Abstract
It was shown recently that Mycobacterium tuberculosis expresses five proteins that are homologous to Rpf (resuscitation promoting factor), which is secreted by growing cells of Micrococcus luteus. Rpf is required to resuscitate the growth of dormant Micrococcus luteus organisms, and its homologues may be involved in mycobacterial reactivation. Mycobacterial Rpf-like products are secreted proteins, which makes them candidates for recognition by the host immune system and anti-Rpf immune responses potentially protective against reactivated tuberculosis. Here we report that the Rpf protein itself and four out of five of its mycobacterial homologues, which were administered as subunit vaccines to C57BL/6 mice, are highly immunogenic. Rpf-like proteins elicit immunoglobulin G1 (IgG1) and IgG2a responses and T-cell proliferation and stimulate production of gamma interferon, interleukin-10 (IL-10), and IL-12 but not IL-4 or IL-5. Both humoral and T-cell responses against these antigens show a high degree of cross-reactivity. Vaccination of mice with Rpf-like proteins results in a significant level of protection against a subsequent high-dose challenge with virulent M. tuberculosis H37Rv, both in terms of survival times and mycobacterial multiplication in lungs and spleens.
Tuberculosis (TB) remains one of the most important causes of morbidity and mortality worldwide (8, 10, 38, 39), and this situation dictates an urgent need for improved measures for controlling TB. The increasing numbers of multidrug-resistant TB cases (6, 37) suggest that the development of innovative vaccine strategies is, perhaps, a method of choice for controlling the spread of TB. Mycobacterium bovis BCG (attenuated M. bovis strain) represents the only vaccine available against TB as yet, although its efficacy in well-controlled clinical trials appears to be highly varied (9, 13, 14). Importantly, it is very likely that BCG vaccination does not protect against adult pulmonary TB in areas where TB is endemic (9, 19), i.e., the vaccine's effect is negligible exactly where it is most needed. An elegant, recent study of mice provided a rationale for the low efficacy of BCG in the regions where there is a high level of exposure to saprophytic mycobacteria (7). A varied BCG performance, as well as the obvious problem of using a live BCG vaccine in populations experiencing a substantial increase in the spread of human immunodeficiency virus (15, 33), validates the development of anti-TB vaccines whose efficacy is not dependent upon the persistence of live mycobacteria in the host.
Among several strategies to replace BCG with novel TB vaccine candidates, e.g., vaccination with a subunit protein, naked DNA, and improved whole bacterial vaccines (for a review, see reference 20), vaccination with a subunit protein is the approach best characterized for animal models (12, 17, 29). The choice of antigens to be included in an experimental subunit vaccine largely relies on the fact that live, as opposed to killed, BCG vaccine displays a high level of protection in animal models. The hypothesis that the response to antigens secreted from live mycobacteria is essential for host protection has been put forward, and several lines of evidence support an important role for these substances in eliciting a protective response against a subsequent TB challenge (2, 3, 17, 31). An important feature of immune responses against extracellular mycobacterial antigens is their early onset in the course of infection and their ability to rapidly stabilize mycobacterial loads in the parenchymal organs of infected animals at levels that are significantly lower than those in nonvaccinated controls (4, 35).
However, none of the TB vaccines studied so far was able to effectively prevent the development of pulmonary TB in animals infected with Mycobacterium tuberculosis. Even if the early mycobacterial multiplication was substantially inhibited due to vaccination, later in the infection course, the mycobacteria recommenced their progressive growth in the lungs, leading to increasing tissue damage and death. Given that the majority of TB cases in humans are thought to be cases of reactivated disease (32), it was suggested (12) that a novel subunit TB vaccine should include antigenic components, e.g., α-crystallin (36), that accumulate in latent (or dormant) mycobacteria during their long survival in the host. Theoretically, an immune response against such dormant-state antigens should protect against late-phase mycobacteria; however, there is no convincing evidence that these substances leave the dormant bacterial cells and become available for recognition by the immune system while metabolically passive microorganisms remain within macrophage phagosomes.
