion of LPS, but not the lipid A portion, is responsible for binding of A. pleuropneumoniae to porcine respiratory tract cells and mucus (15, 32, 35).
Putative receptors for A. pleuropneumoniae LPS have been described. Proteins of approximately 38.5 kDa that are present in swine tracheal epithelial cells showed affinity for LPS of A. pleuropneumoniae (33). A. pleuropneumoniae cells and LPS are also able to recognize saccharide sequences found in different glycosphingolipids such as GalNacβ1-4Gal found in GgO3 and GgO4 molecules (2).
Another class of membrane lipids, the phospholipids—particularly phosphatidylethanolamine (PE)—has been described as a putative receptor for pathogenic bacteria including Chlamydia pneumoniae and Chlamydia trachomatis (24), Helicobacter pylori (27), Helicobacter mustelae (16), Haemophilus influenzae (8), Campylobacter upsaliensis (8), and enteropathogenic and enterohemorrhagic Escherichia coli (12).
The aims of the present study were to determine whether A. pleuropneumoniae binds to commercial phospholipids or lipids extracted from swine lungs and to study whether LPS molecules are implicated in this process.
MATERIALS AND METHODS
Materials.
Thin-layer chromatography (TLC) sheets (SilG, 20 by 20 cm) were purchased from Polygram (Macherly-Nagel, Duren, Germany). Spray reagent molybdenum blue, ninhydrin reagent, p-anisaldehyde, and all antibiotics were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). Lipids were purchased from Sigma-Aldrich and Avanti Polar Lipids (Alabaster, Ala.).
Bacterial strains and growth conditions.
All the strains used for TLC plate assays in this study are described in Table 1. A. pleuropneumoniae reference strains representing serotypes 1 (4074), 5b (L20), and 7 (WF83) were grown on brain heart infusion (BHI; Difco Laboratories, Detroit, Mich.) agar plates supplemented with 15 μg of β-NAD per ml. The nalidixic acid-resistant mutant (4074 Nalr) derived from the A. pleuropneumoniae serotype 1 reference strain was grown on BHI-NAD medium supplemented with 30 μg of nalidixic acid (Nal) per ml. LPS mutants generated by mini-Tn10 mutagenesis were grown on BHI-NAD-Nal plates supplemented with 75 μg of kanamycin per ml (15, 35). For microtiter plate binding assay (MPBA), A. pleuropneumoniae strain 4074 was grown overnight in BHI broth supplemented with 5 μg of NAD per ml.
TABLE 1.
Binding of A. pleuropneumoniae reference strains and various LPS mutants to PE using TLC binding assay
| Strain and mutant | Relevant trait | Putative function of the inactivated protein | Source or reference | Binding to PEb |
|---|---|---|---|---|
| A. pleuropneumoniae reference strains | ||||
| 4074 | Serotype 1 | K. R. Mittala | + | |
| L20 | Serotype 5b | K. R. Mittal | + | |
| WF83 | Serotype 7 | K. R. Mittal | + | |
| A. pleuropneumoniae serotype 1 wild type and mutants | ||||
| 4074 Nalr | Serotype 1 (Nalr), wild type | 35 | + | |
| 27.1 | LPS O-antigen mutant | Undecaprenyl-Gal-1-P transferase | 25, 35 | − |
| 44.1 | LPS O-antigen mutant | Rhamnosyl transferase | 25, 35 | − |
| 51.1 | LPS O-antigen mutant | Rhamnosyl transferase | 25, 35 | − |
| 5.1 | LPS core oligosaccharide mutant | UTP-α-d-glucose-1-phosphate uridyltransferase | 25, 35 | + |
| CG1 | LPS core oligosaccharide mutant | Outer core elongation | 15 | + |
| CG3 | LPS core oligosaccharide mutant | d-glycero-d-manno-Heptosyltransferase | 15 | + |
Faculté de Médecine Vétérinaire, Université de Montréal.
Ten micrograms of PE from egg yolk were used for these assays. GgO4 and GD1a were used as positive and negative controls, respectively. Symbols: +, binding; −, no binding detected.
Lipid extraction.
Lipid extraction from lungs of a 3-month-old piglet was performed as previously described with some modifications (22). After two washes with phosphate-buffered saline (PBS [pH 7.4]; 0.001 M KH2PO4, 0.01 M Na2HPO4, 0.137 M NaCl, 0.0027 M KCl), the pig lung tissue was homogenized in a small volume of PBS. A solution of chloroform-methanol (2:1) was then added to the lung suspension in the following proportion: 3.75 parts of solvent mix to one part of tissue suspension. This preparation was then shaken overnight or vortexed for 2 h at room temperature. PBS was then added to the extracted lipids, shaken vigorously for 30 s, and left overnight at 4°C to allow the formation of the two phases. The two phases were then separated, dried with nitrogen, and the lower phase was redissolved in chloroform-methanol (2:1), whereas the upper phase was redissolved in chloroform-methanol-water (60:36:8) prior to chemical characterization or the overlay assays.
TLC binding assay.
