Abstract
Immunoglobulin G (IgG) antibodies to three vaccine candidate preerythrocytic Plasmodium falciparum antigens were evaluated in children and adults in an epidemic-prone highland area of Kenya during rainy (high-transmission) and dry (low-transmission) seasons. The frequencies and median levels of IgG antibodies to circumsporozoite protein (CSP) and thrombospondin-related adhesive protein (TRAP) were compared to the frequencies and median levels of IgG antibodies to liver-stage antigen 1 (LSA-1) reported previously. The frequencies and median levels of IgG antibodies to CSP and TRAP were similar in children and adults in the rainy season, but they were lower in children than in adults in the dry season. The frequencies and median levels of antibodies to LSA-1 were lower in children than in adults in both the rainy and dry seasons. Antibodies to CSP and LSA-1 were primarily members of the IgG1 and IgG3 subclasses, while antibodies to TRAP were primarily members of the IgG3 and IgG4 subclasses. In a treatment-reinfection study following dry season testing, antibodies to TRAP were associated with a trend toward protection from infection in children (P = 0.051) but not in adults. Antibodies to LSA-1 and CSP did not correlate with protection in children or adults. In this highland area of Kenya with unstable transmission, IgG antibodies to preerythrocytic P. falciparum antigens vary in subjects by age and season, and the protective effects of these antibodies against infection may be different in adults and children.
The preerythrocytic Plasmodium falciparum antigens circumsporozoite protein (CSP), thrombospondin-related adhesive protein (TRAP)/PfSSP2, and liver-stage antigen 1 (LSA-1) are under consideration for inclusion in a multistage malaria vaccine (1, 13, 17). The mechanisms by which these antigens induce protection against malaria in humans have been the subject of multiple investigations. The information that has been obtained to date was based primarily on observations of naturally infected individuals living in areas where there is stable malaria transmission. Residents of such areas generally develop partial protection against severe malaria morbidity and high-density asexual parasitemia with increasing age. Although the protective mechanisms have not been completely defined, they are postulated to involve both cellular and humoral immune responses elicited by preerythrocytic and blood-stage antigens as a consequence of repeated sporozoite and blood-stage infections (3, 5, 7, 9, 11, 15, 18).
It has been suggested that immunoglobulin G (IgG) antibodies to CSP, TRAP, and LSA-1 mediate or represent surrogate markers of resistance to infection and malaria morbidity in areas of Africa where malaria is holoendemic (12, 14). The development of such antibodies is influenced strongly by age and the pattern of transmission (8, 14). Most studies of antibodies to P. falciparum antigens have focused on a single antigen, have exclusively involved children or adults, and have been performed in areas where malaria transmission is stable and perennial. Transmission of P. falciparum is erratic and highly variable in the highlands of western Kenya. Outbreaks of malaria infection, morbidity, and infection tend to occur during periods of heavy rainfall that follow prolonged dry spells (16). The paucity of newly established P. falciparum infections in highland areas during the dry season may lead to waning of protective immune responses to malaria antigens, rendering adults and children susceptible to infection and disease during the subsequent rainy season. It was reported previously that the proportion of people with IgG, IgG1, and IgG3 subclass antibodies to LSA-1 decreased during a period of low transmission in a highland area of Kenya. However, antibodies to LSA-1 did not correlate with time to reinfection (8). In this paper, we describe IgG antibodies to the additional preerythrocytic antigens CSP and TRAP in these children and adults during the dry and rainy seasons and compare the frequencies and levels of these antibodies with those previously described for LSA-1 (8). We also compare the time to reinfection with the presence of these antibodies in children and adults in whom preexisting blood-stage infections were cured with antimalarial chemotherapy.
MATERIALS AND METHODS
Study site and human participants.
Volunteers were recruited from the village of Kabobo in the Uasin Gishu district of Kenya. Volunteers were recruited at the Kabobo Health Centre and followed up at their village residences. Kabobo is located at an altitude of 2,134 m in an isolated rural area where access to health facilities is limited. Transmission of P. falciparum is episodic, and local outbreaks of malaria with high rates of morbidity and mortality have occurred in the past (10). Both P. falciparum infection and Plasmodium malariae infection have been documented in Uasin Gishu (16).
To minimize the confounding effects of travel and acquisition of infection in nearby lowland areas where malaria is holoendemic, only volunteers who lived year-round in Kabobo were recruited. Adults were defined as persons who were ≥18 years old, and children were defined as persons who were ≤8 years old.
