Abstract
Miltefosine (hexadecylphosphocholine [HePC]) is the first drug approved for the oral treatment of visceral leishmaniasis. As part of a study on the mechanisms of action of this drug and on the rates of resistance to this drug, we have been working in vitro with an Leishmania donovani line that was previously shown to be 15-fold more resistant to HePC. We have studied the accumulation of [14C]HePC by L. donovani promastigotes and have found a drastic reduction (>95%) in the ability of the resistant line to internalize the drug. Binding of HePC to the plasma membrane and drug efflux from preloaded cells were similar in both drug-sensitive and -resistant lines, and no [14C]HePC metabolism was evident in either line. Resistant parasites were also unable to take up other short-chain phospholipid analogs, independently of their polar head group, even though endocytosis remained unaltered. Finally, HePC uptake was temperature and energy dependent and sensitive to the thiol-reactive agent N-ethylmaleimide. We propose that inward translocation of a short-chain phospholipid across the plasma membrane may exist in Leishmania promastigotes and that such activity is defective in the resistant line.
The protozoan parasite Leishmania is the causative agent of leishmaniasis, a disease that affects more than 6 million people worldwide, with 400,000 new cases each year (10). There are a number of problems with standard treatment based on pentavalent antimonials, including a lack of efficacy against visceral leishmaniasis and human immunodeficiency virus coinfections, the development of resistance, and high costs. Miltefosine (hexadecylphosphocholine [HePC]), an alkyl-phosphocholine originally developed as an anticancer drug, is the first oral drug that has proved to be highly effective against visceral leishmaniasis (30), including antimony-resistant cases (11), and against cutaneous leishmaniasis (29). Following registration in India in 2002 for the treatment of visceral leishmaniasis, HePC is, it is hoped, about to play an essential role in the control and treatment of this endemic disease (8). However, little is known about the leishmanicidal and trypanocidal mechanisms of HePC and other alkyl-lysophospholipids. Perturbation of the alkyl-lipid metabolism and the biosynthesis of alkyl-anchored glycoproteins (15), as well as damage to the flagellar membrane and phospholipid biosynthesis (14, 25), have been described. The mechanisms of action of this class of drugs against tumor cell lines have been linked to (i) nonspecific ion channel formation, (ii) cell signaling inhibition through phospholipase C inactivation, and (iii) inhibition of de novo phosphatidylcholine (PC) and sphingomyelin synthesis, leading to the accumulation of ceramides and apoptosis (33). The generation of lines resistant in vitro (7, 28, 35) has shown that the ability of the cell to take up the drug is one determinant of its sensitivity, since decreased uptake has been described in these resistant lines. Some other proposed mechanisms of resistance of tumor cell lines to HePC and related ether lipid drugs include faster drug metabolism (7), an increased cholesterol content of the plasma membrane (5), and P-glycoprotein overexpression (24). In Leishmania, P-glycoprotein-mediated resistance to ether lipids has been described (20).
It is well established and appears to be a common feature in eukaryotic cells that aminophospholipids such as fluorescent analogs of phosphatidylethanolamine (PE) and phosphatidylserine (PS) are internalized through inward translocation across the plasma membrane, a process which is energy dependent, protein mediated, and stereospecific for natural l isomers (16, 31). Inward translocation, inward transbilayer movement, and flip are synonyms meaning the transfer of an amphiphilic molecule, i.e., a phospholipid, from the outer leaflet to the inner leaflet of the plasma membrane. On the other hand, a similar translocation activity for short-chain PC has been described in only a few cell lines (9, 21, 34). Little is known about the specific uptake systems for alkyl-lysophospholipids, molecules structurally similar to lysophosphatidylcholine. A similar pathway for the uptake of edelfosine and lysophosphatidylcholine has been proposed in macrophages (35), but whether the same route applies to HePC, a phosphocholine derivative with no glycerol backbone, remains to be investigated.
In the present paper, we describe the accumulation of HePC in wild-type and HePC-resistant Leishmania donovani parasites and provide evidence of a defect in the inward translocation of the drug as one of the main mechanisms of resistance to HePC.
MATERIALS AND METHODS
Chemical compounds.
HePC (miltefosine) was from Zentaris (Frankfurt, Germany). Hexadecylphospho[1,2-ethylene-14C]choline ([14C]HePC; 1,33 MBq/mmol) was synthesized by Amersham Pharmacia Biotech (Little Chalfont, United Kingdom). N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino-PS (C6-NBD-PS), C6-NBD-PC, and C6-NBD-PE were from Avanti Polar Lipids (Birmingham, Ala.). N-(3-Triethylammoniumpropyl)-4-(p-diethylaminophenyl-hexatrienyl)pyridinium dibromide (FM 4-64) was from Molecular Probes (Leiden, The Netherlands). N-Ethylmaleimide (NEM) and sodium azide (NaN3) were from Sigma-Aldrich. Fatty acid-free bovine serum albumin (BSA) was from Calbiochem. All other reagents were from Sigma-Aldrich.