Binding of A. pleuropneumoniae to lipids was assayed as described previously with few modifications (8). TLC plates were prepared by using 10 μg of extracted lipids or commercial phospholipids. The lipids were applied on a TLC plate and separated by using chloroform-methanol-water (60:35:8 by volume) in a TLC chamber. The plate was then dried and blocked for 2 h by incubation with 3% gelatin in PBS with agitation at 37°C. After three 15-min washes with PBS with agitation at 37°C, the plates were overlaid with a bacterial suspension resuspended in PBS at an A540 of 1.8 (equivalent to approximately 3 × 109 CFU/ml) and incubated for 2 h. After three washes in PBS to remove unbound bacteria, the TLC plates were incubated for another 2 h with rabbit polyclonal antibodies raised against whole cells of A. pleuropneumoniae serotype 1, 5b, or 7, kindly supplied by K. R. Mittal (Université de Montréal). The plates were then washed twice and incubated for 1 h with a 1:1,000 goat anti-rabbit immunoglobulin G (heavy plus light chain)-horseradish peroxidase conjugate from Jackson ImmunoResearch Laboratories (Mississauga, Ontario, Canada). After two washes, binding to lipid was revealed by the addition of 4-chloro-1-naphthol and hydrogen peroxide. As controls, TLC plates that had not been overlaid with bacterial cells were incubated with primary and secondary antibodies; these antibodies did not bind directly to the lipids. All the overlay assays were performed at least five times.
MPBA.
To confirm binding of A. pleuropneumoniae to PE, MPBAs were performed according to the methods of Beausoleil and Dubreuil (3) with few modifications. Duplicates of 50 μl of PE (from egg yolk) and phosphatidylserine (PS [from soybean as negative control]; 0.2 μg/μl) diluted in methanol were coated by evaporation for few hours in polystyrene microtiter enzyme-linked immunosorbent assay plates (Falcon 3070 flat-bottom microtest III plate; Becton Dickinson Labware, Lincoln Park, N.J.). Two hundred microliters of PBS-casein (1%, wt/vol) was added in each well for an overnight blocking at 4°C. Wells were then washed once with 200 μl of PBS, and 50 μl of bacterial suspension resuspended in PBS (A540, 0.140) was then added to the wells for 1 h at 37°C. Plates were washed five times with PBS. Then, 50 μl of rabbit polyclonal antibodies raised against whole cells of A. pleuropneumoniae in PBS-casein (1%, wt/vol) was added and left for 1 h at 37°C. Plates were again washed five times as described above, and 50 μl of goat anti-rabbit immunoglobulin in PBS-casein (1%, wt/vol) coupled to horseradish peroxidase was added to each well and incubated at 37°C for 1 h. Plates were washed three times, and 50 μl of the chromogenic substrate ABTS {2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)]; 0.4 mM} dissolved in citrate buffer (pH 4) containing 0.5 mM H2O2 was added. The optical density (OD) was measured with an enzyme-linked immunosorbent assay recorder (MR5000; Dynatech) at 410 nm. Three wells on each plate received methanol without any phospholipid and served as blanks. For analysis, the mean OD obtained for these wells was subtracted from the OD obtained for each of the phospholipids.
Inhibition with MAbs.
The inhibition of binding with monoclonal antibodies (MAbs) was done as described previously (1) by using MAb against A. pleuropneumoniae serotype 1 O antigen (5.1 G8F10) and against the O antigen of serotype 2 (101-G02), kindly supplied by M. Gottschalk (Université de Montréal).
Identification of extracted lipids.
The total lipids extracted from swine lungs were stained either with molybdenum blue, which is specific for phospholipids, or sprayed with ninhydrin, which detects free amino groups contained in PE and PS (14). The lipid of interest was then extracted from the silica gel after migration with the neutral migration solvent as described previously by Rousset et al. (36) with few modifications. Briefly, 50 μg of lower-phase lipid extract was applied in a linear configuration across a 5-cm-wide band on a TLC plate. Commercial PE (egg yolk) was applied at one end of the TLC plate and the chromatography was done as described above. Following the migration, the standard lane was cut out and stained with molybdenum blue. The region that corresponded to the band of PE on the remaining unstained plate was scraped with a scalpel, and the silica gel was collected in a glass tube. We also performed a blank control experiment with silica gel to which no lipid material was applied. Two milliliters of a 2:1 (vol/vol) chloroform-methanol mixture was mixed with the collected silica gel. After 10 min of incubation at room temperature, the mixture was centrifuged at 20,000 × g for 5 min. The supernatant obtained was then transferred to another glass tube. The solvent was then evaporated in a fume hood under a stream of nitrogen. Two milliliters of a solution of chloroform-methanol-water (8:4:3) was then added to the dried lipids, a step which was then followed by a 5-min centrifugation at 22,000 × g. The lower phase obtained was then collected from the glass tube without disturbing the silica pellet if one was present. The extract was put in a preweighed glass tube and then evaporated under a stream of nitrogen. The quantity of lipid was estimated on the basis of dry weight. Lipids were then resuspended in a chloroform-methanol (2:1) solution. Acid migration solvent composed of chloroform-methanol-acetic acid-water (25:15:4:2) and basic migration solvent composed of chloroform-methanol-30% ammonia (65:30:5) were used to compare the migration of extracted lipid of interest to commercial PE. For the mass spectrometry analysis, the extraction of the lipid from silica gel was performed as described above with some modifications. In the first place, ethyl acetate was used instead of chloroform; secondly, the sample analyzed was the supernatant of the first centrifugation. The analysis was done at the biomedical mass spectrometry unit of McGill University.