Signs and symptoms of malaria (fever, headache, vomiting, chills, fatigue, joint pains, splenomegaly, hepatomegaly, jaundice, pallor, and altered mental status) were recorded at the time of enrollment. Prior use of antimalarial medications was ascertained. Blood was collected by venipuncture from adults (10 to 20 ml) and children (5 ml). Thick and thin smears were stained and examined for Plasmodium species by trained microscopists from the Division of Vector Borne Diseases, Ministry of Health, Kenya. Symptomatic individuals whose blood smears were positive for P. falciparum were treated with a single dose of sulfadoxine-pyrimethamine in accordance with the policy of the Kenya Ministry of Health. Two individuals had blood smears that were positive for P. malariae; both of these individuals were also infected with P. falciparum and were treated with chloroquine in addition to sulfadoxine-pyrimethamine. Blood was collected from 21 North American adults who had never traveled to areas where malaria is endemic to obtain control sera.
Repeated cross-sectional study of prevalence of IgG antibodies to P. falciparum antigens.
Blood was collected two times, in July and August 1996, at the end of a long rainy season when transmission of P. falciparum was high, and in April 1997, at the end of a 5-month dry spell. A total of 108 individuals (37 children and 71 adults) were examined in August 1996, and 106 individuals (36 children and 70 adults) were examined in April 1997. Fifty-six of these participants (24 children and 32 adults) were examined at both times. Treatment was administered to any symptomatic individuals with P. falciparum on blood smears after blood was collected. Peripheral blood mononuclear cells and plasma were separated from whole blood by Hypaque-Ficoll density gradient centrifugation as described previously (9). Plasma samples were tested for IgG antibodies to CSP, TRAP, and LSA-1.
Treatment reinfection study.
Eighty-four of the 106 study participants who had undergone testing for IgG antibodies at the end of the long dry season in April 1997 were recruited for a treatment-reinfection study in May 1997. All participants were given a single dose of sulfadoxine-pyrimethamine to clear any blood-stage infection regardless of whether P. falciparum was seen on peripheral blood smears. By 2 weeks after treatment, blood-stage malaria was successfully eliminated in all but two individuals, who were treated with quinine and doxycycline and excluded from the follow-up study. Thick and thin blood smears were obtained weekly for 10 weeks for the remaining 82 individuals (32 children and 50 adults). The time to reinfection was compared with preexisting IgG antibodies to CSP, TRAP, and LSA-1.
Informed consent and ethical approval.
Written informed consent was obtained from all participants and/or their guardians. Ethical approval for the study was granted by the Kenya Medical Research Institute National Ethical Review Committee and the Institutional Review Board for Human Studies at University Hospitals of Cleveland and Case Western Reserve University.
CSP, TRAP, and LSA-1 peptides.
The presence of antibodies to CSP and LSA-1 was tested by using the following central repeat sequence peptides to which individuals from areas where malaria is endemic demonstrate IgG responses: for CSP, the (NANP)5 repeat peptide (4); and for LSA-1, the 17-amino-acid sequence EQQSDLEQERLAKEKLQ (6). The presence of antibodies to TRAP was tested by using the peptide (CHPSDGKCN)2, an epitope previously shown to elicit IgG responses in a population living in an area where malaria is endemic (3). Peptides were synthesized by Genosys Biotechnologies, The Woodlands, Tex., by Fmoc chemistry and were purified so that they were >90% pure.
Antibody measurements.
IgG antibodies were measured by an enzyme-linked immunosorbent assay. CSP, LSA-1, and TRAP peptides were dissolved in 0.05 M sodium bicarbonate (pH 9.6) to a concentration of 10 μg/ml, and 50 μl of a peptide solution was added to Immulon 1 plates pretreated with poly-l-lysine. Following overnight incubation, washing, and blocking in 5% (wt/vol) powdered milk and 1% gelatin in phosphate-buffered saline, duplicate 50-μl samples of serum diluted 1:10 in 5% powdered milk were added to wells, and incubation was continued for 2 h at room temperature. After extensive washing, 50 μl of alkaline phosphatase-conjugated anti-human IgG (Jackson ImmunoResearch, West Grove, Pa.) diluted 1:10,000 in 5% powdered milk was added and then removed after 1 h. The substrate p-nitrophenyl phosphate was added in accordance with the instructions of the manufacturer (Sigma Chemical Co., St. Louis, Mo.). Optical density was measured at 405 nm. Serum samples were diluted 1:10, 1:20, and 1:50, and strong positive responses with a minimal background response were seen only with the 1:10 dilution.
