Abstract
The gene encoding the 56-kDa protein of Orientia tsutsugamushi Shanxi was amplified by a nested PCR and cloned into the expression vector pQE30. The 56-kDa protein of O. tsutsugamushi Shanxi (Sxh56) was expressed as a fusion protein with the His6-binding protein of Escherichia coli by deleting the signal peptide-encoding sequence from the 5′ end of the open reading frame. The recombinant protein formed inclusion bodies when expressed in E. coli M15. The recombinant protein was examined for reactivity with mouse sera against three antigenic prototypes of O. tsutsugamushi by an immunoblot assay. The recombinant Sxh56 reacted only to polyclonal antiserum to O. tsutsugamushi Gilliam in an enzyme-linked immunosorbent assay (ELISA) and in an immunoblot assay. Recombinant Sxh56 was purified by Ni-nitrilotriacetic acid affinity chromatography and injected into mice to evaluate its ability to stimulate immune responses. High levels of immunoglobulin G and T-cell proliferation appeared in mice immunized with the recombinant protein. The recombinant Sxh56 was used in an ELISA to evaluate the ability of the method to detect antibodies to O. tsutsugamushi in human and animal sera. Thirty sera from mice infected with O. tsutsugamushi Gilliam or Shanxi and 55 sera from normal mice were detected in the ELISA with recombinant Sxh56, and the sensitivity and specificity were 96.67 and 100%, respectively. One hundred fifty-one positive sera and 412 negative sera to O. tsutsugamushi Gilliam were detected in an indirect immunofluorescence assay with the recombinant protein, and the sensitivity and specificity were 96.36 and 88.08%, respectively. These results strongly suggest that the recombinant Sxh56 is a suitable type-specific immunodiagnostic antigen and vaccine candidate.
Scrub typhus is an acute, febrile disease caused by Orientia tsutsugamushi (21). The disease is endemic in the Asia-Pacific region, including the People's Republic of China. For poorly understood reasons, the incidence of the disease in humans has increased sharply in China during the past 20 years. It is characterized by fever, rash, and eschar, etc. Diagnosis of scrub typhus is normally based on the clinical presentation and the patient history. However, it is difficult to differentiate scrub typhus from other acute febrile illnesses, such as murine typhus, dengue fever, and viral hemorrhagic fevers, because of the similarities in symptoms. Therefore, underdiagnosis or misdiagnosis of scrub typhus is common and may result in delayed or inappropriate treatment. Confirmatory experimental diagnosis of scrub typhus is generally based on PCR, indirect immunofluorescence assay (IFA), and immunoperoxidase test, etc. (9, 24). However, the shortcomings of these diagnostic methods limit their usefulness. Highly sensitive PCR methods have made it possible to detect O. tsutsugamushi at the onset of illness when antibody titers are not high enough to be detected (4, 8, 18). However, gene amplification requires special instruments and reagents generally not available in most rural hospitals. IFA is highly sensitive and specific, but it also requires an immunofluorescence microscope that may not be available in rural hospitals. Moreover, it requires cultivation of O. tsutsugamushi. The recently developed immunoperoxidase test does not need an immunofluorescence microscope, but purified O. tsutsugamushi antigen is required. However, O. tsutsugamushi is difficult to cultivate. A more practical approach to the development of newer serodiagnostic methods is to clone and express the immunodominant genes of O. tsutsugamushi in Escherichia coli. These recombinant products could then be produced and purified in adequate amounts for use as antigens in developing a convenient and inexpensive diagnostic method that would greatly reduce the cost, transport, and reproducibility problems associated with the present diagnostic tests, which require growth and purification of the orientiae.
O. tsutsugamushi is an antigenically diverse microorganism. Several antigenic variants, such as the representative strains Gilliam, Karp, and Kato, and other isolates have been reported (14). Most isolates of O. tsutsugamushi in China have been identified as serotype Gilliam or Karp. Moreover, seroepidemiological data have shown that O. tsutsugamushi strains endemic in China were of serotype Gilliam or Karp. O. tsutsugamushi strain Shanxi was isolated from a scrub typhus patient's blood in 1995, and it was preliminarily identified as having the serum type of O. tsutsugamushi Gilliam (1). The major surface protein antigen of O. tsutsugamushi is the variable 56-kDa protein, which accounts for 10 to 15% of its total protein (5, 13). This protein is an immunodominant antigen, and its antigenic diversity depends on variation in this molecule. The 56-kDa protein is reactive with group-specific and strain-specific monoclonal antibodies, suggesting the existence of group-specific and strain-specific epitopes in this molecule (5, 11, 12, 17). It is known that sera from most patients with scrub typhus recognize this protein, and mice immunized with the 56-kDa protein could generate neutralizing antibodies and showed increased resistance to homologous O. tsutsugamushi infection (more than 160 times the 50% minimal lethal dose). These data suggest that it is a suitable diagnostic antigen and vaccine candidate (15, 16).
Here we report the molecular cloning and expression of the 56-kDa protein gene of O. tsutsugamushi Shanxi and the investigation of the antigenicity and immunogenicity of the recombinant protein. Finally, the diagnostic potential of this Sxh56 preparation was evaluated by enzyme-linked immunosorbent assay (ELISA) for detection of immunoglobulin G (IgG) in 563 human sera and 88 mouse sera.
MATERIALS AND METHODS
Bacterial strains and vectors.
E. coli M15 was used as the host strain for the pQE30 expression vector. pQE30 was purchased from Qiagen GmbH (Hilden, Germany). Plaque-purified O. tsutsugamushi strain Shanxi was isolated from a patient in Shanxi province, People's Republic of China. O. tsutsugamushi Gilliam, O. tsutsugamushi Karp, and O. tsutsugamushi Kato were kindly supplied by I. S. Kim of the Medical College, Seoul National University.