Strains and parasite culture.
Promastigote forms of wild-type L. donovani (MHOM/ET/67/HU3) and the HePC-resistant line (HePC-R; M-40 R), described previously (26), were maintained at 28°C in the following culture medium: medium 199 (Gibco) supplemented with 40 mM HEPES (Sigma), 100 μM adenosine (Sigma), hemin (0.2% of a 250-μg/ml stock solution; Sigma), and 10% heat-inactivated fetal bovine serum (Gibco). The HePC-R line presents a phenotype of cross-resistance to edelfosine (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine), a related ether lipid drug 12-fold more toxic in the parental wild-type line.
Determination of HePC accumulation.
Unless otherwise specified, logarithmic-phase parasites were incubated at a density of 2 × 107 per ml in culture medium with 0.09 μCi of [14C]HePC per ml (2.5 μM) at 28°C. At the indicated time points (5, 15, 60, 180, and 360 min), 1-ml aliquots were removed and placed on ice. The parasites were spun down in a microcentrifuge and washed two times in 0.2 ml of phosphate-buffered saline (PBS; 1.2 mM KH2PO4, 8.1 mM Na2HPO4, 130 mM NaCl, and 2.6 mM KCl adjusted to pH 7.4) containing 10 mg of BSA per ml for 5 min on ice, followed by a wash in PBS. This washing step with buffer containing albumin (back-exchange) is reported to remove short-chain phospholipid analogs from the external surface of the plasma membrane (16, 17, 27). Finally, the cell pellet was resuspended in 0.1 ml of 1% Triton X-100. Ten microliters of the sample was used for protein determination by use of the Bradford kit (Bio-Rad), and the remaining volume was used to determine cell-associated radioactivity by liquid scintillation counting.
HePC efflux studies.
Parasites (2 × 107 per ml) were loaded at 28°C with different [14C]HePC concentrations in order to allow similar labeling. Wild-type parasites were loaded for 35 min with 0.225 μCi/ml (6.75 μM), whereas HePC-R parasites were loaded for 1 h with 2.7 μCi/ml (75 μM) in culture medium. After the parasites were washed with fresh medium, efflux was initiated at 28°C, and the radioactivity retained was measured at different time points (0, 15, 40, and 60 min), as described above.
Metabolism of HePC.
The parasites were incubated at a density of 4 × 107 per ml in culture medium with 0.18 μCi of [14C]HePC per ml (5 μM). After 6 h at 28°C, two 0.5-ml aliquots were prepared. In one aliquot, both cells and medium were recovered together. In the other aliquot, the medium was removed and the cell pellet was washed with BSA-containing PBS buffer before extraction of total lipids from the samples, as described previously (35). Samples were separated on silica gel 60 thin-layer chromatography plates (Merck) with chloroform-methanol-acetic acid-H2O (75:75:16:8 [vol/vol]). Finally, the plates were exposed to X-ray film at −70°C.
Energy depletion and protein modification experiments.
The parasites were washed two times in HEPES-buffered saline (HBS; 21 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM NaH2PO4, and 20 mM glucose adjusted to pH 7.4) and resuspended in the same buffer at 4 × 107 per ml. For the energy depletion studies, the parasites were preincubated with 20 mM NaN3 for 30 min at 28°C in HBS buffer without glucose. For protein modification, the parasites were treated with either 0.2 or 1 mM NEM for 15 min on ice, centrifuged, and resuspended in fresh HBS. Finally, 1 volume of 0.18 μCi of [14C]HePC per ml (5 μM) in HBS (HBS without glucose for the experiments with NaN3) plus 1% BSA was added, resulting in a final cell density of 2 × 107 per ml. After 1 h of incubation at 28°C or on ice, the amount of drug incorporated into the cells was determined after the back-exchange procedure, as described above. Parasites incubated in HBS at 28°C were used as controls.
Fluorescent phospholipid analog accumulation.
C6-NBD phospholipid accumulation was measured by flow cytometry analysis. Parasites (4 × 106/ml) were preincubated in culture medium with 0.5 μM phenylmethylsulfonyl fluoride for 30 min at 28°C. The parasites were then incubated with the fluorescent phospholipid analogs (3 μM C6-NBD-PC, 3 μM C6-NBD-PE, or 6 μM C6-NBD-PS) for 30 min at 28°C in culture medium. The fluorescent analogs were added directly from an ethanol stock solution. After the samples were washed with cold culture medium to extract the fraction bound to the outer leaflet of the plasma membrane and then with cold PBS, the samples were maintained on ice and the cellular fluorescence was measured by flow cytometry in a FACScan flow cytometer (Becton-Dickinson, San Jose, Calif.) equipped with an argon laser operating at 488 nm. The cells were gated to eliminate dead cells and debris, and the cellular fluorescence was quantified by scanning the emission between 515 and 545 nm (FL-1) by using Cell Quest software. Accumulation ratios were determined as the fluorescence intensity ratio among wild-type and resistant parasites.