IgG1, IgG2, IgG3, and IgG4 subclass antibodies were measured by an enzyme-linked immunosorbent assay, with several adjustments. Mouse anti-human IgG subclass antibodies (Hybridoma Reagent Laboratory, Baltimore, Md.) diluted 1:10,000 in 5% powdered milk were added after incubation of antigen-coated microtiter wells with human sera and washing. Plates were washed again after 2 h, and alkaline phosphatase-conjugated goat anti-mouse immunoglobulin antibody (Jackson ImmunoResearch) diluted 1:1,000 in 5% powdered milk was added. Plates were washed after 1 h of incubation, and p-nitrophenyl phosphate was then added and the optical density at 405 nm was determined as described above.
IgG antibody levels were expressed in arbitrary units (AU). The numbers of AU were calculated by dividing the optical density of a sample by the mean optical density plus 3 standard deviations (SD) for sera from nine North Americans who had never been exposed to malaria. Twenty-one North American sera were tested initially, and the nine serum samples used on each plate had a mean optical density and SD similar to those of the 21 sera tested initially. Values of ≥1.0 AU were considered positive. The overall mean values for optical density plus 3 SD were 0.093, 0.096, and 0.124 for CSP, TRAP, and LSA-1, respectively.
Statistical analysis.
Differences in the proportion of individuals with antibodies to various P. falciparum antigens were evaluated by the χ2 test. Paired samples were evaluated by the McNemar test. Quantitative differences in levels of antibodies to P. falciparum antigens were evaluated by the nonparametric Mann-Whitney U test. Paired samples were evaluated by the nonparametric Wilcoxon matched-pair signed-rank test. Correlations between continuous variables (e.g., levels of antibodies to different peptides) were assessed by Spearman's rank correlation. The Bonferroni correction was used to adjust for multiple comparisons, and the P values for multiple comparisons (e.g., comparisons within and across the data in Tables 1 and 2) incorporated this correction.
TABLE 1.
Frequencies and median levels of IgG antibodies to P. falciparum antigens in children in the rainy and dry seasons
| Antigen | Rainy season
|
Dry season
|
||
|---|---|---|---|---|
| No. of IgG positive/total no. (%)a | Median IgG level (range)b | No. of IgG positive/total no. (%) | Median IgG level (range) | |
| CSP | 16/37 (43.2) | 0.80 (0.14-3.90) | 13/36 (36.1) | 0.88 (0.17-3.87) |
| TRAP | 17/37 (45.9) | 0.96 (0.05-2.95) | 10/36 (27.8) | 0.78 (0.20-3.35) |
| LSA-1 | 4/37 (10.8) | 0.35 (0.02-1.86) | 2/36 (5.6) | 0.28 (0.02-1.61) |
A positive response was defined as an antibody level of ≥1.0 AU.
Individual IgG levels were expressed in AU (see Materials and Methods).
TABLE 2.
Frequencies and median levels of IgG antibodies to P. falciparum antigens in adults in the rainy and dry seasons
| Antigen | Rainy season
|
Dry season
|
||
|---|---|---|---|---|
| No. of IgG positive/total no. (%)a | Median IgG level (range)b | No. of IgG positive/total no. (%) | Median IgG level (range) | |
| CSP | 41/71 (57.7) | 1.10 (0.02-4.80) | 46/70 (65.7) | 1.21 (0.31-3.77) |
| TRAP | 43/71 (60.6) | 1.23 (0.14-4.28) | 45/70 (64.3) | 1.20 (0.36-3.45) |
| LSA-1 | 28/71 (40.8) | 0.84 (0.11-3.81) | 13/70 (18.6)c | 0.54 (0.03-1.76)d |
A positive response was defined as an antibody level of ≥1.0 AU.
Individual IgG levels were expressed in AU (see Materials and Methods).
P < 0.01 for a comparison of the rainy season and the dry season, as determined by a χ2 test.
P < 0.001, for a comparison of the rainy season and the dry season, as determined by a Mann-Whitney U test.