Another option to induce a protective response against reactivated disease is to use so-called therapeutic vaccines, i.e., vaccines that are administered long after the host has been infected with mycobacteria and the early-phase response has already led to a temporary balance between macro- and microorganism. In the study by Lowrie et al. using a murine model (22), therapeutic vaccination with the DNA vaccine encoding mycobacterial protein hsp65 (but not hsp70 or ESAT6) provided some decrease in mycobacterial counts in lungs and spleens. The degree of protection, however, did not reach the levels that are readily obtained with many experimental prophylaxis subunit vaccines. Even more importantly, from the point of view of safety, the therapeutic vaccination approach is difficult to approve in general terms. A well-known Koch phenomenon, i.e., the exacerbation of a balanced-phase TB infection following the administration of mycobacterial substances into the host (see reference 18), was recently rediscovered under well-controlled experimental conditions (25), a result that emphasizes the need for caution in considering postinfection TB vaccination. Thus, tactics relying on simultaneous inductions of protective responses against early-phase and reemerging mycobacteria prior to exposure look more attractive.
Recently, a protein termed Rpf (resuscitation promoting factor) that is encoded by the rpf gene and secreted by growing cells of Micrococcus luteus was discovered (26). It was shown that picomolar concentrations of Rpf were required to resuscitate the growth of dormant Micrococcus luteus organisms and to promote the growth of these bacteria in minimal media from an extremely small inoculum (26, 27). Importantly, genes homologous to rpf are widespread among bacteria with a high G+C content, including mycobacteria (21); in particular, both M. tuberculosis and M. bovis contain five rpf-like genes that share a conserved segment which encodes an Rpf-like domain ∼70 residues long (28). Given the biological activity of Rpf in Micrococcus luteus, the presence of these genes in mycobacteria suggests that one or more corresponding products may be involved in mycobacterial reactivation. Although direct in vivo evidence is lacking, some data supporting this hypothesis were obtained in vitro with Mycobacterium smegmatis and M. bovis BCG as model strains (28). Sequence analysis suggests that at least some of these proteins are secreted by these strains and that all five proteins probably have an extracytoplasmic function(s), making them potential targets for recognition by the host immune system at the stage of reactivated disease.
It is attractive to speculate that a mixed-subunit vaccine which is able to elicit the response against both an early secreted mycobacterial antigen(s) and a resuscitation promoting factor(s) should help the host to combat early as well as reactivated TB. However, apart from the ability of the prototype Rpf protein to give rise to an antibody response in rabbits (28), there is no information concerning the immunogenic, immunoreactive, and vaccine properties of the Rpf-like protein family. In this study, we report for the first time on the immune properties of the Rpf protein and all five of its mycobacterial homologues, as well as on the vaccine properties of some members of this family, as revealed in an experimental model with mice.
MATERIALS AND METHODS
Animals.
C57BL/6JCit (B6) mice were bred under conventional conditions at the animal facilities of the Central Institute for Tuberculosis (Moscow, Russia) in accordance with guidelines from the Russian Ministry of Health (guideline 755; U.S. Office of Laboratory Animal Welfare assurance A5502-01). Water and food were provided ad libitum. Female mice 8 to 10 weeks of age at the beginning of the experiment were used.
Production of recombinant proteins.
The polyhistidine-tagged constructs encoding Micrococcus luteus Rpf and M. tuberculosis strain H37Rv Rpf-like proteins (a kind gift of M. Young and G. Mukamolova, University of Wales, Cardiff, United Kingdom) were expressed in Escherichia coli HMS174 (DE3) using the pET 19b vector. Proteins were purified essentially as described previously, with the additional purification step being performed on a MonoQ column after affinity chromatography on a Ni2+ Sepharose 6B column (26, 28). Each final product provided a single band upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Purified proteins were dialyzed against phosphate-buffered saline (PBS), sterilized by filtration, and stored at 4°C until used.
Immunization.
Each mouse was immunized subcutaneously in the dorsum with 10 μg of Rpf-like proteins in 0.1 ml of PBS mixed with 0.1 ml of incomplete Freund's adjuvant (IFA; Sigma, St.Louis, Mo.) three times at 2-week intervals. Control mice were injected with PBS mixed with IFA.
Antibody response.
Immune and control mice were bled on day 21 after the last immunization to obtain sera. Antibody responses were measured in an enzyme-linked immunosorbent assay (ELISA) using microtiter plates (Costar-Corning, Badhoevedorp, The Netherlands) coated with purified recombinant Rpf-like proteins. Plates were coated overnight with a 10-μg/ml concentration of either antigen in 0.1 M carbonate buffer (pH 9.6). Plates were blocked with 1% bovine serum albumin in 0.05 M Tris-HCl-buffered saline, washed, and incubated for 2 h at 37°C, with serum samples diluted to appropriate concentrations in the blocking solution. After the plates were washed, they were incubated for 2 h at 37°C with alkaline phosphatase-conjugated rat anti-mouse immunoglobulin G1 (IgG1) or IgG2a monoclonal antibodies (Pharmingen, San Diego, Calif.) and then with p-nitrophenylphosphate substrate. The absorbance was measured at 405 nm.