Media and growth conditions.
Luria-Bertani medium was used for routine maintenance of bacterial strains and for transformation experiments. For all strains harboring the recombinant plasmid, ampicillin (50 μg/ml) was added to the culture medium. Broth cultures were grown at 37°C with vigorous shaking (200 rpm) until the mid-logarithmic phase was attained. When noted, isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma) was added to the culture of strain M15 harboring plasmids to induce expression of the gene of interest.
Nucleic acid extraction and PCR amplification.
Orientiae were inoculated into chicken embryos, and the embryos were incubated at 35°C. The orientiae were harvested at 7 to 10 days postinfection, and genomic DNA was phenol-chloroform extracted as described previously (6). A PCR kit was purchased from Gibco (Los Angeles, Calif.). Primers P1, P2, P3, and P4 were synthesized by Shanghai Sangon Company according to the published sxh56 gene sequence (GenBank accession number AF050669) (1), as follows: P1 (56F, positions 8 to 37), 5′-AAA TTA TGT TAA TTG CTA GTG CAA TGT CTG-3′; P2 (56R, positions 1522 to 1548), 5′-CTA GAA GTT ATA GCG TAC ACC TGC ACT TGC-3′; P3 (56F, positions 67 to 88), 5′ CGC GGATCC ATA GAA TTG GGG GAT GAA GGA G; and P4 (56R, positions 1532 to 1548), 5′ CCC AAGCTT CTA GAA GTT ATA GCG TAC AC 3′. Added BamHI and HindIII restriction sites are underlined. The coding sequence was amplified by nested PCR from DNA isolated from O. tsutsugamushi strain Shanxi. The 56-kDa gene was amplified in a mixture of 200 μM (each) deoxynucleoside triphosphate, 0.3 μM (each) primer, and 0.6 U of Taq polymerase (Gibco) in10 mM Tris-HCl buffer (pH 8.3) supplemented with 2.0 mM MgCl2 and 50 mM KCl. The first amplification of the nested PCR was carried out with primers P1 and P2, and the second step used primers P3 and P4. The two PCRs were started with 6 min at 95°C, which was followed by 30 cycles consisting of 45 s at 94°C, 30 s at 55°C, and 90 s at 72°C. A final step of 7 min at 72°C was added to the last cycle. The products of the nested PCR were analyzed on a 1% agarose gel, and the size of the sxh56 gene was determined with the λ DNA/EcoT14I standard molecular weight marker (Sino-America Biotech Company, Luoyang, China).
Construction and identification of recombinant expression plasmid.
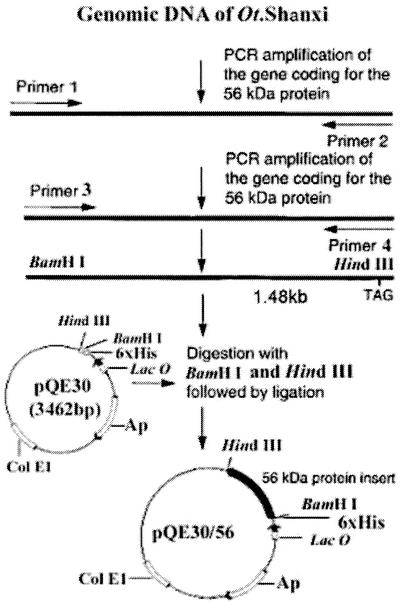
All restriction enzymes were purchased from TaKaRa biotechnology Co., Ltd (Dalian, China). pQE30 and pure PCR products (1.48 kb) were digested with BamHI and HindIII and then ligated overnight at 16°C. E. coli M15 was transformed with the ligation mixture. The recombinant plasmid was constructed as described in Fig. 1. E. coli M15 harboring the recombinant plasmid was screened with penicillin. The recombinant plasmid was also identified by digestion with BamHI and HindIII after repeated PCR, and sequences were further analyzed.
FIG. 1.
Strategy for cloning and construction of pQE30/56, which expresses the recombinant 56-kDa protein of the O. tsutsugamushi Shanxi strain.
Expression and identification of the 56-kDa protein.
E. coli M15 harboring the recombinant plasmid was propagated in LB (A+K) (50 mg of ampicillin, 50 mg of kanamycin, 10 g of Bacto tryptone, 5 g of Bacto yeast extract, and 10 g of NaCl per liter of distilled water [pH 7.0]) at 37°C with shaking. When E. coli M15 transformed with the recombinant plasmid grew to logarithmic phase, the optimum concentration of IPTG was added to induce expression of the sxh56 gene. The expression of the recombinant Sxh56 was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot assay.
SDS-PAGE and immunoblot analysis.
SDS-PAGE analysis was performed with the DYY-5 protein electrophoresis system (12 by 8 by 0.75 cm; Beijing Kesheng). The stacking and separation gels contained 5 and 10% acrylamide, respectively. Electrophoresis was carried out at constant voltage of 160 V for 180 min. The gels were either stained with Coomassie blue R or electroblotted onto nitrocellulose membranes. The molecular masses of proteins were determined with a middle-molecular-mass marker (97, 66, 45, 30, 20.1, and 14.4 kDa). SDS-PAGE and immunoblot analysis were carried out as described by Laemmli (10) and Towbin et al. (22), respectively.
Purification of the 56-kDa protein.
