Abstract
A model for the protracted (30-day) colonization of smooth surfaces by Streptococcus gordonii that incorporates the nutrient flux that occurs in the oral cavity was developed. This model was used to characterize the biphasic expansion of the adherent bacterial population, which corresponded with the emergence of higher-order architectures characteristic of biofilms. Biofilm formation by S. gordonii was observed to be influenced by the presence of simple sugars including sucrose, glucose, and fructose. Real-time PCR was used to quantify changes in expression of S. gordonii genes known or thought to be involved in biofilm formation. Morphological changes were accompanied by a significant shift in gene expression patterns. The majority of S. gordonii genes examined were observed to be downregulated in the biofilm phase. Genes found to be upregulated in the biofilm state were observed to encode products related to environmental sensing and signaling.
Tooth surfaces are persistently colonized by a complex but highly organized biota termed dental plaque, a microbial biofilm with the capacity to adapt to, and to endure, cyclic variation in nutrient availability as well as harsh mechanical and biological forces targeted at its containment and removal. Streptococcus gordonii is among the pioneering species to colonize a tooth surface (29, 30, 34, 39). Binding of these organisms to the tooth enamel creates a template for the subsequent attachment of other bacteria in establishment of the complex oral biofilm (20, 22). As succeeding layers of different bacterial species attach to the plaque, new binding templates and nutritional microenvironments are formed, which may ultimately favor the attachment and residence of periodontal pathogens (6). The net effect is the establishment of an ordered community of heterogeneous microbial species, with each member playing a role in maintaining the vitality and structure of plaque. A key element in the formation and stability of the plaque biofilm, therefore, is the persistent colonization of the smooth surface of the tooth at the base of this complex community.
Several studies have now demonstrated that cells existing in the biofilm state have phenotypic characteristics distinct from those of their planktonic counterparts, with significant changes in the patterns of gene expression (9, 45). This differential expression appears to be governed by communication between bacteria of the same or other species, in addition to cues emanating from the host and the environment (23). Specific intercellular communication mediated by N-(3-oxododecanoyl)-l-homoserine lactone has been shown to be central to the differentiation of the biofilm architecture by Pseudomonas aeruginosa (11).
Little is known of the physiologic changes that accompany persistent colonization of the tooth surface by pioneering species in the formation of the dental plaque biofilm. It was therefore of interest to develop a model of protracted smooth-surface colonization with cyclic variation in nutrient availability as a first approximation of events that occur in the establishment of the dental plaque biofilm by pioneering bacterial species such as S. gordonii. Once the model was established, it was also of interest to determine whether this persistent colonization was accompanied by physiologic changes that may account for the long-term survival and durability of the plaque biofilm. We report the morphological characteristics of S. gordonii Challis DL1 exposed to cyclic variation in nutrient availability and persistently colonizing a smooth surface for as long as a month, and we identify transcriptional changes that accompany the formation of this persistent physiologic state.
MATERIALS AND METHODS
Bacterial strains and media.
To reduce the likelihood of contamination over the protracted cultivation period, a spontaneous streptomycin-resistant mutant of S. gordonii Challis DL1 (a gift from P.E. Kolenbrander, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Md.) was selected by plating approximately 1010 cells on tryptone-yeast extract medium (30 g of tryptone/liter-0.5 g of yeast extract/liter) (TY) supplemented with 10 mmol of sucrose, 200 μg of streptomycin (Sigma-Aldrich Co.)/ml, and 1.5% (wt/vol) agar (2). S. gordonii appeared to preferentially partition into the biofilm phase.
Culture conditions and generation of biofilms.
Streptomycin-resistant S. gordonii Challis DL1 biofilms were cultivated on 18- by 18-mm no. 1 glass coverslips (Fisher Scientific, Pittsburgh, Pa.) in 100-mm-diameter, 15-mm-deep polystyrene petri dishes at 37°C under anaerobic conditions. The biofilm was seeded with 15 ml of a 1:100-diluted overnight planktonic culture of S. gordonii and 200 μg of streptomycin/ml. At 24-h intervals, the spent medium was aspirated from the dish, and the plates and coverslips were washed thoroughly twice with phosphate-buffered saline, pH 7.4 (PBS), to remove adventitiously associated cells. The biofilm was then further cultured in fresh, sterile, appropriately supplemented medium. This cycle of feeding and washing was repeated at 24-h intervals for as long as 30 days.
Confocal microscopy.
For microscopic imaging of biofilms, coverslips were washed in PBS to remove cells nonspecifically associated with the biofilm and were stained with 100 μl of freshly prepared 10-mg/ml aqueous acridine orange, which was applied directly to the coverslip, followed immediately by three sequential PBS rinses. Confocal microscopy was conducted at a magnification of ×63 under PBS immersion by using a model TCS NT confocal microscope (Leica Lasertechnik GmbH, Heidelberg, Germany) equipped with an argon-krypton laser.
Enumeration of adherent cells.
To enumerate cells in the biofilm phase, the biofilms were dislodged from the substratum by scraping and were transferred to a 2-ml Bead Beater tube (BioSpec Products, Bartlesville, Okla.) containing 0.5 ml of 1-mm-diameter glass beads (BioSpec Products) in 1 ml of PBS. The tubes were subsequently transferred to a Bead Beater (BioSpec Products) and horizontally shaken for 1 min to disaggregate the cells from the biofilm matrix, a condition determined experimentally to yield the maximum number of CFU with no detectable loss of viability. Disaggregated biofilm phase cells and planktonic phase cells were then enumerated by quantitative track dilution plating as described previously (18).