Proliferative response of T cells
The proliferative response of the T cells was assessed exactly as described previously (23, 24). Briefly, 2 × 105 cells from auxiliary and inguinal lymph nodes were cultured for 72 h (for the last 18 h, in the presence of 0.5 μCi of [3H]thymidine per ml) in a well of a 96-well flat-bottom plate (Costar) at 37°C and 5% CO2 in the presence of 10 μg of individual Rpf-like antigens per ml. All cultures were performed in triplicate, and wells not stimulated with antigen served as controls. Uptake of the label was measured in a liquid scintillation counter (Wallac, Turku, Finland) after the contents of the wells were harvested with a semiautomatic cell harvester (Scatron, Oslo, Norway) on fiberglass filters. Results were determined with the following equation: Δcpm = (cpmAg − cpmcontr) ± the standard deviation (SD), where Δcpm is the change in counts per minute, cpmAg is that measured in antigen-stimulated wells, and cpmcontr is that measured in control wells. Preliminary titration experiments (results not shown) demonstrated that with the exception of nonimmunogenic RpfC (see below), all Rpf-like proteins showed a Δcpm of >3,000 when the proteins were added to immune lymph node cells at concentrations of 0.5 to 1.0 μg/ml. For individual proteins, a threshold concentration that stimulated a maximum specific response was between 3 and 10 μg/ml. The maximum antigen-specific proliferation plateaued at concentrations up to 30 μg/ml. Since the concentration of 10 μg/ml was a minimal one that provided a maximum response to all Rpf-like proteins, it was used throughout the experiments.
Cytokine production
Cytokine production was assessed exactly as previously described (23). A total of 2 ×106 cells/well were cultured in 24-well plates (Costar) in the presence or absence of 10 μg of individual Rpf proteins per ml. After 48 h, supernatants were harvested and stored at −30°C until used. ELISAs were used to detect interleukin-4 (IL-4), IL-5, IL-10, IL-12, and gamma interferon (IFN-γ) in 48-h culture supernatants. The following capture and biotinylated detection monoclonal antibodies to mouse cytokines were purchased from Pharmingen: for IFN-γ clones, R4-6A2 and XMG1.2; for IL-4 clones, 11B11 and BVD6-24G2; for IL-5 clones, TRFK5 and TRFK4; for IL-10, clones JES5-2A5 and JES5-16E3; and for IL-12 clones, C 17.8 and C 15.6. ELISAs were performed by following the manufacturer's instructions. Standard curves were generated with known concentrations of recombinant IL-4 (rIL-4), rIL-5, and rIL-12 (all from Pharmingen), rIL-10 (Sigma), and recombinant IFN-γ (Genzyme, Boston, Mass.).
Infection with M. tuberculosis.
Six weeks following the third immunization with Rpf-like proteins, B6 mice were challenged intravenously with 106 CFU of M. tuberculosis H37Rv Pasteur (originally a kind gift of G. Marchale, Institute Pasteur). Nonimmunized mice served as controls. The method for establishing and storing clump-free mid-log-phase mycobacterial suspensions for challenge experiments was described previously in detail (24). Mortality in infected mice was monitored daily, and results are expressed as mortality curves and mean survival times following challenge. To determine the CFU levels in infected organs, serial dilutions of 0.1-ml samples of homogenized lungs and spleens in sterile saline were plated on Dubos agar medium (Difco, Detroit, Mich.) 3 weeks following infection. Colonies were counted on days 18 to 20 of incubation at 37°C.
Statistical analysis.
The statistical significance of the differences was estimated by Student's t test. The differences were considered statistically significant at P values of <0.05.
RESULTS AND DISCUSSION
To assess the capacity of Rpf itself and Rpf-like mycobacterial proteins to elicit humoral and cellular immune responses, groups of B6 mice were immunized with a purified, full-length polyhistidine-tagged version of either protein. We estimated specific IgG1 and IgG2a levels in serum, as well as the intensity of antigen-specific-T-cell proliferation and the production of key cytokines in vitro.