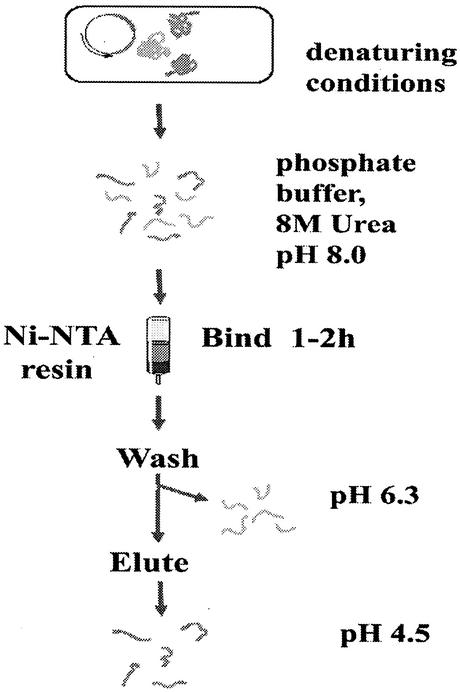
One hundred milliliters of E. coli expressing recombinant Sxh56 induced by IPTG was centrifuged for10 min at 8,000 rpm in a Beckman Allegra 21R centrifuge (F0630 rotor), and the pellets were resuspended in 10 ml of 10 mM Tris-Cl (pH 7.0) containing 1 mM EDTA (buffer A). Disruptions of cells were performed with an Ultrasonic Processor (Kesheng Instrument Corporation, Ningbo, China) at 250 W for 60 min (30 s of sonication and a 30-s pause each time with cooling on ice). The disrupted cell extracts were centrifuged at 8,000 rpm for 30 min. The pellets were vortexed to a homogeneous suspension with buffer A (10 mM Tris-Cl [pH 7.0], 1 mM EDTA) containing 1% (vol/vol) Triton X-100 and shaken at room temperature for an additional 20 min. The suspension was centrifuged for 15 min at 7,000 rpm. The pellets were suspended in buffer A containing 2 M urea, and then the suspension was treated as described above. Finally, the pellets were dissolved in 10 ml of 10 mM Tris-Cl (pH 7.0) containing 100 mM NaH2PO4 and 8 M urea (buffer B) and applied to a Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography column (Qiagen GmbH). The proteins were washed with 10 mM Tris-Cl (pH 6.3) containing 100 mM NaH2PO4and 8 M urea (buffer C) and eluted with 10 mM Tris-Cl (pH 4.5) containing 100 mM NaH2PO4 and 8 M urea (buffer D) (Fig. 2). The purification was done as described in the instructions to the Ni-NTA affinity chromatography purification kit (Qiagen GmbH).
FIG. 2.
Purification of His6-tagged recombinant Sxh56 protein.
Immunization with recombinant Sxh56 protein.
Purified recombinant Sxh56 (200 μg/ml) or phosphate-buffered saline was mixed with an equal volume of paraffin oil, and BALB/c mice and rabbits were immunized according to standard protocols (7). The animal experiments reported here were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (23).
T-cell proliferation assay by a colorimetric method.
Eight days after the first booster, four mice in each group were killed and spleens were ground into single-cell suspensions in RPMI 1640 medium (Gibco, Tulsa, Okla.) supplemented with 10% fetal bovine serum for T-cell proliferation assay. They were seeded in triplicate in flat-bottom 96-well microtiter plates (Costar) at 106 cells per well in 100 μl of culture medium with purified recombinant Sxh56 at either 10, 3, 1, or 0.3 μg/ml. The plates were incubated for 3 days with 5% CO2 at 37°C. Ten microliters of a solution of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was then added to each culture well, and the plates were incubated for 4 h at 37°C. One hundred microliters of lysis buffer containing 10% Triton-50% isopropyl alcohol-0.01 M hydrochloric acid was then added to each well, and the plates were incubated overnight. The optical density at 570 nm (OD570) and the OD630 were measured (19).
Sera.
Mouse polyclonal sera against O. tsutsugamushi Sxh951 were prepared in our laboratory (IFA dilution, 1:5,120). Mouse sera against O. tsutsugamushi Gilliam, O. tsutsugamushi Karp, and O. tsutsugamushi Kato (IFA dilution, 1:5,120, 1:5,120, and 1:2,560, respectively) were supplied by A. Tamura. Mouse polyclonal sera against inclusion of recombinant Sxh56 proteins were prepared in our laboratory (IFA dilution, 1:10,240). Control sera were collected from healthy BALB/c mice. Sera from BALB/c mice infected with O. tsutsugamushi Gilliam were collected after 10 to 15 days. All human sera were collected from 1998 to 2001 in China. All individuals identified as positive cases were positive by IFA.
IFA.
The IFA to detect antibodies in human and mouse sera was performed by using O. tsutsugamushi strain Gilliam propagated in the yolk sacs of embryonated chicken eggs according to established protocols (2, 9, 20, 25).
ELISA.
MaxiSorp plates (96 well; Nalge Nunc International, Roskilde, Denmark) were coated overnight at 4°C with antigens diluted in PBS, blocked with 3% bovine serum albumin for 1 h, and rinsed twice with PBS for 3 min each time. Mouse sera diluted from 1:20 to 1:1,280 with PBS were added to the ELISA plates. The plates were incubated for 1 h at room temperature and washed four times with 0.1% Triton X-100 in PBS. Peroxidase-conjugated goat anti-mouse IgG (Fc specific) (Sihuan Sci-Technics Company, Beijin, China) diluted 1:2,000 was added and incubated for 1 h at 37°C. The plates were washed five times with 0.1% Triton X-100 in PBS before the addition of tetramethyl-benzidine (TMB) substrate (Sihuan Sci-Technics Company), and then the reactions were stopped by adding 3 M NaOH and the OD450s were measured. All reagents were used in a standard volume of 100 μl. A positive control, a negative control, and a blank control were always included on each plate (3). For the detection of human antibodies, all procedures were the same as for the detection of mouse antibodies except that peroxidase-conjugated goat anti-human IgG (Sino-America Bio-Technology Corporation) diluted 1:500 was used. Human sera were diluted 1:80 with PBS.