Individuals with IgG antibodies to CSP, TRAP, and LSA-1 were compared to individuals without antibodies for time to appearance of parasitemia by Kaplan-Meier survival analysis and by Cox proportional hazard analysis with adjustment for age and parasitemia prior to treatment with sulfadoxine-pyrimethamine. Separate Cox analyses were performed for children and adults, and proportional hazard assumptions were tested prior to application of the Cox model. In the cohort of children, the risk of infection by initial P. falciparum infection status did not remain proportional over the time tested. The Cox analysis was therefore stratified by this variable. If it was assumed that the antibody frequency was 50% and the infection rate was 60%, the treatment-reinfection study had 70% power to detect an 80% antibody protective effect in children and a 60% antibody protective effect in adults.
RESULTS
Prevalence of P. falciparum parasitemia in the rainy and dry seasons.
Peripheral blood smears demonstrated that there were P. falciparum blood-stage infections in 23 of 37 children (62.2%) and in 27 of 71 adults (38.0%) in the rainy (high-transmission) season. The values were lower in the dry, low-transmission season; 9 of 36 children (25.0%) and 6 of 70 adults (8.6%) had positive smears at this time.
Frequency and levels of IgG antibodies during the rainy and dry seasons.
Thirty-seven children and 71 adults were evaluated in the rainy season, and 36 children and 70 adults were evaluated in the dry season. A subset of 24 children and 32 adults were tested in both seasons. In children, the frequency and median level of IgG antibodies to TRAP and LSA-1 were lower in the dry season than in the rainy season, but the differences were not statistically significant (Table 1). In adults, the levels of IgG antibodies to LSA-1 were significantly lower in the dry season than in the rainy season, whereas the levels of the IgG antibodies to CSP and TRAP did not differ in the two seasons (Table 2). The frequencies and levels of IgG antibodies to the four antigens in the subset of 24 children and 32 adults tested in both seasons were similar to the frequencies and levels seen in the cross-sectional sample (data not shown). The frequencies and median levels of IgG antibodies to CSP, TRAP, and LSA-1 were not different in individuals who were P. falciparum blood smear positive and individuals who were P. falciparum blood smear negative (data not shown).
Frequencies and levels of IgG antibodies in children and adults.
In the rainy season, the frequencies and median levels of IgG antibodies to CSP and TRAP were similar in adults and children, but the frequency and median level of IgG antibodies to LSA-1 were significantly lower in children than in adults (frequencies, 10.8 and 40.8%, respectively [P = 0.012]; median levels, 0.35 and 0.84 AU, respectively [P < 0.0001]). In the dry season, the frequencies and median levels of IgG antibodies to all three antigens were lower in children than in adults (Tables 1 and 2). In the dry season, in children and adults the frequencies of IgG antibodies to CSP were 36.1 and 65.7% (P = 0.008), respectively, and the median levels of IgG antibodies to CSP were 0.88 and 1.21 AU (P = 0.004), respectively; the frequencies of IgG antibodies to TRAP were 27.8 and 64.3% (P = 0.0008), respectively, and the median levels of IgG antibodies to TRAP were 0.78 and 1.20 AU (P = 0.008), respectively; and the frequencies of IgG antibodies to LSA-1 were 5.6 and 18.6% (P = 0.14), respectively, and the median levels of IgG antibodies to LSA-1 were 0.28 and 0.54 AU (P = 0.006), respectively.
Frequencies of IgG subclasses in the rainy and dry seasons.
The IgG antibodies to CSP and LSA-1 were primarily members of the IgG1 and IgG3 subclasses in both children and adults. The IgG antibodies to TRAP were mostly members of the IgG3 and IgG4 subclasses, particularly in adults (Tables 3 and 4). In the rainy season, children had higher frequencies of IgG1 antibodies to all antigens than adults, most notably to CSP (40.0% in children compared to 9.5% in adults), but children and adults had similar frequencies of IgG3 responses to all antigens. In the dry season, the levels of IgG1 and IgG3 antibodies to all antigens decreased markedly. This change was more pronounced in children than in adults for both the IgG1 and IgG3 subclasses.
TABLE 3.
IgG subclass antibodies to P. falciparum antigens in children in the rainy and dry seasons
| Antigen | IgG1
|
IgG2
|
IgG3
|
IgG4
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainy seasona
|
Dry seasonb
|
Rainy season
|
Dry season
|
Rainy season
|
Dry season
|
Rainy season
|
Dry season
|
|||||||||
| No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | |
| CSP | 8 | 40.0 | 0 | 0.0d | 0 | 0.0 | 0 | 0.0 | 8 | 40.0 | 2 | 5.5e | 0 | 0.0 | 0 | 0.0 |
| TRAP | 2 | 10.0 | 2 | 5.5 | 1 | 5.0 | 0 | 0.0 | 6 | 30.0 | 1 | 2.8e | 2 | 10.0 | 2 | 5.5 |
| LSA-1 | 8 | 40.0 | 0 | 0.0d | 0 | 0.0 | 0 | 0.0 | 6 | 30.0 | 3 | 8.3 | 1 | 5.0 | 4 | 11.1 |
The total number of children tested in the rainy season was 20.
