Abstract
The pharmacokinetics of [14C]viramidine, a prodrug of ribavirin, were studied in rats (30 mg/kg of body weight) and monkeys (10 mg/kg) following intravenous (i.v.) and oral administration. The levels of oral absorption and bioavailabilities were 61.7 and 9.91%, respectively, in rats and 43.9 and 13.6%, respectively, in monkeys. Following i.v. administration, the elimination half-lives were 2.7 h in rats and 28.9 h in monkeys. Total body clearances were 14.0 liters/h/kg in rats and 1.23 liters/h/kg in monkeys; the apparent volumes of distribution were 15.6 liters/kg in rats and 18.6 liters/kg in monkeys. Following oral administration, viramidine was extensively converted to ribavirin, followed by further metabolism of ribavirin in both species, with a faster rate of metabolism in rats than in monkeys. In rats, excretion of total radioactivity in urine accounted for 77.0% of the i.v. dose and 60.8% of the oral dose, while in monkeys it accounted for 44.4% of the i.v. dose and 39.0% of the oral dose. The amount of unchanged viramidine and ribavirin in urine was small in both species after i.v. and oral administration of viramidine.
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxam-ide) is a purine nucleoside analog with activity against a variety of DNA and RNA viral infections (10, 11). The clinical efficacies of interferon alfa-2b and pegylated interferon alfa-2b in combination with ribavirin are about 40% (6, 9) and 54% (5), respectively, in terms of a sustained virologic response when they are used to treat chronic hepatitis C virus infection. At present, combination therapy with ribavirin and pegylated interferon alfa-2b is the “gold standard” for the treatment of chronic hepatitis C. However, ribavirin has a dose-limiting side effect, hemolytic anemia. After absorption into the circulation, a significant portion of ribavirin is transported into red blood cells (RBCs) (2) and phosphorylated into the triphosphate form (8). Due to the lack of phosphatase activity in erythrocytes, phosphorylated derivatives of ribavirin are retained intracellularly and accumulate over time, leading to hemolytic anemia (1, 3). This side effect is dose limiting and necessitates dose reduction and withdrawal in some patients. A new ribavirin which retains those properties deemed critical in the treatment of chronic hepatitis C, but with less potential for hemolytic anemia, would be highly desirable.
Viramidine is a prodrug of ribavirin. Preliminary studies in our laboratories indicated that viramidine can be retained and converted to ribavirin in the liver (J. Lau et al., Abstr. 52nd Am. Assoc. Study Liver Dis., abstr. 1020, 2001). Following daily oral dosing (30 mg/kg of body weight) of cynomolgus monkeys with [14C]viramidine and [14C]ribavirin for 10 days, radioactivity levels in the liver were three times higher in those treated with [14C]viramidine than in those treated with [14C]ribavirin, but the radioactivity levels in RBCs and plasma were 50% lower (C. Lin et al., Abstr. 52nd AASLD, abstr. 1123, 2001). Since the liver is the target for hepatitis C virus infection and since RBCs are the target for ribavirin toxicity, the use of viramidine may provide an opportunity to improve the efficacy and reduce the toxicity associated with ribavirin.
The aim of this study was to determine the absorption, pharmacokinetics, metabolism, and excretion of viramidine in rats and monkeys.
MATERIALS AND METHODS
Compound.
The compound [5-14C]viramidine (Fig. 1) was synthesized by using [14C]barium carbonate as a precursor. The labeled nucleoside was extensively purified by column chromatography and repetitive recrystallization. The chemical identity and purity were verified by mass spectrometry and proton magnetic resonance spectrometry. The radiopurity (>98%) of the preparation was confirmed by high-pressure liquid chromatography (HPLC) coupled with radioflow detection. Ribavirin, triazole carboxamide (TCONH2), and triazole carboxylic acid nucleoside (RTCOOH) were obtained from Ribapharm, Inc.