RESULTS
Molecular cloning of sxh56.
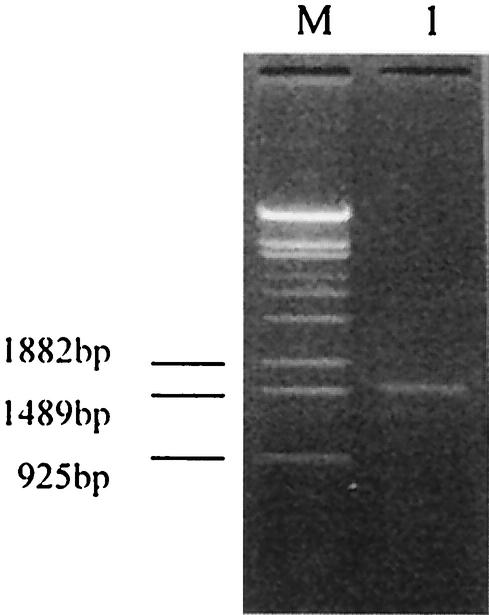
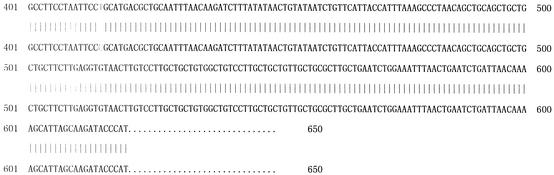
The sxh56 gene size was determined after amplification by nested PCR. A PCR product was obtained, and the sxh56 gene size was about 1.5 kb (Fig. 3). The results of the PCR and the restriction fragment length polymorphism reaction showed that the O. tsutsugamushi strain Sxh951 56-kDa protein gene had been cloned into the expression plasmid pQE30 (Fig. 4). The sequence of the inserted gene was 99% homologous with the sequence reported previously (Fig. 5) (1).
FIG. 3.
Amplified product of the 56-kDa protein gene from genomic DNA of O. tsutsugamushi Shanxi by nested PCR. Lane M, λ DNA/EcoT14I standard marker; lane 1, product of O. tsutsugamushi Shanxi mature 56-kDa protein gene amplified by nested PCR.
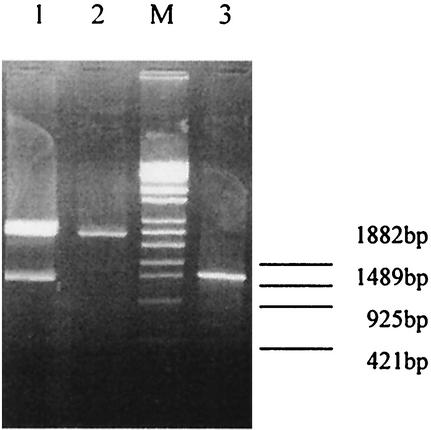
FIG. 4.
Identification of the recombinant plasmid by digestion with BamHI plus HindIII and PCR. Lane M, λ DNA/EcoT14I standard marker; lane 1, recombinant plasmid pQE30/56 digested with BamHI plus HindIII; lane 2, pQE30 digested with BamHI plus HindIII; lane 3, PCR product of recombinant plasmid pQE30/56.
FIG. 5.
Comparison of 5′and 3′ terminal sequences of the inserted fragment with the sequence of the O. tsutsugamushi Sxh951 56-kDa protein gene.
Expression and purification of the recombinant 56-kDa protein.
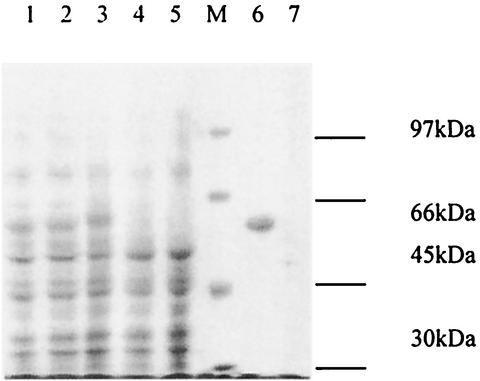
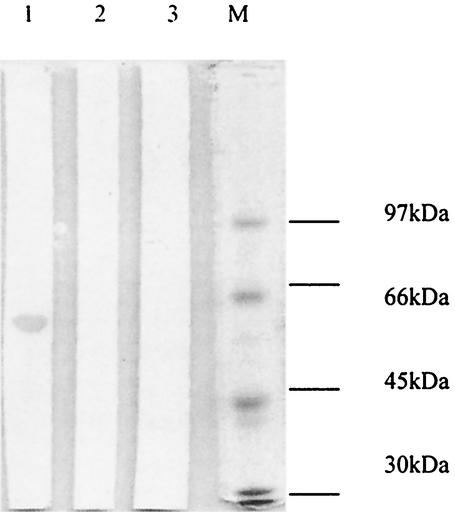
In order to optimize conditions for the expression of Sxh56, E. coli M15 transformed with pQE30/sxh56 was propagated at 37°C in medium containing 50 mg of ampicillin and kanamycin per liter to an OD550 of 0.6 to 0.7, induced with 1.0 or 2.0 mM IPTG, and grown further for 2, 3, 4, or 16 h. Although the expression level of Sxh56 increased with the time of incubation, no significant differences were observed with different levels of IPTG. Recombinant Sxh56 protein was verified to react to polyclonal antiserum to O. tsutsugamushi Sxh951. In the pQE30 expression system, highly expressed recombinant proteins were inclusion bodies, as was observed in SDS-PAGE (Fig. 6 and 7). The inclusion bodies of recombinant Sxh56 were subjected to Ni-NTA affinity chromatography for purification. The elution fractions detected by SDS-PAGE and immunoblot assay contained only recombinant Sxh56 (Fig. 8 and 9). Approximately 20 mg of recombinant Sxh56 could be purified from a 1-liter culture.