RNA extraction.
RNA was rapidly purified from planktonic cells or 10-day-old biofilm cells as previously described (35, 36), with minor modifications. Cells from the biofilms were disaggregated by scraping the biofilm into a 2-ml microcentrifuge tube (Biospec Products) containing 0.5 ml of 1-mm-diameter glass beads and 1.5 ml of PBS. The tube was placed in the Mini Beadbeater (BioSpec Products) and shaken twice at 5,000 rpm for 60 s. The tube was immediately transferred to ice, and the beads were allowed to settle out. The suspended cells were removed from the beads and transferred to a 17- by 100-mm snap-cap tube (VWR International, Westchester, Pa.). To recover additional cells interspersed within the bead bed, the beads were washed with an additional 1.5 ml of PBS, and fractions were combined. Disaggregated biofilm or planktonic phase cells were pelleted by centrifugation at 2,500 × g for 4 min at 4°C and then resuspended in 1.5 ml of Tri-Reagent (Sigma-Aldrich). The suspension was immediately transferred to a 2-ml microcentrifuge tube containing approximately 0.5 ml of packed 100-μm-diameter zirconia-silica beads, which was placed in a high-speed reciprocating shaker (BioSpec Products) and horizontally shaken at 5,000 rpm for 1 min to lyse the cells. RNA was recovered from the cell lysate, treated with RQ1 RNase-free DNase (Promega, Madison, Wis.) as described previously (39, 40), and stored in diethyl pyrocarbonate (DEPC)-treated water. The integrity of the RNA was assessed by electrophoresis of 2 μl of each sample through a 1.2% agarose-0.66 M formaldehyde gel in MOPS running buffer (20 mM morpholinepropanesulfonic acid [pH 7.0] [MOPS], 8 mM sodium acetate, 1 mM EDTA [pH 8.0]) at 3 to 4 V/cm (40). The RNA concentration was determined spectrophotometrically by measuring the A260/A280 ratio of a 1:50 dilution in DEPC-treated water.
Real-time quantitative PCR.
Genes for which expression in biofilm and planktonic cultures was compared included those known or previously suggested to be involved in biofilm formation or in S. gordonii adhesion or coaggregation and are listed in Table 1. Amplification, detection, and analysis were performed using the ABI Prism 7700 Sequence Detection system (Applied Biosystems, Foster City, Calif.) as described previously (36). Briefly, primers were developed by using the algorithms provided in Primer Express (version 1; Applied Biosystems) for uniformity in size (approximately 100 bp) and melting temperature. Primer sequence data are provided in Table 1.
TABLE 1.
Genes whose expression in biofilm and planktonic cultures was compared
| Gene | Description | Source or reference | Primer sequence
|
|
|---|---|---|---|---|
| Forward | Reverse | |||
| scaR | Manganese-dependent repressor of scaCBA | 16 | TTCTTGCCAAGGAACTTGAACAT | GGTCACATGAGGCAGTGGAA |
| sgg | GTP-binding protein | 19 | TCGGTAAAGGCGGATCCA | CCCCCAGCATAAGCTCAATAT |
| ropA | Chaperone-trigger factor (prolyl isomerase) | This study | CCTTCAGCTGCTGGACCTTC | TGCACGTATCGAACGCGA |
| dltA | d-Alanine-d-Alanyl carrier protein ligase | 5 | TTGCCTGCTTATCATACTGGAGAT | GAAATCCATTCGACCACCGTAA |
| scaA | Coaggregation-mediating adhesin | 21 | TGTCCTGGGCTTGAGGAGTT | CTCTGCGGTTCGCAATACAAC |
| hhpH | Oligopeptide-binding lipoprotein (hexa- or heptapeptide) | 17 | ACCCGACAAATGGTGGATTATT | AGAGCTGACACAACATCTTGGTTT |
| arcB | Omithine carbamoyltransferase | 3 | CGTACGCGTGCAGCCTT | TCATTAGCACCAAGATATTCTGGATGC |
| abpA | Amylase binding protein | 33 | AGTTGAAGGTGGAAGCCACAAT | ACGTACAGCGTTGAAAGCGTT |
| htgX | Putative heat shock protein | 41 | GGTCGCCTTCTTTGCTCTTTT | CACCCAGAGGAGAATTCATCCA |
| LDH | Lactate dehydrogenase | This study | CATGATGTAGGCGTGCACTGA | AGCTCGTTTCCGTCAAGCA |
| tRNA-arg | tRNA for Arg | 14 | GGTCCCATAGCTCAGCTGGATA | TCCCAGCAAGATTCGAACTTG |
| hsa | Streptococcal hemagglutinin | 38 | CGTGGGACCCTTCAGGAAAT | TTGACCGTAAGGACAAATCTTTCTAG |
| rpoC | DNA-directed RNA polymerase beta subunit | 24 | TGTCCTGGGCTTGAGGAGTT | CTCTGCGGTTCGCAATACAAC |
| int/CoA | Intrageneric coaggregation-relevant adhesin | 46 | TCCAGCAGTCTGTGGCAGATA | TTAGCGACACGGTGGTGGT |
| scaA orf | Hydrophobic membrane protein | 21 | TGATTACAGCCATTGTGATTGGA | TGCCCCGCAGAATGATAAACT |
| ddl | d-Alanine: d-alanine ligase | 13 | CCCGTCTTCACCAAACCTTCTA | CGCAGTTCTTCCTGATTATCAGAT |