FIG. 1.
Chemical structures of viramidine and ribavirin.
Drug administration and sample collection for rats.
Following an overnight fast, six male Sprague-Dawley rats received an intravenous (i.v.) dose of 30 mg of [14C]viramidine per kg via a tail vein and six rats received an oral dose of 30 mg of [14C]viramidine (75 μCi) per kg via oral gavage. Serial blood samples from three rats receiving drug by each dose route were collected directly in heparinized Vacutainer tubes and immediately centrifuged to harvest the plasma. In a separate study, urine and fecal samples from three rats receiving drug by each dose route were collected for analysis.
Drug administration and sample collection for cynomolgus monkeys.
Following an overnight fast, four male cynomolgus monkeys received an i.v. bolus dose of 10 mg of [14C]viramidine per kg via a saphenous vein or an oral dose of 10 mg of [14C]viramidine (0.5 mCi) per kg via oral gavage. Serial blood samples from each monkey were collected directly in heparinized Vacutainer tubes and immediately centrifuged to harvest the plasma. Urine and fecal samples were collected from each monkey for analysis.
Measurement of radioactivity.
The levels of radioactivity in plasma (0.5 ml) and urine (0.2 ml) were measured by using Ultima Gold XR scintillation cocktail and a liquid scintillation counter (model 1900TR; Packard Instrument Company, Meriden, Conn.). Fecal samples were combusted in a sample oxidizer (model 306; Packard Instrument Company), and the resulting 14CO2 was trapped in a mixture of Perma Fluor E+ and Carbo-Sorb E, followed by liquid scintillation counting. Scintillation counting data were automatically corrected for counting efficiency, based on an external standard, and an instrument-stored quench curve generated from a series of sealed quenched standards.
LC-MS-MS method for determination of viramidine and ribavirin in plasma.
The analytical liquid chromatography (LC)-mass spectrometry (MS)-MS method involved the addition of an internal standard (acyclovir), protein precipitation with acetonitrile, solvent evaporation, reconstitution of residue, and separation on an Inertsil Silica column, followed by MS-MS detection. A Perkin-Elmer Sciex API 3000 instrument in the multiple-reaction monitoring mode with positive electrospray ionization was used to monitor the transitions from m/z 245 to 113 and 259 to 128 for ribavirin and the internal standard.
For viramidine, the limit of quantitation was 10 ng/ml, with a coefficient of variation (CV) of 9% and a bias of 1.3%. Linear regression of the concentration data (range, 10 to 5,000 ng/ml) yielded a correlation coefficient of >0.999. The LC-MS-MS method was accurate (bias, <6%) and reproducible (CV, <8.2%).
For ribavirin, the limit of quantitation was 10 ng/ml, with a CV of 8.5% and a bias of 1.8%. Linear regression of the concentration data (range, 10 to 5,000 ng/ml) yielded a correlation coefficient of >0.999. The LC-MS-MS method was accurate (bias, <5%) and reproducible (CV, <8.0%).
HPLC procedure for studying the metabolic profiles of the drug in plasma and urine.
Plasma and urine were mixed with an equal volume of acetonitrile. The mixtures were centrifuged, and the supernatant was injected into the HPLC apparatus coupled with a radioactivity detector (β-Ram model 2; INU Systems, Inc., Tampa, Fla.). The high-pressure liquid chromatograph (model SCL 10VP; Shimadzu) was equipped with an Amide-80 column (4.6 mm by 110 cm; TosoHaas). The column was eluted with a solvent mixture consisting of 95% of an organic mobile phase (acetonitrile) and 10% of an aqueous phase (25 mM ammonium acetate) at a flow rate of 1.2 ml/min. Immediately after the injection, the solvent mixture was switched to 70% organic phase and 30% aqueous phase. Identification of radioactive peaks in rat and monkey plasma and urine as ribavirin, TCONH2, and RTCOOH was based on the retention times and LC-MS-MS of authentic standards.