Measurements of endocytosis.
The uptake of the endocytosis marker FM 4-64 was measured by flow cytometry. A total of 4 × 106 parasites/ml were incubated with 5 μM FM 4-64 in HBS plus 0.5% BSA at 28°C or on ice for 30 min. Afterward, the parasites either were washed one time with cold PBS to remove the label bound to the plasma membrane and flagellar pocket and then resuspended in PBS or were directly analyzed by measuring their cellular fluorescence as described above, but by scanning the emission between 615 and 680 nm (FL-3).
Statistical analysis.
Results are expressed as means ± standard errors of the means or as proportions. Statistical significance was calculated by using Student's t test.
RESULTS
Uptake and binding of HePC.
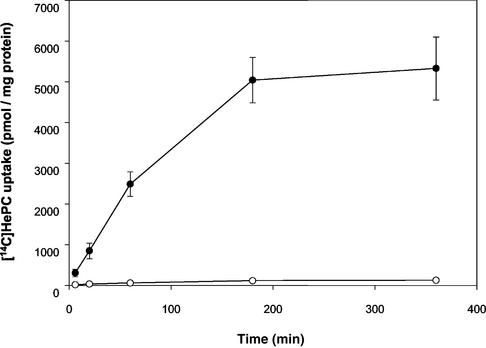
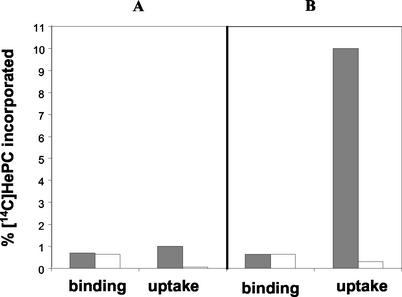
HePC-R promastigotes showed a >95% reduction in their ability to take up [14C]HePC from the medium (Fig. 1). Under in vitro culture conditions, approximately 10% of the radioactivity added was taken up within 1 h by wild-type cells, and saturation was reached in about 6 h, with 22% accumulation. Following incubation on ice, a condition that blocks endocytosis but that keeps a residual translocation activity (9), [14C]HePC accumulation was about 90% higher by wild-type parasites than resistant ones at different time points (data not shown). Removal of the drug fraction bound to the plasma membrane by back-exchange with BSA allowed us to measure the total amount of HePC incorporated (by washing with PBS only) and the fraction internalized (the radioactivity remaining after back-exchange) independently. The levels of binding (the total amount incorporated minus the internalized fraction) of [14C]HePC were similar in both parasite lines (P > 0.05) and constant through the time course of the study, showing that the equilibrium between the HePC bound to BSA and the fraction partitioned within the cell membranes is constant under given conditions (Fig. 2). About 0.7% of the total radioactivity added was bound to the cell surface under the conditions used.
FIG. 1.
Time-dependent uptake of [14C]HePC. Labeling of wild-type (solid circles) and HePC-R (open circles) promastigotes was performed as described in Materials and Methods. All values represent the means ± standard errors of the means of three independent experiments, each of which was performed in duplicate.
FIG. 2.
Binding and uptake of [14C]HePC. Wild-type (solid bars) and HePC-R (open bars) parasites were incubated with [14C]HePC for 5 min (A) and 60 min (B), as described in Materials and Methods. The uptake (internalized HePC) data were measured after two washes with BSA-containing buffer to remove the drug bound to the plasma membrane, whereas the binding (BSA exchangeable fraction) data were determined as the total amount of HePC incorporated after two washings in PBS without BSA minus the corresponding uptake amount. The data shown are the mean values of three independent experiments, each of which was performed in duplicate. Standard errors were below 10%. Results are expressed as the percentage of the total radioactivity incorporated normalized to the protein concentration.
Efflux of HePC.
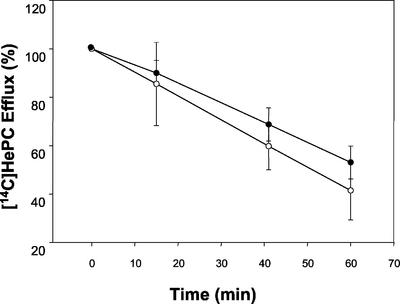
To study whether the reduced level of accumulation of HePC in the resistant parasites was due to HePC efflux, wild-type and HePC-R parasites were loaded under conditions that yielded similar amounts of intracellular drug, and the amount of drug retained in parasites maintained in drug-free culture medium was measured at different time points. The efflux of [14C]HePC was linear in both parasite lines (Fig. 3) and was only slightly faster for resistant parasites. Thus, differences in levels of HePC accumulation are not due to increased efflux activity but are due to a defect in drug uptake.