Antibody response.
Table 1 shows levels in serum of specific antibodies to all six members of the Rpf protein family under study. For convenience, trivial synonyms of the nomenclature designations of mycobacterial Rpf-like products are used hereinafter as they appeared in a previous publication (28). All proteins except RpfC elicited a high level of IgG1 (results are depicted for 1:500 serum dilutions; 1:100 dilutions provided optical densities corresponding to the saturation plateau of the titration curve) and an appreciable level of IgG2a response. RpfC protein was poorly immunogenic.
TABLE 1.
Levels in sera of antibodies to the proteins of the Rpf familya
| Immunization protein | IgG subclassb
|
|
|---|---|---|
| IgG1 (1/500) | IgG2a (1/100) | |
| Rpf | 1.326 ± 0.03 | 0.527 ± 0.058 |
| RpfA (Rv0867c) | 1.260 ± 0.031 | 0.282 ± 0.041 |
| RpfB (RV1009) | 1.493 ± 0.024 | 0.278 ± 0.063 |
| RpfC (Rv1884c) | 0.146 ± 0.022 | 0.045 ± 0.006 |
| RpfD (Rv2389c) | 1.414 ± 0.024 | 0.167 ± 0.068 |
| RpfE (Rv2450c) | 1.194 ± 0.173 | 0.408 ± 0.08 |
Mice were immunized three times at 2-week intervals with 10 μg of either Rpf or an Rpf-like protein in IFA and bled at day 21 following the last immunization; serum antibody levels were assessed by ELISA, as described in Materials and Methods.
Sera from five animals in each group were evaluated individually at the dilutions indicated. Results are mean optical densities ± SDs for the group from one out of two similar experiments.
Given that all the proteins carry a highly conservative Rpf domain structure, sharing ∼65% of the amino acid sequence within the family (28), it was of interest whether there is an immune cross-reactivity between individual members of the family. To address this issue, the levels of cross-reactive antibodies were estimated in the sera of mice immunized with a particular Rpf-like protein, by means of an ELISA in which different members of the family served as the covering antigen. All members of the family, except the poorly immunogenic RpfC protein, demonstrated a significant degree of IgG1 and IgG2a cross-reactivity, with antibody cross-binding varying from 30 to 80% of that with an immunizing antigen.
T-cell proliferation.
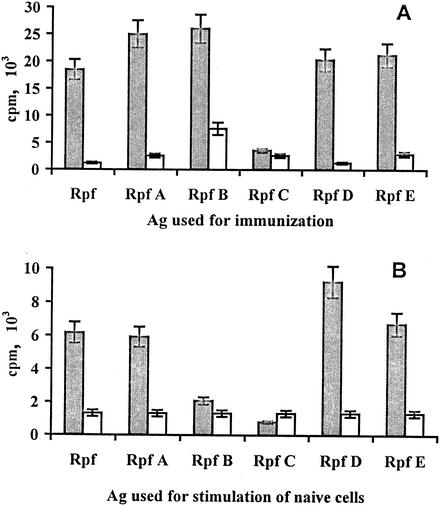
The capacity of mycobacterial products to induce a high level of antibody response in mice does not always correlate with their immunogenicity for T cells. For example, a 19-kDa mycobacterial antigen administered in IFA is a good inducer of IgG antibodies as opposed to T-cell proliferation (our unpublished observations). Bearing in mind future vaccination studies, we considered it important to demonstrate that Rpf-like proteins are able to induce a T-cell response following their administration in vivo, since this response is pivotal in providing TB protection (12, 34). The proliferative response of lymph node T cells was measured following immunization of the mice with individual Rpf-like proteins and a secondary stimulation of a mixture of cells from auxiliary and inguinal lymph nodes in vitro with corresponding antigens. As shown in Fig. 1A, five out of six Rpf-like proteins were able to stimulate significant levels of specific T-cell proliferation. In addition, four members of the family—Rpf, RpfA, RpfD, and RpfE—revealed the properties of weak mitogens, able to excite in vitro a moderate increase in [3H]thimidine uptake by lymph node cells from nonimmune control mice (6,000 to 8,000 cpm in the presence of Rpf-like proteins compared to 1,200 to 1,300 cpm in medium alone) (Fig. 1B). It is unlikely that the mitogenic effect was due to the stimulation of B cells by contaminating lippopolysaccharides since (i) highly purified (>98% purity) lymph node T cells from these mice mixed with irradiated antigen-presenting cells from intact mice readily demonstrated a mitogenic effect and (ii) the sera of mice immunized with any Rpf-like protein contained no lippopolysaccharide-specific antibodies (data not shown). In addition, the RpfB product elicited a high level of specific response (Fig. 1) but was not mitogenic (≃2,000 cpm in RpfB-stimulated cultures containing nonimmune lymphocytes). As in the case of the antibody response, the RpfC protein was practically nonimmunogenic and nonreactive.