Mass spectrometry.
The analysis was performed with a Quattro II (Micromass, Manchester, United Kingdom) triple quadrupole mass spectrometer by using electrospray in positive mode. The sample was introduced by direct infusion at a flow rate of 3 μl/min. Capillary voltage was set at 3.4 kV, cone voltage was set at 50 V, source temperature was kept at 80°C, and the collision cell energy was 31 eV with argon pressure at 1.7 × 10−3 mbar. The mass spectrometer was programmed to scan for a neutral loss of 141 m/z, which is characteristic for PE ions (10).
RESULTS
Binding of A. pleuropneumoniae to commercial phospholipids.
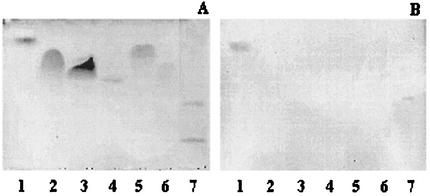
Binding of A. pleuropneumoniae serotype 1 strain 4074 to various commercial phospholipids from several sources separated on TLC plates was evaluated (Fig. 1). A. pleuropneumoniae serotype 1 bound only to PE (from egg yolk). No binding to PS (bovine brain or egg yolk), phosphatidylinositol (bovine liver or soybean), phosphatidylcholine (bovine brain or egg yolk), phosphatidylglycerol (egg yolk) or phosphatidic acid (egg yolk) was observed. The controls used in this study were glycolipids GgO4 and GD1a, which were positive and negative, respectively, for the binding with A. pleuropneumoniae serotype 1 in a previous study (1). Binding of A. pleuropneumoniae to PE (from egg yolk) was confirmed by a MPBA. A mean OD of 0.576 ± 0.093 was obtained for PE. No binding was observed for PS (mean OD of 0.002 ± 0.001). PS was chosen as a negative control due to our previous results (Fig. 1) and to its similarity to PE—both of them have a primary amino group in their polar heads. A. pleuropneumoniae serotype 1 was also able to bind to lyso-PE from egg yolk (data not shown), which is a normal degradation product of PE. This molecule results from the removal of a monoacyl from PE. Binding to PE and lyso-PE by A. pleuropneumoniae is consistent with previous studies, which showed that organisms that recognize PE also recognize lyso-PE (16, 23, 39).
FIG. 1.
TLC plates stained with anisaldehyde (A) and immunostained chromatogram obtained by overlay with A. pleuropneumoniae serotype 1 cells (B). Lane 1, PE (egg yolk); lane 2, PS (soybean); lane 3, phosphatidylinositol (soybean); lane 4, phosphatidylcholine (bovine brain); lane 5, phosphatidylglycerol (egg yolk); lane 6, phosphatidic acid (egg yolk); lane 7, GgO4 (upper band, positive control), GD1a (lower band, negative control). Ten micrograms was used for each lipid.
Binding of A. pleuropneumoniae to commercial PE and glycosphingolipids.
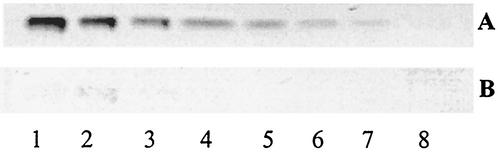
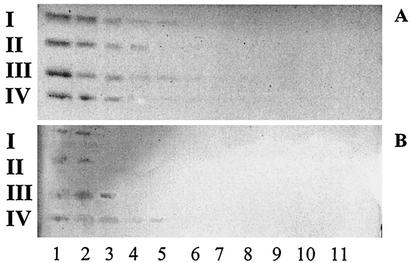
The relative binding affinity of A. pleuropneumoniae serotype 1 to PE on TLC plates was evaluated (Fig. 2) and compared to the relative binding affinity to GlcCer, LacCer, GgO3, and GgO4 (Fig. 3). Different quantities of lipids were applied on a TLC plate (20, 10, 5, 2.5, and 1.25 μg and 630, 310, 160, 80, 40, and 20 ng). The smallest amounts of lipids recognized by A. pleuropneumoniae serotype 1 were 630 ng for GgO4, 2.5 μg for GgO3 and PE, and 5 μg for GlcCer and LacCer.
FIG. 2.
TLC stained with anisaldehyde (A) and immunostained chromatogram obtained by overlay with A. pleuropneumoniae serotype 1 cells (B). Lanes contain different concentrations of PE as follows: lane 1, 10 μg; lane 2, 5 μg; lane 3, 2.5 μg; lane 4, 1.25 μg; lane 5, 0.63 μg; lane 6, 0.31 μg; lane 7, 0.16 μg; lane 8, 0.08 μg.
FIG. 3.
TLC stained with anisaldehyde (A) and immunostained chromatogram obtained by overlay with A. pleuropneumoniae serotype 1 cells (B). Lanes contain different concentrations of GlcCer (I), LacCer (II), GgO3 (III), or GgO4 (IV) as follows: lane 1, 10 μg; lane 2, 5 μg; lane 3, 2.5 μg; lane 4, 1.25 μg; lane 5, 0.63 μg; lane 6, 0.31 μg; lane 7, 0.16 μg; lane 8, 0.08 μg; lane 9, 0.04 μg; lane 10, 0.02 μg; lane 11, 0.01 μg.