Pharmacokinetic analysis.
The concentrations of radioactivity, viramidine, and ribavirin in plasma were used to determine the values of the pharmacokinetic parameters by noncompartmental methods (Win Nonlin-2; Pharsight Corp. Mountain View, Calif.). The maximum concentration in plasma (Cmax) and the time to Cmax (Tmax) were observed values. The area under the concentration-time curve (AUC) to the last quantifiable sampling time (tf), AUCtf, was computed by using the linear trapezoidal rule. The AUC to infinity, AUCI, was calculated as the sum of AUCtf and the quotient of the last measurable concentration (Ctf) and the elimination rate constant (K). K was estimated as the negative slope of the regression of the log concentration versus time. The half-life (t1/2) was calculated by dividing 0.693 by K. The apparent total body clearance (CL) was calculated as the ratio of the dose to AUCI. The volume of distribution (V) was calculated as the ratio of CL to K.
RESULTS
Concentrations of viramidine in rat plasma.
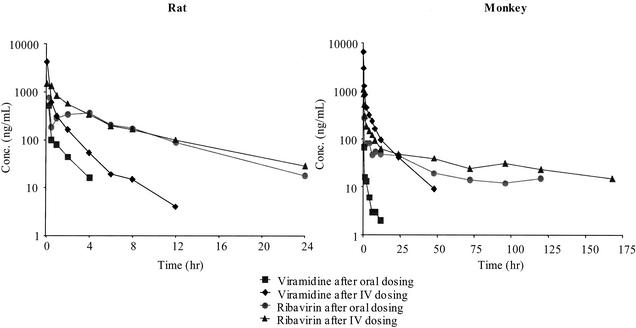
Following i.v. administration of viramidine, plasma viramidine levels decreased with time (Fig. 2), with an elimination t1/2 of 2.7 h. The mean V was 15.6 liters/kg, and the mean CL was 14.0 liters/h/kg. Following oral dosing of viramidine, viramidine was rapidly absorbed, with a Tmax of 0.50 h and a Cmax of 0.10 μg/ml. By comparison of the AUCI of viramidine obtained after oral dosing to that obtained after i.v. dosing, the absolute bioavailability of viramidine was calculated to be 9.91% (Table 1).
FIG. 2.
Concentrations of viramidine and ribavirin in rat and monkey plasma following i.v. (IV) and oral dosing of viramidine
TABLE 1.
Pharmacokinetic parameters for rats and monkeys after i.v. and oral dosing of [14C]viramidinea
| Route | Viramidine in plasma
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (mg/liter) | Tmax (h) | AUCtf (μg · h/ml) | AUCI (μg · h/ml) | Bioavailability (%) | t1/2 (h) | CL (liters/h/kg) | V (liters/kg) | |
| Rat (n = 3) | ||||||||
| i.v. | 6.32 (5.43-7.71) | 0 | 2.27 (2.12-2.38) | 2.32 (2.18-2.42) | NAb | 2.7 (2.1-3.5) | 14.0 (13.3-14.5) | 15.6 (11.0-19.7) |
| Oral | 0.10 (0.09-0.11) | 0.50 | 0.20 (0.17-0.21) | 0.23 (0.20-0.26) | 9.91 | 1.4 (1.2-1.8) | 142 (125-168) | NA |
| Monkey (n = 3) | ||||||||
| i.v. | 9.66 (6.08-14.9) | 0 | 7.31 (4.47-12.0) | 7.90 (4.63-12.9) | NA | 28.9 (5.9-56.3) | 1.23 (0.69-1.82) | 18.6 (8.78-31.2) |
| Oralc | 0.156 (0.153-0.157) | 1 | 0.992 (0.913-1.07) | 1.14 (1.08-1.20) | 13.6 | 8.4 (6.1-10.7) | 7.22 (6.87-7.57) | NA |
Rats received a dose of 30 mg/kg, and monkeys received a dose of 10 mg/kg. The values are means (ranges).
