Abstract
To determine if stimulation of Th1-cell-associated immune responses, mediated by interleukin 12 (IL-12) and gamma interferon (IFN-γ), enhance the antileishmanial effect of amphotericin B (AMB), Leishmania donovani-infected BALB/c mice were first treated with (i) exogenous IL-12 to induce IFN-γ, (ii) agonist anti-CD40 monoclonal antibody (MAb) to maintain IL-12 and induce IFN-γ, or (iii) anti-IL-10 receptor (IL-10R) MAb to blockade suppression of IL-12 and IFN-γ. In animals with established visceral infection, low-dose AMB alone (two injections of 1 mg/kg of body weight; total dose, 2 mg/kg) killed 15 to 29% of liver parasites; by themselves, the immunointerventions induced 16 to 33% killing. When the interventions were combined, the leishmanicidal activities increased 3.4-fold (anti-CD40), 6.3-fold (anti-IL-10R), and 9-fold (IL-12) compared with the activities of AMB plus the control preparations; and overall killing (76 to 84%) approximated the 84 to 92% killing effect of 7.5-fold more AMB alone (three injections of 5 mg/kg; total dose, 15 mg/kg). These results suggest that strengthening the host Th1-cell response may be a strategy for the development of AMB-sparing regimens in visceral leishmaniasis.
Macrophages infected in vitro with the protozoan Leishmania donovani respond to treatment with either pentavalent antimony (Sb) or amphotericin B (AMB), with rapid elimination of intracellular amastigotes (3, 21). For both drugs, this in vitro leishmanicidal effect is direct, in that it is expressed in unstimulated resident macrophages and does not require the presence of host T cells or exogenously applied, macrophage-activating cytokines. Nevertheless, in mice experimentally infected with L. donovani, an agent of visceral leishmaniasis, the in vivo efficacies of Sb and AMB are quite different, depending upon the state of the host's antileishmanial immune response (21, 22). This response, which culminates in macrophage activation and intracellular killing, is T cell dependent, is primarily regulated by Th1-cell-type cytokines (interleukin 12 [IL-12] and gamma interferon [IFN-γ]), and involves mononuclear cell recruitment and granuloma assembly at infected tissue sites (27, 28).
Expression of the in vivo leishmanicidal effect of Sb in parasitized tissues is not direct, but, instead, requires the preceding mechanism with interdigitation of (i) T (Th1) cells and T-cell costimulation via CD40L and CD40, (ii) IFN-γ and allied activating, inflammatory cytokines (IL-12 and tumor necrosis factor), and (iii) influxing blood monocytes as primary effector cells within epithelioid granulomas (21, 25-27; H. W. Murray, C. M. Lu, E. B. Brooks, R. E. Fichtl, J. L. DeVecchio, and F. P. Heinzel, submitted for publication). In clear contrast, AMB retains its leishmanicidal effect in animals deficient in T cells as well as those devoid of each of the host immune factors or mechanisms mentioned above (22, 25-27; Murray et al., submitted). Thus, while AMB's fungicidal action may be supported by or involve induction of cytokine expression, inflammatory mediators, and cell activation (4-6, 16, 19, 33, 39-41), its experimental intracellular antileishmanial effect is independent of the host immune response and therefore is apparently direct.
Previous studies took advantage of Sb's dependence on immunologic responses, particularly those mediated by IFN-γ (26), by pairing Sb with treatments which raise the level of T-cell reactivity, enhance Th1-cell-type cytokine effects, and/or stimulate target cell (macrophage) activation. Such treatments included coadministration of (i) exogenous IFN-γ to induce macrophage activation (20), (ii) exogenous IL-12 to induce endogenous IFN-γ (as well as IFN-γ-independent effects) (25), and (iii) monoclonal antibodies (MAbs) which stimulate endogenous IL-12 and IFN-γ secretion via different mechanisms: anti-IL-10 receptor (IL-10R) MAb (which blockades IL-10's suppressive effects on Th1-cell responses [29]) and agonist anti-CD40 MAb (which ligates CD40 and mimics CD40-CD40L costimulation [Murray et al., submitted]). Each of the preceding treatments synergistically increased Sb's efficacy in mice with established L. donovani infections, and one of these approaches, the use of Sb plus IFN-γ, has been tested clinically (27).