FIG. 3.
[14C]HePC efflux. Wild-type (solid circles) and HePC-R (open circles) parasites were preincubated with [14C]HePC at 28°C as described in Materials and Methods, and the decay in radioactivity was monitored at different times (15, 40, and 60 min). The data are expressed as the percentage of the initial amount of [14C]HePC incorporated (in counts per minute per microgram of protein) and represent the means ± standard errors of three independent experiments, each of which was performed in duplicate.
Metabolism of HePC.
Previous studies have demonstrated that HePC, as well as alkyl-lysophospholipids, are only very slowly metabolized in mammalian cells (2); and this assumption is also accepted for Leishmania parasites, although it has not been tested yet. However, the possibility of an increased breakdown or modification of HePC could explain the resistance of the HePC-R line to this compound. Consequently, we looked into [14C]HePC metabolism in wild-type and HePC-R parasites. The label in [14C]HePC is present in the ethyl group of the choline. The action of phospholipase C or D would release a labeled choline that would not appear within the lipid extract. Incorporation of this choline into phospholipids would be reflected by a lower band in the thin-layer chromatograph. As observed in Fig. 4, there is no metabolism of [14C]HePC by either the wild-type or the HePC-R line after 6 h of incubation. Under the conditions used, the majority of the label was cell associated in wild-type parasites, but almost none of it was cell associated in resistant ones (lanes 4 and 5, respectively). Furthermore, when cells and medium were harvested together, the signal was a unique band similar for wild-type and resistant parasites (lanes 2 and 3), ruling out any significant breakage of the drug in any of the cell lines. Thus, it is clear that the reduced HePC load in resistant parasites is not due to metabolism.
FIG. 4.
Lack of metabolism of [14C]HePC. Cells were incubated for 6 h at 28°C in the presence of [14C]HePC. Both cells and medium (lanes 2 and 3) or just the cell pellets (lanes 4 and 5) were recovered, and lipids were extracted for analysis by thin-layer chromatography as described in Materials and Methods. Lane 1, 0.9 μCi of [14C]HePC from an ethanol stock solution, lanes 2 and 4, wild-type parasites; lanes 3 and 5, HePC-R parasites.
Energy, protein, and temperature dependence of HePC uptake.
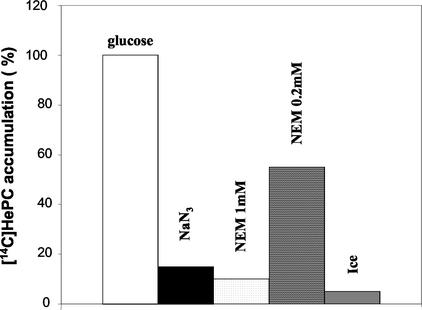
We studied the energy, protein, and temperature dependence of the [14C]HePC uptake process in the wild-type line. To deplete intracellular ATP pools, the parasites were pretreated with 20 mM NaN3. Treatment with NEM has been described to chemically modify sulfhydryl residues, leading to protein inactivation. The uptake of HePC seems to be a protein-mediated active process, since both ATP depletion and protein inactivation greatly reduced the level of HePC accumulation in wild-type promastigotes (P < 0.01) (Fig. 5). No more than 15% of the accumulation measured for the glucose-treated control was observed after preincubation with 20 mM NaN3 for 30 min at 28°C. Treatment with 1 mM NEM prevented HePC accumulation, reaching no more than 10% of that measured for the controls, whereas a lower NEM concentration (0.2 mM) decreased the level of accumulation to 55% of that for the controls. Finally, incubation on ice instead of 28°C also decreased the level of HePC uptake to about 5% of that for the controls. Under the same conditions, the level of HePC accumulation was also significantly reduced in the resistant line (data not shown), probably due to inhibition of endocytosis.
FIG. 5.
Effects of temperature, energy depletion, and protein inactivation on [14C]HePC uptake in the wild-type L. donovani line. Wild-type parasites were incubated with 20 mM NaN3 in HBS buffer without glucose or 0.2 and 1 mM NEM in HBS buffer, as described in Materials and Methods. After 1 h of incubation in the presence of [14C]HePC and 0.5% BSA at 28°C or on ice, the amount of drug incorporated in the cells was determined. The data shown are the means of three independent experiments, each of which was performed in duplicate, and are expressed as the percentage of HePC accumulation compared to that for the controls incubated in HBS buffer only at 28°C. Standard errors were below 15%.
Uptake of C6-NBD-labeled phospholipids.