The total number of children tested in the dry season was 36.
Number of children with IgG subclass antibodies (≥1.0 AU).
P < 0.001 for a comparison of the rainy season and the dry season, as determined by a χ2 test.
P < 0.01 for a comparison of the rainy season and the dry season, as determined by a χ2 test.
TABLE 4.
IgG subclass antibodies to P. falciparum antigens in adults in the rainy and dry seasons
| Antigen | IgG1
|
IgG2
|
IgG3
|
IgG4
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainy seasona
|
Dry seasonb
|
Rainy season
|
Dry season
|
Rainy season
|
Dry season
|
Rainy season
|
Dry season
|
|||||||||
| No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | No.c | % | |
| CSP | 6 | 9.5 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 25 | 39.7 | 10 | 14.3d | 1 | 1.6 | 1 | 1.4 |
| TRAP | 3 | 4.8 | 4 | 5.7 | 1 | 1.6 | 1 | 1.4 | 21 | 33.3 | 4 | 5.7d | 15 | 23.8 | 13 | 18.6 |
| LSA-1 | 17 | 27.0 | 0 | 0.0d | 0 | 0.0 | 1 | 1.4 | 21 | 33.3 | 7 | 10.0d | 3 | 4.8 | 3 | 4.3 |
The total number of adults tested in the rainy season was 63.
The total number of adults tested in the dry season was 70.
Number of adults with IgG subclass antibodies (≥1.0 AU).
P < 0.001 for a comparison of the rainy season and the dry season, as determined by a χ2 test.
Correlation between levels of IgG antibodies to various P. falciparum antigens.
There were strong correlations between IgG antibodies to CSP and TRAP in both children and adults (Spearman's φ, 0.807 and 0.883, respectively; P < 0.0001 for both comparisons), and there were weaker correlations between antibodies to LSA-1 and CSP and between antibodies to LSA-1 and TRAP (Spearman's φ, 0.322 to 0.559; P < 0.05 for all comparisons). All children and 91% of the adults with anti-LSA-1 antibodies also had anti-TRAP antibodies, and all children and 81% of the adults with anti-TRAP antibodies had anti-CSP antibodies. Thus, the individuals with antibodies to LSA-1 were a subset of the individuals with antibodies to TRAP, who in turn were a subset of the individuals with antibodies to CSP.
IgG antibodies to P. falciparum antigens and time to reinfection.
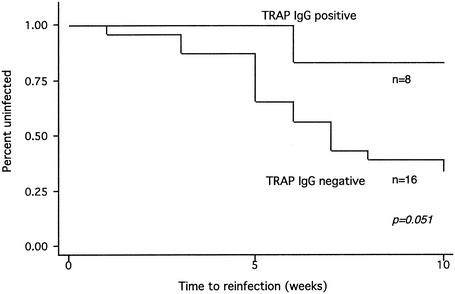
Thirty-two children were enrolled in the treatment-reinfection study. IgG antibodies to TRAP were associated with a decreased risk of reinfection in children (Fig. 1), although this association weakened slightly after we controlled for antibodies to other antigens, age, and initial P. falciparum infection (Table 5). There was no correlation between antibodies to CSP and risk of reinfection. Antibodies to LSA-1 were too infrequent in children to allow a comparison of times to reinfection in individuals with and without antibodies (Table 5).
FIG. 1.
Time to reinfection with P. falciparum in children with and without IgG antibodies to TRAP, as determined by a Kaplan-Meier survival analysis.
TABLE 5.
Risk of P. falciparum reinfection in children with IgG antibodies to P. falciparum antigens
| Antigen | Hazard ratioa | SE | P value | 95% Confidence interval limits |
|---|---|---|---|---|
| CSP | 2.062 | 1.425 | 0.295 | 0.532, 7.989 |
| TRAP | 0.133 | 0.133 | 0.108 | 0.011, 1.551 |
| LSA-1 | —b | — | — | — |
Determined by the Cox proportional hazard model, with control for age and initial P. falciparum blood smear status.
—, hazard ratio could not be calculated because only two children had LSA-1 antibodies.