Binding of different A. pleuropneumoniae strains to PE.
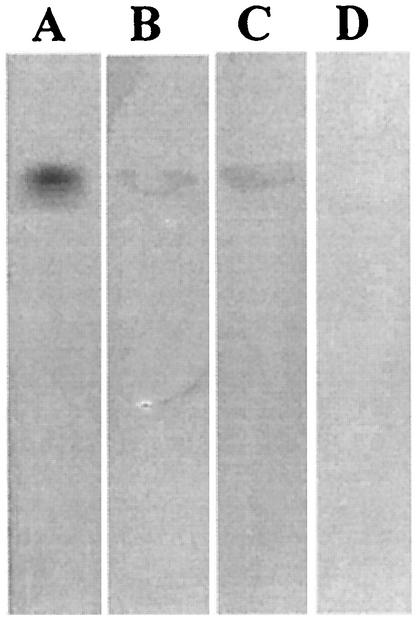
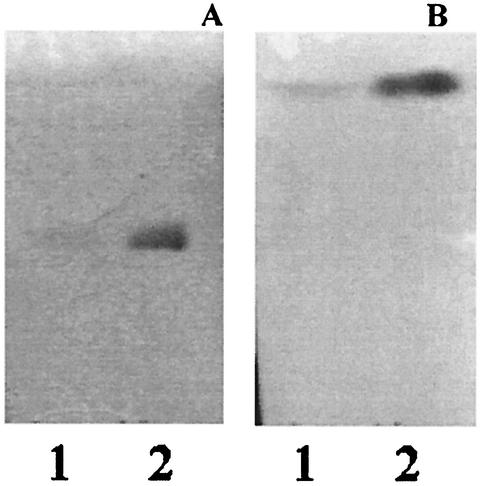
Binding to PE was then evaluated for various A. pleuropneumoniae strains and mutants (Table 1 and Fig. 4). Reference strains representing serotypes 5b and 7 also bound to PE. The mutants used were LPS isogenic mutants from A. pleuropneumoniae serotype 1 (4074 Nalr) created by mini-Tn10 mutagenesis (15, 25, 35). The saccharidic moiety of the LPS molecule is known to be responsible for the binding property of the molecule (32). We therefore used different mutants for the polysaccharide portion of serotype 1 LPS to determine whether or not the molecule was implicated in the binding of the bacteria to PE. We tested rough mutants devoid of O antigen as well as mutants with a modified core oligosaccharide but still expressing O antigens in order to determine the influence of different regions of the LPS molecule toward binding to PE. Binding was observed with all core mutants, thus indicating that a complete core is not essential for binding to PE. However, rough mutants 27.1, 44.1, and 51.1 were unable to bind to PE. All the strains were able to bind to GgO4 (positive control), but none of them was able to bind to GD1a (negative control).
FIG. 4.
TLC plate stained with anisaldehyde (A) and immunostained chromatogram obtained by overlay with A. pleuropneumoniae serotype 1 wild-type cells (B), CG1 (LPS core oligosaccharide mutant) (C), and 27.1 (LPS O antigen mutant) (D). Ten micrograms of PE was applied to each lane.
Inhibition of binding by MAbs specific for LPS O antigens.
The binding of A. pleuropneumoniae to PE was inhibited by O-antigen-specific MAbs. After incubation of bacteria with specific MAbs against A. pleuropneumoniae serotype 1 O antigen, the bacterial cells failed to recognize PE. As a negative control, cells were incubated with MAb raised against A. pleuropneumoniae serotype 2 O antigen. With this antibody, no inhibition of binding to PE was detected (data not shown).
Binding of A. pleuropneumoniae serotype 1 to a lipid extracted from swine lungs.
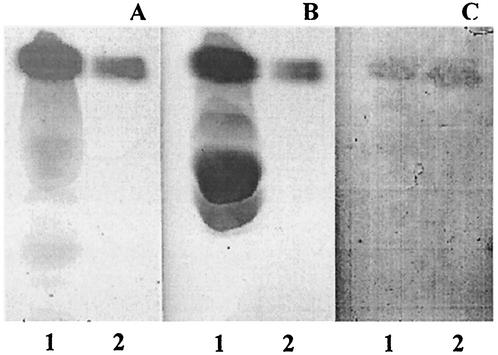
A. pleuropneumoniae serotype 1 was able to bind to a lipid present in the lower phase of a Folch extraction performed on swine lungs (Fig. 5). This phase normally contains all tissue lipids other than gangliosides. The lipid comigrated with commercial PE in a neutral solvent system (Fig. 5A and B) as well as in acidic and basic solvent systems (Fig. 6). The unknown lipid, like the commercial PE, stained with ninhydrin, which is specific for free amino groups (Fig. 5A), and with molybdenum blue, which stains phospholipids (Fig. 5B). We did not observe any binding in the upper phase, which contains the nonlipid moieties as well as most of the gangliosides and only negligible amounts of the other lipids.
FIG. 5.