Even though AMB's killing of L. donovani does not require a host immune response, we reasoned that similar targeting of the Th1-cell mechanism might increase its efficacy and permit lower doses to be used with comparable activities. Since AMB has emerged as effective, albeit arduous, treatment for human visceral leishmaniasis (kala azar) (27), it seemed worthwhile to determine if combination immunochemotherapy with AMB might have therapeutic potential for the treatment of visceral infection.
MATERIALS AND METHODS
Mice.
Female BALB/c mice (weight, 20 to 30 g) were purchased from Charles Rivers Laboratory (Wilmington, Mass.). Some of these mice were injected subcutaneously once weekly for 4 weeks with 0.2 ml of saline containing 1.5 × 107 heat-killed L. major promastigotes (HKLMP). This procedure induces a cross-reacting Th2-cell-associated response upon subsequent challenge with viable L. donovani (24). BALB/c IL-10−/− and BALB/c IL-10 (human IL-10) transgenic mice were produced and bred at DNAX Research Institute of Molecular and Cellular Biology (Palo Alto, Calif.), as described previously (29). These mutant mice were 10 to 17 weeks old when they were challenged with L. donovani, and males and females were used in a random fashion (29).
Visceral infection.
Groups of three to five mice were infected via the tail vein with 1.5 × 107 hamster spleen-derived L. donovani amastigotes (1 Sudan strain) (29). Visceral infection was monitored microscopically by using Giemsa-stained liver imprints, in which liver parasite burdens were measured by blind counting of the number of amastigotes per 500 cell nuclei × liver weight (in milligrams) (Leishman-Donovan units [LDU]) (29).
AMB treatment.
The liver parasite burdens were determined 2 weeks after infection (day +14), and a parallel group of mice then received no treatment or intraperitoneal injections of AMB (Gensia Laboratories Ltd., Irvine, Calif.). Optimal treatment consisted of 5 mg/kg of body weight, given on days +14, +16, and +18 (total dose, 15 mg/kg) (22); in the suboptimal regimen, 1 mg/kg was given on days +14 and +17 (total dose, 2 mg/kg). The mice were killed on day +21.
Combination treatments.
Immunoenhancing treatments, whose antileishmanial effects have already been characterized in this model (25, 29, 30; Murray et al., submitted), were started on day +12, 2 days before AMB treatment. This 48-h period was selected to allow induction of some immunologic effect at the time that AMB treatment was begun. With the exception of anti-IL-4 (17, 24), each of the treatments tested induces a leishmanicidal effect by itself (25, 29; Murray et al., submitted); therefore, except for anti-IL-4, previously defined suboptimal doses were used in combination with low-dose (suboptimal) AMB. Murine recombinant IL-12 (2.7 × 106 U/mg; Genetics Institute/Wyeth-Ayerst Research, Andover, Mass.), diluted in saline containing 0.1% bovine serum albumin, was administered continuously for 7 days at 0.1 μg/day by an osmotic pump (Alzet model 2001; Alza Corp., Palo Alto, Calif.) (25). The pumps were implanted subcutaneously on day +12; control pumps contained saline plus 0.1% bovine serum albumin. MAbs were injected once intraperitoneally on day +12 in 0.5 ml of saline containing the following rat anti-mouse immunoglobulin G (IgG) preparations: 0.1 mg of anti-CD40 (FGK 45) (13; Murray et al., submitted), 0.1 mg of anti-IL-10R (1B1.3A; DNAX Research Institute) (29), or 5 mg of anti-IL-4 (11.B.11; Biologic Response Modifers Program, National Cancer Institute, Frederick, Md.) (17). Control injections contained the same amounts of purified rat IgG (Sigma Chemical Co., St. Louis, Mo.). The liver parasite burdens (in LDU) in all mice were measured on day +21. The LDU on day +21 were compared to the LDU on day +14 in untreated mice to determine the percent parasite killing; differences between mean LDU were analyzed by a two-tailed Student's t test.
Cytokine assays.
Blood was obtained from mice before and up to 21 days after infection. Serum from each mouse was assayed for IL-12p40 and IFN-γ by enzyme-linked immunosorbent assay in duplicate by using fourfold dilutions of serum and pairs of antibodies from Becton Dickinson-Pharmagen (San Diego, Calif.), as described previously (1). The lower limits of detection were 40 pg/ml for IL-12p40 and 6 pg/ml for IFN-γ.