Fifty adults were enrolled in the treatment-reinfection study. IgG antibodies to CSP, TRAP, and LSA-1 did not correlate with protection from infection in adults (Table 6). There were not enough children or adults with IgG subclass antibodies to the antigens tested to allow an analysis of times to reinfection in individuals with and without subclass antibodies.
TABLE 6.
Risk of P. falciparum reinfection in adults with IgG antibodies to P. falciparum antigens
| Antigen | Hazard ratioa | SE | P value | 95% Confidence interval limits |
|---|---|---|---|---|
| CSP | 0.917 | 0.557 | 0.887 | 0.279, 3.016 |
| TRAP | 1.408 | 0.839 | 0.565 | 0.750, 5.920 |
| LSA-1 | 2.107 | 1.110 | 0.157 | 0.967, 7.577 |
Determined by the Cox proportional hazard model, with control for age and initial P. falciparum blood smear status.
DISCUSSION
Malaria outbreaks in the highlands of Kenya usually occur during the rainy season, after several relatively dry months when the rate of malaria transmission is very low. The outbreaks typically affect both children and adults (16). Outbreaks of clinical malaria in these areas may be due in part to a loss of protective immune responses to P. falciparum antigens during prolonged periods of low rates of transmission. The results of the present study performed in a highland area of Kenya suggest that both age and prevailing transmission conditions are important in determining the persistence of serologically detectable IgG antibodies to P. falciparum antigens and that the effects of these conditions on IgG antibodies differ according to antigen. The results also suggest that IgG antibodies to TRAP, whose levels decrease in the dry season in children, may be associated with protection from P. falciparum infection in children.
In the rainy season, the frequencies and levels of IgG antibodies to CSP and TRAP were similar in children and adults, but, as described previously, the frequencies and levels of IgG antibodies to LSA-1 were significantly lower in children than in adults (8). In the dry season, the frequencies and levels of antibodies to all three antigens were lower in children than in adults. These findings suggest that in areas where there is unstable transmission, age and/or multiple exposures to P. falciparum are important in the acquisition of IgG antibodies to LSA-1 but not in the acquisition of IgG antibodies to CSP or TRAP and in the maintenance of antibody responses to all three antigens. A recent report from a nearby holoendemic region of Kenya documented that infants were able to mount and maintain strong LSA-1 antibody responses in the first year of life (19), suggesting that exposure may be a more important factor than age in development of IgG antibodies to LSA-1. We found that the IgG responses to CSP and LSA-1 were primarily responses of the IgG1 and IgG3 subclasses. Findings for IgG subclass antibodies to LSA-1 have been reported previously (8), and our IgG subclass findings for CSP are similar to those obtained by other workers (11). We did not find any association between antibodies to CSP or LSA-1 and protection from infection. Although the peptides which we used are well-documented B-cell epitopes for CSP and LSA-1, testing for these single peptides, instead of multiple peptides or recombinant antigens, may have affected our ability to detect an association with protection from infection. In addition, the low levels of antibodies to these antigens, particularly LSA-1, may also have precluded detection of an association.
A trend toward protection from infection was observed for children with preexisting IgG antibodies to TRAP (Fig. 1). A study conducted in Mali similarly demonstrated that the levels of anti-TRAP antibodies decreased in young children when the malaria transmission rate was low but were maintained in older children and adults (14). Anti-TRAP antibodies were associated with protection against high-level parasitemia in children only, despite the higher frequency of such antibodies in adults. These findings and the present findings suggest that anti-TRAP antibodies may be more important in mediating resistance to infection in children than in adults. Different IgG subclass responses to TRAP in children and adults may be partially responsible for the differences in resistance to infection; anti-TRAP antibodies in adults during the dry season were primarily members of the IgG4 subclass, while in children they were primarily members of the IgG1 and IgG3 subclasses. It is unclear why IgG1 and IgG3 subclass responses to preerythrocytic antigens protect more effectively against infection than IgG2 or IgG4 subclass responses. For asexual blood-stage antigens, the IgG1 and IgG3 subclasses are cytophilic and may interact with peptides displayed on the surface of infected red blood cells and enhance antibody-dependent monocyte-mediated inhibition of growth (2). However, this mechanism should not affect responses to preerythrocytic antigens. The finding that IgG subclass antibodies to the preerythrocytic antigens CSP, LSA-1, and TRAP are primarily IgG1 and IgG3 antibodies suggests that there is either an antigen-induced bias toward production of these subclasses or that there are functional differences between the subclass antibodies to these antigens. The small number of children and adults with IgG subclass antibodies to CSP, LSA-1, and TRAP precluded detection of an association between subclass antibodies to these antigens and time to reinfection. Further research is required to determine if particular subclass antibodies are associated with protection from infection and to elucidate the potential mechanisms for this protection.