FIG. 1.
Proliferative response of lymph node cells to Rpf-like proteins. Mice were immunized with individual proteins of the Rpf family as described in Materials and Methods (A) or left unimmunized (B). Two weeks after the last immunization, cells were isolated from auxiliary and inguinal lymph nodes of three mice in each group, mixed, and stimulated in vitro with 10 μg of an individual antigen (Ag) (shaded bars) per ml or left unstimulated (open bars). All cultures were performed in triplicate. Cultures were pulsed with [3H]thymidine (0.5 μCi/ml) for the last 18 of 72 h of incubation. Results of one out of two similar experiments are expressed as mean counts per minute ± SDs from triplicate cultures.
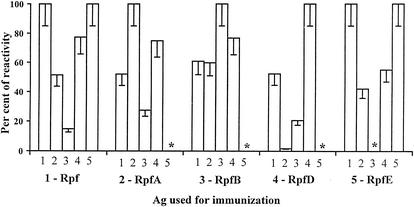
Like Rpf-specific serum antibodies, T cells immune to particular members of the Rpf family showed significant levels of cross-reactivity. The level of T-cell proliferation in response to cross-reactive Rpf, RpfA, RpfD, and RpfE products varied between 50 and 100% of that of the immunizing protein (Fig. 2). One notable difference between humoral and T-cell responsiveness concerned the RpfB antigen: while cross-reactivity with RpfB of antibodies developed against other members of the family exceeded 50% of the specific response (data not shown), corresponding T cells were only weakly cross-reactive with RpfB (Fig. 2). Thus, in terms of T-cell response, RpfB showed the highest degree of immunological specificity; being highly immunogenic, this product was neither cross-reactive nor mitogenic.
FIG. 2.
Cross-reactivity of T cells immune to individual Rpf-like proteins. Lymph node cells were obtained from immune mice, and their capacity to proliferate in vitro in response to the immunizing as well as to relative Rpf-like proteins was assessed as described in Materials and Methods. Results of two similar experiments are summarized and expressed as percentages of the antigen (Ag)-specific (Δcpm = cpmAg - cpmcontr) reactivity. Asterisk, not determined.
Cytokine production.
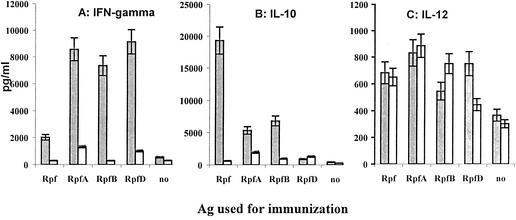
We also assessed the capacity of lymph node cells to produce the key type 1 and type 2 cytokines following immunization of the mice with four different Rpf-like proteins and the subsequent culturing of cells in the presence or absence of corresponding antigens. As shown in Fig. 3A, all Rpf-like proteins tested elicited antigen-specific IFN-γ production, with mycobacterial RpfA, RpfB, and RpfD proteins stimulating higher levels of response than that of the micrococcal Rpf protein. In contrast, Rpf-stimulated cells produced a large amount of IL-10 in the antigen-specific manner, whereas mycobacterial Rpf-like products stimulated only low to moderate IL-10 production (Fig. 3B). One may conclude that there is a kind of bias towards a type 1 or type 2 response following immunization with, respectively, mycobacterial or micrococcal Rpf-like products. However, since the differences in IFN-γ and IL-10 production were purely quantitative and since neither antigen tested induced measurable production of IL-4 and IL-5 (data not shown), this bias is only mildly pronounced and incomplete. This result is not unexpected, since the response to mycobacterial antigens, being biased towards type 1 in general and in B6 mice in particular (5, 11), usually includes certain features of type 2 reactivity, e.g., IgG1 and/or IL-10 production (1, 16, 24).
FIG. 3.