RESULTS AND DISCUSSION
Treatment with AMB was begun 2 weeks after L. donovani challenge. At this stage of infection, normal BALB/c mice show both suppressive Th2-cell-associated cytokine responses (IL-4 and IL-10) and activating Th1 cell-associated cytokine responses (IL-12 and IFN-γ) and parasite replication in the liver is logarithmic (17). Liver parasite burdens peak by week 4 and then decline in this model, as the IL-12-driven, IFN-γ-mediated Th1-type mechanism predominates, resistance is acquired, and the self-curing phenotype is expressed (27, 28). To determine if stimulating emerging Th1-cell responses enhance AMB's efficacy in established infection, two approaches were applied in conjunction with low-dose AMB: direct stimulation by administration of IL-12 or agonist anti-CD40 MAb (13, 25; Murray et al., submitted) or indirect stimulation by inhibition of the suppressive effect of IL-4 or IL-10 (17, 29, 30). In all experiments, mice were treated with AMB during the third week of infection (starting on day +14), before spontaneous decreases in liver parasite burdens were evident.
Effect of IL-12 coadministration.
IL-12 likely initiates and maintains the antileishmanial Th1-cell-type response (8, 25, 35) and was used here to induce endogenous IFN-γ and downstream macrophage activation (23). As shown in Table 1, treatment with low-dose AMB (two injections of 1 mg/kg; total dose, 2 mg/kg) alone resulted in 19% killing of liver parasites on day +21. Use of this AMB regimen in combination with a suboptimal dose of IL-12, which by itself contributed a 33% leishmanicidal effect, increased the level of parasite killing to 82%. This leishmanicidal activity was ninefold higher than that in mice treated with saline-loaded pumps plus AMB (9% killing) and was comparable to the 92% killing in control mice given the higher-dose AMB regimen. The latter involved the administration of 7.5-fold more drug (three injections of 5 mg/kg; total dose, 15 mg/kg).
TABLE 1.
Effect of combining IL-12 with low-dose AMB treatmenta
| Treatment | Low-dose AmB | Liver parasite burden (LDU)
|
% Killing | |
|---|---|---|---|---|
| Day +14 | Day +21 | |||
| None | − | 1,346 ± 68 | 1,514 ± 148 | 0 |
| None | + | 1,086 ± 79 | 19 | |
| IL-12 | − | 893 ± 126 | 33 | |
| IL-12 | + | 242 ± 52b | 82 | |
| Saline | + | 1231 ± 129 | 9 | |
| AMB (optimal) | 107 ± 28b | 92 | ||
At 12 days after infection, mice received no therapy or were started on pump-delivered IL-12 or saline treatment. Low-dose AMB (1 mg/kg) was given on days +14 and +17; optimal AMB (5 mg/kg) was given on days +14, +16, and +18. The results are from two experiments and indicate the mean ± standard errors of the means for a total of 7 to 11 mice per group at each time point.
P < 0.05 versus the value on day +14.
CD40 ligation.
T-cell costimulation via CD40-CD40L signaling is a primary stimulus for IL-12 generation by antigen-presenting cells (38), and CD40L is required for a Th1-cell-associated response to and control over visceral L. donovani infection (Murray et al., submitted). CD40 ligation, induced by injection of agonist anti-CD40 MAb, enhances IL-12 and IFN-γ secretion (9, 13; Murray et al., submitted) and in normal mice provokes leishmanicidal activity dependent upon both cytokines (Murray et al., submitted). Therefore, low-dose AMB was next tested with anti-CD40-treated BALB/c animals (Table 2). Use of a single injection of a suboptimal dose of anti-CD40 in combination with low-dose AMB increased the level of killing to 84%, higher than that induced by either MAb (22%) or AMB (29%) alone and 3.4-fold higher than the 25% killing in mice treated with control IgG plus AMB.
TABLE 2.