TLC plates stained with ninhydrin (A), molybdenum blue (B), and immunostained chromatogram obtained by overlay with A. pleuropneumoniae serotype 1 cells (C) Lane 1, lower phase from a Folch extraction performed on porcine lungs; lane 2, commercial PE.
FIG. 6.
TLC plates stained with molybdenum blue showing migration of PE with a basic solvent containing chloroform-methanol-30% ammoniac (65:30:5) (A) or an acidic solvent containing chloroform-methanol-acetic acid-water (25:15:4) (B). Lane 1, lipids extracted from swine lung; lane 2, commercial PE.
Mass spectrometry.
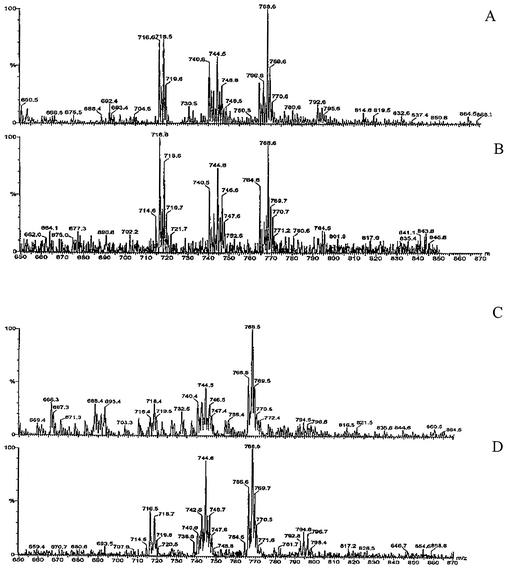
To confirm the identity of the putative PE extracted from swine lungs, the lipid was analyzed by electrospray mass spectrometry in positive mode. According to Cole and Enke (10), when PE undergoes collision-induced dissociation, the major reaction that occurs is cleavage of the phosphate-glycerol bond, which results in the loss of the polar head group as a neutral molecule, while the rest of the ion retains its charge. For PE, this cleavage represents a loss of a 141 m/z from the parent ion and allows an unequivocal identification of the compound. The spectra shown in Fig. 7A and C are scans of the ions present in the standard sample (panel A) and in the swine sample (panel C) before collision-induced dissociation. Since the mass spectrometer was programmed to scan for a neutral loss of 141 m/z, Fig. 7B and D show the parent ions of the molecules that undergo this characteristic loss due to collision-induced dissociation. Some of the peaks present in scan A are also present in scan B, thus confirming the presence of PE molecules in the commercial standard. Similarly, the presence of PE molecules is also confirmed in the swine lung sample according to scans shown in panels C and D. Interestingly, the peaks present in Fig. 7B and D have similar masses, which shows that the two samples share similar species of PE. According to Cole and Enke (10), the different PE molecules differ from each other by the lengths of their fatty acids and the hydroxylation of the fatty acids. Thus, the analysis of the mass of some of the major peaks, for example, peak 744.6, indicates that this particular peak represents molecules (with two fatty acids) that contain a total of 32 carbon atoms, 62 hydrogen atoms, and two unsaturations, whereas molecules in peak 768.6 contain 36 carbon atoms, 66 hydrogen atoms, and four unsaturations.
FIG. 7.
Electrospray mass spectrometry analysis in positive mode of commercial PE from egg yolk (A and B) and phospholipids from lungs of swine (C and D). The mass spectrometer was programmed to scan for a neutral loss of 141 m/z (characteristic for PE ions). Scan of the ions present in the sample before collision-induced dissociation (panels A and C) and parent ions present after a neutral loss of 141 m/z collision-induced dissociation experiment (panels B and D).
DISCUSSION
Adherence to host epithelial cells and/or the mucous layer of the mucosal surfaces is well-known to be the initial step of colonization by microorganisms (2, 30). The mechanisms of adhesion of A. pleuropneumoniae to porcine respiratory tract cells are poorly understood. Several reports indicate that LPS is an important adhesin for different organisms, including A. pleuropneumoniae (9, 20, 28, 34). Our research group had previously identified some putative receptors for A. pleuropneumoniae that were either proteinaceous or lipidic in nature (1, 33). The aim of the present study was to investigate the ability of A. pleuropneumoniae to bind to phospholipids, which are abundant in host plasma membranes and act as receptors for many bacterial pathogens (8, 12, 19, 26, 27, 37, 39).
Our results indicate that A. pleuropneumoniae can bind to PE but not to the five other phospholipids tested. This adds A. pleuropneumoniae to the growing list of pathogens that have affinity for PE but not for other phospholipids. The list includes other respiratory tract pathogens such as C. pneumoniae as well as H. influenzae, which is another member of the Pasteurellaceae family (8, 24). The importance of the polar ethanolamine head of the PE molecule in binding to these bacteria is suggested by the fact that a closely related phospholipid such as PS does not show any affinity to A. pleuropneumoniae or any of the other aforementioned pathogens. Reference strains representing the other prevalent A. pleuropneumoniae serotypes in North America (serotypes 5b and 7) also demonstrated binding to PE, which shows that binding of A. pleuropneumoniae to PE is not restricted to one serotype.