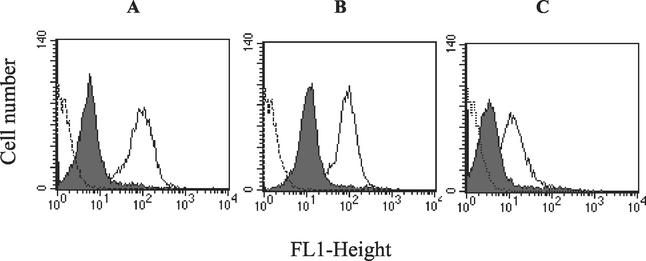
It is unknown whether HePC and edelfosine uptake follows the same biochemical routes used for other short-chain phospholipids. To study whether the uptake of other phospholipid derivatives was also altered in the HePC-R line, we determined the accumulation of fluorescent C6-NBD-phospholipid analogs in culture medium by flow cytometry assays. Interestingly, the reduced uptake by the HePC-R line was not specific to just HePC. The level of accumulation of the acyl-phosphatidylcholine derivative C6-NBD-PC was also reduced. C6-NBD-PC, a compound that chemically resembles edelfosine, showed an accumulation ratio between wild-type and HePC-R parasites of 14 (Fig. 6A). We then studied the effect of the polar head group in the uptake profile. HePC-R promastigotes were also less able to take up an ethanolamine phospholipid analog, C6-NBD-PE, with an accumulation ratio of 8 (Fig. 6B). This phospholipid was taken up by wild-type promastigotes with an efficiency similar to that for the choline analog. Finally, we tested the uptake of the serine phospholipid analog, C6-NBD-PS, but at higher concentrations, due to the less efficient accumulation of this analog by wild-type parasites. The level of uptake was also significantly reduced in resistant parasites, with an accumulation ratio of 5 (Fig. 6C).
FIG. 6.
Cellular accumulation of C6-NBD-labeled phospholipids. Fluorescence intensity histograms were obtained by flow cytometry analysis after incubation of wild-type (open profiles) and HePC-R (solid profiles) parasites with 3 μM C6-NBD-PC (A), 3 μM C6-NBD-PE (B), or 6 μM C6-NBD-PS (C) for 30 min at 28°C in culture medium, as described in Materials and Methods. Parasite autofluorescence (broken profiles) is also shown. A total of 10,000 cells were counted for each histogram. Experiments were repeated three times and gave essentially the same profiles as the ones shown here. All values were significantly different (P < 0.01) between wild-type and resistant parasites.
Endocytosis function.
To determine whether a defect in endocytosis is one of the factors preventing HePC and NBD analog uptake in the HePC-R line, we studied the accumulation of a fluorescent endocytosis marker. FM 4-64 has previously been used as an endocytosis marker in Leishmania parasites (18). This compound binds to the plasma membrane and the flagellar pocket, from which it is introduced by endocytosis to the multivesicular network, the Leishmania lysosomes. The uptake of FM 4-64 was similar for both wild-type and HePC-R lines. After 30 min of incubation at 28°C, the fraction internalized was 23% of the total marker bound to the parasites (the amount of compound internalized plus the fraction bound to the plasma membrane and the flagellar pocket, measured without washing). When the incubation was performed on ice for 30 min, conditions in which endocytosis is supposed to be blocked, no more than 3% of the marker bound was taken up inside the cells.
DISCUSSION
In this work we have studied the accumulation of HePC in wild-type and HePC-R Leishmania promastigotes, suggesting a defect in HePC inward translocation and accumulation as one of the main mechanisms of resistance to alkylphosphocholines in our Leishmania line with drug-induced resistance.
The development and initial characterization of this HePC-R L. donovani line have been described elsewhere (26). The stability of the resistant phenotype, the absence of DNA amplification as a drug response, and the lack of involvement of P-glycoprotein-like transporters in the mechanism of resistance suggested that the presence of stable mutations accounts for the resistance phenotype. Considering that one of the main determinants of cell sensitivity to alkylphosphocholines and alkyl-lysophospholipids is drug uptake (3, 6, 7, 28, 32, 35), we studied the accumulation of [14C]HePC by wild-type and HePC-R Leishmania promastigotes. Interestingly, the resistant parasites show a significantly reduced level of uptake of the drug, as seen in Fig. 1. It is well established that short-chain phospholipids such as HePC or C6-NBD-PC bind to the albumin of the culture medium, which acts as a reservoir from which the phospholipids are partitioned to the outer leaflet of the plasma membrane. It is considered that no receptor mediates binding of HePC to the plasma membrane (28). This idea is also supported by our data, as observed in Fig. 2, in which the rates of binding of HePC to both wild-type and resistant parasites were similar and constant over time. In fact, the defective HePC accumulation in the resistant parasites is caused not by differences in drug binding but by a reduction in its level of uptake. Since drug efflux is similar for both wild-type and resistant parasites (Fig. 3) and no metabolism is observed for HePC (Fig. 4), the differential uptake must be due to an internalization defect in HePC-R parasites.