FIG. 6.
SDS-10% PAGE analysis of the expression products of E. coli M15 harboring pQE30/sxh56. Lane M, protein molecular weight marker; lanes 1 to 3, E. coli M15 harboring pQE30/sxh56 induced by IPTG; lane 4, E. coli M15 harboring pQE30 induced by IPTG; lane 5, E. coli M15 induced by IPTG; lane 6, protein expressed as inclusion bodies; lane 7, supernatant of supersonic crash.
FIG. 7.
Immunoblot analysis of the recombinant Sxh56 protein produced by the pQE30/sxh56 clone. Crude cell extracts were obtained from E. coli cells containing recombinant pQE30/sxh56 plasmids, separated by SDS-10% PAGE, transferred to nitrocellulose membranes, and reacted with anti-O. tsutsugamushi Sxh951 antibodies (see Materials and Methods). Molecular masses are indicated on the left. Lane M, protein molecular weight marker; lane 1, E. coli M15 harboring pQE30/sxh56 induced by IPTG; lane 2, E. coli M15 harboring pQE30 induced by IPTG; lane 3, E. coli M15 induced by IPTG.
FIG. 8.
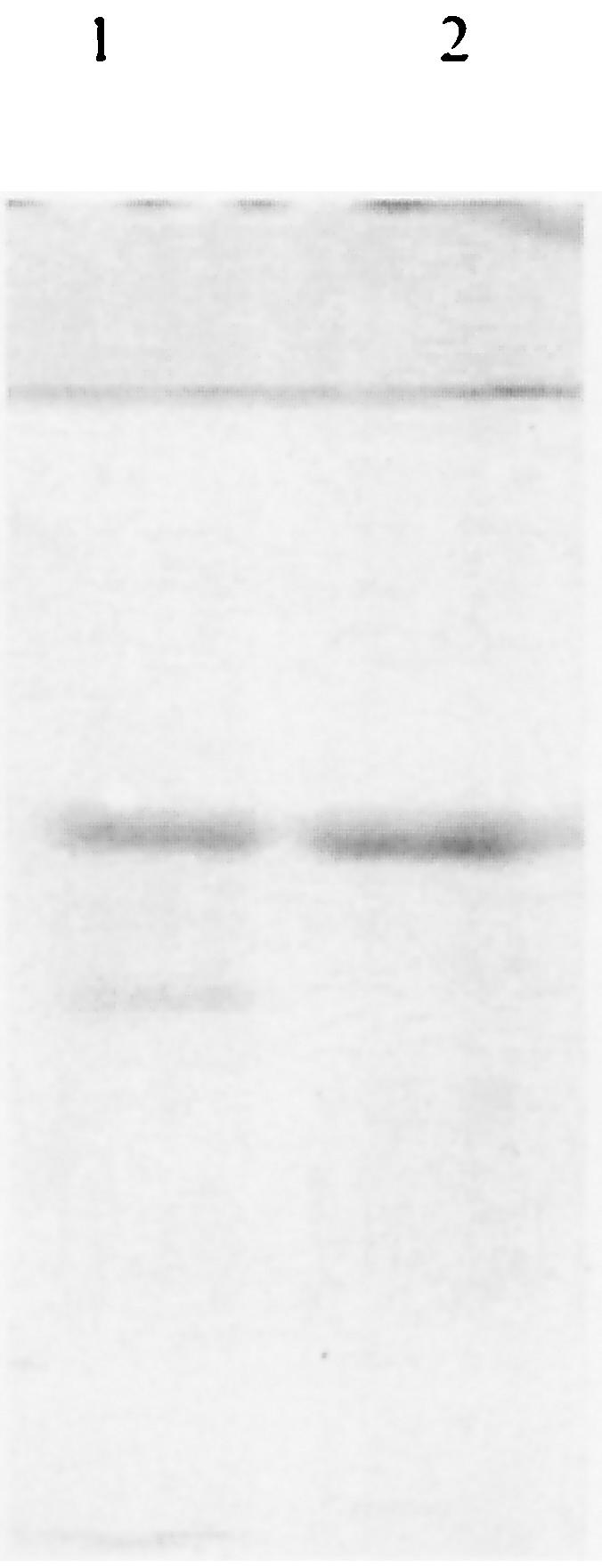
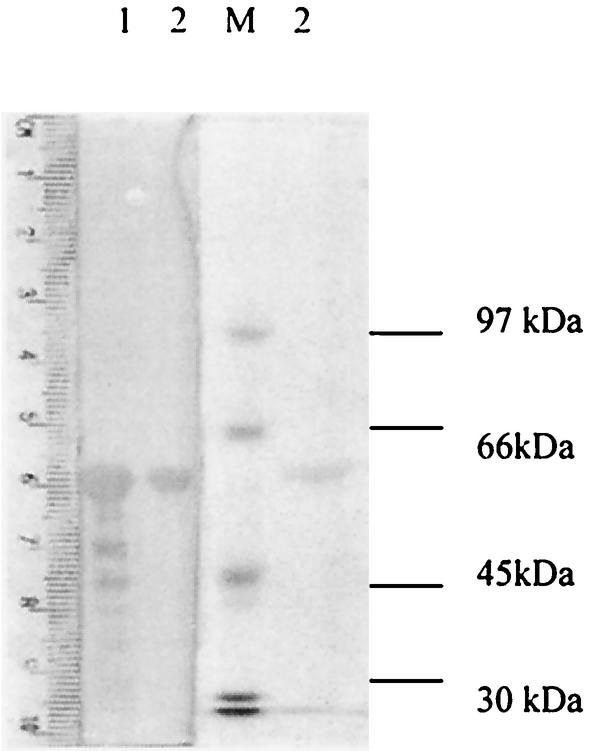
SDS-PAGE analysis of purified recombinant Sxh56 proteins. Lane 1, inclusion of recombinant Sxh56 proteins; lane 2, purified recombinant Sxh56 proteins.
