Abstract
Streptococcus pneumoniae is a common cause of respiratory tract infections, its main entry route being the nasal mucosa. The recent development of pneumococcal polysaccharide conjugate vaccines has led to a dramatic improvement in protection against invasive disease in infants and children, but these vaccines have been found to be only 50 to 60% protective against bacterial carriage. In this study, we investigated the efficacy of intranasal (i.n.) conjugate vaccine delivery using interleukin-12 (IL-12) as a mucosal adjuvant. Immunized mice treated with IL-12 demonstrated increased expression of lung and splenic gamma interferon and IL-10 mRNAs; high levels of antibody, particularly serum immunoglobulin G2a (IgG2a) and respiratory IgA; and significantly increased opsonic activity. After intraperitoneal challenge with type 3 pneumococci, there was 75% survival of i.n. vaccinated mice compared to 0% survival of unvaccinated mice. In addition, after i.n. challenge with type 14 pneumococci, vaccinated mice possessed fewer bacterial colonies in the upper respiratory tract than unvaccinated mice. However, no significant difference in type 14 carriage was observed between vaccinated and unvaccinated groups following intramuscular vaccination, the typical route of vaccination in humans. Using mice with a genetic disruption in IgA expression, it was found that pneumococcus-specific IgA played a significant role in the clearance of bacteria from the upper respiratory tract. We conclude that i.n vaccination in the presence of IL-12 is able to enhance systemic and mucosal immune responses to pneumococci and efficiently protect against both invasive infection and bacterial carriage.
Streptococcus pneumoniae colonizes the human nasopharynx and is a common etiologic agent of respiratory tract infection. In addition, infection with the pneumococcus frequently results in bacteremia and sepsis because of its capacity to invade the bloodstream (19, 30). Consequently, vaccination strategies against these pathogens, whose main entry route is the mucosal layer, need to involve both systemic and mucosal immune responses. The recently introduced pneumococcal conjugate vaccine, which is administered via the intramuscular (i.m.) route, effectively stimulates systemic immunity but is only partially effective against nasal colonization (10, 23). Administering the vaccine via the intranasal (i.n.) route could offer several advantages: (i) it would stimulate both mucosal and systemic immunity and thus offer effective protection against both invasive disease and nasal carriage, (ii) it would be easily administered, and (iii) it would be noninvasive and thus avoid the use of needles and the associated risks of transmitting hepatitis B and human immunodeficiency virus infection.
Host protection against S. pneumoniae is mediated mainly by opsonin-dependent phagocytosis, and the opsonic activities of antibodies to pneumococcal capsular polysaccharides are believed to correlate with protection (1). It has been demonstrated (5, 7, 13, 32) that treatment of mice with interleukin-12 (IL-12) during vaccination with model T-independent and T-dependent antigens significantly enhances protective antibody production against a variety of pathogens. The present study was designed to compare the protective efficacies of pneumococcal conjugate vaccine in mice following i.n. and i.m. vaccination in the presence of IL-12. Th1/Th2 type cytokine expression, serum and respiratory antibody production, and protection against systemic disease and nasal carriage were examined to determine whether i.n. vaccination would lead to augmented protection.
MATERIALS AND METHODS
Mice and immunization.
BALB/cAnNCr mice, 4 to 6 weeks old, were purchased from Charles River Laboratories (Raleigh, N.C.) through a contract with the National Cancer Institute (Bethesda, Md.) and maintained at the Albany Medical College. (B6 × 129)F1 immunoglobulin A (IgA) knockout (IgA−/−) mice (generated by deletion of the entire α Ig heavy-chain switch region and the 5′ half of the constant region) (15) were bred at Albany Medical College, and wild-type control mice were purchased from Taconic Farms Inc., Germantown, N.Y. The mice were inoculated i.n. with 1 μg of conjugate vaccine (type 3 or type 14; Wyeth Vaccines, Pearl River, N.Y.) on day 0 and i.n. with 1 μg of IL-12 (Genetics Institute, Cambridge, Mass.) on days 0, 1, 2, and 3. The conjugates consisted of pneumococcal polysaccharide (PPS) covalently linked to CRM197, a mutated diphtheria toxin (type 3 [PPS3] was conjugated to 0.256 mg of CRM197/ml, and type 14 [PPS14] was conjugated to 0.468 mg of CRM197/ml). The preparations were given in phosphate-buffered saline (PBS) containing 1% normal mouse serum (PBS-NMS), while control mice received PBS-NMS vehicle only. For some experiments, mice were boosted i.n. on day 28 with 5 μg of PPS3 (American Type Culture Collection, Manassas, Va.) prepared in PBS-NMS. For i.m. immunization, the vaccines were mixed with 2 mg of alum (Rehydrogel Low Viscosity Gel; Reheis Inc,, Berkeley Heights, N.J.)/ml and given together with 1 μg of IL-12 i.m. on day zero. Further i.m. treatments with IL-12 in PBS-NMS were performed on days 1, 2, and 3. The mice were boosted i.m. with PPS as described above. Sera were obtained by bleeding the mice from the orbital plexus.
Collection of bronchoalveolar lavage (BAL) fluid.
For collection of BAL fluid, the tracheas of euthanized mice were intubated using a 0.58-mm (outside diameter) polyethylene catheter (Becton Dickinson, Sparks, Md.). The lungs were then lavaged two or three times with PBS containing 5 mM EDTA. The recovered BAL fluids were centrifuged at 350 × g for 5 min at 4°C, and the supernatants were stored at −70°C until they were used.
Bacterial strains, growth conditions, and growth media.
S. pneumoniae strain A66.1, which expresses PPS3, and strain TJO983, which expresses PPS14, were grown overnight on blood agar plates and cultured at 37°C in Todd-Hewitt broth supplemented with 0.5% yeast extract. The identities of the pneumococci were confirmed by colony morphology on blood agar plates and by sensitivity to optochin (Sigma, St. Louis, Mo.). Bacteria were harvested by centrifugation and washed twice in sterile PBS. The bacteria were resuspended in Todd-Hewitt broth containing 0.5% yeast extract and 15% glycerol and stored in aliquots at −70°C.