NA, not applicable.
Two monkeys had significant carryover from previous i.v. dosing, and so data for those two monkeys were not included in the calculation.
Concentrations of ribavirin in rat plasma.
After i.v. administration of viramidine, plasma ribavirin levels decreased with time (Fig. 2), with an elimination t1/2 of 6.6 h. After oral dosing of viramidine, plasma ribavirin levels reached a maximum at 4 h, with a Cmax of 0.362 μg/ml. Thereafter, plasma ribavirin levels decreased with time, with an elimination t1/2 of 5.0 h, which is longer than that of viramidine after either i.v. dosing (2.7 h) or oral dosing (1.4 h) of viramidine. The ribavirin AUCIs were 5.33 μg · h/ml after i.v. dosing of viramidine and 3.39 μg · h/ml after oral dosing of viramidine (Table 1).
Radioactivity levels in rat plasma.
Following i.v. administration of [14C]viramidine, the plasma radioactivity level declined, with a t1/2 of approximately 10.4 h. The AUCtfs for radioactivity in plasma were 43.5 μg equivalents · h/ml after oral dosing and 32.6 μg equivalents · h/ml after i.v. dosing (Table 1).
Concentrations of viramidine in monkey plasma.
Following i.v. administration of viramidine, plasma viramidine levels decreased with time (Fig. 2), with an elimination t1/2 of 28.9 h. The mean V was 18.6 liters/kg, and the mean CL was 1.23 liters/h/kg. After oral administration of viramidine, viramidine was rapidly absorbed, with a Tmax of 1 h and a Cmax of 0.156 μg/ml. By comparison of the viramidine AUCI obtained after oral dosing to that obtained after i.v. dosing, the absolute bioavailability of viramidine was calculated to be 13.6% (Table 1).
Concentrations of ribavirin in monkey plasma.
Following i.v. administration of viramidine, plasma ribavirin levels decreased with time (Fig. 2), with an elimination t1/2 of 80.9 h. Following oral dosing of viramidine, plasma ribavirin levels reached a maximum at 3.0 h, with a Cmax of 0.089 μg/ml. Thereafter, plasma ribavirin levels declined with time, with an elimination t1/2 of 62.2 h. The ribavirin AUCIs were 9.68 μg · h/ml after i.v. administration of viramidine and 4.62 μg · h/ml after oral dosing of viramidine.
Radioactivity levels in monkey plasma.
Following i.v. administration of [14C]viramidine, the plasma radioactivity levels declined, with a t1/2 of 88.7 h. The AUCtfs for radioactivity in plasma were 25.0 μg equivalents · h/ml after oral administration and 28.7 μg equivalents · h/ml after i.v. administration.
Urinary and fecal excretion of radioactivity in rats and monkeys.
In rats, 77.0% of the i.v. dose and 60.8% of the oral dose were excreted in urine (0 to 96 h). A total of 1.89% of the i.v. dose and 33.5% of the oral dose were excreted in rat feces (Table 1). These data demonstrate that biliary excretion does not play a significant role in the elimination of viramidine in rats (Table 1). On the basis of the urinary excretion and the total recovery of radioactivity after oral dosing in rats, the level of absorption was estimated to be 61.7%.
In monkeys, 44.4% of the i.v. dose and 39.0% of the oral dose were excreted in urine (0 to 168 h). A total of 0.82% of the i.v. dose and 42.1% of the oral dose were excreted in monkey feces. The data indicate that biliary excretion does not play a significant role in the elimination of viramidine in monkeys. On the basis of urinary excretion and the total recovery of radioactivity after oral dosing in monkeys, the level of absorption was estimated to be 43.9%.