Effect of anti-CD40 MAb plus low-dose AMB treatmenta
| Treatment | Low-dose AMB | Liver parasite burden (LDU)
|
% Killing | |
|---|---|---|---|---|
| Day +14 | Day +21 | |||
| None | − | 1,493 ± 116 | 1,589 ± 87 | 0 |
| None | + | 1,060 ± 107 | 29 | |
| Anti-CD40 | − | 1,165 ± 99 | 22 | |
| Anti-CD40 | + | 236 ± 61b | 84 | |
| Rat IgG | + | 1,127 ± 99 | 25 | |
| AmB (optimal) | 149 ± 38b | 90 | ||
At 12 days after infection, mice received no therapy or a single injection of anti-CD40 or rat IgG. Low-dose AMB (1 mg/kg) was given on days +14 and +17; optimal AMB (5 mg/kg) was given on days +14, +16, and +18. The results are from two experiments and indicate the means ± standard errors of the means for a total of seven to eight mice per group at each time point.
P < 0.05 versus the value on day +14.
Effect of AMB in a predominant Th2-cell-type cytokine environment.
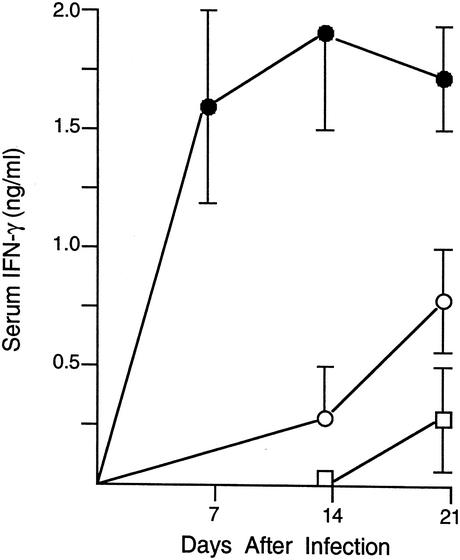
The downregulating Th2-cell-associated cytokines, IL-4 and IL-10, disable both the afferent and efferent limbs of the Th1 response, suppressing IL-12 and IFN-γ and deactivating macrophages (18, 27, 29, 36). In experiments conducted before determination of whether inhibition of IL-4 or IL-10 enhances drug efficacy in normal animals, AMB was tested in BALB/c mice manipulated to express a predominant Th2-cell-type response and a noncuring phenotype: (i) normal mice immunized with HKLMP, which cross-react to challenge with L. donovani with exaggerated IL-4- and IL-10-dependent responses (24), and (ii) IL-10 transgenic mice, which show the effects of a sustained IL-10 response alone (10, 29). The latter included suppressed IFN-γ levels (Fig. 1); and no increase in the serum IL-12p40 level on day +14 (3.8 ± 0.2 ng/ml) relative to that on day 0 (3.6 ± 0.3 ng/ml) was found (1 experiment, n = 3 to 4 mice), whereas an increase from 3.1 ± 0.4 to 8.1 ± 1.1 ng/ml was found in normal controls (two experiments; n = 10 mice per time point). HKLMP-treated and IL-10 transgenic mice were given optimal AMB treatment (three injections of 5 mg/kg) starting on day +14. Both groups of animals responded normally, with 82 to 93% parasite killing demonstrated on day +21 (two experiments; n = 6 to 9 mice per group; data not shown).
FIG. 1.
Serum IFN-γ levels before (day 0) and after L. donovani infection in normal mice (open circles), IL-10 transgenic mice (open squares), and IL-10−/− BALB/c mice (filled circles). The result for each time point indicates the mean ± standard error of the mean from single experiments performed with IL-10−/− mice (n = 4 mice) and transgenic mice (n = 3 to 4) and from two experiments performed with normal mice (n = 6 to 10 mice in total).
Neutralization of IL-4 and blockade of IL-10R in normal mice.
The preceding results indicated that suppressed Th1-cell-type responses and the noncuring phenotype induced by excess IL-4 and/or excess IL-10 did not impair AMB's efficacy, consistent with AMB's direct antileishmanial action (27). However, these results left open the possibility that inhibition of endogenous IL-4 or IL-10, leading to enhanced Th1-cell responses (18, 24, 29, 30, 32, 36), could be combined with and increase the effect of the drug. To test this hypothesis, normal BALB/c mice were injected with anti-IL-4 or anti-IL-10R MAb on day +12 and were then treated with low-dose AMB on days +14 and +17.