Cytokine production by lymph node cells from mice immunized with individual Rpf-like proteins. The mixture of cells from three mice was either additionally stimulated in vitro with a corresponding antigen (Ag) (shaded bars) or left unstimulated (open bars), and the amounts of IFN-γ (A), IL-10 (B), and IL-12 (C) in supernatants were assessed by ELISA as described in Materials and Methods. The amounts of IL-4 and IL-5 were always below the sensitivities of the corresponding assay. Results (in picograms per milliliter) are expressed as the means of results from three similar experiments ± standard errors of the means.
Lymph node cells from mice immunized with any of the four antigens produced more IL-12 than their counterparts from control intact mice, but its synthesis did not depend upon secondary antigen stimulation in vitro, with the only exception being the RpfD product (Fig. 3C). This antigen seems to be the best inducer of a type 1 cytokine response, eliciting IFN-γ and IL-12 production in an antigen-specific manner and no IL-10 response.
TB protection studies.
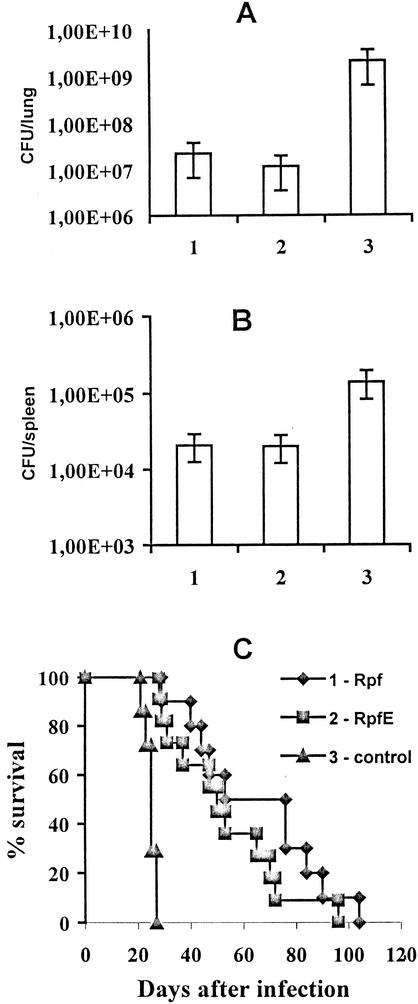
The experiments described above demonstrate that Rpf-like proteins are immunogenic and are able to elicit specific IFN-γ synthesis and to increase constitutive IL-12 production by lymph node cells, i.e., to stimulate the type of response that is generally considered protective against mycobacteria (see references 30 and 34 for reviews). In order to assess the vaccine properties of these proteins, groups of mice were vaccinated with Rpf and RpfE proteins in IFA (see Materials and Methods), challenged with a high dose of virulent M. tuberculosis H37Rv, and compared with nonvaccinated control animals with respect to mycobacterial loads in organs and postinfection survival times. In addition to a typical mycobacterial Rpf-like product, RpfE, we decided to include Rpf from Micrococcus luteus in protection studies because it was of interest to find out whether the response against a nonmycobacterial Rpf protein is sufficient to protect mice against a mycobacterial challenge, i.e., whether the response against a conservative Rpf domain which is shared by all members of the family is protective.
As shown in Fig. 4, vaccination with either antigen resulted in a significant degree of protection against a TB challenge. In control animals mycobacterial numbers were 2 logs higher in the lungs (Fig. 4A) and 1 log higher in the spleens (Fig. 3B) than the levels in vaccinated mice. Moreover, the survival times of animals vaccinated with either antigen were significantly prolonged (P < 0.01, Student's t test) compared to that of the control group (Fig. 4C). Taken together, the differences between the results for vaccinated and control mice with respect to mycobacterial multiplication in organs and survival times indicate that the levels of protection achieved by immunization with Rpf-like proteins are comparable to those provided in this challenge model by short-term culture filtrate components, e.g., Ag85B and ESAT-6 (29; our unpublished observations), or by vaccination with a low dose (103 CFU) of live BCG but are lower than the level achieved by vaccination with 104 to 106 CFU of BCG (40). One preliminary experiment (data not shown) performed with outbred guinea pigs also indicates the protective capacity of vaccination with Rpf-like proteins against a TB challenge.
FIG. 4.