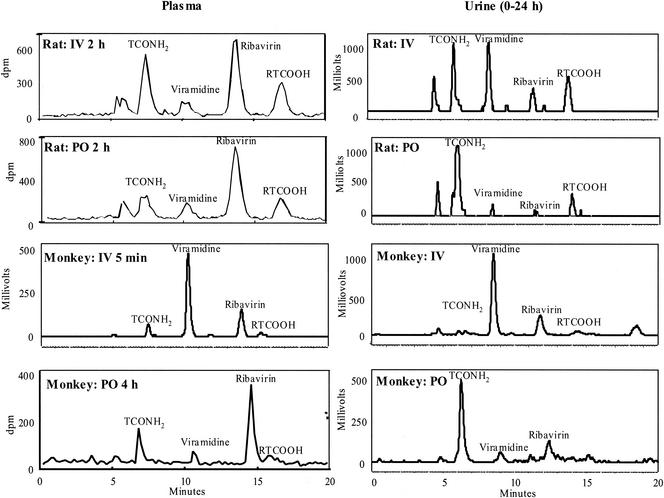
Metabolic profiles in plasma and urine of rats and monkeys.
In rat plasma 2 h after i.v. administration of viramidine, ribavirin and TCONH2 were the two major radioactive peaks, with RTCOOH and viramidine being minor peaks (Fig. 3). However, at 2 h after oral administration of viramidine, ribavirin was the only major radioactive peak, with small amounts of TCONH2, viramidine, and RTCOOH detected. In monkey plasma 5 min after i.v. administration of viramidine, viramidine was the predominant radioactive peak, with small amounts of ribavirin and TCONH2 detected (Fig. 3), indicating a lesser extent of metabolism in monkeys than in rats. At 4 h after oral administration of viramidine, ribavirin was the only major radioactive peak, indicating a significant first-pass effect in monkeys.
FIG. 3.
Metabolic profile of [14C]viramidine in rat and monkey plasma and urine following i.v. (IV) and oral (PO) dosing of viramidine.
In rat urine collected from 0 to 24 h after i.v. administration of viramidine, viramidine and TCONH2 were the major radioactive peaks, while after oral administration of viramidine, TCONH2 was the predominant radioactive peak, with very small amounts of RTCOOH detected (Fig. 3). In monkey urine collected from 0 to 24 h after i.v. administration of viramidine, viramidine was the major radioactive peak, while after oral administration of viramidine, TCONH2 was the major radioactive peak (Fig. 3). These urinary metabolic data are in good agreement with the plasma metabolic data, in that there is more extensive metabolism of viramidine in rats than in monkeys.
DISCUSSION
In rats following oral administration of viramidine, the AUCI of ribavirin (3.39 μg · h/ml) was 15 times the AUCI of viramidine (0.23 μg · h/ml). Similarly, in monkeys following oral administration of viramidine, the AUCI of ribavirin (4.62 μg · h/ml) was four times the AUCI of viramidine (1.14 μg · h/ml). Furthermore, following oral administration of viramidine, the ratios of the viramidine AUCtf to the radioactivity AUCtf were 0.005 for rats and 0.040 for monkeys, whereas the ratios of the ribavirin AUCtf to the radioactivity AUCtf were 0.075 for rats and 0.136 for monkeys. These data suggest that in both rats and monkeys viramidine is orally absorbed and rapidly converted to ribavirin, followed by further metabolism to RTCOOH and TCONH2. Ribavirin has been reported to undergo hydrolytic deamination in vivo and is converted to RTCOOH, a metabolite that does not possess any antiviral activity (4, 7). Recently, Wu et al. (12) reported that the conversion of viramidine to ribavirin was catalyzed by adenosine deaminase. No deamination was observed in the absence of the deaminase or in the presence of 1 nM deoxycoformycin (Calbiochem, La Jolla, Calif.).