The use of anti-IL-4 in combination with low-dose AMB did not increase the level of parasite killing on day +21 (two experiments; data not shown), an observation consistent with prior findings that, even though IL-4 is induced (17), IL-4 exerts little or no suppressive role in the mouse model of L. donovani infection (7, 10, 11, 14, 15, 17, 27, 34). In contrast, endogenous IL-10 is a primary regulator of the outcome of L. donovani infection (29, 31). IL-10 acts in a pansuppressive fashion to deactivate macrophages by inhibiting virtually all components of the activating Th1-cell mechanism and may directly impair the macrophage effector function as well (18, 29). Treatment of infected BALB/c mice with anti-IL-10R MAb, used to block the effects of IL-10, maintains IL-12p40 secretion in vivo, stimulates a 55-fold increase in serum IFN-γ levels, and induces IL-12- and IFN-γ-dependent parasite killing (30). As shown in Table 3, while a suboptimal dose of anti-IL-10R alone produced some leishmanicidal effect (16% killing), the use of anti-IL-10R in combination with low-dose AMB produced 76% parasite killing, a level 6.3-fold higher than the 12% killing induced by treatment with control IgG plus AMB.
TABLE 3.
Effect of anti-IL10R MAb plus low-dose AMB treatmenta
| Treatment | Low-dose AMB | Liver parasite burden (LDU)
|
% Killing | |
|---|---|---|---|---|
| Day +14 | Day +21 | |||
| None | − | 1,257 ± 55 | 1,236 ± 132 | 0 |
| None | + | 1,078 ± 147 | 15 | |
| Anti-IL-10R | − | 1,058 ± 126 | 16 | |
| Anti-IL-10R | + | 302 ± 90b | 76 | |
| Rat IgG | + | 1,074 ± 99 | 12 | |
| AmB (optimal) | 201 ± 30b | 84 | ||
At 12 days after infection, mice received no therapy or a single injection of anti-IL-10R or rat IgG. Low-dose AMB (1 mg/kg) was given on days +14 and +17; optimal AMB (5 mg/kg) was given on days +14, +16, and +18. The results are from two experiments and indicate the means ± standard errors of means for a total of 9 to 11 mice per group at each time point.
P < 0.05 the value on day +14.
Response to AMB in IL-10-deficient mice.
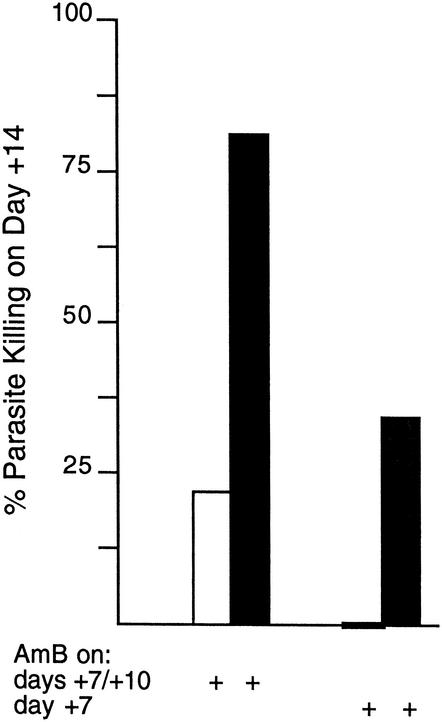
In view of the effect of IL-10R blockade in normal mice, we completed this analysis by testing AMB in mice devoid of IL-10. These IL-10−/− BALB/c mice exert extraordinarily rapid control over L. donovani (29, 31), an effect dependent upon IL-12 and IFN-γ (31) and associated with increased levels of both IL-12p40 (7.9 ± 0.3 on day +14 versus 3.6 ± 0.4 ng/ml on day 0 [one experiment; n = 4 mice]) and IFN-γ in serum (Fig. 1). Since accelerated parasite killing is shown in IL-10−/− mice (29, 31), AMB was started earlier (on day +7) and tested in two low-dose regimens (two injections of 1 mg/kg versus a single injection of 1 mg/kg). The data in Fig. 2 show that the two-injection regimen was considerably more active in IL-10−/− mice than in control mice and that measurable effects could also be achieved in IL-10-deficient animals by a single injection of a low dose of drug.
FIG. 2.