| sodA | Manganese-dependent superoxide dismutase | 32 | GCTGACATTGATGCTACTTTTGGT | CAAAACGAGTTGTTGCTGCAG |
| flpA | Fibronectin-binding protein-like protein A | 27 | TCTGCTTTCTCCGTTGCTAGG | CCGCCGAGTAGAAAATGAGTTG |
| xdhA | Extracellular glyceraldehyde-3-phosphate dehydrogenase | 28 | TCGGTCGTATCGGTCGTCTT | AGGTCGTTGATGCGAGTAACTTC |
| tdkF | Thymidine kinase | 26 | ATGACCAGCGCAGTTGACAC | GCCTGGCGTTTCATACCAATT |
| cysK | Cysteine kinase | A. J. M. Vriesema, unpublished data | TTTTCAATGGTAAAGTTCAGTTAAGCA | GCTATGGCTATCATAGCTTTTTTTTATATC |
| comYA | ABC transporter subunit | 24 | TCCGGACTTGCTCATCATTG | AAACTGTAGCTCCCGTCAAGCT |
| sspA | Surface adhesin A | 12 | AAGAGTTGGCTGAGTATCCGACTAA | AGTTCTACAAGTGCCGCCTTAATT |
| spaA | spaA homolog adhesin protein I/II V-region | 4 | ACAACTCCAATACCAAGAACACCA | TGGTGTGTCAGGTTTGTCAGG |
| msrA | Methionine sulfoxide reductase | 44 | TTGCCGGTGACAAGTTTGAG | CTCTGGCAATAGGCCGACTAA |
| orf pH | Neutral pH-inducible promoter region and unknown gene | 43 | GGCCTTCAGATTTTTCAGAGATTAA | CGTGTCCGAAATACAGGTCATTT |
| gtfG | Glucosyltransferase | 42 | AGAGCGTTTGCCAGAACCA | CCAACACATCGTCATCATGCT |
| rggD | gtfG regulator (positive) | 37 | TCGTAAAGTCGTCGGGAAAAA | CGAGACAGCTGGGCAACAG |
| pbg | Phospo-beta-glucosidase | 44 | CGGCGGCAAATCAATATGA | AGACAGGCCTTTTCCCATTAGAT |
| comD | Competence pheromone receptor (histidine kinase) | 14 | AAATGCACATCTTAATAGCTTTGCTAGT | CATATTGTTCACGAGCAGACTTCAG |
| comE | Competence pheromone regulator | 14 | TTGAGTCAGACGAGGTAAATCAACTT | GCATAGGGATTATGTTGGCGTATA |
RNA concentrations were normalized by using amplification of the 23S rRNA gene of S. gordonii as an internal standard. For each experimental reaction, cDNA synthesis and PCR amplification were performed in a two-step reaction. Reverse transcriptase reactions were performed using the Taqman reverse transcription (RT) reagent kit (Applied Biosystems). Each 50-μl reaction mixture contained the following in a 1× RT buffer: 5.5 mM MgCl2, 500 μM each deoxynucleoside triphosphate, 0.4 U of RNase inhibitor/μl, 1.25 U of reverse transcriptase/μl, 2.5 μM reverse primer, and 10 ng of total RNA. The RT reaction was incubated at 48°C for 30 min and then at 95°C for 5 min.
Real-time PCR amplifications were performed in 50-μl reaction mixtures that contained 1× SYBR Green I PCR master mix (Applied Biosystems), 300 nM forward primer, 50 nM additional reverse primer, and 5 μl of cDNA template. PCR conditions included an initial denaturation at 95°C for 10 min, followed by a 40-cycle amplification consisting of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min. All primer pairs were checked for primer-dimer formation by using the two-step protocol described above without the addition of RNA template. As an additional control for each primer pair and each RNA sample, the cDNA synthesis reaction was carried out without reverse transcriptase in order to identify contamination of RNA samples by residual genomic DNA.
The value used for quantitation and comparison among the samples was the threshold cycle (CT), or the number of cycles required to cross the midpoint of the detectable amplification curve, which was normalized to a passive reference dye (carboxy-x-rhodamine [ROX]) included in each reaction. Real-time PCR analysis was performed on three independent RNA preparations from three separate planktonic and biofilm cultures. The CT values for RNA obtained from biofilm cultures were compared to the CT values of the same products amplified from planktonic-culture RNA in order to determine the fold difference. Fold difference was calculated by dividing the larger of the mean CT values for a particular gene (biofilm or planktonic) by the smaller. If the larger mean (which indicates more cycles of amplification required to cross the midpoint of the visible range of detection, and hence a smaller starting RNA concentration) derived from the planktonic culture, a plus sign was affixed to the fold difference to indicate an increase in RNA abundance in the biofilm sample. If the reverse was true, a minus sign was affixed to indicate a decrease in the abundance of that specific RNA in the biofilm-derived sample. Student's t test was used to calculate the significance of the difference between the mean expression of a given gene in a 10-day biofilm and its mean expression in planktonic culture. A P value of <0.05 was considered significant.