It is noteworthy that both the rate of conversion of viramidine to ribavirin and the rate of conversion of ribavirin to other metabolites were much higher in rats than in monkeys. These observations are in good agreement with the findings that the t1/2 of viramidine after i.v. administration in rats (2.7 h) was shorter than that in monkeys (28.9 h) and that the CL of viramidine in rats (14.0 liters/min/kg) was much higher than that in monkeys (1.23 liters/min/kg). These findings are also in good agreement with the observation that following oral dosing of [14C]viramidine, viramidine was the only major radioactive peak in monkey urine collected over 0 to 24 h, whereas viramidine, TCONH2, ribavirin, and RTCOOH were all major radioactive peaks in rat urine collected over 0 to 24 h. The differences in conversion may be related to the presence of higher levels of deaminase and hydroxylase activity in rats compared to those in monkeys.
On the basis of the CL of viramidine for rats and monkeys, allometric scaling (log CL versus log body weight) was used to estimate the CL of viramidine in humans, which was estimated to be about 0.15 liter/h/kg. Given the phylogenetic proximity of monkeys and humans, it may be anticipated that the pharmacokinetic profile and metabolism of viramidine in humans are likely to be more similar to those in monkeys than to those in rats. If this were the case, viramidine would be converted to ribavirin at a rate that will allow the conversion of ribavirin to its phosphorylated metabolites, which are then retained in the liver. A too rapid conversion of viramidine to ribavirin could saturate the phosphorylation processes and favor the redistribution of the converted ribavirin to RBCs.
The t1/2s of ribavirin were previously found to be 9.9 h in rats and 130 h in monkeys following i.v. dosing of ribavirin (4). In this study, the t1/2s of ribavirin after i.v. dosing of viramidine were 6.6 h in rats and 80.9 h in monkeys. These t1/2s represent the net formation of ribavirin from viramidine and the elimination of ribavirin by both hepatic and renal clearance. These t1/2s were slightly shorter than the viramidine t1/2s after i.v. dosing of viramidine in rats (2.7 h) and monkeys (28.9 h), since viramidine can be eliminated from the systemic circulation by urinary excretion as unchanged drug, in addition to its conversion to ribavirin. These data indicate that rats had higher rates of conversion of viramidine to ribavirin than monkeys and higher rates of metabolism of ribavirin to other metabolites.
Percent absorption can be estimated from the percentage of radioactivity excreted in urine after oral dosing, corrected for total recovery. On the basis of this approach, the levels of absorption of viramidine were estimated to be 61.7% in rats and 43.9% in monkeys, which were slightly lower than the levels of absorption of ribavirin in rats (80.9%) and monkeys (79.1%) determined previously (4). By comparing the plasma viramidine AUCI obtained after oral dosing of viramidine to that obtained after i.v. administration of viramidine, the bioavailabilities of viramidine were estimated to be 9.91% in rats and 13.6% in monkeys. This low bioavailability is expected, since viramidine is a ribavirin prodrug and is rapidly converted to ribavirin after oral dosing.
Despite the lower level of absorption of viramidine compared to that of ribavirin, the plasma ribavirin AUCI after oral dosing of viramidine in rats (3.39 μg · h/ml) was similar to or slightly higher than the plasma ribavirin AUCI after oral dosing of ribavirin (3.04 μg · h/ml) detected previously (4). Again, this is in good agreement with the rapid conversion of viramidine to ribavirin in rats. In monkeys, however, the plasma ribavirin AUCI following oral viramidine dosing (4.62 μg · h/ml) was lower than the plasma ribavirin AUCI following oral ribavirin dosing (13.1 μg · h/ml) detected previously (4). This is probably related to either the lower level of absorption of viramidine compared to that of ribavirin in monkeys and/or the slower rate of conversion of viramidine to ribavirin.