Interestingly, our resistant line is not the first organism in which the inability to take up alkyl-lysophospholipids induces resistance. Remarkably, the generation of RAW macrophage-like cells resistant to edelfosine by mutagenesis followed by selection against the drug (35) produced a phenotype very similar to that found in our resistant Leishmania parasites. The investigators proved by hybridization studies that reversion of the resistance phenotype was possible in heterozygous lines, suggesting that a defect in just one gene is probably responsible for this phenotype.
The protein, energy, and temperature dependence of the uptake in wild-type parasites (Fig. 5) also suggests a loss of function in the resistant parasites. This dependence is in agreement with previous results describing the uptake of C6-NBD-PC and C6-NBD-PE from the plasma membranes of yeasts (13), the flippase activity dependent on the Ros3 protein in yeasts (12), and the inward translocation activity of short-chain PC in MDCK epithelial cells (21), all of which strongly depend on ATP.
As shown in the C6-NBD-phospholipid accumulation studies by flow cytometry (Fig. 6), resistant parasites also present with an impairment in the uptake of short-chain phospholipids, independently of the polar head group. Furthermore, their uptake also depends on temperature, energy, and membrane proteins, just as HePC uptake does (data not shown). These results suggest that all these phospholipids are taken up in L. donovani promastigotes by one similar route or through pathways that require at least one common factor that is defective in resistant parasites. It is of interest to draw attention to the chemical differences between HePC and glycerophospholipids such as C6-NBD-PC, C6-NBD-PE, and edelfosine. Even though HePC is more hydrophilic and such a simple molecule, it must be recognized by the same protein(s) responsible for the uptake of short-chain glycerophospholipids. Whether a similar activity for the uptake of long-chain physiological phospholipids is also present in Leishmania remains to be investigated. In order to be internalized inside the cells, HePC as well as other water-soluble phospholipids needs to either flip from the outer to the inner side of the plasma membrane or be endocytosed together with a fraction of the membrane. In any case, the action of one or more proteins is likely to be needed, since short-chain phospholipids flip in synthetic bilayers with half-lives of hours (22). We propose that transbilayer movement across the plasma membrane, the nonendocytic pathway, or inward translocation must be the main determinant of HePC uptake in wild-type Leishmania promastigotes and that such activity is absent in the resistant line. In fact, endocytosis of the bulk-phase marker FM 4-64 is similar in both wild-type and resistant parasites. If endocytosis were the main factor affecting HePC uptake and accumulation, resistant parasites would not behave normally in the uptake of FM 4-64, or the defect in HePC and C6-NBD derivative uptake would not be so severe. Moreover, studies of the accumulation of [14C]HePC at 0°C, conditions under which endocytosis is prevented, still showed a significant translocation of the drug in wild-type parasites but none in resistant ones. Studies with mammalian cell lines suggested that choline phospholipids enter cells through endocytosis after membrane binding (19), whereas aminophospholipids enter mainly through transbilayer movement (16), and this general opinion had been extrapolated to ether lipid uptake (3, 28). However, Hofmann and coauthors (23) showed in a panel of tumor cell lines that endocytosis does not correlate with HePC accumulation. Moreover, recent studies with yeast mutants defective in either vesicular trafficking or endocytosis (9) have proven that C6-NBD-PC and C6-NBD-PE are internalized mainly through transbilayer movement across the plasma membrane and not by endocytosis. Thus, it is likely that those cell types able to internalize C6-NBD-PC through inward translocation are also likely to transport edelfosine and HePC by the same mechanism. The molecular identities of the proteins involved in the inward-directed translocation of PC were unknown until recently, with the identification of Ros3p as a membrane protein essential for the internalization of C6-NBD-PE and C6-NBD-PC in yeasts (12), although no flippase protein has been identified so far. It will be interesting to study whether possible Ros3 protein analogs are present in Leishmania and whether their functionality is related to HePC sensitivity.