Response to low-dose AMB in IL-10−/− mice. Seven days after infection, normal mice (open bars) and IL-10−/− mice (filled bars) were injected with 1 mg of AMB per kg either on days +7 and +10 or on day+ 7 only, and liver parasite burdens (LDU) were determined on day +14. On day +7, the liver parasite burdens in normal and IL-10−/− mice were 726 ± 92 and 642 ± 45 LDU, respectively. The results are from two experiments (n = 6 to 7 total mice per group) and the indicate percent parasite killing on day +14 compared to that on day +7, calculated as [(mean LDU on day +7 − mean LDU on day +14)/mean LDU on day + 7] × 100.
While AMB's experimental leishmanicidal activity does not require participation of the host immune response (27), the results presented here indicate that interventions which stimulate curative Th1-cell-type mechanisms (exogenous IL-12 and agonist anti-CD40) (25; Murray et al., submitted) or which free up curative Th1-cell-type mechanisms (anti-IL-10R) (30) enhance parasite killing in the livers of AMB-treated animals. Since the experiments were carried out with normal mice in the midst of developing a Th1-cell response (27), the therapeutic usefulness of these three interventions is likely predicated on the host's capacity to satisfactorily produce IL-12 and IFN-γ (9, 23, 24, 30; Murray et al., submitted). Since AMB's antifungal effect can also be successfully amplified by coadministration of IFN-γ, IL-12, tumor necrosis factor, or an IL-4 antagonist (12, 16, 37), this immunochemotherapeutic approach may have wider application.
In comparison to the killing induced by drug alone, IL-12 or agonist anti-CD40 in combination with AMB produced additive and possibly synergistic effects, while cotreatment with AMB and anti-IL-10R acted synergistically. The latter effect was particularly well illustrated in one of the three experiments whose results are summarized in Table 3, in which the levels of parasite killing on day +21 in mice treated with anti-IL-10R, AMB, or the combination were 0, 0, and 56%, respectively. It would also be of interest to determine the effects of treatment with anti-IL-10R (as well as IL-12 and anti-CD40) plus AMB in the spleen, an organ in which L. donovani replication may not be spontaneously controlled (8, 15).
The overall killing induced by combining the selected immunointerventions with low-dose AMB (total dose, 2 mg/kg) was high (76 to 84%) and in the range of that achieved by 7.5-fold more AMB used alone at a total dose of 15 mg/kg. Therefore, in an experimental infection, manipulation of the host's immune response in favor of the Th1-cell-associated mechanism may provide the opportunity to use AMB-sparing regimens with lower doses of drug, fewer injections, and/or a shorter treatment duration. Such an immunochemotherapeutic approach with these potential benefits has clinical appeal for three reasons: (i) AMB's well-recognized toxicity (e.g., anemia, azotemia, and electrolyte imbalances) is related to the cumulative dose (2); (ii) in human visceral leishmaniasis (kala azar), AMB is given for a prolonged 20- or 30-day period and is not necessarily well tolerated (27); and (iii) this approach takes advantage of available, endogenous host defense mechanisms which can separately control L. donovani when they are fully expressed in an unimpeded fashion.
Acknowledgments
Joseph P. Sypek (Genetics Institute/Wyeth-Ayerest Research) generously provided the murine recombinant IL-12. Amy Beebe and Robert L. Coffman (DNAX Research Institute of Molecular and Cellular Biology, supported by Schering Plough Corp.) provided the anti-IL-10R MAb and IL-10−/− and IL-10 transgenic mice, and we also acknowledge their help.
This study was supported by NIH grant AI 16393 (to H.W.M.) and NIH grants AI 35979 and AI 45602 (to F.P.H.).
REFERENCES
- 1.Balkhy, H. H., and F. P. Heinzel. 1999. Endotoxin fails to induce interferon-γ in endotoxin tolerant mice: deficiencies in both IL-12 heterodimer production and IL-12 responsiveness. J. Immunol. 162:3633-3638. [PubMed] [Google Scholar]
- 2.Bennett, J. E. 2001. Amphotericin B, p. 1295-1299. In J. C. Hardman and L. E. Limbird (ed.), Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed. McGraw-Hill, New York, N.Y.