Cytokine RT-PCR.
Total RNA isolation from snap-frozen spleens and lungs was performed with the Trizol reagent kit (Life Technologies, Gaithersburg, Md.) according to the manufacturer's instructions. Briefly, the frozen tissues were homogenized with a mortar and pestle and immediately transferred to tubes containing 1 ml of Trizol reagent per 50 to 100 mg of tissue. After chloroform extraction, the homogenized samples were centrifuged at 11,400 × g for 15 min at 4°C. The aqueous phase containing the RNA was precipitated with isopropanol, washed with 75% ethanol, and dissolved in diethylpyrocarbonate-treated water. Two micrograms of total RNA was reverse transcribed into cDNA using a reverse transcription (RT) kit (Life Technologies) and oligo(dT)16-18 primers. The cDNA was amplified using primers specific for gamma interferon (IFN-γ), IL-5, and IL-10, with hypoxanthine phosphoribosyl transferase (HPRT) serving as a housekeeping control for nucleic acid loading. The sense and antisense primers had the following sequences: IFN-γ, 5′-TGAACGCTACACACTGCATCTTGG-3′ and 5′-GACTCCTTTTCCGCTTCCTGAG-3′; IL-10, 5′-ATGCAGGACTTTAAGGGTTACTTGGGTT-3′ and 5′-ATTTCGGAGAGAGGTACAAACGAGGTTT-3′; IL-5, 5′-GACAAGCAATGAGACGATGAG-3′ and 5′-GTTATCCTTGGCTACATTACC-3′; and HPRT, 5′-GTTGATACAGGCCAGACTTTGTTG-3′ and 5′-GATTCAACTTGCGCTCATCTAGGC-3′. PCRamplification was performed by mixing 2 μl of cDNA, 0.25 mM deoxynucleoside triphosphates (Invitrogen, San Diego, Calif.), 0.8 μM primer, and 2.5 U of Taq DNA polymerase (Life Technologies) in a final volume of 50 μl in 60 mM Tris-HCl, pH 8.5, 15 mM (NH4)2SO4, and 0.4 mM MgCl2. The mixtures were incubated at 95°C for 5 min and then subjected to the following amplification profile: 1 min at 95°C, 1 min at 56°C, and 1 min at 72°C for 35 cycles. This was followed by a final extension for 10 min at 72°C. The PCR products were separated on a 2.5% agarose gel, stained with ethidium bromide, and visualized by UV transillumination.
Measurement of antibody levels by enzyme-linked immunosorbent assay (ELISA).
To assess antibody responses to type 14 and type 3 pneumococcal conjugate vaccines, 96-well Nunc-Immuno Polysorp plates (Krackler Scientific, Albany, N.Y.) were coated overnight at 4°C with 15 μg of PPS14 or PPS3/ml in 0.05 M carbonate-bicarbonate buffer, pH 9.6 (Sigma). After being washed with PBS containing 0.05% Tween 20 and 0.25% gelatin, the plates were blocked for 2 h with PBS containing 1% gelatin and then incubated at 4°C overnight with serial twofold dilutions of mouse sera or BAL in PBS containing 0.05% Tween 20 and 0.25% gelatin. After the plates were washed, 50 μl of alkaline phosphatase-conjugated goat anti-mouse antibody reactive with whole immunoglobulin or specific isotypes (Southern Biotechnology, Birmingham, Ala.) were added at room temperature for 2 h. Bound enzyme was detected by adding p-nitrophenyl substrate and measuring absorbance at 405 nm with a microplate reader (Bio-Tek Instruments, Winooski, Vt.).
Opsonophagocytosis assay.
Type 3 pneumococci were grown in Todd-Hewitt broth overnight, washed, and incubated at 56°C for 30 min (to inactivate pneumococcal autolysins). The pneumococci (1.5 × 109 CFU) were mixed with 1 mg of Lucifer Yellow (LY) dye (Sigma)/ml in 0.1 M sodium bicarbonate (pH 9.5) for 2 h at room temperature with vortexing every 30 min. The labeled bacteria were subsequently washed, resuspended in sterile PBS, and stored at −20°C. An opsonophagocytosis assay was then performed as previously described (3). Briefly, 106 bacteria were incubated with heat-inactivated immune serum and 2% guinea pig complement (Rockland, Gilbertsville, Pa.) in a round-bottom microtiter plate for 15 min at 37°C with shaking by means of a microtiter plate agitator. The opsonized bacteria were then incubated for an additional 30 min at 37°C with the J774A.1 macrophage cell line (2 × 105 J774A.1 cells/well; American Type Culture Collection). Cells containing bacteria were washed by differential centrifugation to remove unbound bacteria and then resuspended in 50 μl of 0.2-mg/ml trypan blue to quench membrane-bound bacteria. The reaction mixtures were analyzed by flow cytometry using a Becton Dickinson FACScan, and the mean fluorescence intensity of macrophages containing fluorescent bacteria was used as a measure of phagocytic activity.
Confocal microscopy.
Confocal microscopy was performed to ensure that opsonized bacteria were actually internalized and not simply bound to the macrophage cell surface membrane. Following incubation with LY-labeled bacteria, J774A.1 cells were washed with PBS to remove unbound bacteria. The cells were then incubated with a lipophilic carbocyanine dye, SP-DilC18, for 5 min at room temperature to stain the cell membranes. The cells were washed with PBS, and images of optical sections, taken at 0.4-μm intervals in the z direction, were collected on a Nikon (Melville, N.Y.) Diaphot inverted microscope attached to a Noran-Oz laser scanning confocal microscope system (Noran Instruments, Middleton, Wis.). LY staining was detected using a 488-nm-wavelength laser for excitation and a 500 to 550-nm-wavelength bandpass filter for emission. SP-DilC18 was detected using a 568 nm-wavelength laser for excitation and a 590 nm-wavelength longpass filter for emission. The images were analyzed using Noran InterVision acquisition and 2D analysis software.