RESULTS
Microscopic characterization of the persistent biofilm model.
To simulate the cyclic exposure to nutrients experienced by S. gordonii in the oral cavity over protracted periods, a batch cycling and rinsing model was developed, where S. gordonii Challis DL1 was cultured in sucrose-supplemented 0.5× TY medium on glass coverslips in petri dishes as the substratum. A visible film of adherent bacteria was observable 24 h after inoculation in PBS-rinsed dishes. Initially, the adherent cells took the form of a smooth, uniform coating of the support substratum. However, by 8 days, a visibly rough, coherent, sheet-like structure developed, which was maintained and continued to evolve over the duration of the study. There was no observable difference between the biofilm that formed on the glass coverslips and that on the polystyrene surfaces of the petri dishes that contained them.
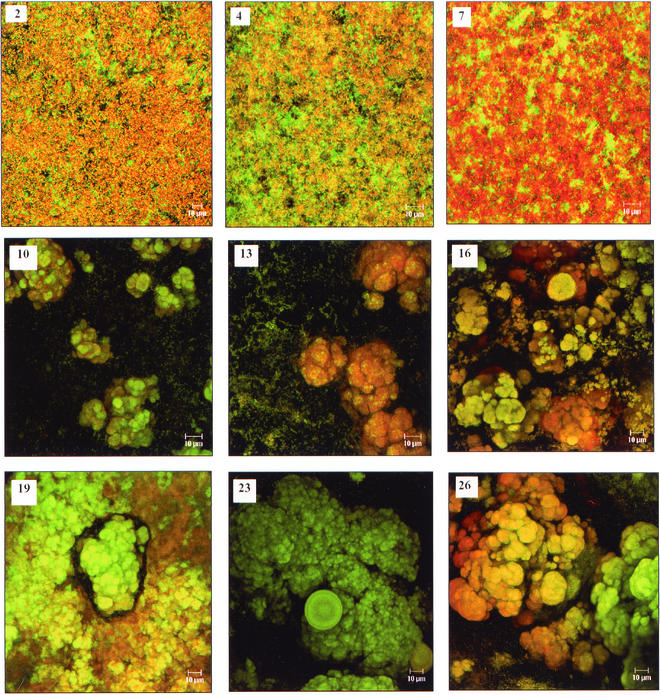
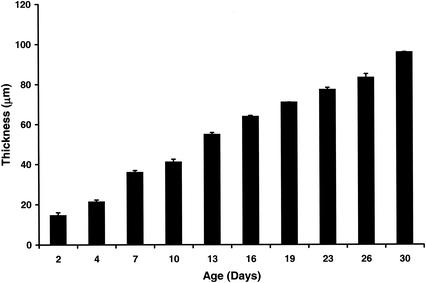
To characterize the developing S. gordonii Challis DL1 biofilm in greater detail, its evolution was monitored throughout development by confocal microscopy, with concurrent enumeration of the adherent population. At regular intervals over a period of approximately 1 month, biofilms developing on the glass coverslips were stained and examined (Fig. 1). Initially and through the first 4 days of cyclic feeding and washing, adherent cells formed a thin, confluent layer of uniform thickness, with no observable differentiation of higher-order architectures, confirming observations made visually. However, following 8 to 9 days of cyclic feeding and washing, adherent cells began to differentiate into discrete microcolonies. By 10 days, a stippled mat of S. gordonii Challis DL1 covered the surface, with obvious differentiation into microcolony and channel architectures (Fig. 1), similar to those reported during maturation of P. aeruginosa biofilms (7, 8). These complex structures continued to develop throughout continued cycles of feeding and washing (Fig. 1), achieving a thickness of 100 μm by day 30 (Fig. 2).
FIG. 1.
Confocal scanning laser micrographs of the development of S. gordonii Challis DL1 biofilms over 26 days. The biofilms were generated on glass coverslips in 0.5× TY medium supplemented with 10 mM sucrose and were stained immediately prior to microscopy with acridine orange. Numbers indicate the age of the biofilm in days. Magnification, ×63.
FIG. 2.
Change in thickness of biofilms with time, as measured by confocal microscopy. The depth of the biofilm represents the mean of five randomly chosen sites within each biofilm.
Growth kinetics of S. gordonii Challis DL1 biofilms.
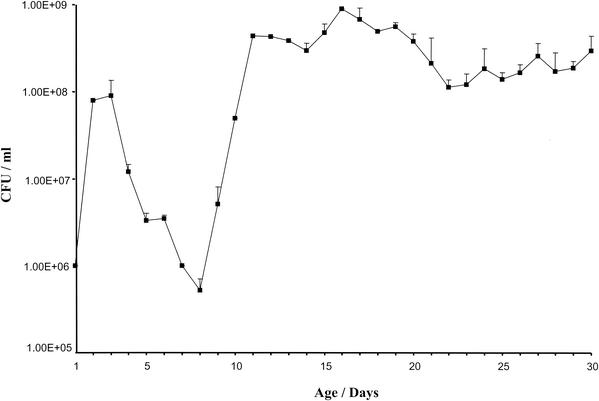
Because of the observable evolution of higher-order architecture in the growing biofilm over its monthlong development, it was of interest to determine the relationship between biofilm structure and the population dynamics of the cultivable bacteria comprising the biofilm. Therefore, adherent cells were harvested in triplicate, disaggregated with glass beads, and enumerated by track dilution quantitation (18) (Fig. 3).