FIG. 9.
Immunoblot analysis of the purified recombinant Sxh56 protein produced by the pQE30/sxh56 clone. The purified recombinant Sxh56 protein were separated by SDS-10% PAGE, transferred to nitrocellulose membranes, and reacted with sera against inclusion of recombinant Sxh56 proteins (see Materials and Methods). Molecular masses are indicated on the left. Lane M, protein molecular weight marker; lane 1, inclusion of recombinant Sxh56 proteins; lane 2, purified recombinant Sxh56 proteins.
Immunogenicity of recombinant Sxh56 protein.
In order to evaluate the ability of recombinant Sxh56 to stimulate rabbit and mouse immune responses, 8 days after the first booster, sera from mice and rabbits were collected. Sera were also collected about every 10 days for detection of IgG antibody to O. tsutsugamushi Gilliam (Table 1). For each panel, four mice were killed, and spleen cell suspensions were prepared for detection of specific T cells (Table 2).
TABLE 1.
Anti-recombinant Sxh56 protein antibody responses of BALB/c mice and rabbits immunized with recombinant Sxh56 proteinsa
| Animals | IgG titer (by IFA) of sera collected on the following day after the first booster
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 18 | 28 | 38 | 48 | 58 | 68 | 100 | 114 | 128 | |
| BALB/c mice | 40,960 | 10,240 | 5,120 | 2,560 | 1,280 | 640 | 320 | 80 | 40 | 20 |
| Rabbits | 5,120 | 5,120 | 2,560 | 640 | 640 | 640 | 40 | 40 | 0 | 0 |
BALB/c mice and rabbits were immunized with recombinant Sxh56 proteins. At various times after the first booster, mice and rabbits were bled and IgG anti-recombinant Sxh56 protein antibody titers were determined by IFA.
TABLE 2.
In vitro proliferation in response to recombinant Sxh56 proteins of T cells from mice immunized with recombinant Sxh56 proteina
| Group | Proliferation (OD570 − OD630) with the following concn (μg/ml) of recombinant Sxh56 protein:
|
||||
|---|---|---|---|---|---|
| 10 | 3 | 1 | 0.3 | 0 | |
| PBS control | 0.423 | 0.437 | 0.476 | 0.420 | 0.407 |
| Recombinant Sxh56 | 0.453 | 0.565 | 0.643 | 0.604 | 0.414 |
BALB/c mice (four per group) were immunized subcutaneously with PBS or recombinant Sxh56 protein. Eight days after the first booster, spleens of four mice per group were harvested and T-cell cultures were stimulated in vitro for 3 days with medium or with various concentrations of purified recombinant Sxh56 proteins. Significant differences were found between the groups of mice immunized with Sxh56 or PBS (P < 0.05 [0.045] by t test).
Cross-reactivity of recombinant Sxh56 protein.
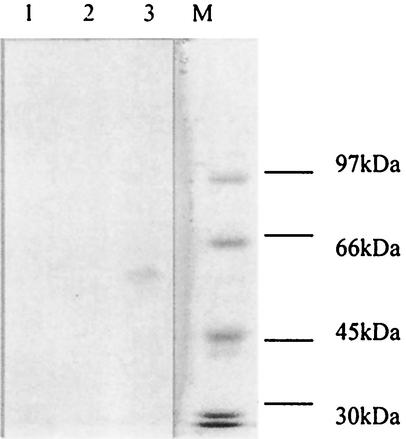
The recombinant Sxh56 protein was incubated with sera from mice infected with O. tsutsugamushi Sxh951, O. tsutsugamushi Gilliam, O. tsutsugamushi Karp, or O. tsutsugamushi Kato. Only antisera to O. tsutsugamushi Sxh951 and O. tsutsugamushi Gilliam were positive by the immunoblot assay and ELISA with the recombinant Sxh56 (Fig. 10). Additionally, antiserum to O. tsutsugamushi Sxh951 was incubated with recombinant 56-kDa proteins of O. tsutsugamushi Gilliam, O. tsutsugamushi Karp, and O. tsutsugamushi Kato. Only the recombinant 56-kDa protein of O. tsutsugamushi Gilliam was positive by the immunoblot assay (Fig. 11).
FIG. 10.
Immunoblot analysis of reactivity of recombinant Sxh56 on antibodies against different strains of O. tsutsugamushi. The purified recombinant Sxh56 protein were separated by SDS-10% PAGE, transferred to nitrocellulose membranes, and reacted with antibodies against different strains of O. tsutsugamushi. Molecular masses are indicated on the left. Lane 1, antiserum to O. tsutsugamushi Sxh951; lane 2, antiserum to O. tsutsugamushi Gilliam; lane 3, antiserum to O. tsutsugamushi Karp; lane 4, antiserum to O. tsutsugamushi Kato; lane 5, normal mouse serum.