Survival studies and pneumococcal carriage.
For survival studies, groups of 4- to 6-week-old BALB/c mice (eight mice/group) were vaccinated i.n. or i.m. with type 3 pneumococcal conjugate vaccine on day 0 together with PBS-NMS or IL-12 on days 0, 1, 2, and 3. Five weeks later, the mice were challenged intraperitoneally (i.p.) with type 3 pneumococcal bacteria (5 × 104 CFU/mouse in 100 μl of PBS) and monitored daily for survival. Bacteremia was assessed by plating samples of blood and spleen homogenates on blood agar plates, and pneumococcal disease was confirmed by testing for sensitivity to optochin.
For carriage studies, groups of 4- to 6-week-old BALB/c or (B6 × 129)F1 mice (six mice/group) were vaccinated i.n. or i.m. with type 14 pneumococcal conjugate vaccine on day 0 together with PBS-NMS or IL-12 on days 0, 1, 2, and 3. Fourteen days later, the mice were challenged i.n. with type 14 pneumococcal bacteria (107 CFU/mouse in 10 μl of Ringer's solution). Seventy-two hours after infection, the mice were sacrificed, 100 μl of Ringer's solution was injected into their tracheas, and 50 μl was collected from the tips of the noses. Serial dilutions were then plated onto blood agar plates containing gentamicin alone or gentamicin plus optochin to determine nasal colonization.
Statistical analysis.
All experiments were performed a minimum of two times. The opsonophagocytosis assay was performed three times. For comparison between groups, the Mann-Whitney rank sum test was used. A P value of <0.05 was considered statistically significant.
RESULTS
Enhancement of splenic and lung IFN-γ and IL-10 expression by i.n. vaccination in the presence of IL-12.
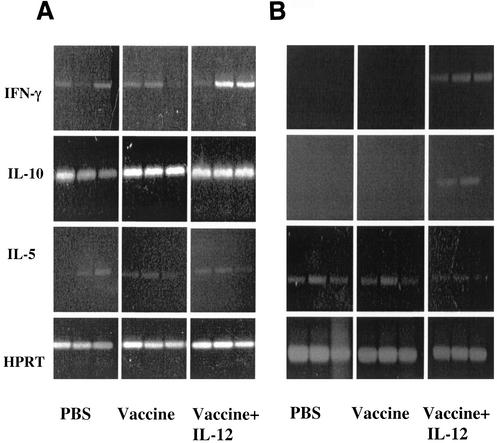
IL-12 is a regulatory cytokine that activates Th1 and NK cells to induce IFN-γ production (12, 26). To determine the effects of i.n. administration of vaccine and IL-12, cytokine gene expression was examined in the spleens (Fig. 1A) and lungs (Fig. 1B) of BALB/c mice 48 h after immunization. The 48-h time point has been shown to yield optimal cytokine expression after IL-12 expression, and the cycle number used was optimal to obtain linearity (3-6). Mice that received vehicle or vaccine alone demonstrated low expression of splenic IFN-γ mRNA that was enhanced in immunized mice treated with IL-12. In the lungs, IFN-γ mRNA was not expressed in mice that received vehicle or vaccine alone. However, IFN-γ expression was clearly observed in immunized mice treated with IL-12. IL-10 was found to be expressed in the spleen but was only expressed in the lungs of mice treated with IL-12 (Fig. 1B). There was no difference between the levels of expression of IL-5 mRNA in the spleens or lungs of any of the mice studied. Simultaneous amplification of HPRT cDNA confirmed that equal amounts of nucleic acid were used in all RT-PCR assays. Previous studies in our laboratory demonstrated similar effects on splenic cytokine expression after i.p. administration of pneumococcal vaccine with or without IL-12 (8), as well as induction of cytokine expression after administration of IL-12 alone.
FIG. 1.
Expression of IFN-γ, IL-10, and IL-5 in the spleens (A) and lungs (B) of BALB/c mice. The mice were immunized i.n. with type 3 pneumococcal conjugate vaccine plus IL-12 or PBS vehicle. Control mice received PBS vehicle only. The mice were sacrificed 48 h after treatment, total RNA was isolated from individual spleen and lungs, and expression of IFN-γ, IL-10, and IL-5 was analyzed by RT-PCR. HPRT was used as a housekeeping control.
Enhancement of systemic and respiratory antibody levels by i.n. vaccination in the presence of IL-12.
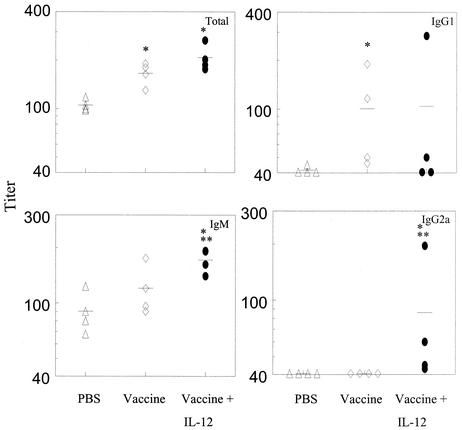
The levels of anti-PPS3 antibodies in mice primed i.n. with pneumococcal conjugate vaccine were measured in serum and BAL 14 days after i.n. boosting with PPS3. Since capsular polysaccharide is the primary target of protective antibody responses, the goal was to characterize antibody induction after boosting with free polysaccharide as a mimic of live bacterial challenge. Significant levels of IgM and IgG2a antibodies were observed in the sera of immunized mice treated with IL-12, which were higher than those of mice that received vaccine alone (Fig. 2). However, levels of total and IgG1 antibodies were not significantly different in the two groups of mice. Small amounts of IgG3 were detected in i.n. immunized mice treated with IL-12 but not in mice that received vaccine only (data not shown). Pneumococcus-specific IgA levels were not detected in the sera of any vaccinated mice.