FIG. 3.
Change in CFU within S. gordonii Challis DL1 biofilms cultured in 0.5× TY medium supplemented with sucrose, which was replaced at 24-h intervals. Cells were enumerated over 30 days of cultivation. Numbers of CFU for biofilms harvested at each time point are expressed as means of triplicate determinations. Error bars represent the standard errors of the means and are not detectable where the error is smaller than the symbol.
Bacterial growth was found to be biphasic. Over the first 2 days, the bacterial population increased in number; after that point, the population of cultivable cells declined until day 8. This change in population appears to parallel the log, stationary, and decline phases characteristically observed for planktonic organisms. From day 8 through day 10, however, there was a resurgence in the adherent cell population, which reached a second plateau by day 11. Cell numbers remained remarkably constant over the remainder of the 30-day study period. Interestingly, the period corresponding to the initial decline in population (days 4 to 8) followed by resurgence (days 8 to 10) (Fig. 3) corresponded precisely with the emergence of highly organized channel and microcolony architectures (Fig. 1).
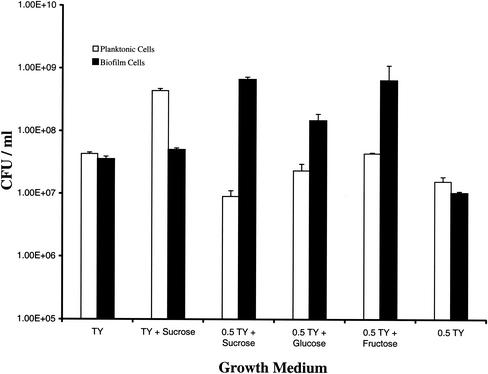
Because the nutrient content of the medium has been found to regulate the development of biofilms by other organisms (31, 47), we tested global nutrient components for their influence on biofilm formation by S. gordonii DL1. The availability of simple carbohydrates and nitrogenous nutrients was varied as follows: (i) unsupplemented TY, (ii) 0.5× unsupplemented TY, (iii) TY plus 10 mmol of sucrose, (iv) 0.5× TY plus 10 mmol of sucrose, (v) 0.5× TY plus 10 mmol of glucose, and (vi) 0.5× TY plus 10 mmol of fructose. The extent of partitioning between adherent populations and planktonic cells was determined after 4 days of cyclic feeding and washing, a time found in preliminary experiments to yield substantial populations of cells in both phases. As shown in Fig. 4, when the medium was not supplemented with simple carbohydrates (i.e., sucrose, glucose, or fructose), similar numbers of cells were found in the planktonic and biofilm phases (culture in TY or 0.5× TY). The addition of sucrose to TY resulted in numbers of adherent-population cells comparable to, or nominally higher than, those observed with unsupplemented TY but, unexpectedly, even higher numbers of cells in the planktonic phase at this relatively early point in biofilm development. On the other hand, when the concentration of nitrogenous medium components was reduced by 50% (i.e., 0.5× TY) and the medium was supplemented with sucrose, glucose, or fructose, the cells appeared to preferentially partition into the biofilm phase, indicating that nitrogen limitation in the presence of excess carbohydrate promotes biofilm formation by this species.
FIG. 4.
Effects of medium composition on partitioning of S. gordonii DL1 into biofilm and planktonic phases after 4 days of cultivation. Reduction in the yeast extract and tryptone contents of the medium resulted in increased numbers of CFU occurring in the biofilm phase in all cases where the medium was supplemented with 10 mmol of a simple sugar (sucrose, glucose, or fructose). Lack of carbohydrate supplementation of either TY or 0.5× TY resulted in comparable numbers in the biofilm and planktonic phases, and supplementation of TY with sucrose resulted in an unexpected increase in the number of planktonic cells, with no net change in numbers in the biofilm phase.
Differential gene expression.
To assess the expression of genes known or thought to be involved in biofilm formation by S. gordonii, real-time PCR was used to quantify gene expression in planktonic cells for comparison to that in 10-day-old biofilms. In general, most genes tested were observed to be downregulated in the biofilm (Table 2). The greatest reduction in gene expression in the biofilm phase was observed for scaR, which codes for a metalloregulator of the manganese uptake system in S. gordonii (16). Levels of mRNA encoding ScaR were observed to be reduced approximately 45-fold in biofilm-derived cells. This reduction in expression was accompanied by an eightfold reduction in the abundance of mRNA encoding ScaA, one component of the putative manganese transport system. The second greatest reduction in mRNA abundance in the biofilm phase was observed for that encoding Sgg, a GTP-binding protein shown to be a member of the G protein superfamily in streptococci (19). The third greatest reduction in biofilm gene expression occurred in a homolog of ropA in group A streptococci, encoding a putative peptidyl-prolyl isomerase and chaperone (25). Genes observed to be expressed at increased levels in the biofilm phase were largely limited to comD and comE, which were observed to occur at four- and ninefold-increased abundances, respectively. The comD and comE genes encode the histidine kinase and response regulator for the S. gordonii competence pathway for DNA uptake (14).