FIG. 11.
Immunoblot analysis of reactivities of anti-O. tsutsugamushi Sxh951 sera on 56-kDa recombinant proteins from different strains of O. tsutsugamushi. The recombinant 56-kDa proteins of O. tsutsugamushi Kato , O. tsutsugamushi Karp, and O. tsutsugamushi Gilliam were separated by SDS-10% PAGE, transferred to nitrocellulose membranes, and reacted with anti-O. tsutsugamushi Sxh951 sera. Molecular masses are indicated on the left. Lane M, protein molecular weight marker; Lane 1, recombinant 56-kDa protein of O. tsutsugamushi Kato ; lane 2, recombinant 56-kDa protein of O. tsutsugamushi Karp; lane 3, recombinant 56-kDa protein of O. tsutsugamushi Gilliam.
Sensitivity and specificity of ELISA with recombinant Sxh56.
Fifty-five normal sera from healthy BALB/c mice were collected. Thirty sera from mice infected with O. tsutsugamushi Gilliam were positive for the presence of IgG antibody against O. tsutsugamushi Gilliam in the IFA. Twenty-nine of the 30 sera from mice infected with O. tsutsugamushi Gilliam were positive in the ELISA. All of 55 normal sera were negative. Compared with the clinical presentation, the sensitivity and specificity of the ELISA with recombinant Sxh56 were 96.67 and 100%, respectively (Table 3).
TABLE 3.
Mouse serum IgG antibody against O. tsutsugamushi Gilliam determined by recombinant Sxh56 ELISAa
| Mice | No.
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Infected | 29 | 1 | 30 |
| Normal | 0 | 55 | 55 |
| Total | 29 | 56 | 85 |
Sensitivity = 29/(29 + 1) × 100% = 96.67%; specificity = 55/(55 + 0) × 100% = 100%
Comparison of ELISA and IFA with human sera.
The results of ELISA with recombinant Sxh56 and 563 human sera from China were compared with the IgG titers determined by an IFA method with the Gilliam prototype of Orientia at a 1:80 serum dilution (9, 20). The specificity and sensitivity of the ELISA with recombinant Sxh56 were 96.36 and 88.08%, respectively (Table 4).
TABLE 4.
Comparison of recombinant Sxh56 ELISA with IFA to detect human serum IgG antibodya
| IFA result | No. with ELISA result
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 133 | 18 | 151 |
| Negative | 15 | 397 | 412 |
| Total | 148 | 415 | 563 |
Sensitivity = 133/(133 + 18) × 100% = 88.08%; specificity = 397/(15 + 397) × 100% = 96.36%.
DISCUSSION
Scrub typhus, which is transmitted by trombiculid mites, occurs mainly in rural areas of the Asia-Pacific area. The diagnosis of scrub typhus is generally based on the clinical presentation and the patient history. However, an increasing number of cases having no typical symptoms have been reported, and some drug-resistant strains were isolated recently. It is very difficult to differentiate scrub typhus from other acute febrile illnesses, such as murine typhus, dengue fever, and viral hemorrhagic fevers, etc., because of the similarities in signs and symptoms. Underdiagnosis or misdiagnosis of scrub typhus is common and may result in delayed or inappropriate treatment. High-cost diagnostic methods are not available in developing countries. Therefore, development of a rapid, effective diagnostic test that could be conveniently used in rural areas is badly needed now. O. tsutsugamushi is an antigenically diverse microorganism. Confirmatory serological experimental diagnosis of scrub typhus is based mainly on IFA. This requires cultivation of some antigenic variants of O. tsutsugamushi, such as the representative strains Gilliam, Karp, and Kato, and other isolates. However, O. tsutsugamushi is difficult to cultivate, so developing suitable recombinant antigens to substitute for cell antigens is a more practical approach. The recombinant protein antigen Sxh56 offers a considerable advantage over the antigens derived directly from O. tsutsugamushi. Compared with cell antigens, it is more stable, and its quantity and purity can be more easily assessed. The 56-kDa protein of O. tsutsugamushi is a major polypeptide that determines serotype specificity. To analyze the cross-reactivity of recombinant Sxh56, denaturing recombinant Sxh56 was incubated with sera to O. tsutsugamushi Sxh951, O. tsutsugamushi Gilliam, O. tsutsugamushi Karp, and O. tsutsugamushi Kato. The results showed that denaturing Sxh56 had little cross-reactivity with the mouse antisera against O. tsutsugamushi Karp and O. tsutsugamushi Kato in the immunoblot assay and ELISA. In addition, antiserum to O. tsutsugamushi Sxh951 was incubated with recombinant 56-kDa proteins of O. tsutsugamushi Gilliam, O. tsutsugamushi Karp, and O. tsutsugamushi Kato. Only the recombinant 56-kDa protein of O. tsutsugamushi Gilliam was positive in the immunoblot assay. The results suggest that the denaturing recombinant Sxh56 can be a type-specific diagnostic antigen. The results of the ELISA with recombinant Sxh56 show high sensitivity and specificity. Compared to the IFA of O. tsutsugamushi Gilliam, the ELISA has good sensitivity and specificity to detect human antibodies to O. tsutsugamushi Gilliam. This also suggests that recombinant Sxh56 is a good candidate as a diagnostic reagent to substitute for the cell antigens of O. tsutsugamushi Gilliam. Furthermore, recombinant Sxh56 showed good immunogenicity, as mice and rabbits inoculated with recombinant Sxh56 generated strong humoral and cellular immune responses.