Vaccination efficacy of Rpf-like proteins. Mycobacterial multiplication in lungs (A) and spleens (B) at week 3 postinfection, and survival rates (C) were estimated for groups of B6 mice vaccinated with Rpf (group 1), RpfE (group 2), or PBS (group 3, control) in IFA and challenged intravenously with 106 M. tuberculosis H37Rv CFU (see Materials and Methods). (C) For the mortality experiment whose results are shown, mean survival times ± standard errors of the means were 64.3 ± 6.9 (10 mice), 52.2 ± 6.3 (11 mice), and 24.7 ± 1.0 (7 repetitions) for groups 1, 2, and 3, respectively. In each experiment, 5 mice per group were used to assess mean numbers of CFU ± SDs in organs, and 7 to 12 mice per group were used to assess mortality. Results of one out of two similar experiments are depicted; the protective effect of the Rpf protein in terms of mortality was estimated with consistent results in three independent experiments.
In conclusion, the immunogenicity and protective capacity of the members of the Rpf-like protein family demonstrated herein, along with their possible involvement in support of mycobacterial growth under restrictive conditions (28), suggest that these products should be included in combined-subunit TB vaccines. Arming the host immune system with the capacity to respond to both early-phase proteins, such as short-term culture filtrate proteins, and late-phase proteins, such as Rpf-like proteins and sigma factors (39), the addition of extracytoplasmic mycobacterial antigens may significantly improve the performance of a subunit vaccine, a possibility which is presently under study in our laboratories.
Acknowledgments
We thank M. Young and G. Mukamolova for their kind gift of the constructs for the expression of Rpf-like proteins.
This work was supported by International Science and Technology Center (ISTC) grants 1879 and 2201, Russian Foundation for Basic Research (RFBR) grants 04-48691 and 04-49095, and by the Wellcome Trust.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abou-Zeid, C., M.-P. Gares, J. Inwald, R. Janssen, Y. Zhang, D. B. Young, C. Hetzel, J. R. Lamb, S. L. Baldwin, I. M. Orme, V. Yeremeev, B. V. Nikonenko, and A. S. Apt. 1997. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect. Immun. 65:1856-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P. 1994. The T cell response to secreted antigens of Mycobacterium tuberculosis. Immunobiology 191:537-547. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 5.Arriaga, A. K., E. H. Orozco, L. D. Aguilar, G. A. W. Rook, and R. Hernandez-Pando. 2002. Immunological and pathological comparative analysis between experimental latent tuberculosis infection and progressive pulmonary tuberculosis. Clin. Exp. Immunol. 128:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom, B. R., and C. L. Murray. 1992. Tuberculosis: commentary on re-emergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 7.Brandt, L., J. F. Cunha, A. W. Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chintu, C., and A. Mwinga. 1999. An African perspective on the threat of tuberculosis and HIV/AIDS—can despair be turned to hope. Lancet 353:997-1001. [DOI] [PubMed] [Google Scholar]
- 9.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Feinberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 10.Dolin, P. J., M. Raviglione, and A. Kochi. 1994. Global tuberculosis incidence and mortality during 1990-2000. Bull. W. H. O. 72:213-220. [PMC free article] [PubMed] [Google Scholar]
- 11.Druilhe, R., P. Hagan, and G. A. W. Rook. 2002. The importance of models of infection in the study of disease resistance. Trends Microbiol. 10:S38-S46. [DOI] [PubMed]
- 12.Elhay, M., and P. Andersen. 1997. Immunological requirements for a subunit vaccine against tuberculosis. Immunol. Cell Biol. 75:595-603. [DOI] [PubMed] [Google Scholar]
- 13.Fine, P. E. M. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 14.Fine, P. E. M. 2000. BCG vaccines and vaccination, p. 503-522. In L. R. Reichmann and E. S. Hershfield (ed.), Tuberculosis. A comprehensive international approach. Marcel Dekker, New York, N.Y.