FIG. 2.
Levels of anti-pneumococcal capsular polysaccharide serum antibodies in immunized mice treated with IL-12 or PBS vehicle or mice primed with PBS vehicle only. Mice were inoculated i.n. with type 3 pneumococcal conjugate vaccine on day 0 and treated i.n. with IL-12 or PBS vehicle on days 0, 1, 2, and 3. All mice were injected i.p. with PPS3 on day 28. Total, IgM, IgG1, and IgG2a anti-pneumococcal antibody levels were assayed by ELISA 14 days after PPS3 injection. Serum titers for individual mice (four mice per group) are shown, and mean titers for the groups are indicated by the bars. Levels of total antibody were significantly different (*, P < 0.05) between the vaccinated groups and the PBS group. However, no difference in levels of total antibody was observed between the vaccinated group treated with IL-12 and the group that was primed with vaccine alone. Levels of IgG1 in the vaccinated groups were significantly different (*, P < 0.05) from those in the PBS group. IgM and IgG2a levels were significantly higher in the vaccinated group treated with IL-12 than in the mice primed with PBS only (*, P < 0.05) or vaccine only (**, P < 0.05).
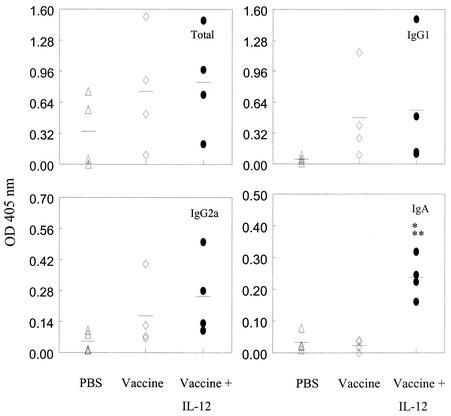
In BAL fluids, increased levels of IgA PPS3-specific antibody levels were detected in vaccinated mice treated with IL-12 (Fig. 3). Antibody levels in the BAL fluid tended to be lower than those present in serum, partly because the lavage process diluted the antibodies in the respiratory fluid. Thus, optical density values are shown rather than titers. Mice receiving IL-12 alone in the absence of vaccine behaved like unvaccinated mice, i.e., there was no induction of serum or BAL pneumococcal antibody in mice exposed only to IL-12.
FIG. 3.
Mucosal antibody levels in BALB/c mice immunized with type 3 pneumococcal conjugate vaccine plus IL-12 or PBS vehicle. The mice were immunized i.n. as described in the legend to Fig. 2 and sacrificed 14 days after i.p. injection with PPS3, and BAL fluid was assayed by ELISA for anti-PPS3 antibody (total, IgG1, IgG2a, and IgA). Optical density (OD) values at 405 nm for individual mice (four mice per group) are shown, and the mean values for the groups are indicated by the bars. IgA levels were significantly enhanced in vaccinated mice treated with IL-12 compared to mice that were primed with PBS vehicle (*, P < 0.05) or vaccine alone (**, P < 0.05).
Increased opsonophagocytosis activity induced by i.n. vaccine plus IL-12.
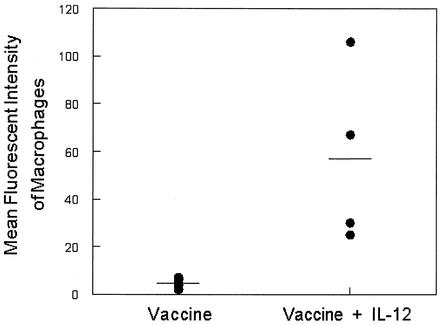
The primary mechanism of clearance of the pneumococcus is opsonophagocytosis (2). As shown above, i.n. vaccination in the presence of IL-12 effectively enhanced antibody production in both serum and the respiratory tract. We thus analyzed the opsonic activities of sera from the immunized mice using an in vitro opsonophagocytosis assay. The assay was performed by opsonizing fluorescent type 3 S. pneumoniae with heat-treated immune serum, followed by incubation with J774A.1 mouse macrophage cells. Flow cytometry analysis showed that serum from immunized mice treated with IL-12 mediated more efficient opsonophagocytosis of S. pneumoniae than serum from mice that received vaccine alone (Fig. 4). The increased opsonic activity observed in immunized mice treated with IL-12 was also demonstrated by confocal microscopy (Fig. 5A), which confirmed that the labeled bacteria were in fact internalized by the macrophage cell line. Serum from mice that received vaccine alone (Fig. 5B) or IL-12 alone (data not shown) failed to internalize the labeled bacteria. Although total antibody levels were not significantly different in the vaccinated groups, IgG2a titers were significantly enhanced in the IL-12-treated group, and this most likely explains the increased opsonophagocytic activity.
FIG. 4.
Opsonic activities of sera from mice immunized i.n. with type 3 pneumococcal conjugate vaccine plus IL-12 or PBS vehicle and boosted 28 days later with PPS3. Opsonic activities of antisera were determined by incubating LY-labeled pneumococci in the presence of antiserum, guinea pig complement, and J774A.1 macrophages. After phagocytosis, unbound bacteria were removed by washing, and any surface-bound fluorescence was quenched with trypan blue. The mean fluorescence intensity values of macrophages incubated with immune sera from individual mice (four mice per group) are shown by the symbols, and the mean values for the groups are indicated by the bars. The low values obtained after the incubation of cells with NMS have been subtracted. The mean fluorescence intensity obtained with sera from vaccinated mice treated with IL-12 was significantly higher (P < 0.05) than the mean fluorescence intensity obtained with sera from mice that received vaccine alone.