TABLE 2.
mRNA abundance in biofilm relative to the planktonic culture
| Gene | Fold differencea |
|---|---|
| Reduced expression in biofilm | |
| scaR | −45.68 |
| sgg | −33.21 |
| ropA | −11.50 |
| dltA | −8.08 |
| scaA | −8.01 |
| hhpH | −5.89 |
| arcB | −5.79 |
| abpA | −5.50 |
| htgX | −5.02 |
| 2856 LDH | −4.57 |
| tRNA-arg | −3.93 |
| hsa | −3.76 |
| rpoC | −3.68 |
| int/CoA | −3.05 |
| scaA orf | −2.75 |
| ddl | −2.60 |
| sodA | −2.45 |
| flpA | −2.40 |
| xdhA | −2.30 |
| tdkF | −1.97 |
| cysK | −1.82 |
| No significant difference | |
| comYA | −1.52 |
| sspA | −1.44 |
| spaA | −1.23 |
| msrA | −1.14 |
| orf pH | 1.26 |
| gtfG | 1.58 |
| Increased expression in biofilm | |
| rggD | 1.95 |
| pbg | 2.29 |
| comD | 4.23 |
| comE | 9.40 |
Except for genes in the group labeled “No significant difference,” comparisons of means were statistically significantly different (P < 0.05).
DISCUSSION
Using a cyclic feeding and washing model of persistent colonization, we have shown that S. gordonii Challis DL1 forms highly differentiated biofilms capable of supporting stable populations of organisms over a protracted period. The differentiated architectures that developed over the 30-day experimental period were reminiscent of the microcolony and channel structures seen in biofilms of Pseudomonas fluorescens and Streptococcus mutans (1, 31). An interesting bimodality of growth was observed during S. gordonii biofilm development. Confocal microscopic observation, accompanied by quantitative determination of population size, revealed an initial cycle of growth, spanning days 0 to 8, which appears to represent the conventional log, stationary, and decline phases of growth commonly observed for bacteria grown in planktonic culture. This early period was characterized by adherence to glass coverslips in a fine, confluent, and evenly distributed pattern, with little evidence of community organization. The second phase initiated with a resurgence in bacterial numbers after day 8 and achieved population stability by day 11, which corresponded in time with the appearance of organization within the adherent community. Characteristic mushroom and pillar formations emerged, and channels appeared to be cleared in place of the uniformly distributed individual cells that initially attached to the substratum. In many ways, the emergence of organization, involving growth, remodeling, and potentially specialization within the community, from an initial mass of uniform cells resembles early stages in the tissue differentiation of higher organisms.
Following the emergence of organization over days 8 to 11 postinoculation, the population remained remarkably stable through the end of the 30-day experimental period. However, the biofilm did not remain static. Biofilm thickness was observed to increase steadily to a maximum of 100 μm. The increase in biofilm thickness without an observable increase in culturable CFU may represent the continuing deposition of the extracellular polysaccharide matrix, the embedding of dead cells and their replacement by live progeny, or perhaps other physiological changes which render some members of the community less capable of reversion to a mode of growth that permits colony formation on an agar surface (i.e., viable but not culturable).
The physiology of biofilm formation has become a topic of considerable interest and investigation. Biofilms involving several different species have been studied, perhaps none more intensively than those of P. aeruginosa (7, 8, 9, 45). The pattern of biofilm development in P. aeruginosa has been dissected into distinct phases: initial attachment to a solid surface and microcolony formation, followed by maturation of microcolonies into exopolysaccharide-encased biofilms (7, 8). Cook et al. (6) recently analyzed biofilm formation by S. gordonii Challis DL1 on saliva-coated glass coverslips in a flow cell system similar to that used successfully for analysis of P. aeruginosa biofilm development. Biofilm development by S. gordonii Challis DL1, as determined by the formation of microcolonies around adherent bacteria, was assessed after 4 h. It was noted that under these conditions, S. gordonii Challis DL1 failed to establish a definable biofilm in this brief monoculture but did form a template for attachment of the periodontal pathogen Porphyromonas gingivalis.
Differences in the ability of S. gordonii Challis DL1 to form a biofilm are clearly model dependent. The results of the present study show that the culture medium exerts an important influence on the partitioning of cells between planktonic and biofilm phases. It is not known whether this partitioning is the simple result of a shift in physiology resulting from quorum-sensing cues, which have been shown to be important for P. aeruginosa biofilm development (11), or whether there is an underlying genetic switch, such as that seen with phase variation by some species facilitating adaptation to new ecological conditions (15) (a phenomenon known to occur in S. gordonii and to affect glucan production by cell surface glucosyltransferases [40]), with the subsequent selection and outgrowth of adherent-phase variants in a time- and population size-dependent manner.