Quantitative whole-body autoradiography has been used to evaluate the tissue drug distribution in rats following oral dosing of viramidine and ribavirin (C. Lin et al., Abstr. 52nd AASLD., abstr. 1021, 2001). The highest tissue drug level was found in the liver after either viramidine or ribavirin dosing. However, viramidine dosing yielded liver drug concentrations 44% higher than those obtained after ribavirin dosing. The distribution in tissue has also been evaluated in monkeys at 24 h after administration of the 10th oral daily dose (10 mg/kg) of [14C]viramidine or [14C]ribavirin (Lin et al., Abstr. 52nd AASLD., abstr. 1123, 2001). The highest level of radioactivity was found in the liver after oral dosing of viramidine. Ribavirin dosing gave a distribution in tissue similar to that after viramidine dosing, except that viramidine was distributed to the liver at higher levels than ribavirin and to RBCs, the eyes, and fat at lower levels than ribavirin. Although viramidine dosing gave higher drug levels in the monkey liver than ribavirin dosing, viramidine showed a much better safety profile than ribavirin, probably due to the lower plasma and RBC drug levels after viramidine dosing. A 28-day toxicity study was conducted with rats and oral doses of 0, 30, 60, and 120 mg/kg and with monkeys and oral doses of 0, 100, 300, and 600 mg/kg. Ribavirin at 300 mg/kg/day induced significant hematological changes in monkeys, whereas viramidine at 600 mg/kg/day had only a slight effect on hematological parameters (C. Lin et al., Abstr. 53rd AASLD, abstr. 508, 2002).
We have previously demonstrated (Lin et al., Abstr. 52nd AASLD., abstr. 1123, 2001) that viramidine dosing in monkeys gave liver drug levels about three times higher than those achieved after ribavirin dosing but 50% lower RBC and plasma drug levels. Recently, Aroa et al. (S. Aroa et al., Abstr. 53rd AASLD, abstr. 773, 2002) reported that with an oral dose of 600 mg in humans, viramidine dosing gave about 50% lower plasma and RBC ribavirin levels than ribavirin dosing. Although liver drug levels after either viramidine or ribavirin dosing are not yet available, it appears that the monkey, not the rat, is a good model for prediction of the pharmacokinetics and tissue distribution of viramidine in humans.
Table 1a.
| Ribavirin in plasma
|
Radioactivity in plasma
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (mg/liter) | Tmax (h) | AUCtf (μg · h/ml | AUCI (μg · h/ml) | t1/2 (h) | Cmax (mg/liter) | Tmax (h) | AUCtf (μg · h/ml) | t1/2 (h) |
| 1.51 (1.44-1.59) | 0 | 5.05 (4.55-5.33) | 5.33 (4.86-5.57 | 6.6 (6.1-7.4) | 13.0 (11.0-14.2) | 0 | 32.6 (31.0-33.5) | 10.4 (8.90-12.7) |
| 0.362 (0.312-0.458) | 4 | 3.26 (3.11-3.54) | 3.39 (3.23-3.60) | 5.0 (4.6-5.4) | 5.60 (3.94-6.62) | 4 | 43.5 (36.5-4.80) | NA |
| 1.38 (1.06-1.89) | 0 | 6.25 (4.51-8.49) | 9.68 (9.13-10.9) | 80.9 (43.3-112) | 15.3 (10.2-21.9) | 0 | 28.7 (21.8-34.6) | 88.7 (60.4-115) |
| 0.089 (0.070-0.108) | 3 | 3.41 (2.84-3.98) | 4.62 (3.75-5.49) | 62.2 (50.1-74.3) | 0.69 (0.42-0.96) | 24 | 25.0 (14.9-35.1) | NA |
Continued on following page
Table 1b.