Editor: B. B. Finlay
REFERENCES
- 1.Chen, X., H. Niu, Y. Zhang, X. Zhang, and Q. Yu. 1998. Typing of R. tsutsugamushi isolated in Shanxi province and determination of nucleotide sequence encoding 56-kDa protein gene. Chinese J. Zoonoses 14(3):21-23. (In Chinese.)
- 2.Du, P. 1982. Medical experimental virology, p. 182-183. Shanghai Association of Microbiology, Shanghai, China. (In Chinese.)
- 3.Du, P. 1982. Medical experimental virology, p. 211-212. Shanghai Association of Microbiology, Shanghai, China. (In Chinese.)
- 4.Furuya, Y., Y. Yoshida, T. Katayama, F. Kawamori, S. Yamamoto, N. Ohashi, A. Kamura, and A. Kawamura, Jr. 1991. Specific amplification of Rickettsia tsutsugamushi DNA from clinical specimens by polymerase chain reaction. J. Clin. Microbiol. 29:2628-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson, B. 1985. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect. Immun. 50:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin, D., and M. Li. 1992. Molecular cloning: a laboratory manual, 2nd ed., p. 955-956. Press of Science, Beijing, China. (In Chinese.)
- 7.Jin, D., and M. Li. 1992. Molecular cloning: a laboratory manual, 2nd ed., p. 854-855. Press of Science, Beijing, China. (In Chinese.)
- 8.Kelly, D. J., D. Marana, C. Stover, E. Oaks, and M. Carl. 1990. Detection of Rickettsia tsutsugamushi by gene amplification using polymerase chain reaction techniques. Ann. N.Y. Acad. Sci. 590:564-571. [DOI] [PubMed] [Google Scholar]
- 9.Kelly, D. J., P. W. Wong, E. Gan, and G. E. Lewis, Jr. 1988. Comparative evaluation of the indirect immunoperoxidase test for the serodiagnosis of rickettsial disease. Am. J. Trop. Med. Hyg. 38:400-406. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Murata, M., Y. Yoshida, M. Osono, N. Ohashi, M. Oyanagi, H. Urakami, A. Tamura, S. Nogami, H. Tanaka, and A. Kawamura, Jr. 1986. Production and characterization of monoclonal strain-specific antibodies against prototype strains of Rickettsia tsutsugamushi. Microbiol. Immunol. 30:599-610. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi, N., A. Tamura, H. Sakurai, and T. Suto. 1988. Immunoblotting analysis of anti-rickettsial antibodies produced in patients of tsutsugamushi disease. Microbiol. Immunol. 32:1085-1092. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi, N., A. Tamura, M. Ohta, and K. Hayashi. 1989. Purification and partial characterization of a type-specific antigen of Rickettsia tsutsugamushi. Infect. Immun. 57:1427-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi, N., Y. Koyama, H. Urakami, M. Fukuhara, A. Tamura, F. Kawamori, S. Yamamoto, S. Kasuya, and K. Yoshimura. 1996. Demonstration of antigenic and genotypic variation in Orientia tsutsugamushi which were isolated in Japan, and their classification into type and subtype. Microbiol. Immunol. 40:627-638. [DOI] [PubMed] [Google Scholar]
- 15.Seong, S. Y., M. S. Huh, W. J. Jang, S. G. Park, J. G. Kim, S. G. Woo, M. S. Choi, I. S. Kim, and W. H. Chang. 1997. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect. Immun. 65:1541-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seong, S. Y., H. R. Kim, M. S. Huh, et al. 1997. Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine 15:1741-1747. [DOI] [PubMed] [Google Scholar]
- 17.Stover, C. K., D. P. Marana, J. M. Carter, B. A. Roe, E. Mardis, and E. V. Oaks. 1990. The 56-kilodalton major protein antigen of Rickettsia tsutsuga-mushi: molecular cloning and sequence analysis of the sta56 gene and precise identification of a strain-specific epitope. Infect. Immun. 58:2076-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugita, Y., T. Nagatani, K. Okuda, Y. Yoshida, and H. Nakajima. 1992. Diagnosis of typhus infection with Rickettsia tsutsugamushi by polymerase chain reaction. J. Med. Microbiol. 37:357-360. [DOI] [PubMed] [Google Scholar]
- 19.Sun, S., et al. 2000. Nuclear vaccine, p. 114. Press of the Second Military Medicine University, Shanghai, China. (In Chinese.)
- 20.Suwanabun, N., C. Chouriyagune, C. Eamsila, P. Watcharapichat, G. A. Dasch, R. S. Howard, and D. J. Kelly. 1997. Evaluation of an enzyme-linked immunosorbent assay in Thai scrub typhus patients. Am. J. Trop. Med. Hyg. 56:38-43. [DOI] [PubMed] [Google Scholar]
- 21.Tamura, A., N. Ohashi, H. Urakami, and S. Miyamura. 1995. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int. J. Syst. Bacteriol. 45:589-591. [DOI] [PubMed] [Google Scholar]
- 22.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. 1985. Guide for the care and use of laboratory animals. Publication 86-23. Institute of Laboratory Animal Resources, National Research Council. U.S. Department of Health and Human Services, Washington, D.C.
- 24.Yamamoto, S., and Y. Minamishima. 1982. Serodiagnosis of tsutsugamushi fever (scrub typhus) by the indirect immunoperoxidase technique. J. Clin. Microbiol. 15:1128-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto, S., N. Kawabata, A. Tamura, H. Urakami, N. Ohashi, M. Murata, Y. Yoshida, and A. Kawamura, Jr. 1986. Immunological properties of Rickettsia tsutsugamushi, Kawasaki strain, isolated from a patient in Kyushu. Microbiol. Immunol. 30:611-620. [DOI] [PubMed] [Google Scholar]