A. pleuropneumoniae serotype 1 cells were able to bind to a phospholipid in a swine lung extract identified as PE. This lipid was first stained by molybdenum blue, which stains lipids containing phosphate groups. It was also stained with ninhydrin, which is specific for the free amino group. Ninhydrin can therefore stain only two types of phospholipids—PE and PS. With the neutral solvent used, we could see that this phospholipid comigrated with commercial PE, which migrates differently from PS. We then compared the migration of the potential PE and of the commercial PE in both acidic and basic solvent systems and observed that these two phospholipids migrate similarly in these two solvent systems. Although its staining and migration were similar to those of PE, we additionally confirmed the identity of this phospholipid by using mass spectrometry analysis. We were first able to observe that commercial PE standard as well as PE extracted from a pig lung were composed of different species of PE that differ from each other at the level of their fatty acid chains. Interestingly, the PE species found in the two samples were very similar and both allow the binding of A. pleuropneumoniae serotype 1.
A. pleuropneumoniae serotype 1 also showed binding to lyso-PE (from egg yolk), which is a normal degradation product of PE. A similar binding was also observed with other pathogens such as C. upsaliensis, H. mustelae, and H. pylori (16, 39). These data show that the fatty acid attached to the second carbon of the glycerol backbone of PE is probably not essential for the recognition of PE by these bacteria. However, the second fatty acid chain seems to be critical for the optimal presentation of the phospholipid to the bacterial adhesin since, among all the commercial sources of PE tested (i.e., sheep brain, bovine brain, egg yolk, soybean, and E. coli), there was no binding to PE from sheep and bovine brain and very faint binding to PE from E. coli and soybean (data not shown). The strongest binding was to PE extracted from egg yolk, which is likely the result of variations in the fatty acids (27, 38). This phenomenon was also observed with other pathogens like C. upsaliensis and E. coli and can be explained by the fact that the length and degree of saturation of fatty acids may vary between the different sources of the phospholipids. All PEs are therefore not equally bound by bacterial pathogens (38).
To see whether LPS molecules were involved in the binding to PE, we first used isogenic O antigen and core LPS mutants of A. pleuropneumoniae serotype 1 generated by mini-Tn10 transposon mutagenesis (15, 25, 35). The data obtained showed that three core mutants as well as the wild-type parent strain were able to bind to PE. On the other hand, the O antigen rough mutants tested in binding assays (27.1, 44.1, and 51.1) were unable to bind PE.
To confirm that O antigens were implicated in the binding of A. pleuropneumoniae serotype 1 to PE, we then preincubated the bacteria with MAbs against LPS O antigen as described previously (1). These results provided another indirect proof that O antigen might be implicated in binding to PE. The same kind of inhibition was also previously observed with other putative lipidic receptors (mono- and disaccharide glycosphingolipids) like GlcCer, GalCer, LacCer, and sulfatide—but not with longer glycolipids like GgO3 and GgO4 (1). The O antigen of A. pleuropneumoniae serotype 1, which is the terminal part of the LPS, seems to be implicated in binding of short lipids such as PE, while the core region might be responsible for the binding to GgO3 and GgO4 (1). Interestingly, the binding data shown in Fig. 2 and 3 indicate that A. pleuropneumoniae serotype 1 demonstrates poorer recognition of short lipids like LacCer, GlcCer, and PE than longer lipids like GgO3 and GgO4. This phenomenon seems to be the same for A. pleuropneumoniae serotype 2 (1). The O antigen seems to have weaker affinity with its lipidic receptors compared to the core region with longer lipids. Since rough mutants of serotypes 2, 5b, and 7 are not yet available, further studies are needed in order to determine whether the O antigens of these serotypes are also involved in binding.
Interestingly, like several other bacterial pathogens (e.g., H. pylori, C. pneumoniae, C. trachomatis, H. influenzae, and Pseudomonas aeruginosa), A. pleuropneumoniae shows a common binding specificity for GgO3, GgO4 (1), and PE (the present study). To the best of our knowledge, A. pleuropneumoniae is the only known bacterium for which LPS is implicated in the binding to these three lipids.
In conclusion, A. pleuropneumoniae is able to bind to PE both from commercial sources and extracted from lungs of swine. The use of isogenic rough LPS mutants as well as monoclonal antibodies against O antigen indicate that the O antigen of A. pleuropneumoniae serotype 1 could be implicated in this interaction. It is tempting to speculate that, through a multiple-step binding process, A. pleuropneumoniae first uses low-affinity binding between O antigen and phospholipids (PE) or short glycolipids (LacCer and GlcCer) and then relies on the core oligosaccharide of LPS and/or surface proteins (55-kDa OMP, fimbriae) to interact more avidly with other larger lipidic (GgO3 and GgO4) or proteinic receptors.
Acknowledgments
This work has been supported by grants from Natural Sciences and Engineering Research Council of Canada (grant number RGPIN0003428 to M.J.) and from Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (grant number FCAR 2002-ER-71900). M.-E.J. is the recipient of a studentship from FCAR.
We are grateful to M. Gottschalk and K. R. Mittal for monoclonal and polyclonal antibodies. We also thank O. A. Mamer, Director of the McGill University biomedical mass spectrometry unit, for the mass spectrometry analysis and Josée Labrie and Vincent Labrie for technical assistance.