| Ratio of viramidine in plasma/ radioactivity in plasma
|
Ratio of ribavirin in plasma/ radioactivity in plasma
|
Excretion of radioactivity (% of dose)
|
% Absorption of radioactivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax | AUCtf | Cmax | AUCtf | Urine | Feces | Others | Total | ||
| 0.486 | 0.696 | 0.116 | 0.155 | 77.0 (76.0-78.1) | 1.89 (1.49-2.24) | 20.4 | 99.6 (99.5-99.7) | NA | |
| 0.018 | 0.005 | 0.065 | 0.075 | 60.8 (52.8-68.2) | 33.5 (27.9-39.6) | 4.21 | 98.5 (96.2-99.8) | 61.7 | |
| 0.631 | 0.255 | 0.090 | 0.218 | 44.4 (39.8-48.1) | 0.82 (0.65-0.95) | 9.43 | 54.4 (48.0-59.3) | NA | |
| 0.226 | 0.040 | 0.129 | 0.136 | 39.0 (22.3-55.7) | 42.1 (22.4-61.8) | 7.93 | 88.8 (85.4-92.2) | 43.9 | |
REFERENCES
- 1.Connor, E., S. Morrison, J. Lane, J. Oleske, R. L. Sonke, and J. Connor. 1993. Safety and tolerance and pharmacokinetics of systemic ribavirin in children with human immunodeficiency virus infections. Antimicrob. Agents Chemother. 37:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laskin, O. L., J. A. Longstreth, C. C. Hart, D. Scavuzzo, C. M. Connor J. D. Kalman, and R. B. Robert. 1987. Ribavirin disposition in high-risk patients for acquired immunodeficiency syndrome. Clin. Pharmacol. Ther. 41:546-555. [DOI] [PubMed] [Google Scholar]
- 3.Lertora, J. J., A. B. Rege, J. T. Lacour, N. Ferencz, W. J. George, R. B. VanDyke, K. C. Agrawal, and N. E. Hyslop. 1991. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin. Pharmacol. Ther. 50:442-449. [DOI] [PubMed] [Google Scholar]
- 4.Lin, C.-C., L. T. Yeh, T. Luu, D. Lourenco, and J. Y. Lau. 2003. Pharmacokinetics and metabolism of [14C]ribavirin in rats and cynomolgus monkeys. Antimicrob. Agents Chemother. 47:1395-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Goodman Z. D. Reindollar, K. Koury, M. Ling, J. K. Albrecht, and the International Hepatitis Interventional Therapy Group. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C; a randomized trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 6.McHutchison, J. G., S. Gordon, E. R. Schiff, M. D. Mitchell, M. L. Shiffmann, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, and J. K. Albrecht. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 7.Miller, J. P., J. Kigwana, D. G. Streeter, R. K. Robin, L. N. Simon, and J. Roboz. 1977. The relationship between the metabolism of ribairin and its proposed mechanism of action. Ann. N. Y. Acad. Sci. 284:211-229. [DOI] [PubMed] [Google Scholar]
- 8.Page, T., and J. D. Connor. 1990. The metabolism of ribavirin in erythrocytes and nucleated cell. Int. J. Biochem. 22:379-383. [DOI] [PubMed] [Google Scholar]
- 9.Poynard, T., P. Marcellin, S. S. Lee, C. Nieerau, G. Minuk, G. Ideo, and V. H. Bain. 1998. Randomized trial of interferon alfa-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alfa-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C Virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 10.Sidwell, R. W., J. Huffman, L. B. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of virazole: 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 11.Stephen, E., C. J. Jones, C. J. Peters, G. A. Eddy, P. S. Loizeaux, and P. B. Jahrling. 1980. Ribavirin treatment of toga-, arena-, and bunyavirus infections in subhuman primates and other laboratory species. In R. A. Smith and W. Kirkpatrick (ed.), Ribavirin: a broad spectrum antiviral agent. Academic Press, Inc., New York, N.Y.
- 12.Wu, J., H. Walker, J. Y. N. Lau, and Z. Hong. 2003. Activation and deactivation of a broad-spectrum antiviral drug by a single enzyme: adenosine deaminase catalyzes two consecutive deamination reactions. Antimicrob. Agents Chemother. 47:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]