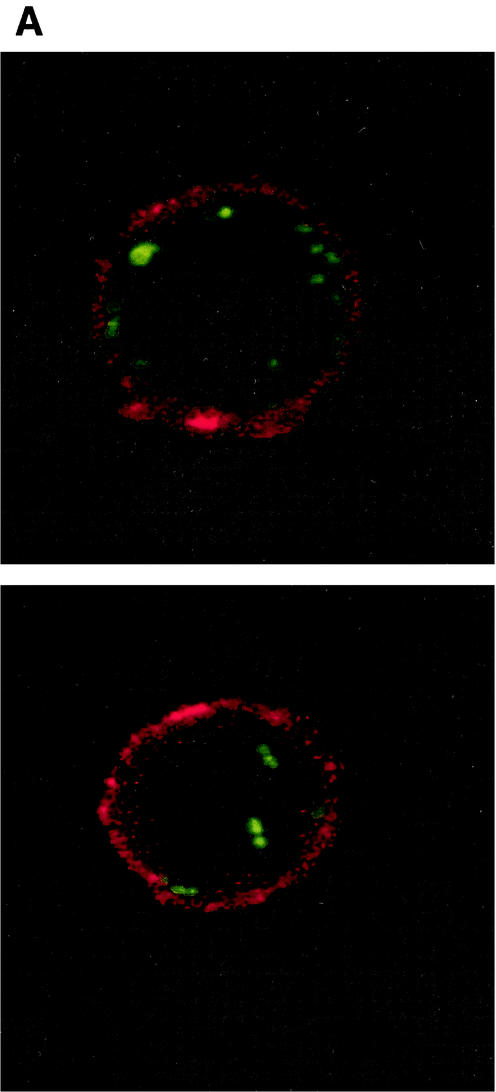
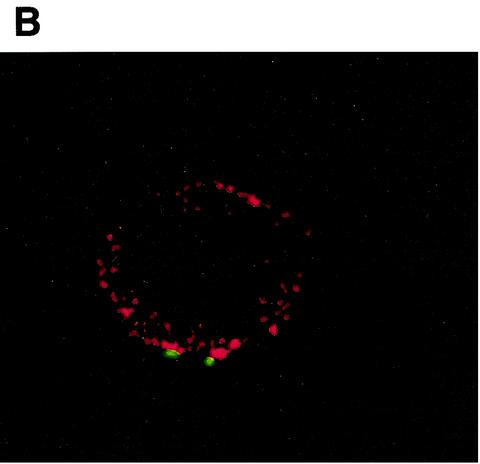
FIG. 5.
Opsonization of bacteria by J774A.1 cells determined by confocal microscopy. Confocal optical sections taken from a 51-step Z series with 0.4-μm steps showing internalization of bacteria in macrophages after incubation in sera from immunized mice treated with IL-12 (A) versus sera from mice that received vaccine alone (B).
Increased survival against invasive S. pneumoniae type 3 infection induced by i.n. vaccine plus IL-12.
Our laboratory has recently shown that intraperitoneal administration of polysaccharide conjugate vaccines and IL-12 induces protection in mice against invasive disease caused by S. pneumoniae (8). In the present study, we compared the level of protection achieved by immunization with the pneumococcal vaccine conjugate and IL-12 by the i.n. and i.m. routes. Alum was also used as an adjuvant for i.m. immunization. On day 28 after vaccination by either route, mice were challenged i.p. with type 3 S. pneumoniae and monitored for survival.
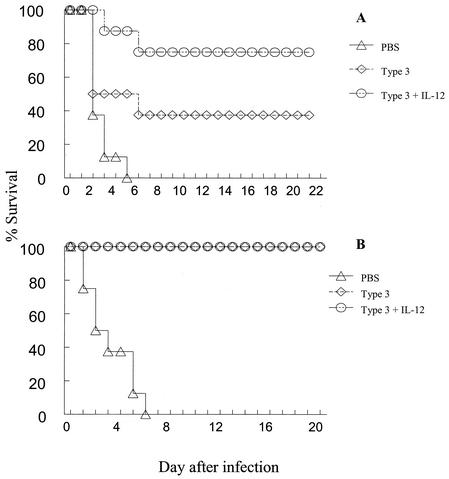
The results showed that 75% of mice immunized i.n. with the vaccine and IL-12 survived invasive infection compared to only 37.5% in the group that received type 3 conjugate vaccine plus PBS vehicle (P < 0.05) (Fig. 6A). Thus, the use of IL-12 as a mucosal vaccine adjuvant significantly increased survival, and hence systemic protection, after i.p. challenge with S. pneumoniae. In mice immunized i.m. with type 3 conjugate vaccine in alum, either alone or together with IL-12, there was 100% survival (Fig. 6B). All mice immunized i.m. with alum produced significantly higher levels of antibody to PPS3 than mice immunized with soluble antigen i.n. (approximately fourfold-higher mean antibody titers in serum were present in i.m. versus i.n. vaccinated mice). This observation likely explains the greater systemic protection observed after i.m. versus i.n. immunization.
FIG. 6.
Survival of mice after challenge i.p. with type 3 pneumococci. BALB/c mice (eight per group) were immunized i.n. (A) or i.m. (B) with type 3 pneumococcal conjugate vaccine on day 0 plus IL-12 or PBS vehicle on days 0, 1, 2, and 3. Eight mice received PBS vehicle only. Twenty-eight days after treatment, the mice were challenged i.p. with type 3 pneumococci (5 × 105 CFU per mouse). The mice were monitored daily for survival. The survival rate of the group of mice vaccinated i.n. plus IL-12 was significantly greater than the group that received vaccine alone (P < 0.05).
Reduction in pneumococcal colonization by i.n. vaccine plus IL-12.
The effect of i.n. vaccination on nasopharyngeal carriage of type 14 pneumococci was next examined in groups of mice primed with type 14 pneumococcal conjugate vaccine. Mice were challenged i.n. with type 14 pneumococcal bacteria (107 CFU/mouse) 28 days after vaccination, and nasal colonization was determined 72 h after infection. The results showed that fewer pneumococci were recovered from mice immunized with type 14 pneumococcal conjugate vaccine than from mice that received PBS vehicle alone (threefold reduction) (Fig. 7). Thus, i.n. vaccination is able to significantly modify the mucosal immune response and cause efficient clearance of S. pneumoniae from the nasopharynx.