The number of children in the present study was small, and the trend toward protection with anti-TRAP antibodies in children requires further confirmation in future studies with larger numbers of subjects. We were not able to measure (and therefore were not able to control for) potential confounding factors, such as heterogeneity of exposure to mosquitoes, which relate to risk of infection. In addition, some new infections may have represented recrudescence of infection rather than reinfection, although the very low clinical failure rate of sulfadoxine-pyrimethamine treatment in this population at the time of this study (unpublished data) argue against a high frequency of sulfadoxine-pyrimethamine resistance.
A limitation of any immunologic study in areas where there is unstable transmission is that frequencies of protective immune responses in the period of greatest vulnerability are likely to be low, so fairly large numbers may be required to detect an association of these responses with protection. In addition, in areas where there is unstable transmission, like the highlands of Kenya, the timing of seasonal increases in malaria incidence and the occurrence of outbreaks are not easily predictable, and correlation of responses with protection from infection or disease cannot occur in the absence of significant infection in the population. The initial collection in the present study was performed shortly after a malaria outbreak, and the second collection was performed just before another outbreak associated with the El Niño southern oscillation. The second outbreak occurred during our treatment-reinfection study, in which almost 50% of the individuals were infected over a 10-week period. This unique set of circumstances allowed us to detect with relatively small numbers the effects of high infection rates on the development of antigen-specific IgG antibodies in this population, the effects of a marked decrease in transmission on antibody levels and frequencies, and the relationship between the presence of specific antibodies and protection from infection. Our study could detect only very strong (>60%) antibody protective effects, however, and repeated studies with larger numbers are required to assess whether less-high-level protective effects are associated with IgG antibodies to CSP, TRAP, and LSA-1.
Future studies of these issues should include longitudinal analysis of in vitro B-cell IgG responses to these and other antigens, including blood-stage antigens, in children and adults. This should enable direct assessment of the effects of transmission and blood-stage infection on acquisition and maintenance of B-cell responses independent of the confounding effects of the in vivo clearance of IgG.
Acknowledgments
This work was supported by NIH grants AI-01572 and AI-43906.
This work is published with the permission of the Office of the Director of the Kenya Medical Research Institute. We thank John Ouma for a review of the study design and study site, Venkatachalam Udhayakumar and Bernard Nahlen for the use of the Centers for Disease Control and Prevention laboratory in Kisian, Fred Hazlett and Elkana Gichana Ondere for assistance with laboratory work, and David Koech and Johana Milgo of the Division of Vector Borne Diseases for inspection of blood smears.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bharadwaj, A., P. Sharma, S. K. Joshi, B. Singh, and V. S. Chauhan. 1998. Induction of protective immune responses by immunization with linear multiepitope peptides based on conserved sequences from Plasmodium falciparum antigens. Infect. Immun. 66:3232-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 60:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charoenvit, Y., V. Fallarme, W. Rogers, J. J. Sacci, M. Kaur, J. Aguiar, L. Yuan, G. Corradin, E. Andersen, B. Wizel, R. Houghten, A. Oloo, L. V. P. De, and S. Hoffman. 1997. Development of two monoclonal antibodies against Plasmodium falciparum sporozoite surface protein 2 and mapping of B-cell epitopes. Infect. Immun. 65:3430-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chougnet, C., J. Lepers, P. Astagneau, M. Rason, J. Savel, and P. Deloron. 1991. Lymphoproliferative responses to synthetic peptides from merozoite ring-infected erythrocyte surface antigen and circumsporozoite protein: a longitudinal study during a falciparum malaria episode. Am. J. Trop. Med. Hyg. 45:560-566. [DOI] [PubMed] [Google Scholar]