Editor: J. N. Weiser
REFERENCES
- 1.Abul-Milh, M., S. E. Paradis, J. D. Dubreuil, and M. Jacques. 1999. Binding of Actinobacillus pleuropneumoniae lipopolysaccharides to glycosphingolipids evaluated by thin-layer chromatography. Infect. Immun. 67:4983-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, Y. H., R. B. Dickinson, and R. J. Doyle. 2000. Mechanisms of bacterial adhesion and pathogenesis of implant and tissue infections, p. 1-28. In Y. H. An and R. J. Frieman (ed.), Handbook of bacterial adhesion—principles, methods, and applications. Humana Press, Totowa, N.J.
- 3.Beausoleil, H. -E., and J. D. Dubreuil. 2001. In vitro binding characteristics and affinity for sulfatide of Escherichia coli STb enterotoxin. Receptors Channels 7:401-411. [PubMed] [Google Scholar]
- 4.Bélanger, M., D. Dubreuil, J. Harel, C. Girard, and M. Jacques. 1990. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect. Immun. 58:3523-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bélanger, M., S. Rioux, B. Foiry, and M. Jacques. 1992. Affinity for porcine respiratory tract mucus is found in some isolates of Actinobacillus pleuropneumoniae. FEMS Microbiol. Lett. 76:119-125. [DOI] [PubMed] [Google Scholar]
- 6.Blackall, P. J., H. L. Klaasen, H. Van Den Bosch, P. Kuhnert, and J. Frey. 2002. Proposal of a new serovar of Actinobacillus pleuropneumoniae: serovar 15. Vet. Microbiol. 84:47-52. [DOI] [PubMed] [Google Scholar]
- 7.Bosse, J. T., H. Janson, B. J. Sheehan, A. J. Beddek, A. N. Rycroft, J. S. Kroll, and P. R. Langford. 2002. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect. 4:225-235. [DOI] [PubMed] [Google Scholar]
- 8.Busse, J., E. Hartmann, and C. A. Lingwood. 1997. Receptor affinity purification of a lipid-binding adhesin from Haemophilus influenzae. J. Infect. Dis. 175:77-83. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, P. S., J. C. Arruda, T. J. Williams, and D. C. Laux. 1985. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect. Immun. 48:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, M. J., and C. G. Enke. 1991. Direct determination of phospholipid structures in microorganisms by fast atom bombardment triple quadrupole mass spectrometry. Anal. Chem. 63:1032-1038. [DOI] [PubMed] [Google Scholar]
- 11.Dubreuil, J. D., M. Jacques, K. R. Mittal, and M. Gottschalk. 2000. Actinobacillus pleuropneumoniae surface polysaccharides: their role in diagnosis and immunogenicity. Anim. Health Res. Rev. 1:73-93. [DOI] [PubMed] [Google Scholar]
- 12.Foster, D. B., D. Philpott, M. Abul-Milh, M. Huesca, P. M. Sherman, and C. A. Lingwood. 1999. Phosphatidylethanolamine recognition promotes enteropathogenic E. coli and enterohemorrhagic E. coli host cell attachment. Microb. Pathog. 27:289-301. [DOI] [PubMed] [Google Scholar]
- 13.Frey, J. 1995. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 3:257-261. [DOI] [PubMed] [Google Scholar]
- 14.Fried, B. 1994. Lipids. In J. Fried and J. Sherma (ed.), Thin-layer chromatography: techniques and applications, 3rd ed., vol. 66. M. Dekker, New York, N.Y.
- 15.Galarneau, C., S. Rioux, and M. Jacques. 2000. Core oligosaccharide mutants of Actinobacillus pleuropneumoniae serotype 1 obtained by mini-Tn10 mutagenesis. Pathogenesis 1:253-264.
- 16.Gold, B. D., M. Huesca, P. M. Sherman, and C. A. Lingwood. 1993. Helicobacter mustelae and Helicobacter pylori bind to common lipid receptors in vitro. Infect. Immun. 61:2632-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haesebrouck, F., K. Chiers, I. Van Overbeke, and R. Ducatelle. 1997. Actinobacillus pleuropneumoniae infections in pigs: the role of virulence factors in pathogenesis and protection. Vet. Microbiol. 58:239-249. [DOI] [PubMed] [Google Scholar]
- 18.Hitchcock, P. J., L. Leive, P. H. Makela, E. T. Rietschel, W. Strittmatter, and D. C. Morrison. 1986. Lipopolysaccharide nomenclature—past, present, and future. J. Bacteriol. 166:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huesca, M., A. Goodwin, A. Bhagwansingh, P. Hoffman, and C. A. Lingwood. 1998. Characterization of an acidic-pH-inducible stress protein (Hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori. Infect. Immun. 66:4061-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacques, M. 1996. Role of lipo-oligosaccharides and lipopolysaccharides in bacterial adherence. Trends Microbiol. 4:408-410. [DOI] [PubMed] [Google Scholar]
- 21.Jacques, M., and S. Paradis. 1998. Adhesin-receptor interactions in Pasteurellaceae. FEMS Microbiol. Rev. 22:45-59. [DOI] [PubMed] [Google Scholar]
- 22.Kates, M. 1986. Techniques of lipidology: isolation, analysis, and identification of lipids, 2nd ed. Elsevier, Amsterdam, The Netherlands.