The active uptake of short-chain phospholipids has been explained physiologically for different mammalian cell types. For instance, the protein-mediated transport of water-soluble phospholipids across the brush border cells of the rabbit intestine (34) may be relevant for the absorption of phospholipid digestion products. Similarly, the uptake of lysophospholipids and edelfosine by macrophages (35) could be important for the clearance of compounds able to induce biological responses, such as platelet-activating factor or lysophosphatidylcholine. L. donovani promastigotes are able to take up ether lysophospholipids rapidly (1), and these are extensively metabolized within 1 h, mainly through incorporation into physiological plasmalogens. Such an active uptake of lysophospholipids may constitute an advantage for the parasites that could synthesize plasmalogens and normal phospholipids through this pathway instead of from de novo synthesis. Ether lipids such as edelfosine and perifosine are metabolically stable and nondegraded in mammalian cells (2, 4) due to their stable ether linkages. Similarly, HePC is not degraded in Leishmania parasites (Fig. 4). It is tempting to suggest that Leishmania parasites have an active uptake of short-chain phospholipids in order to feed more effectively. Consequently, they incorporate HePC and other ether lipid analogs, which can then exert their toxic actions. Since resistant parasites have the same duplicating time and morphology as their parental line, it is clear that this uptake activity is not essential for promastigotes grown in vitro, but we cannot rule out its importance in intracellular amastigotes in vivo. It will be interesting to test the infectivity of the HePC-R line for macrophages and animal models and their response to HePC under those conditions, as well as to study whether the generation of amastigote lines resistant to HePC by continuous drug pressure is possible.
In summary, we have shown that an impairment in HePC uptake is likely to be the main mechanism of resistance in our HePC-R line. We have also described a new activity in Leishmania characterized by the rapid transbilayer movement of short-chain phospholipids across the plasma membrane, an energy-dependent and protein-mediated activity defective in the resistant line. Whether this loss of function is due to a defect in some of the proteins directly involved in flippase activity, a defect in vesicular trafficking, or any other defect remains to be investigated. We are approaching these questions through proteomic and functional genomic experiments, since Leishmania is an organism amenable to genetic manipulation.
Acknowledgments
This work was supported by EC grant QLRT-2000-01404 and by Spanish grant SAF2001-4562-E. F.J.P.-V. was the recipient of a fellowship from the Ministerio de Educación y Cultura.
We thank Simon L. Croft for critical reading of the manuscript. We also thank Pilar Navarro for expert technical assistance. We are grateful to Zentaris (Frankfurt, Germany) for providing the HePC used in this study.
REFERENCES
- 1.Achterberg, V., and G. Gercken. 1987. Metabolism of ether lysophospholipids in Leishmania donovani promastigotes. Mol. Biochem. Parasitol. 26:277-287. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, G., and R. Bittman. 1998. The inhibition of cell signaling pathways by antitumor ether lipids. Biochim. Biophys. Acta 1390:85-102. [DOI] [PubMed] [Google Scholar]
- 3.Bazill, G. W., and T. M. Dexter. 1990. Role of endocytosis in the action of ether lipids on WEHI-3B, HL60, and FDCP-mix A4 cells. Cancer Res. 50:7505-7512. [PubMed] [Google Scholar]
- 4.Berdel, W. E. 1991. Membrane-interactive lipids as experimental anticancer drugs. Br. J. Cancer 64:208-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diomede, L., F. Colotta, B. Piovani, F. Re, E. J. Modest, and M. Salmona. 1993. Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine. A possible basis for its selective action. Int. J. Cancer 53:124-130. [DOI] [PubMed] [Google Scholar]
- 6.Fleer, E. A., D. Berkovic, H. Eibl, and C. Unger. 1993. Investigations on the cellular uptake of hexadecylphosphocholine. Lipids 28:731-736. [DOI] [PubMed] [Google Scholar]
- 7.Fleer, E. A., D. Berkovic, U. Grunwald, and W. Hiddemann. 1996. Induction of resistance to hexadecylphosphocholine in the highly sensitive human epidermoid tumour cell line KB. Eur. J. Cancer 32:506-511. [DOI] [PubMed] [Google Scholar]
- 8.Ganguly, N. K. 2002. Oral miltefosine may revolutionize treatment of visceral leishmaniasis. TDR News W. H. O. 68:2. [Google Scholar]
- 9.Grant, A. M., P. K. Hanson, L. Malone, and J. W. Nichols. 2001. NBD-labeled phosphatidylcholine and phosphatidylethanolamine are internalized by transbilayer transport across the yeast plasma membrane. Traffic 2:37-50. [DOI] [PubMed] [Google Scholar]
- 10.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 11.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 12.Kato, U., K. Emoto, C. Fredriksson, H. Nakamura, A. Ohta, T. Kobayashi, K. Murakami-Murofushi, T. Kobayashi, and M. Umeda. 2002. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J. Biol. Chem. 277:37855-37862. [DOI] [PubMed] [Google Scholar]