Direct comparison of the gene expression profiles of cells in biofilm and planktonic phases consistently demonstrates that biofilm formation is accompanied by significant shifts in cell physiology. Others have shown that in P. aeruginosa, algC and algD, required for the synthesis of the exopolysaccharide matrix, alginate, are upregulated upon adhesion to an inanimate surface (10). More recently, it was shown by use of a microarray that in comparatively shorter term biofilms, approximately 0.5% of P. aeruginosa genes were upregulated and 0.5% were downregulated at significant levels (45). Because of the sensitivity of real-time PCR and its amenability to the performance of replicates for individual statistical determination, we were able to measure changes that achieved statistical significance for a larger fraction of the genes tested, although our results are in good agreement with the finding that changes of large magnitude occurred only in a minority of the genes examined. Also, in the absence of a completed genome, our experiments focused only on factors previously known or suspected of being involved in biofilm formation and adhesion, which may be a selected group more likely to be environmentally responsive.
In conclusion, a simple system for the analysis of biofilm formation by S. gordonii Challis DL1 was developed that permits study of the persistent colonization of smooth surfaces by this organism. Biofilm formation by this organism was influenced by the availability of several simple carbohydrates, importantly including two other than sucrose, demonstrating the importance of bacterial traits, potentially in addition to surface glucosyltransferases, in biofilm establishment. More-global differential gene expression studies to characterize S. gordonii biofilm formation will be greatly facilitated by the availability of genome sequence information.
Acknowledgments
This research was supported by U.S. Public Health Service grants DE13244 and EY11648 and by an unrestricted grant from Research to Prevent Blindness.
We gratefully acknowledge Crissandra Womack, Brett Shepard, Lynn Hancock, Phil Coburn, and Michael Engelbert for helpful advice in manuscript preparation and Paul Kolenbrander for many helpful discussions in initiating this project.
Editor: J. N. Weiser
REFERENCES
- 1.Burne, R. A., Y. Y. Chen, and J. E. Penders. 1997. Analysis of gene expression in Streptococcus mutans in biofilms in vitro. Adv. Dent. Res. 11:100-109. [DOI] [PubMed] [Google Scholar]
- 2.Burne, R. A., K. Schilling, W. H. Bowen, and R. E. Yasbin. 1987. Expression, purification, and characterization of an exo-β-d-fructosidase of Streptococcus mutans. J. Bacteriol. 169:4507-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldelari, I., B. Loeliger, H. Langen, M. P. Glauser, and P. Moreillon. 2000. Deregulation of the arginine deiminase (arc) operon in penicillin-tolerant mutants of Streptococcus gordonii. Antimicrob. Agents Chemother. 44:2802-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatenay-Rivauday, C., I. Yamodo, M. A. Sciotti, N. Troffer-Charlier, J. P. Klein, and J. Ogier. 2000. TNF-α release by monocytic THP-1 cells through cross-linking of the extended V-region of the oral streptococcal protein I/II. J. Leukoc. Biol. 67:81-89. [DOI] [PubMed] [Google Scholar]
- 5.Clemans, D. L., P. E. Kolenbrander, D. V. Debabov, Q. Zhang, R. D. Lunsford, H. Sakone, C. J. Whittaker, M. P. Heaton, and F. C. Neuhaus. 1999. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect. Immun. 67:2464-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook, G. S., J. W. Costerton, and R. J. Lamont. 1998. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J. Periodont. Res. 33:323-327. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., S. S. Philip, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 10.Davies, D. G., A. M. Chakrabarty, and G. G. Geesey. 1993. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 59:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 12.Demuth, D. R., Y. Duan, W. Brooks, A. R. Holmes, R. McNab, and H. F. Jenkinson. 1996. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol. Microbiol. 20:403-413. [DOI] [PubMed] [Google Scholar]
- 13.Garnier, F., G. Gerbaud, P. Courvalin, and M. Galimand. 1997. Identification of clinically relevant viridans group streptococci to the species level by PCR. J. Clin. Microbiol. 35:2337-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havarstein, L. S., P. Gaustad, I. F. Nes, and D. A. Morrison. 1996. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 21:863-869. [DOI] [PubMed] [Google Scholar]
- 15.Henderson, I. R., P. Owen, and J. P. Nataro. 1999. Molecular switches—the ON and OFF of bacterial phase variation. Mol. Microbiol. 33:919-932. [DOI] [PubMed] [Google Scholar]
- 16.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38:140-153. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson, H. F., R. A. Baker, and G. W. Tannock. 1996. A binding-lipoprotein-dependent oligopeptide transport system in Streptococcus gordonii essential for uptake of hexa- and heptapeptides. J. Bacteriol. 178:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata, S., Y. Terao, T. Andoh, and S. Hamada. 1997. Nucleotide sequence and molecular characterization of a gene encoding GTP-binding protein from Streptococcus gordonii. FEMS Microbiol. Lett. 156:211-216. [DOI] [PubMed] [Google Scholar]
- 20.Kolenbrander, P. E., and J. London. 1992. Ecological significance of coaggregation among oral bacteria. Adv. Microb. Ecol. 12:183-217. [Google Scholar]