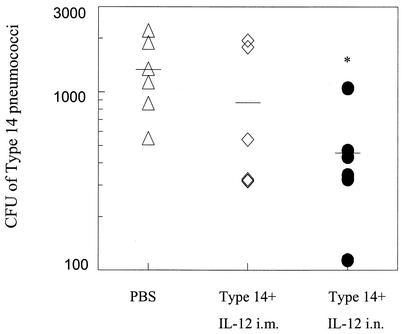
FIG. 7.
Clearance of pneumococci from the upper respiratory tract after i.n. challenge with type 14 pneumococci. BALB/c mice (six per group) were immunized i.n. or i.m. with type 14 conjugate vaccine on day 0 plus IL-12 or PBS vehicle on days 0, 1, 2, and 3. The mice were challenged i.n. 72 h later with 107 CFU of pneumococci, and nasal washes were performed to determine the number of pneumococci present. Statistical significance was achieved in mice vaccinated i.n. compared to unvaccinated mice (*, P < 0.05). No difference was observed between mice vaccinated i.m. and unvaccinated mice. The data shown are representative of two experiments. The mean values for the groups are indicated by the bars.
The effect of vaccination on carriage was also determined following i.m. immunization with type 14 vaccine conjugate. It was found that although mice immunized i.m. generated higher titers of pneumococcus-specific antibodies in their sera than mice immunized i.n., there was only minimal reduction in carriage (approximately one-third reduction in CFU in i.m. immunized compared to unimmunized mice) (Fig. 7). In addition, only neglible of amounts of antibody restricted to the IgM isotype were detected in the BAL fluids of i.m. immunized mice (data not shown). There was no difference in carriage between normal unvaccinated mice and unvaccinated mice treated with IL-12 only (data not shown). These results show that i.n. vaccination in the presence of IL-12 is effective at reducing colonization of bacteria expressing the vaccine serotype. Mice given vaccine alone (i.n. or i.m) in the absence of IL-12 showed no differences from unvaccinated mice in the ability to clear pneumococci from the upper respiratory tract.
Clearance of pneumococci from the upper respiratory tract is mediated by IgA.
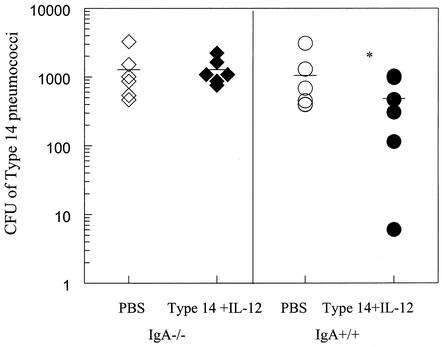
As shown above, respiratory IgA levels and protection against carriage were increased after immunization in the presence of IL-12. Since IgA is the predominant antibody isotype at mucosal surfaces, it was important to determine the contribution of this isotype to clearance of pneumococci from the upper respiratory tract. IgA+/+ and IgA−/− mice were immunized i.n. with type 14 conjugate vaccine plus IL-12 and challenged i.n. 2 weeks later with type 14 pneumococci. Examination of nasal washes showed that IgA−/− mice failed to clear bacteria as efficiently as IgA+/+ mice (Fig. 8). In fact, there was no difference in carriage between immunized and unimmunized IgA−/− mice. This study demonstrates the importance of pneumococcus-specific IgA in protection against nasal colonization.
FIG. 8.
Clearance of pneumococci in immunized IgA−/− mice challenged i.n. with type 14 pneumococci. IgA−/− and IgA+/+ mice were immunized i.n. as described in the legend to Fig. 7. Nasal washes were performed 72 h after challenge. Clearance of pneumococci was significantly greater (*, P < 0.05) in vaccinated IgA+/+ mice than in vaccinated IgA−/− mice or unvaccinated mice. The mean values for the groups are indicated by the bars.
DISCUSSION
The nasal mucosa is the first point of contact for inhaled antigens, and as a consequence, i.n. immunization has emerged as potentially the most effective route of vaccination for both peripheral and mucosal immunity (20, 24). The work described in this paper demonstrates the effectiveness of i.n. vaccination in the presence of IL-12 for enhancing mucosal and systemic protective immunity against pneumococcal infection. The results showed that i.n. vaccination induced the production of IFN-γ and the generation of pneumococcus-specific opsonizing antibodies. Furthermore, it enhanced protection against invasive infection, as well as nasopharyngeal carriage.
We found that coadministration of pneumococcal conjugate vaccine and IL-12 increased IFN-γ mRNA expression in the lungs and spleen within 48 h. IL-10 mRNA expression was also increased in the lungs by IL-12 treatment. These effects were due to the IL-12 adjuvant and not the vaccine. IL-10 is an important regulator of T cells and is suggested to be involved in a feedback mechanism that modulates the Th1 pathway and down-regulates the inflammatory effects of IFN-γ. IL-12, however, had no effect on expression of the Th2-type cytokine, IL-5. Similar findings were observed in previous studies of IL-12-treated mice immunized with various antigens (5, 8, 21).