- 15.Goletti, D., D. Weissman, R. W. Jackson, N. M. H. Graham, D. Vlahov, R. S. Klein, S. S. Munsiff, L. Ortona, R. Cauda, and A. S. Fauci. 1996. Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J. Immunol. 157:1271-1278. [PubMed] [Google Scholar]
- 16.Hernandez-Pando, R., E. H. Orozco, A. Sampieri, L. Pavon, C. Velasquillo, J. Larriva-Sahol, J. M. Alcocei, and M. V. Madrid. 1996. Correlation between the kinetics of Th-1/Th-2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 89:26-33. [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz, M. A., B. W. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iseman, M. D. 2000. A physician's guide to tuberculosis. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 19.Karonga Prevention Trial Group. 1996. Randomized controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 20.Kaufmann, S. H. E. 2000. Is the development of a new tuberculosis vaccine possible? Nat. Med. 6:955-960. [DOI] [PubMed] [Google Scholar]
- 21.Kell, D. B., and M. Young. 2000. Bacterial dormancy and culturability: the role of autocrine growth factors. Curr. Opin. Microbiol. 3:238-243. [DOI] [PubMed] [Google Scholar]
- 22.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccioli, E. Stavropoulos, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 23.Lyadova, I. V., E. B. Eruslanov, S. V. Khaidukov, V. V. Yeremeev, K. B. Majorov, A. V. Pichugin, B. V. Nikonenko, T. K. Kondratieva, and A. S. Apt. 2000. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant and hyperresistant to Mycobacterium tuberculosis-triggered disease. J. Immunol. 165:5921-5931. [DOI] [PubMed] [Google Scholar]
- 24.Lyadova, I., V. Yeremeev, K. Majorov, B. Nikonenko, S. Khaidulkov, T. Kondratieva, N. Kobets, and A. Apt. 1998. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect. Immun. 66:4981-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreira, A. L., L. Tsenova, M. H. Aman, L.-G. Bekker, S. Freeman, B. Mangaliso, U. Schröder, J. Jagirdar, W. N. Rom, M. G. Tovey, V. H. Freedman, and G. Kaplan. 2002. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect. Immun. 70:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukamolova, G. V., A. S. Kaprelyants, D. I. Yound, M. Yong, and D. B. Kell. 1998. A bacterial cytokine. Proc. Natl. Acad. Sci. USA 95:8916-8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukamolova, G. V., O. A. Turapov, D. I. Young, A. S. Kaprelyants, D. B. Kell, and M. Young. 2002. A family of autocrine growth factors in Mycobacterium tuberculosis. Mol. Microbiol. 46:623-635. [DOI] [PubMed] [Google Scholar]
- 28.Mukamolova, G. V., O. A. Turapov, K. A. Kazarian, M. Telkov, A. S. Kaprelyants, D. B. Kell, and M. Young. 2002. The rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Mol. Microbiol. 46:611-621. [DOI] [PubMed] [Google Scholar]
- 29.Olsen, A. W., L. A. H. van Pinxteren, L. M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orme, I., and A. M. Cooper. 1999. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol. Today 20:307-312. [DOI] [PubMed] [Google Scholar]
- 31.Pal, P. G., and M. A. Horwitz. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrish, N. M., J. D. Dick, and W. R. Bishai. 1998. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6:107-112. [DOI] [PubMed] [Google Scholar]
- 33.Reynes, J., C. Perez, I. Lamaury, F. Janbon, and A. Bertrand. 1989. Bacille Calmette-Guerin adenitis 30 years after immunization in a patient with AIDS. J. Infect. Dis. 160:727-730. [DOI] [PubMed] [Google Scholar]
- 34.Schluger, N. W., and W. N. Rom. 1998. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157:679-691. [DOI] [PubMed] [Google Scholar]
- 35.Sørensen, A. L., S. Nagai, G. Houen, P. Andersen, and Å. B. Andersen. 1995. Purification and characterization of a low molecular mass T cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbon, A., R. A. Hartskeerl, A. Schuitema, A. H. J. Kolk, D. B. Young, and R. Lathigra. 1992. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J. Bacteriol. 174:1352-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. 1997. Anti-tuberculosis drug resistance in the world. The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. World Health Organization, Geneva, Switzerland.
- 38.World Health Organization.1998. The world health report. World Health Organization, Geneva, Switzerland.
- 39.Wu, Q. L., D. Kong, K. Lam, and R. N. Husson. 1997. A mycobacterial extracytoplasmic function sigma factor involved in survival following stress. J. Bacteriol. 179:2922-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeremeev, V. V., G. R. Stewart, O. Neyrolles, K. Skrabal, V. G. Avdienko, A. S. Apt, and D. B. Young. 2000. Deletion of the 19kDa antigen does not alter the protective efficacy of BCG. Tuber. Lung Dis. 80:243-247. [DOI] [PubMed] [Google Scholar]