- 3.Berman, J. D., and D. J. Wyler. 1980. An in vitro model for chemotherapeutic agents in leishmaniasis. J. Infect. Dis. 142:83-86. [DOI] [PubMed] [Google Scholar]
- 4.Bistoni, F., A. Vecchiarelli, R. Mazzolla, P. Puccetti, P. Marconi, and E. Geraci. 1985. Immunoadjuvant activity of amphotericin B as displayed in mice infected with Candida albicans. Antimicrob. Agents Chemother. 27:625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanke, T. J., J. R. Little, S. F. Shirley, and R. G. Lynch. 1977. Augmentation of murine immune responses by amphotericin B. Cell. Immunol. 33:180-190. [DOI] [PubMed] [Google Scholar]
- 6.Cenci, E., A. Mencacci, G. Del Sero, F. Bistoni, and L. Romani. 1997. Induction of protective Th1 responses to Candida albicans by antifungal therapy alone or in combination with an interleukin-4 antagonist. J. Infect. Dis. 176:217-226. [DOI] [PubMed] [Google Scholar]
- 7.Cotterell, S. E., C. Engwerda, and P. M. Kaye. 1999. Leishmania donovani infection initiates T cell-independent chemokine responses which are subsequently amplified in a T cell-dependent manner. Eur. J. Immunol. 29:203-214. [DOI] [PubMed] [Google Scholar]
- 8.Engwerda, C. R., M. L. Murphy, S. E. Cotterell, S. C. Smelt, and P. M. Kaye. 1998. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur. J. Immunol. 28:669-680. [DOI] [PubMed] [Google Scholar]
- 9.Ferlin, W. G., T. von der Weid, F. Cottrez, D. A. Ferrick, R. L. Coffman, and M. C. Howard. 1998. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 MAb. Eur. J. Immunol. 28:525-531. [DOI] [PubMed] [Google Scholar]
- 10.Groux, H., F. Cottrez, M. Rouleau, S. Mauze, S. Aantonenko, S. Hurst, T. McNeil, M. Bigler, M.-G. Roncarolo, and R. L. Coffman. 1999. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 62:1723-1729. [PubMed] [Google Scholar]
- 11.Lehmann, J., K. H. Enssle, I. Lehmann, A. Emmendorfer, and M. L. Lohmann-Matthes. 2000. The capacity to produce IFN-gamma rather than the presence of interleukin-4 determines the resistance and the degree of susceptibility to Leishmania donovani infection in mice J. Interferon Cytokine Res. 20:63-77. [DOI] [PubMed] [Google Scholar]
- 12.Louie, A., A. L. Baltch, and R. P. Smith. 1995. Fluconazole and amphotericin B antifungal therapies do not negate the protective effect of endogenous tumor necrosis factor in a murine model of fatal disseminated candidiasis. J. Infect. Dis. 171:406-415. [DOI] [PubMed] [Google Scholar]
- 13.Martin, D. L., C. L. King, E. Pearlman, E. Strine, and F. P. Heinzel. 2000. IFN-γ is necessary but not sufficient for anti-CD40 antibody-mediated inhibition of the Th2 response to Schistosoma mansoni eggs. J. Immunol. 64:779-785. [DOI] [PubMed] [Google Scholar]
- 14.Melby, P. C., Y.-Z. Yang, J. Cheng, and W. Zhao. 1998. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect. Immun. 66:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melby, P. C., A. Tabares, B. I. Restrepo, A. E. Cardona, H. S. McGuff, and J. M. Teale. 2001. Leishmania donovani: evolution and architecture of the splenic cellular immune response related to control of infection. Exp. Parasitol. 99:17-25. [DOI] [PubMed] [Google Scholar]
- 16.Mencacci, A., E. Cenci, A. Bacci, F. Bistroni, and L. Romani. 2000. Host immune response determines the efficacy of combination immunotherapy and antifungal chemotherapy in candidiasis. J. Infect. Dis. 181:686-694. [DOI] [PubMed] [Google Scholar]
- 17.Miralles, G. D., M. Y. Stoeckle, D. F. McDermott, F. D. Finkelman, and H. W. Murray. 1994. Induction of Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect. Immun. 62:1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian, N., J. W. Berman, and A. Casadevall. 1997. Enhancement of nitric oxide synthesis by macrophages represents an additional mechanism of action for amphotericin B. Antimicrob. Agents Chemother. 41:1825-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray, H. W., J. D. Berman, and S. D. Wright. 1988. Immunochemotherapy for intracellular Leishmania donovani infection: interferon-gamma plus pentavalent antimony. J. Infect. Dis. 157:973-978. [DOI] [PubMed] [Google Scholar]