- 5.Connelly, M., C. L. King, K. Bucci, S. Walters, B. Genton, M. P. Alpers, M. Hollingdale, and J. W. Kazura. 1997. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect. Immun. 65:5082-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidock, D. A., H. Gras-Masse, J. P. Lepers, K. Brahimi, L. Benmohamed, S. Mellouk, C. Guerin-Marchand, A. Londono, L. Raharimalala, J. F. Meis, et al. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 153:190-204. (Erratum, 153:5347.) [PubMed] [Google Scholar]
- 7.Flanagan, K. L., M. Plebanski, P. Akinwunmi, E. A. Lee, W. H. Reece, K. J. Robson, A. V. Hill, and M. Pinder. 1999. Broadly distributed T cell reactivity, with no immunodominant loci, to the pre-erythrocytic antigen thrombospondin-related adhesive protein of Plasmodium falciparum in West Africans. Eur. J. Immunol. 29:1943-1954. [DOI] [PubMed] [Google Scholar]
- 8.John, C. C., J. H. Ouma, P. O. Sumba, M. R. Hollingdale, J. W. Kazura, and C. L. King. 2002. Lymphocyte proliferation and antibody responses to Plasmodium falciparum liver-stage antigen-1 in a highland area of Kenya with seasonal variation in malaria transmission. Am. J. Trop. Med. Hyg. 66:372-378. [DOI] [PubMed] [Google Scholar]
- 9.John, C. C., P. O. Sumba, J. H. Ouma, B. L. Nahlen, C. L. King, and J. W. Kazura. 2000. Cytokine responses to Plasmodium falciparum liver-stage antigen 1 vary in rainy and dry seasons in highland Kenya. Infect. Immun. 68:5198-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan, B., A. Ofulla, D. Kariuki, J. Githure, E. Kabiru, and S. Martin. 1992. Drug sensitivity studies during a highland malaria epidemic in Kenya. Trans. R. Soc. Trop. Med. Hyg. 86:371-372. [DOI] [PubMed] [Google Scholar]
- 11.Kitua, A. Y., H. Urassa, M. Wechsler, T. Smith, P. Vounatsou, N. A. Weiss, P. L. Alonso, and M. Tanner. 1999. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol. 21:307-317. [DOI] [PubMed] [Google Scholar]
- 12.Migot-Nabias, F., P. Deloron, P. Ringwald, B. Dubois, J. Mayombo, T. N. Minh, N. Fievet, P. Millet, and A. Luty. 2000. Immune response to Plasmodium falciparum liver stage antigen-1: geographical variations within Central Africa and their relationship with protection from clinical malaria. Trans. R. Soc. Trop. Med. Hyg. 94:557-562. [DOI] [PubMed] [Google Scholar]
- 13.Ockenhouse, C. F., P. F. Sun, D. E. Lanar, B. T. Wellde, B. T. Hall, K. Kester, J. A. Stoute, A. Magill, U. Krzych, L. Farley, R. A. Wirtz, J. C. Sadoff, D. C. Kaslow, S. Kumar, L. W. Church, J. M. Crutcher, B. Wizel, S. Hoffman, A. Lalvani, A. V. Hill, J. A. Tine, K. P. Guito, C. de Taisne, R. Anders, W. R. Ballou, et al. 1998. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J. Infect. Dis. 177:1664-1673. [DOI] [PubMed] [Google Scholar]
- 14.Scarselli, E., R. Tolle, O. Koita, M. Diallo, H. Muller, K. Fruh, O. Doumbo, A. Crisanti, and H. Bujard. 1993. Analysis of the human antibody response to thrombospondin-related anonymous protein of Plasmodium falciparum. Infect. Immun. 61:3490-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snow, R., J. Omumbo, B. Lowe, C. Molyneux, J. Obiero, A. Palmer, M. Weber, M. Pinder, B. Nahlen, C. Obonyo, C. Newbold, S. Gupta, and K. Marsh. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650-1654. [DOI] [PubMed] [Google Scholar]
- 16.Some, E. 1994. Effects and control of highland malaria epidemic in Uasin Gishu District, Kenya. East Afr. Med. J. 71:2-8. [PubMed] [Google Scholar]
- 17.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, and M. Marchand. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 18.Udhayakumar, V., D. Anyona, S. Kariuki, Y. Shi, P. Bloland, O. Branch, W. Weiss, B. Nahlen, D. Kaslow, and A. Lal. 1995. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J. Immunol. 154:6022-6030. [PubMed] [Google Scholar]
- 19.Zhou, Z., L. Xiao, O. H. Branch, S. Kariuki, B. L. Nahlen, and A. A. Lal. 2002. Antibody responses to repetitive epitopes of the circumsporozoite protein, liver stage antigen-1, and merozoite surface protein-2 in infants residing in a Plasmodium falciparum-hyperendemic area of western Kenya. XIII. Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 66:7-12. [DOI] [PubMed] [Google Scholar]