- 23.Khursigara, C., M. Abul-Milh, B. Lau, J. A. Giron, C. A. Lingwood, and D. E. Foster. 2001. Enteropathogenic Escherichia coli virulence factor bundle-forming pilus has a binding specificity for phosphatidylethanolamine. Infect. Immun. 69:6573-6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krivan, H. C., B. Nilsson, C. A. Lingwood, and H. Ryu. 1991. Chlamydia trachomatis and Chlamydia pneumoniae bind specifically to phosphatidylethanolamine in HeLa cells and to GalNAcβ1-4Galβ1-4Glc sequences found in asialo-GM1 and asialo-GM2. Biochem. Biophys. Res. Commun. 175:1082-1089. [DOI] [PubMed] [Google Scholar]
- 25.Labrie, J., S. Rioux, M. M. Wade, F. R. Champlin, S. C. Holman, W. W. Wilson, C. Savoye, M. Kobisch, M. Sirois, C. Galarneau, and M. Jacques. 2002. Identification of genes involved in biosynthesis of Actinobacillus pleuropneumoniae serotype 1 O-antigen and biological properties of rough mutants. J. Endotoxin Res. 8:27-38. [PubMed] [Google Scholar]
- 26.Lingwood, C. 1993. H. pylori adhesins and receptors, p. 209-222. In S. Goodwin and B. Worsley (ed.), Helicobacter pylori: biology and clinical practice. CRC Press, Boca Raton, Fla.
- 27.Lingwood, C. A., M. Huesca, and A. Kuksis. 1992. The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect. Immun. 60:2470-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McSweegan, E., and R. I. Walker. 1986. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect. Immun. 53:141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen, R. 1986. Serological characterization of Actinobacillus pleuropneumoniae strains and proposal of a new serotype: serotype 12. Acta Vet. Scand. 27:453-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ofek, I., and R. J. Doyle. 1994. Bacterial adhesion to cells and tissues. Chapman & Hall, New York, N.Y.
- 31.Overbeke, I. V., K. Chiers, G. Charlier, I. Vandenberghe, J. V. Beeumen, R. Ducatelle, and F. Haesebrouck. 2002. Characterization of the in vitro adhesion of Actinobacillus pleuropneumoniae to swine alveolar epithelial cells. Vet. Microbiol. 88:59-74. [DOI] [PubMed] [Google Scholar]
- 32.Paradis, S. E., D. Dubreuil, S. Rioux, M. Gottschalk, and M. Jacques. 1994. High-molecular-mass lipopolysaccharides are involved in Actinobacillus pleuropneumoniae adherence to porcine respiratory tract cells. Infect. Immun. 62:3311-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paradis, S. E., J. D. Dubreuil, M. Gottschalk, M. Archambault, and M. Jacques. 1999. Inhibition of adherence of Actinobacillus pleuropneumoniae to porcine respiratory tract cells by monoclonal antibodies directed against LPS and partial characterization of the LPS receptors. Curr. Microbiol. 39:313-320. [DOI] [PubMed] [Google Scholar]
- 34.Porat, N., M. A. Apicella, and M. S. Blake. 1995. A lipooligosaccharide-binding site on HepG2 cells similar to the gonococcal opacity-associated surface protein Opa. Infect. Immun. 63:2164-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rioux, S., C. Galarneau, J. Harel, J. Frey, J. Nicolet, M. Kobisch, J. D. Dubreuil, and M. Jacques. 1999. Isolation and characterization of mini-Tn10 lipopolysaccharide mutants of Actinobacillus pleuropneumoniae serotype 1. Can. J. Microbiol. 45:1017-1026. [DOI] [PubMed] [Google Scholar]
- 36.Rousset, E., J. Harel, and J. D. Dubreuil. 1998. Sulfatide from the pig jejunum brush border epithelial cell surface is involved in binding of Escherichia coli enterotoxin b. Infect. Immun. 66:5650-5658. [DOI] [PMC free article] [PubMed]
- 37.Schumacher, U., M. Maennel, and H. Werner. 2000. Adherence of Bacteroides species and Bilophila wadsworthia to phospholipids and glycolipids. Anaerobe 6:61-63. [Google Scholar]
- 38.Slater, S. J., C. Ho, F. J. Taddeo, M. B. Kelly, and C. D. Stubbs. 1993. Contribution of hydrogen bonding to lipid-lipid interactions in membranes and the role of lipid order: effects of cholesterol, increased phospholipid unsaturation, and ethanol. Biochemistry 32:3714-3721. [DOI] [PubMed] [Google Scholar]
- 39.Sylvester, F. A., D. Philpott, B. Gold, A. Lastovica, and J. F. Forstner. 1996. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, Campylobacter upsaliensis. Infect. Immun. 64:4060-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tascon, R. I., J. A. Vazquez-Boland, C. B. Gutierrez-Martin, J. I. Rodriguez-Barbosa, and E. F. Rodriguez-Ferri. 1996. Virulence factors of the swine pathogen Actinobacillus pleuropneumoniae. Microbiologia 12:171-184. [PubMed] [Google Scholar]
- 41.Taylor, D. J. 1999. Actinobacillus pleuropneumoniae, p. 343-354. In B. E. Straw, S. D'Allaire, W. I. Mengeling, and D. J. Taylor (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames.