Abstract
Binding of immunoglobulin M (IgM) antibodies from normal human serum to the surface of Plasmodium falciparum-infected red blood cells (iRBC) has previously been demonstrated only in parasites that form rosettes with uninfected red cells. We show that natural, nonspecific IgM but not IgG, IgA, IgD, or IgE also binds to the surface of iRBC selected for adhesion to chondroitin sulfate A (CSA), a placental receptor for parasites associated with malaria in pregnancy. The protease sensitivity of IgM-binding appears to match that of CSA binding, suggesting that the two phenotypes may be mediated by the same parasite molecule. We also show that a wide range of mouse monoclonal antibodies of the IgM class bind nonspecifically to CSA-selected iRBC, an important consideration in the interpretation of immunological assays performed on these parasite lines.
Variant antigens of Plasmodium falciparum located on the surface of infected red blood cells (iRBC) allow malaria parasites to sequester via receptors at specific sites within the host (21). In pregnancy-associated malaria, sequestration in the placenta may be due to iRBC adhering to the glycosaminoglycan chondroitin sulfate A (CSA) (4, 17), specific low-sulfated forms of which are found at the placental syncytiotrophoblast surface and within the intervillous spaces (1). Selection for parasites that bind to CSA in the placenta is thought to be a major factor leading to the pathology associated with malaria in pregnancy (6).
Antibodies directed against CSA-binding parasites correlate with increasing gravidity and increased protection from malaria pathology in women from regions where malaria is endemic, suggesting that development of a protective vaccine against pregnancy-associated malaria may be possible (18, 26, 41). P. falciparum erythrocyte surface antigens of the PfEMP-1 class, encoded by the var multigene family (3, 42), are candidates for vaccinating antigens to induce such protective antibodies. The Duffy binding-like (DBL) γ and cysteine-rich interdomain region domains of certain PfEMP-1 variants have been proposed to mediate CSA binding (7, 13, 25). However, some of the genes associated with CSA binding are commonly expressed in many P. falciparum lines, including many nonplacental and non-CSA-binding isolates (20, 32, 34). Definitive identification of the candidate sequence(s) for inclusion in a vaccine to protect against malaria in pregnancy therefore has not yet been achieved.
Other adhesion properties of P. falciparum, such as the sticking of mature parasitized cells to endothelial cells and rosetting adhesion between infected and uninfected red blood cells, also appear to be mediated by specific PfEMP-1 variants (2, 31). Some of these adhesive interactions involve host serum proteins. For example, rosetting parasites, associated with severe malaria in African children (8, 30), frequently bind nonspecific human immunoglobulin M (IgM) (11, 33, 37, 39, 43). Nonspecific IgG binding has also recently been reported to be involved in adherence of iRBC to placental sections (16). To study the involvement of host immunoglobulin binding in parasite attachment to placental receptors, we selected six parasite lines for the CSA-binding phenotype. These selected parasites, together with the unselected parental lines, were tested for their capacity to bind immunoglobulin from normal human serum.
MATERIALS AND METHODS
Parasites and culture.
The parasites used in this study are listed in Table 1. All parasite lines originated from peripheral blood samples with the exception of Gb337, which was washed from an infected placenta delivered at the Albert Schweitzer Hospital, Lambarene, Gabon. Parasite lines SD2H3 and SD202 were from women from the Sudanese village of Daraweesh. The Busua line was from a Danish male who contracted malaria in the village of Busua in Ghana. HB3 and FCR3 are long-established laboratory clones from Honduras and the Gambia, respectively. The selected clone FCR3CSA (36) was obtained from the Malaria Research and Reference Reagent Resource Centre (American Type Culture Collection). Clone TM284S2 was obtained from the Karolinska Institute, Stockholm, Sweden (16). All isolates had been grown in culture for several months before testing. Parasites were grown in group O-positive erythrocytes under standard conditions (29), in RPMI 1640 medium containing sodium bicarbonate and 25 mM HEPES, supplemented with 20 mM glucose, 2 mM glutamine, 25 μg of gentamicin per ml, and 10% pooled normal human serum, with the pH adjusted to between 7.2 and 7.4 with 1 M NaOH (complete medium). Cultures were gassed with a mixture of 1% oxygen, 3% carbon dioxide, and 96% nitrogen.
TABLE 1.
Binding of nonspecific human IgM to CSA-selected parasitesa
| Parasite isolate | Origin | Selected for CSA binding | Recognized by female but not male antibodies | % of iRBC binding IgM |
|---|---|---|---|---|
| SD2H3 | Sudan | No | No | 1 |
| SD2H3CSA | Yes | Yes | 74 | |
| SD202 | Sudan | No | No | 4 |
| SD202CSA | Yes | Yes | 94 | |
| Busua | Ghana | No | No | 9 |
| BusuaCSA | Yes | Yes | 91 | |
| Gb337 | Gabon | No | No | 2 |
| Gb337CSA | Yes | Yes | 79 | |
| HB3 | Honduras | No | No | 4 |
| HB3CSA | Yes | No | 47 | |
| FCR3 | Gambia | No | No | 4 |
| FCR3CSA | Yes | Yes | 88 |
Results are given as the percentage of IgM-positive iRBC observed in a representative experiment on each line. All lines were tested at least three times, some as many as 10 times. Consistent results were obtained in all experiments. There was variation in the time at which each culture reached sufficient density to allow testing and also in the rate at which different lines lost their selected adhesion property (27). This precluded averaging results, because the timing of each test postselection will not be strictly comparable.
CSA selection.
In vitro selection for binding to CSA was carried out by multiple rounds of panning with protocols similar to those published (9). Trophozoite-stage parasite cultures were incubated in plastic petri dishes (Falcon 1008) which had been coated with 10 μg of bovine tracheal CSA (Sigma) per ml in phosphate-buffered saline (PBS) overnight and then preblocked for 2 h with 2% bovine serum albumin (BSA)-PBS. The cultures were incubated at 37°C with gentle agitation for 1 h. Uninfected and unbound iRBC were washed off gently until the plate was homogeneously clear and microscopic examination showed only static, bound iRBC. Uninfected red blood cells in complete medium at 5% hematocrit were then added to the plates, which were kept under normal culture conditions, harvested 24 h later, and maintained in culture. All parental parasite lines were selected at least 10 times to give the high CSA-binding lines tested here.
Differential recognition of CSA-selected parasites by serum from multigravid women.
All the parasite lines were tested by flow cytometry for their ability to differentially recognize antibodies in serum from multigravid women relative to serum from males from regions where malaria is endemic and to nonexposed European patient controls (40). Erythrocytes were labeled with 0.1 mg of ethidium bromide solution per ml per 105 erythrocytes to differentiate between uninfected cells and the nucleic acid-containing parasitized cells. After washing, the cells were incubated in 1 to 5 μl of pooled adult male serum, pooled multigravid female serum from a region where malaria is endemic (Ghana), or control serum from Danish malaria-naïve donors. Cells were again washed and incubated with goat anti-human immunoglobulin G (Dako, Glostrup, Denmark) diluted 1:200, followed by washing and incubation with 100 μl of fluorescein isothiocyanate-conjugated rabbit anti-goat immunoglobulin (Dako) diluted 1:25 in PBS. All incubations were for 30 min at room temperature, followed by three washes in 3 ml of PBS between each incubation step (twice before the last step). Samples were kept overnight at 5°C before analysis on a Coulter Epics XL-MCL flow cytometer (Coulter Electronics, Luton, United Kingdom). Uninfected and infected cells were gated according to ethidium bromide fluorescence, and the median value was used for the quantification of fluorescein isothiocyanate fluorescence. Recognition of antibodies specific to the pool of serum from multigravid women was scored relative to antibodies recognized in the pool of male serum and from control serum from the malaria-naïve patients. All data were analyzed with Win MDI software (http://facs.scrips.edu/software.html).
Immunofluorescence assay to detect human immunoglobulin.
The immunofluorescence assay (IFA) to detect human immunoglobulin on the surface of iRBCs was carried out with unfixed parasite cultures at the mature pigmented trophozoite stage (33). Because of known cross-reactivity in these assays between some animal sera and infected and uninfected human cells (33), particular attention was paid to the reagents used. Only monoclonal antibodies (MAbs) and affinity-purified polyclonal antisera were used, and extensive isotype controls were included. Briefly, aliquots of culture suspension at 2% hematocrit were washed in PBS and resuspended in PBS-1% immunoglobulin-free BSA (Sigma). Mouse MAbs to human IgG(Fc), IgG(Fab), IgM, IgA, IgD, IgE, IgG1, IgG2, IgG3, and IgG4 (all Serotec) and matched isotype controls were added to give a final concentration of 1 μg/ml. These were incubated for 1 h on ice, with gentle resuspension of the cells every 10 min.
All mouse MAbs used were of the IgG class. A polyclonal antiserum to human IgG (Dako) was also tested at 1:20 dilution as described (33). After two washes with 750 μl of PBS, detection was carried out with highly cross-absorbed Alexa Fluor 488-conjugated goat anti-mouse IgG (Molecular Probes) at 1:500 dilution in PBS-1% BSA plus 4′,6′-diamidino-2-phenylindole (DAPI) at 1 μg/ml for 45 min on ice, in the dark, with gentle mixing every 10 min. After washing, cells were resuspended at 30% hematocrit in PBS-1% BSA and used to make a thin smear on a clean microscope slide. After air drying, the smear was overlaid with a drop of 1.25 mg of diazabicyclo-(2, 2,2)octane (DABCO) per ml in 50% glycerol-50% PBS and a coverslip (22 by 22 mm). The edges of the coverslip were sealed with nail varnish. Each set of assays was performed at least three times and read each time by two independent observers. Slides were viewed on an Olympus BX-50 microscope, and images were recorded with a digital camera and OpenLab software. The percentage of iRBCs binding IgM was determined by counting at least 200 infected cells. Each culture was assessed for rosette-forming capacity immediately prior to the IFA tests.
Assessment of rosetting.
Parasite culture aliquots at 2% hematocrit were stained with 25 μg of ethidium bromide per ml, and wet preparations were viewed by fluorescence microscopy with UV and white light simultaneously to visualize both stained and unstained cells. Two hundred mature infected cells were counted, with the binding of two or more uninfected cells constituting a rosette. The rosette frequency is the percentage of mature iRBCs that formed rosettes.
IFA to detect mouse IgM binding.
To determine whether IgM from species other than humans also binds to CSA-selected parasite lines, the culture medium-derived human serum IgM was stripped from the iRBC surface by incubation at 2% hematocrit with 200 mg of chloroquine diphosphate (pH 5.0) per ml for 1 h at 20°C (15). Preliminary experiments showed that this treatment removed all detectable human IgM from the iRBC surface (data not shown). The cells were then washed twice with 750 μl of PBS and incubated for 1 h as above with 10 μg of mouse IgM MAbs raised to a variety of different antigens per ml (Table 2). None of these IgM MAbs reacted with normal human red blood cells, as assayed by IFA (data not shown). Cells were washed and incubated with a 1:200 dilution of Alexa Fluor 488-conjugated goat anti-mouse IgM (Molecular Probes) for 45 min. Negative controls were incubated with PBS-1% BSA followed by the above secondary antibody. Positive controls were incubated with 10% normal human serum followed by mouse anti-human IgM MAb followed by Alexa Fluor 488-conjugated goat anti-mouse IgG. Cells were washed, prepared, and viewed as described for the IFA assays with human immunoglobulin.
TABLE 2.
Binding of mouse IgM MAbs to the CSA-selected parasite line Gb337CSA
| MAb | Specificity | Source or reference | % IgM positivea |
|---|---|---|---|
| Human IgM-positive control | Not applicable | Human serum | 78 |
| Negative control (goat anti-mouse IgM secondary antibody) | Not applicable | Molecular Probes | 0 |
| MAb 2040 | Heparan sulfate | Chemicon | 80 |
| SM phage antibody | CD36 | Sigma | 74 |
| MAb CS-56 | Chondroitin sulfate | Sigma | 77 |
| MAb MOPC-104E | IgM isotype control | Sigma | 78 |
| MAb TCN-1 | Toxocara canis | 24 | 60 |
| MAb TCN-4 | Toxocara canis | 24 | 82 |
| MAb TCN-8 | Toxocara canis | 24 | 82 |
Data from a representative experiment are shown. Three further experiments gave consistent results. The unselected parental line Gb337 was also tested, and all MAbs showed less than 2% positive infected cells by IFA.
Protease treatments.
Aliquots of parasite cultures were treated with 1 μg, 10 μg, and 100 μg of TPCK-treated trypsin (Sigma) per ml for 5 min at 37°C, followed by 1 mg of soybean trypsin inhibitor per ml for 5 min at 37°C. Controls were treated in the same way with PBS substituting for the enzyme. Cells were washed three times in complete RPMI medium prior to IFA and binding assays.
CSA binding assays.
Spots (3 μl) of 10-μg/ml CSA in PBS were placed on marked positions on plastic petri dishes (Falcon 1007), interspersed with 3-μl spots of PBS-1% BSA as negative controls. Plates were incubated overnight in a humid box at 4°C. Next morning, the spots were removed by suction, and the plates were blocked by incubating with PBS-1% BSA for 2 h at 37°C. Then 2 ml of parasite culture suspension at 5 to 10% parasitemia and 2% hematocrit in a binding medium (pH 7.2) of RPMI 1640 without sodium bicarbonate and human serum but with 1% immunoglobulin-free BSA was added to each plate. This was incubated for 1 h at 37°C, with gentle resuspension of the cells every 10 min. Plates were then washed gently with PBS 6 to 10 times with a plastic Pasteur pipette, swirling the plate to remove unbound cells. Washing continued until all background binding had been removed. Bound cells were fixed with 1% glutaraldehyde and stained with 5% Giemsa. Plates were viewed with an Olympus BX-2 microscope, with oil immersion and a 100× objective. The number of cells bound in 10 high-power fields in each spot was counted.
RESULTS AND DISCUSSION
Six parasite lines demonstrating high levels of CSA binding were selected from parental lines by multiple rounds of panning on CSA. After selection, each line with the exception of HB3CSA showed enhanced recognition by serum from multigravid women relative to serum from males from regions where malaria is endemic (Table 1). This confirms earlier observations that most CSA-selected lines are recognized by specific antibodies in serum from women who have had children in areas where malaria is endemic. This specific recognition is also correlated with the acquisition of protection from the adverse outcomes of malaria in pregnancy (26, 41).
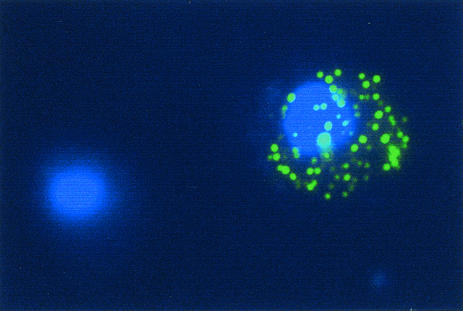
The six CSA-selected and corresponding unselected parental lines were tested for natural antibody binding by IFA on unfixed cells. Surprisingly, all six CSA-selected lines showed a marked increase in binding of nonspecific human IgM to the iRBC surface relative to the unselected parental lines (Table 1). This IgM, which was derived from normal human serum in the parasite culture medium, was defined in a punctate pattern (Fig. 1), characteristic of the reaction of antibodies with the parasite-encoded knob-associated erythrocyte surface antigens (3). We did not detect any binding to human IgG, IgA, IgD, or IgE in any of the parasite lines tested (data not shown).
FIG. 1.
Photomicrograph of parasites of P. falciparum line Gb337CSA binding nonspecific human IgM. Immunofluorescence was detected with mouse anti-human IgM (Serotec) and Alexa Fluor 488-conjugated goat anti-mouse IgG secondary antibody (green). Parasite nuclei are stained blue with DAPI. An IgM-positive and an IgM-negative infected human erythrocyte are shown.
Nonspecific IgM binding has previously been reported to be a common property of parasite isolates with the rosetting phenotype (11, 33, 37, 39, 43). All of these experimental lines were therefore tested for rosetting. Neither the CSA-selected nor the parental unselected parasites formed rosettes. This is consistent with reports that the rosetting phenotype is rarely seen in P. falciparum isolates from pregnant women (23, 28). These results indicate that CSA binding is a second, unrelated parasite adhesion phenotype closely associated with the ability to bind nonspecific IgM from normal human serum. To our knowledge, this is the first report of apparent coselection for CSA binding and nonspecific IgM binding on the surface of P. falciparum-infected erythrocytes. Interestingly, nonspecific IgM has recently been reported to bind to the surface of erythrocytes infected with another Apicomplexan parasite, Babesia bigemina (14). It will be important in future work to find out whether nonspecific binding of IgM is also a property of fresh placental isolates and, if so, whether this property correlates with their CSA-binding capacity.
In searching for potential malaria parasite adhesion receptors in the placenta, it has been reported that clone TM284S2, which does not bind CSA but binds to placental tissue, binds nonspecific IgG and may use the Fcγ receptors on the syncytiotrophoblast layer as a binding receptor (16). We found no evidence for nonspecific IgG binding in any of the CSA binding isolates we studied. Nor have we been able to repeat the observation of an IgG binding phenotype in the TM284S2, although we confirmed that this clone binds IgM and forms large rosettes. Differences in technique and/or reagents used may account for this discrepancy (see (33) for further discussion). In particular, it was found that the use of polyclonal antisera that are not affinity purified may give false-positive results in IFA with P. falciparum (12, 33, 35). The IgG detected in the initial report on TM284S2 (16) could be a false-positive signal resulting from the use of such a polyclonal reagent.
In view of the nonspecific nature of human IgM binding, we investigated whether IgM of other species might act in a similar manner. We found that a variety of mouse monoclonal antibodies of the IgM class raised against nonmalarial antigens also bound to the CSA-selected but not the unselected iRBC (Table 2). Nonspecific binding of mouse IgG MAbs was never detected (data not shown). The ability of CSA-selected iRBC to cross-react nonspecifically with both human and mouse IgM has important implications for the use of IgM reagents in assays with CSA-binding parasites. It has recently been reported that mouse MAbs raised against the CSA-binding domain of a PfEMP-1 variant were pan-reactive with several CSA-binding parasite lines (22). However, all of the MAbs described experimentally in this report were of the IgM class. There is therefore a possibility that the apparent pan-reactivity between IgM monoclonal antibodies and CSA-binding isolates might be due to the nonspecific cross-reaction of mouse IgM to the selected CSA-binding iRBC as shown here.
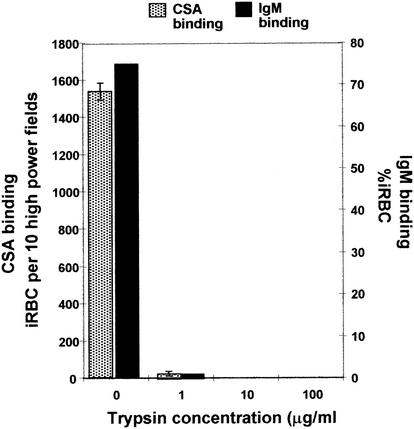
Previous studies on the IgM binding phenotype of rosetting P. falciparum clones have suggested that the ligand for IgM on the iRBC is the trypsin sensitive PfEMP-1 surface antigen (10, 16). PfEMP-1 proteins, particularly certain DBLγ domains of these molecules, are also proposed to be the CSA-binding ligands of iRBC (7, 13, 25). To determine whether PfEMP-1 is involved in IgM binding to CSA-selected parasites, the protease sensitivity of IgM and CSA-binding was analyzed. With the parasite line SD202CSA, we found that both IgM binding and CSA binding could be abolished by treatment of iRBC with low concentrations (1 μg/ml) of trypsin (Fig. 2). Our results are consistent with, though not proof of, the hypothesis that PfEMP-1 can mediate both IgM and CSA binding. The concentration of trypsin required to abolish CSA binding in this parasite was somewhat lower than in some other reports (5, 19). This might be due to the use of different forms of CSA, in selection and binding assays, between the various studies. It could also mean that antigenically and presumably conformationally distinct parasite variants may have different trypsin sensitivities.
FIG. 2.
Abolition of IgM-binding capacity and CSA-binding capacity of parasites of P. falciparum line SD2O2CSA upon incubation with increasing concentrations of trypsin protease. Data from a representative experiment are shown. Binding to negative control (BSA) spots was less than 5 iRBC per 10 high-power fields. Two further experiments gave consistent results.
Among rosetting parasites, it has been suggested that a function of nonspecific IgM binding is to enhance interactions between infected and uninfected cells in the host bloodstream, for example, via IgM binding to complement receptors (11, 33, 37, 39, 43). Placental IgM binding could function in a similar way, perhaps through binding to other surface receptors in addition to CSA. An Fc receptor for human IgM has recently been described (38), although it is not yet known whether this receptor is present on the placenta. Adhesion to the multimeric 900-kDa IgM molecules could also permit multiple interactions with the large, free, CSA-rich syncytial ‘knots’ abundant in the placental circulation (1, 44). Cross-linking with syncytial knots might inhibit clearance of such large bodies through the venular exits from the placenta, confining large numbers of mature parasites to the placental environment.
Study of the mechanisms of placental adhesion, together with identification of the parasite antigens targeted by protective antibodies, is urgently needed to elucidate the pathology of placental malaria and ultimately allow protective immunity to be induced in women prior to their first pregnancy.
Acknowledgments
We are grateful for the donation of parasite line Gb337 from Mo Klinkert and Ayman Khattab, Tubingen University and TM284S2 from Mats Wahlgren, Karolinska Institute, Stockholm. The parasite line FCR3CSA, obtained from ATTC, was originally deposited by Artur Scherf, Pasteur Institute, Paris. We also thank Rick Maizels, University of Edinburgh, for the gift of several mouse MAbs.
This work was funded by Wellcome Trust Senior Fellowship grant 067431 to J.A.R. and a Wellcome Trust University Fellowship and the Commission of the European Union, through grant QLK2-CT-2001-01302 (PAMVAC), to D.E.A.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Achur, R. N., M. Valiyaveettil, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Baruch, D. I., X. C. Ma, H. B. Singh, X. Bi, B. L. Pasloske, and R. J. Howard. 1997. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood 90:3766-3775. [PubMed] [Google Scholar]
- 3.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 4.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeson, J. G., S. J. Rogerson, B. M. Cooke, J. C. Reeder W. Chai, A. M. Lawson, M. E. Molyneaux, and G. V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beeson, J. G., J. C. Reeder, S. J. Rogerson, and G. V. Brown. 2001. Parasite adhesion and immune evasion in placental malaria. Trends Parasitol. 17:331-337. [DOI] [PubMed] [Google Scholar]
- 7.Buffet, P. A., B. Gamain, C. Scheidig, D. Baruch, J. D. Smith, R. Hernandez-Rivas, B. Pouvelle, S. Oishi, N. Fujii, T. Fusai, D. Parzy, L. H. Miller, J. Gysin, and A. Scherf. 1999. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl. Acad. Sci. USA 96:12743-12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson, J., H. Helmby, A. V. Hill, D. Brewster, B. M. Greenwood, and M. Wahlgren. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336:1457-1460. [DOI] [PubMed] [Google Scholar]
- 9.Chaiyaroj, S. C., P. Angkasekwinai, A. Buranakiti, S. Looareesuwan, S. J. Rogerson, and G. V. Brown. 1996. Cytoadherence characteristics of Plasmodium falciparum isolates from Thailand: evidence for chondroitin sulfate A as a cytoadherence receptor. Am. J. Trop. Med. Hyg. 55:76-80. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Q., A. Heddini, A. Barragan, V. Fernandez, S. F. Pearce, and M. Wahlgren. 2000. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 192:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clough, B., F. A. Atilola, J. Black, and G. Pasvol. 1998. Plasmodium falciparum: the importance of IgM in the rosetting of parasite-infected erythrocytes. Exp. Parasitol. 89:129-132. [DOI] [PubMed] [Google Scholar]
- 12.Crandall, I., N. Guthrie, and I. W. Sherman. 1996. Plasmodium falciparum: naturally occurring rabbit immunoglobulins recognize human band 3 peptide motifs and malaria-infected red cells. Exp. Parasitol. 82:45-53. [DOI] [PubMed] [Google Scholar]
- 13.Degen, R., N. Weiss, and H.-P. Beck. 2000. Plasmodium falciparum: cloned and expressed CIDR domains of PfEMP1 bind to chondriotin sulfate A. Exp. Parasitol. 95:113-121. [DOI] [PubMed] [Google Scholar]
- 14.Echaide, I. E., S. A. Hines, T. F. McElwain, C. E. Suarez, T. C. McGuire, and G. H. Palmer. 1998. In vivo binding of immunoglobulin M to the surfaces of Babesia bigemina-infected erythrocytes. Infect. Immun. 66:2922-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards, J. M., J. J. Moulds, and W. J. Judd. 1982. Chloroquine dissociation of antigen-antibody complexes. A new technique for typing red blood cells with a positive direct antiglobulin test. Transfusion 22:59-61. [DOI] [PubMed] [Google Scholar]
- 16.Flick, K., C. Scholander, Q. Chen, V. Fernandez, B. Pouvelle, J. Gysin, and M. Wahlgren. 2001. Role of nonimmune IgG bound to PfEMP1 in placental malaria. Science 293:2098-2100. [DOI] [PubMed] [Google Scholar]
- 17.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 18.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 19.Fried, M., R. M. Lauder, and P. E. Duffy. 2000. Plasmodium falciparum: adhesion of placental isolates modulated by the sulfation characteristics of the glycosaminoglycan receptor. Exp. Parasitol. 95:75-78. [DOI] [PubMed] [Google Scholar]
- 20.Fried, M., and P. E. Duffy. 2002. Two DBLγ subtypes are commonly expressed by placental isolates of Plasmodium falciparum. Mol. Biochem. Parsitol. 122:201-210. [DOI] [PubMed] [Google Scholar]
- 21.Ho, M., and N. J. White. 1999. Molecular mechanisms of cytoadherence in malaria. Am. J. Physiol. 276:C1231-C1242. [DOI] [PubMed] [Google Scholar]
- 22.Lekana Douki, J. B., B. Traore, F. T. Costa, T. Fusai, B. Pouvelle, Y. Sterkers, A. Scherf, and J. Gysin. 2002. Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood 100:1478-1483. [DOI] [PubMed] [Google Scholar]
- 23.Maubert, B., N. Fievet, G. Tami, C. Boudin, and P. Deloron. 1998. Plasmodium falciparum isolates from Cameroonian pregnant women do not rosette. Parasite 5:281-283. [DOI] [PubMed] [Google Scholar]
- 24.Page, A. P., A. J. Hamilton, and R. M. Maizels. 1992. Toxocara canis: monoclonal antibodies to carbohydrate epitopes of secreted (TES) antigens localize to different secretion-related structures in infective larvae. Exp. Parasitol. 75:56-71. [DOI] [PubMed] [Google Scholar]
- 25.Reeder, J. C., A. F. Cowman, K. M. Davern, J. G. Beeson, J. K. Thompson, S. J. Rogerson, and G. V. Brown. 1999. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl. Acad. Sci. USA 96:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 27.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogerson, S. J., J. G. Beeson, C. G. Mhango, F. K. Dzinjalamala, and M. E. Molyneux. 2000. Plasmodium falciparum rosette formation is uncommon in isolates from pregnant women. Infect. Immun. 68:391-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe, A., A. R. Berendt, K. Marsh, and C. I. Newbold. 1994. Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocyte rosettes. Exp. Parasitol. 79:506-516. [DOI] [PubMed] [Google Scholar]
- 30.Rowe, A., J. Obeiro, C. I. Newbold, and K. Marsh. 1995. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect. Immun. 63:2323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe, J. A., J. M. Moulds, C. I. Newbold, and L. H. Miller. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388:292-295. [DOI] [PubMed] [Google Scholar]
- 32.Rowe, J. A., S. A. Kyes, S. J. Rogerson, H. A. Babiker, and A. Raza. 2002. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J. Infect. Dis. 185:1207-1211. [DOI] [PubMed] [Google Scholar]
- 33.Rowe, J. A., J. Shafi, O. K. Kai, K. Marsh, and A. Raza. 2002. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am. J. Trop. Med. Hyg. 66:692-699. [DOI] [PubMed] [Google Scholar]
- 34.Salanti, A., A. T. Jensen, H. D. Zornig, T. Staalsoe, L. Joergensen, M. A. Nielsen, A. Khattab, D. E. Arnot, M. Q. Klinkert, L. Hviid, and T. G. Theander. 2002. A sub-family of common and highly conserved Plasmodium falciparum var genes. Mol. Biochem. Parasitol 122:111-115. [DOI] [PubMed] [Google Scholar]
- 35.Saul, A., W. L. Maloy, E. P. Rock, and R. J. Howard. 1998. A portion of the Pf155/RESA antigen of Plasmodium falciparum is accessible on the surface of infected erythrocytes. Immunol. Cell Biol. 66:269-276. [DOI] [PubMed] [Google Scholar]
- 36.Scherf, A., R. Hernandez-Rivas, P. Buffet, E. Bottius, C. Benatar, B. Pouvelle, J. Gysin, and M. Lanzer. 1998. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 17:5418-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholander, C., C. J. Treutiger, K. Hultenby, and M. Wahlgren. 1996. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat. Med. 2:204-208. [DOI] [PubMed] [Google Scholar]
- 38.Shibuya, A., N. Sakamoto, Y. Shimizu, K. Shibuya, M. Osawa, T. Hiroyama, H. J. Eyre, G. R. Sutherland, Y. Endo, T. Fujita, T. Miyabayashi, S. Sakano, T. Tsuji, E. Nakayama, J. H. Phillips, L. L. Lainer, and H. Nakauchi. 2000. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 1:441-446. [DOI] [PubMed] [Google Scholar]
- 39.Somner, E. A., J. Black, and G. Pasvol. 2000. Multiple human serum components act as bridging molecules in rosette formation by Plasmodium falciparum-infected erythrocytes. Blood 95:674-682. [PubMed] [Google Scholar]
- 40.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 41.Staalsoe, T., R. Megnekou, N. Fievet, C. H. Ricke, H. D. Zornig, R. Leke, D. W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 42.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 43.Treutiger, C. J., C. Scholander, J. Carlson, K. P. McAdam, J. G. Raynes, L. Falksveden, and M. Wahlgren. 1999. Rouleaux-forming serum proteins are involved in the rosetting of Plasmodium falciparum-infected erythrocytes. Exp. Parasitol. 93:215-224. [DOI] [PubMed] [Google Scholar]
- 44.Yamada, M., R. Steketee, C. Abramowsky, M. Kida, J. Wirima, D. Heymann, J. Rabbege, J. Breman, and M. Aikawa. 1989. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am. J. Trop. Med. Hyg. 41:161-168. [DOI] [PubMed] [Google Scholar]