A single dose of pneumococcal conjugate vaccine followed by i.n. challenge with PPS3 28 days later resulted in augmented antibody responses compared to mice that received PPS3 alone. Similarly, antibody levels were increased in mice treated i.n. with IL-12 compared to mice that received the vaccine conjugate alone, demonstrating the ability of IL-12 to enhance humoral responses to specific polysaccharide antigens. Mice immunized i.n. and treated with IL-12 showed enhanced levels of serum IgG2a pneumococcus-specific antibodies, but this treatment had no effect on IgG1 antibody levels. In previous studies, examination of serum anti-meningococcal group C polysaccharide antibody levels 14 days after i.p. immunization with conjugate vaccine showed that IL-12 treatment at the time of vaccination enhanced levels of total IgG2a and IgG3 antibodies (8). Similarly, studies showed that levels of serum IgG2a anti-influenza virus antibodies were significantly elevated in mice immunized i.n. with influenza subunit vaccine plus IL-12 (4). However, in the latter case, levels of IgG1 antibodies were also increased by IL-12 treatment, unlike the results with polysaccharide vaccine. Thus, the nature of the antigen seems to play an important role in influencing the production of specific antibody isotypes during IL-12 treatment.
Host defense against encapsulated bacteria, such as S. pneumoniae, depends on the presence of opsonic antibodies specific for capsular polysaccharide (22). Therefore, antibody levels measured by ELISA may not adequately reflect the presence of protective antibodies that are capable of triggering leukocyte effector functions (14, 22, 29). We found that mice immunized i.n. and treated with IL-12 exhibited significant opsonic capacity compared to mice that received vaccine i.n. alone. In addition, these animals were protected against lethal i.p. challenge with live pneumococci. The enhanced levels of opsonizing antibodies are likely related to the presence of IgG2a, detected in IL-12-treated mice. In previous studies, it was demonstrated that mice immunized i.n. with pneumococcal surface protein A (PspA) and treated with IL-12 generated significantly increased levels of serum IgG2a and were 100% protected against systemic infection (3). In this study, the levels of antibody generated in mice immunized i.m. with vaccine in alum were fourfold higher than in i.n. vaccinated mice. There was 100% survival in these mice treated with IL-12 or untreated following systemic challenge with pneumococci compared to survival of mice immunized i.n. with or without IL-12 (75 and 37.5% survival, respectively). The 100% survival in mice vaccinated i.m. was due to the production of significant levels of antibody types and subtypes and significant functional opsonic capacity. Alum is a well-known and effective delivery system for soluble vaccines, which could explain the significant levels of antibody generated in i.m. vaccinated mice.
Other investigators have suggested that IgG2a and IgG3 are protective during infection with encapsulated bacteria, including pneumococcal infection (27). It is possible that under conditions of limiting expression of antibody, such as might be seen following i.n. vaccination, the requirement for IgG2a is more apparent than after parenteral vaccination, which induces significantly larger amounts of antibody. In the mouse, IgG2a and IgG3 are highly effective at fixing complement and promoting opsonophagocytosis (9, 11), and IgG2a binds with highest affinity to the macrophage FcγRI receptor (28). These considerations suggest that the changes in levels of anti-polysaccharide antibody isotypes and titers that are associated with IL-12 treatment enhance host protection against pneumococcal infection.
In addition to the protective functions of IgG antibody, secretory IgA is considered to be an important first line of defense at mucosal surfaces, and it is generally assumed that this antibody isotype inhibits adherence and invasion of mucosal pathogens (25). The present results, like previous findings in an influenza vaccination model (3), showed that i.n. immunization coupled with IL-12 treatment resulted in significant increases in respiratory IgA levels compared to mice that received the vaccine conjugate alone. In addition, increased protection against carriage of type 14 pneumococci was demonstrated in mice given type 14 vaccine conjugate and IL-12 i.n. Significantly fewer colonies were present in the nasal washes of immunized mice than in those of mice that received PBS vehicle alone. However, there were no significant differences in the ability of i.m. immunized mice to clear colonies from the upper respiratory tract compared to mice given the PBS vehicle alone.
The importance of pneumococcus-specific IgA in clearance of pneumococci from the upper respiratory tract was confirmed using IgA−/− mice. It has been shown that IgA−/− mice have similar or even enhanced levels of other Ig isotypes in serum and BAL fluid compared to IgA+/+ mice but lack the ability to express IgA and thus have increased susceptibility to influenza virus infection (6).
In humans, protection from carriage following conjugate immunization appears to correlate with IgG antibody levels, but it is evident that both IgA and IgG are necessary to effectively reduce carriage. Similarly, a recent study (18) showed that i.n. administration of pneumococcal conjugate vaccine in the presence of an Escherichia coli heat-labile enterotoxin mutant (LT-K63) resulted in enhanced levels of pneumococcus-specific IgA and IgG levels and protective efficacy. In further animal studies, it has been shown that parenteral immunization with PspA is less able to elicit protection against carriage than mucosal immunization, even though higher serum antibody titers are elicited by parenteral immunization (31). In our studies, we also found that, despite the significantly increased levels of antibodies expressed in i.m. compared to i.n. immunized mice, the mean number of pneumococcal colonies isolated from the nasopharynx was lower in the i.n. vaccinated group than in the i.m. vaccinated group, though the difference was not statistically significant.
The results of this study demonstrate that PPS conjugate vaccine given i.n. together with IL-12 significantly enhances anti-polysaccharide antibody responses and induces protective immunity against both systemic disease and nasal carriage. Recent clinical trials investigating the effect of subcutaneous IL-12 treatment as an adjuvant for human PPS vaccination reported significant toxic effects of the IL-12 treatment (16). However, it has now been found that i.n. inoculation of IL-12 induces much less toxicity than parenteral administration (17). Thus, i.n. vaccination may be a new approach that could be combined with standard vaccination strategies to give optimal protection both systemically and at mucosal surfaces.
Acknowledgments
This work was supported by National Institutes of Health grants A141715 and HL62120.
We are grateful to Victor Huber and Bernard Arulanandam for advice, and we also thank the Genetics Institute for providing rIL-12. In addition, we thank Joseph Mazurkiewicz and the Albany Medical College Imaging Facility for assistance with confocal microscopy.