- 21.Murray, H. W., M. J. Oca, A., M. Granger, and R. D. Schreiber. 1989. Successful response to chemotherapy in experimental visceral leishmaniasis: requirement for T cells and effect of lymphokines. J. Clin. Investig. 83:1254-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray, H. W., J. Hariprashad, and R. Fichtl. 1993. Treatment of experimental visceral leishmaniasis in a T cell-deficient host: response to amphotericin B and pentamidine. Antimicrob. Agents Chemother. 37:1504-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray, H. W., and J. Hariprashad. 1995. Interleukin 12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J. Exp. Med. 181:387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, H. W., J. Hariprashad, and R. L. Coffman. 1997. Behavior of visceral Leishmania donovani in an experimentally-induced Th2 cell-associated response model. J. Exp. Med. 185:867-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray, H. W., C. Montelibano, R. Peterson, and J. P. Sypek. 2000. Interleukin 12 regulates the response to chemotherapy in experimental visceral leishmaniasis. J. Infect. Dis. 182:1497-1502. [DOI] [PubMed] [Google Scholar]
- 26.Murray, H. W., and S. Delph-Etienne. 2000. Role of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect. Immun. 68:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray, H. W. 2001. Clinical and experimental advances in treatment in visceral leishmaniasis. Antimicrob. Agents Chemother. 45:2185-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray, H. W. 2001. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 82:249-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray, H. W., C. M. Lu, S. Mauze, S. Freeman, A. L. Moreira, G. Kaplan, and R. L. Coffman. 2002. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 70:6284-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray, H. W., A. L. Moreira, C. M. Lu, J. L. DeVecchio, M. Matsuhashi, X. Ma, and F. P. Heinzel. Determinants of response to interleukin 10 receptor blockade immunotherapy in experimental visceral leishmaniasis. J. Infect. Dis., in press. [DOI] [PubMed]
- 31.Murphy, M. L., U. Wille, E. N. Villegas, C. A. Hunter, and J. P. Farrell. 2001. IL-10 mediates susceptibility to Leishmania donovani infection. Eur. J. Immunol. 31:2848-2856. [DOI] [PubMed] [Google Scholar]
- 32.Nabors, G. S. 1997. Modulating ongoing Th2-cell responses in experimental leishmaniasis. Parasitol. Today 13:76-79. [DOI] [PubMed] [Google Scholar]
- 33.Rogers, P. D., J. K. Jenkins, S. W. Chapman, K. Ndebele, B. A. Chapman, and J. D. Cleary. 1998. Amphotericin B activation for human genes encoding for cytokines. J. Infect. Dis. 178:1726-1733. [DOI] [PubMed] [Google Scholar]
- 34.Satoskar, A., H. Bluethmann, and J. Alexander. 1995. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect. Immun. 63:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoskar, A. R., S. Rodig, S. R. Telford, A. A. Satoskar, S. K. Ghosh, F. von Lichtenberg, and J. R. David. 2000. IL-12 gene-deficient C57BL/6 mice are susceptible to Leishmania donovani but have diminished hepatic pathology. Eur. J. Immunol. 30:834-839. [DOI] [PubMed] [Google Scholar]
- 36.Spellberg, B., and J. E. Edwards. 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 37.Stevens, D. A. 1998. Combination immunotherapy and antifungal chemotherapy. Clin. Infect. Dis. 26:1266-1269. [DOI] [PubMed] [Google Scholar]
- 38.Stout, R. D., and J. Suttles. 1996. The many roles of CD40 in cell-mediated inflammatory responses. Immunol. Today 17:487-492. [DOI] [PubMed] [Google Scholar]
- 39.Tokuda, Y., M. Tsuji, M. Yamazaki, S. Kimura, S. Abe, and H. Yamaguchi. 1993. Augmentation of murine tumor necrosis factor production by amphotericin B in vitro and in vivo. Antimicrob. Agents Chemother. 37:2228-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vonk, A. G., M. G. Netea, N. E. Denecker, I. C. Verschueren, J. W. van der Meer, and B. J. Kullberg. 1998. Modulation of the pro- and anti-inflammatory cytokine balance by amphotericin B. J. Antimicrob. Chemother. 42:469-474. [DOI] [PubMed] [Google Scholar]
- 41.Wolf, J. E., and S. E. Massof. 1990. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infect. Immun. 58:1296-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]