- 13.Kean, L. S., R. S. Fuller, and J. W. Nichols. 1993. Retrograde lipid traffic in yeast: identification of two distinct pathways for internalization of fluorescent-labeled phosphatidylcholine from the plasma membrane. J. Cell Biol. 123:1403-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lira, R., L. M. Contreras, R. M. Rita, and J. A. Urbina. 2001. Mechanism of action of anti-proliferative lysophospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. J. Antimicrob. Chemother. 47:537-546. [DOI] [PubMed] [Google Scholar]
- 15.Lux, H., N. Heise, T. Klenner, D. Hart, and F. R. Opperdoes. 2000. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Mol. Biochem. Parasitol. 111:1-14. [DOI] [PubMed] [Google Scholar]
- 16.Martin, O. C., and R. E. Pagano. 1987. Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. Evidence for a protein-mediated and ATP-dependent process(es). J. Biol. Chem. 262:5890-5898. [PubMed] [Google Scholar]
- 17.Mohandas, N., J. Wyatt, S. F. Mel, M. E. Rossi, and S. B. Shohet. 1982. Lipid translocation across the human erythrocyte membrane. J. Biol. Chem. 257:6537-6543. [PubMed] [Google Scholar]
- 18.Mullin, K. A., B. J. Foth, S. C. Ilgoutz, J. M. Callaghan, J. L. Zawadzki, G. I. McFadden, and M. J. McConville. 2001. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol. Biol. Cell 12:2364-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagano, R. E., and R. G. Sleight. 1985. Defining lipid transport pathways in animal cells. Science 229:1051-1057. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Victoria, J. M., F. J. Perez-Victoria, A. Parodi-Talice, I. A. Jimenez, A. G. Ravelo, S. Castanys, and F. Gamarro. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45:2468-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pomorski, T., A. Herrmann, P. Muller, G. van Meer, and K. Burger. 1999. Protein-mediated inward translocation of phospholipids occurs in both the apical and basolateral plasma membrane domains of epithelial cells. Biochemistry 38:142-150. [DOI] [PubMed] [Google Scholar]
- 22.Raggers, R. J., T. Pomorski, J. C. Holthuis, N. Kalin, and G. van Meer. 2000. Lipid traffic: the ABC of transbilayer movement. Traffic 1:226-234. [DOI] [PubMed] [Google Scholar]
- 23.Rybczynska, M., M. Spitaler, N. G. Knebel, G. Boeck, H. Grunicke, and J. Hofmann. 2001. Effects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivities. Biochem. Pharmacol. 62:765-772. [DOI] [PubMed] [Google Scholar]
- 24.Rybczynska, M., R. Liu, P. Lu, F. J. Sharom, E. Steinfels, A. D. Pietro, M. Spitaler, H. Grunicke, and J. Hofmann. 2001. MDR1 causes resistance to the antitumour drug miltefosine. Br. J. Cancer 84:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santa-Rita, R. M., H. Santos Barbosa, M. N. Meirelles, and S. L. de Castro. 2000. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 75:219-228. [DOI] [PubMed] [Google Scholar]
- 26.Seifert, K., S. Matu, F. J. Pérez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents, in press. [DOI] [PubMed]
- 27.Sleight, R. G., and M. N. Abanto. 1989. Differences in intracellular transport of a fluorescent phosphatidyl choline analog in established cell lines. J. Cell Sci. 939:363-374. [DOI] [PubMed] [Google Scholar]
- 28.Small, G. W., J. C. Strum, and L. W. Daniel. 1997. Characterization of an HL-60 cell variant resistant to the antineoplastic ether lipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine. Lipids 32:715-723. [DOI] [PubMed] [Google Scholar]
- 29.Soto, J., J. Toledo, P. Gutierrez, R. S. Nicholls, J. Padilla, J. Engel, C. Fischer, A. Voss, and J. Berman. 2001. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect. Dis. 33:57-61. [DOI] [PubMed] [Google Scholar]
- 30.Sundar, S., A. Makharia, D. K. More, G. Agrawal, A. Voss, C. Fischer, P. Bachmann, and H. W. Murray. 2000. Short-course of oral miltefosine for treatment of visceral leishmaniasis. Clin. Infect. Dis. 31:1110-1113. [DOI] [PubMed] [Google Scholar]
- 31.Tang, X., M. S. Halleck, R. A. Schlegel, and P. Williamson. 1996. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 272:1495-1497. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsumi, T., A. Tokumura, and S. Kitazawa. 1998. Undifferentiated HL-60 cells internalize an antitumor alkyl ether phospholipid more rapidly than resistant K562 cells. Biochim. Biophys. Acta 1390:73-84. [DOI] [PubMed] [Google Scholar]
- 33.Wieder, T., W. Reutter, C. E. Orfanos, and C. C. Geilen. 1999. Mechanisms of action of phospholipid analogs as anticancer compounds. Prog. Lipid Res. 38:249-259. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Z., and J. W. Nichols. 1994. Protein-mediated transfer of fluorescent-labeled phospholipids across brush border of rabbit intestine. Am. J. Physiol. 267:80-86. [DOI] [PubMed] [Google Scholar]
- 35.Zoeller, R. A., M. D. Layne, and E. J. Modest. 1995. Animal cell mutants unable to take up biologically active glycerophospholipids. J. Lipid Res. 36:1866-1875. [PubMed] [Google Scholar]