- 21.Kolenbrander, P. E., R. N. Andersen, and N. Ganeshkumar. 1994. Nucleotide sequence of the Streptococcus gordonii PK488 coaggregation adhesin gene, scaA, and ATP-binding cassette. Infect. Immun. 62:4469-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolenbrander, P. E., R. N. Andersen, and L. V. H. Moore. 1990. Intergeneric coaggregation among strains of human oral bacteria: potential role in primary colonization of the tooth surface. Appl. Environ. Microbiol. 56:3890-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolter, R., and R. Losick. 1998. One for all and all for one. Science 280:226-227. [DOI] [PubMed] [Google Scholar]
- 24.Lunsford, R. D., and A. G. Roble. 1997. comYA, a gene similar to comGA of Bacillus subtilis, is essential for competence-factor-dependent DNA transformation in Streptococcus gordonii. J. Bacteriol. 179:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon, W. R., C. M. Gibson, and M. G. Caparon. 1998. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 17:6263-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNab, R. 1996. Cloning and sequence analysis of thymidine kinase from the oral bacterium Streptococcus gordonii. FEMS Microbiol. Lett. 135:103-110. [DOI] [PubMed] [Google Scholar]
- 27.McNab, R., H. F. Jenkinson, D. M. Loach, and G. W. Tannock. 1994. Cell-surface-associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol. Microbiol. 14:743-754. [DOI] [PubMed] [Google Scholar]
- 28.Nelson, D., J. M. Goldstein, K. Boatright, D. W. Harty, S. L. Cook, P. J. Hickman, J. Potempa, J. Travis, and J. A. Mayo. 2001. pH-regulated secretion of a glyceraldehyde-3-phosphate dehydrogenase from Streptococcus gordonii FSS2: purification, characterization, and cloning of the gene encoding this enzyme. J. Dent. Res. 80:371-377. [DOI] [PubMed] [Google Scholar]
- 29.Nyvad, B., and M. Killian. 1987. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 95:369-380. [DOI] [PubMed] [Google Scholar]
- 30.Nyvad, B., and M. Killian. 1990. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 24:267-272. [DOI] [PubMed] [Google Scholar]
- 31.O'Toole, G. A., and R. Kolter. 1998. The initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 32.Poyart, C., G. Quesne, S. Coulon, P. Berche, and P. Trieu-Cuot. 1998. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 36:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers, J. D., E. M. Haase, A. E. Brown, C. W. Douglas, J. P. Gwynn, and F. A. Scannapieco. 1998. Identification and analysis of a gene (abpA) encoding a major amylase-binding protein in Streptococcus gordonii. Microbiology 144:1223-1233. [DOI] [PubMed] [Google Scholar]
- 34.Rosan, B., C. H. Lai, and M. A. Listgarten. 1976. Streptococcus sanguis: a model in the application of immunochemical analysis for the in situ localization of bacteria in dental plaque. J. Dent. Res. 55:124-141. [DOI] [PubMed] [Google Scholar]
- 35.Shepard, B. D., and M. S. Gilmore. 1999. Identification of aerobically and anaerobically induced genes of Enterococcus faecalis using random arbitrarily primed PCR. Appl. Environ. Microbiol. 65:1470-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepard, B. D., and M. S. Gilmore. 2002. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect. Immun. 70:4344-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulavik, M. C., G. Tardif, and D. B. Clewell. 1992. Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the Spp phenotype of Streptococcus gordonii Challis. J. Bacteriol. 174:3577-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi, Y., K. Konishi, J. O. Cisar, and M. Yoshikawa. 2002. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect. Immun. 70:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Houte, J., R. J. Gibbons, and A. J. Pulkkinen. 1971. Adherence as an ecological determinant for streptococci in the human mouth. Arch. Oral Biol. 16:1131-1141. [DOI] [PubMed] [Google Scholar]
- 40.Vickerman, M. M., D. B. Clewell, and G. W. Jones. 1991. Ecological implications of glucosyltransferase phase variation in Streptococcus gordonii. Appl. Environ. Microbiol. 57:3648-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vickerman, M. M., N. M. Mather, P. E. Minick, and C. A. Edwards. 2002. Initial characterization of the Streptococcus gordonii htpX gene. Oral Microbiol. Immunol. 17(1):22-31. [DOI] [PubMed]
- 42.Vickerman, M. M., M. C. Sulavik, and D. B. Clewell. 1995. Molecular analysis of Streptococcus gordonii glucosyltransferase phase variants. Dev. Biol. Stand. 85:309-314. [PubMed] [Google Scholar]
- 43.Vriesema, A. J. M., R. Brinkman, J. Kok, J. Dankert, and S. A. J. Zaat. 2000. Broad-host-range shuttle vectors for screening of regulated promoter activity in viridans group streptococci: isolation of a pH-regulated promoter. Appl. Environ. Microbiol. 66:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vriesema, A. J. M., J. Dankert, and S. A. J. Zaat. 2000. A shift from oral to blood pH is a stimulus for adaptive gene expression of Streptococcus gordonii CH1 and induces protection against oxidative stress and enhanced bacterial growth by expression of msrA. Infect. Immun. 68:1061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 46.Whittaker, C. J., D. L Clemans, and P. E. Kolenbrander. 1996. Insertional inactivation of an intrageneric coaggregation-relevant adhesin locus from Streptococcus gordonii DL1 (Challis). Infect. Immun. 64:4137-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wimpenny, J. W. T., and R. Colasanti. 1997. A unifying hypothesis for the structure of microbial biofilms based on cellular automaton models. FEMS Microbiol. Ecol. 22:1-16. [Google Scholar]