Editor: J. N. Weiser
REFERENCES
- 1.AlonsoDeVelasco, E., B. A. Dekker, A. F. Verheul, R. G. Feldman, J. Verhoef, and H. Snippe. 1995. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J. Infect. Dis. 172:562-565. [DOI] [PubMed] [Google Scholar]
- 2.AlonsoDeVelasco, E., A. F. Verheul, J. Verhoef, and H. Snippe. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 59:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180:940-949. [DOI] [PubMed] [Google Scholar]
- 5.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 2000. Neonatal administration of IL-12 enhances the protective efficacy of antiviral vaccines. J. Immunol. 164:3698-3704. [DOI] [PubMed] [Google Scholar]
- 6.Arulanandam, B. P., R. H. Raeder, J. G. Nedrud, D. J. Bucher, J. Le, and D. W. Metzger. 2001. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J. Immunol. 166:226-231. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan, R. M., B. P. Arulanandam, and D. W. Metzger. 1998. IL-12 enhances antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J. Immunol. 161:5525-5533. [PubMed] [Google Scholar]
- 8.Buchanan, R. M., D. E. Briles, B. P. Arulanandam, M. A. Westerink, R. H. Raeder, and D. W. Metzger. 2001. IL-12-mediated increases in protection elicited by pneumococcal and meningococcal conjugate vaccines. Vaccine 19:2020-2028. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, L. J., J. C. Schimenti, D. D. Glass, and N. S. Greenspan. 1991. H chain C domains influence the strength of binding of IgG for streptococcal group A carbohydrate. J. Immunol. 146:2659-2663. [PubMed] [Google Scholar]
- 10.Dagan, R., R. Melamed, M. Muallem, L. Piglansky, D. Greenberg, O. Abramson, P. M. Mendelman, N. Bohidar, and P. Yagupsky. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271-1278. [DOI] [PubMed] [Google Scholar]
- 11.Ey, P. L., G. J. Russell-Jones, and C. R. Jenkin. 1980. Isotypes of mouse IgG. I. Evidence for ′non-complement-fixing' IgG1 antibodies and characterization of their capacity to interfere with IgG2 sensitization of target red blood cells for lysis by complement. Mol. Immunol. 17:699-710. [DOI] [PubMed] [Google Scholar]
- 12.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 13.Germann, T., M. Bongartz, H. Dlugonska, H. Hess, E. Schmitt, L. Kolbe, E. Kolsch, F. J. Podlaski, M. K. Gately, and E. Rude. 1995. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b, and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 25:823-829. [DOI] [PubMed] [Google Scholar]
- 14.Giebink, G. S., J. E. Foker, Y. Kim, and G. Schiffman. 1980. Serum antibody and opsonic responses to vaccination with pneumococcal capsular polysaccharide in normal and splenectomized children. J. Infect. Dis. 141:404-412. [DOI] [PubMed] [Google Scholar]
- 15.Harriman, G. R., M. Bogue, P. Rogers, M. Finegold, S. Pacheco, A. Bradley, Y. Zhang, and I. N. Mbawuike. 1999. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 162:2521-2529. [PubMed] [Google Scholar]
- 16.Hedlund, J., B. Langer, H. B. Konradsen, and A. Ortqvist. 2001. Negligible adjuvant effect for antibody responses and frequent adverse events associated with IL-12 treatment in humans vaccinated with pneumococcal polysaccharide. Vaccine 20:164-169. [DOI] [PubMed] [Google Scholar]
- 17.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. S. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801-809. [DOI] [PubMed]
- 18.Jakobsen, H., S. Bjarnarson, G. Del Giudice, M. Moreau, C. A. Siegrist, and I. Jonsdottir. 2002. Intranasal immunization with pneumococcal conjugate vaccines with LT-K63, a nontoxic mutant of heat-labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice. Infect. Immun. 70:1443-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 20.McGhee, J. R., C. Czerkinsky, and J. Mestecky. 1999. Mucosal vaccines: an overview, p. 741-757. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, San Diego, Calif.
- 21.Metzger, D. W., R. M. McNutt, J. T. Collins, J. M. Buchanan, V. H. Van Cleave, and W. A. Dunnick. 1997. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-gamma-dependent and -independent mechanisms. Eur. J. Immunol. 27:1958-1965. [DOI] [PubMed] [Google Scholar]
- 22.Musher, D. M., A. J. Chapman, A. Goree, S. Jonsson, D. Briles, and R. E. Baughn. 1986. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 154:245-256. [DOI] [PubMed] [Google Scholar]
- 23.Obaro, S. K., R. A. Adegbola, W. A. Banya, and B. M. Greenwood. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348:271-272. [DOI] [PubMed] [Google Scholar]
- 24.Renauld-Mongenie, G., N. Mielcarek, J. Cornette, A. M. Schacht, A. Capron, G. Riveau, and C. Locht. 1996. Induction of mucosal immune responses against a heterologous antigen fused to filamentous hemagglutinin after intranasal immunization with recombinant Bordetella pertussis. Proc. Natl. Acad. Sci. USA 93:7944-7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinmetz, I. 1997. Comparative in vivo analysis of IgA- and IgG-mediated mucosal defense against bacterial pathogens. Behring Inst. Mitt. 53-55. [PubMed]
- 26.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 27.Tuomanen, E. I., R. Austrian, and H. R. Masure. 1995. Pathogenesis of pneumococcal infection. N. Engl. J. Med. 332:1280-1284. [DOI] [PubMed] [Google Scholar]
- 28.Unkeless, J. C., E. Scigliano, and V. H. Freedman. 1988. Structure and function of human and murine receptors for IgG. Annu. Rev. Immunol. 6:251-281. [DOI] [PubMed] [Google Scholar]
- 29.Vitharsson, G., I. Jonsdottir, S. Jonsson, and H. Valdimarsson. 1994. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 170:592-599. [DOI] [PubMed] [Google Scholar]
- 30.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 32.Wynn, T. A., A. Reynolds, S. James, A. W. Cheever, P. Caspar, S. Hieny, D. Jankovic, M. Strand, and A. Sher. 1996. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J. Immunol. 157:4068-4078. [PubMed] [Google Scholar]