Abstract
In vitro screening of thiacetazone derivatives indicated that two derivatives, SRI-286 and SRI-224, inhibited a panel of 25 Mycobacterium avium complex (MAC) isolates at concentrations of 2 μg/ml or lower. In mice, SRI-224 and thiacetazone had no significant activity against the MAC in livers and spleens, but treatment with SRI-286 resulted in significant reduction of bacterial loads in livers and spleens. A combination of SRI-286 and moxifloxacin was significantly more active than single drug regimens in liver and spleen.
Mycobacterium avium is an environmental organism that can cause opportunistic infection in immunosuppressed individuals, such as patients with AIDS or with specific abnormalities in the immune system (9, 12). In addition, disease-causing organisms of the M. avium complex (MAC) are isolated from patients with chronic lung diseases and individuals without predisposing conditions (14-16). One of the characteristics of M. avium is its resistance to several first-line antimicrobials used in the treatment of tuberculosis (11).
The treatment and prophylaxis of M. avium infection were remarkably improved with the introduction of the new macrolides, clarithromycin (5), azithromycin (17), and roxithromycin, although for the latter no clinical trials have been reported (3). However, once resistance to macrolides develops, the options for treatment of M. avium infection are limited.
Thiacetazone is a thiosemicarbazole antimicrobial that has been widely used for treatment of tuberculosis in Africa and South America (6). The drug is bacteriostatic against Mycobacterium tuberculosis but has been found to be quite useful in combination with isoniazid (8). Because thiacetazone is associated with dermatologic side effects and Stevens-Johnson syndrome in AIDS patients, it is not available in the United States (8). Thiacetazone has been demonstrated to have anti-M. avium activity in vitro, with MICs ranging from 0.02 to 0.15 μg/ml (7), but evidence for in vivo activity is lacking.
Recently, a number of analogs have been synthesized, with some showing promising results in vitro against M. tuberculosis (unpublished data). In this report we show the results obtained in vitro and in vivo with two thiacetazone analogs.
Mycobacteria.
MAC 101 is a well-characterized human isolate that has been used in many previous in vivo studies (4). MAC strains 100, 108, 109, and 116 were also isolated from the blood of AIDS patients. MAC organisms were cultured on Middlebrook 7H11 agar (Difco) supplemented with oleic acid-albumin-dextrose-catalase for 10 days at 37°C. For animal experiments, transparent colony morphotypes were harvested and suspended in Hanks' buffered salt solution at a concentration of 3 × 108 CFU/ml for infection of mice. The size of the inoculum was confirmed by colony plating onto 7H11 agar.
Antibiotics.
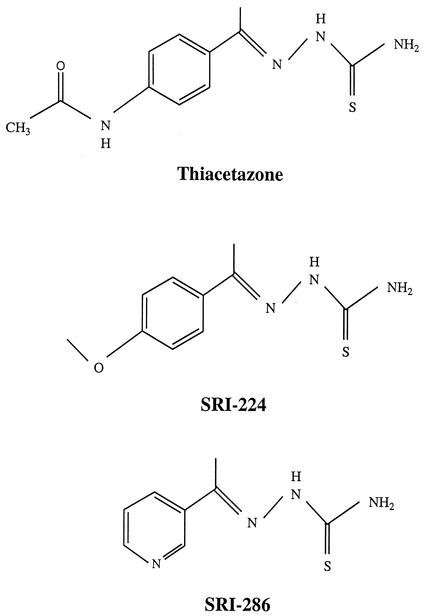
Antimicrobial preparations for therapy were made by suspending the agent with 2.5% gum arabic (Sigma) in 0.1% Tween 80 (Sigma). SRI-224, SRI-286, and thiacetazone were synthesized at Southern Research Institute (Fig. 1). Moxifloxacin was kindly provided by Bayer Corp.
FIG. 1.
Structures of thiacetazone, SRI-224, and SRI-286.
In vitro susceptibility testing.
MICs were determined by a radiometric broth macrodilution method and the T100 method of data analysis (10). The inoculum was prepared as previously reported (2). It was placed into 7H9 broth and frozen at −70°C. The concentration was adjusted to approximately 5 × 104 CFU/ml as previously described (2). Controls included the inoculum undiluted without added drug, the inoculum diluted 1:100 (99% control), and the inoculum diluted 1:1,000 (99.9% control). The period of observation and the end point were determined by daily monitoring of the control and test cultures. Seven days was sufficient for most isolates.
In vivo testing.
C57BL/6J-Lystbg-J/Lystbg-J female mice, aged 8 to 10 weeks, were obtained from Jackson Laboratories for challenge studies. Mice were infected through the caudal veins with 3 × 107 CFU of MAC 101 in a volume of 0.1 ml. After 7 days, treatment was initiated with SRI-224, SRI-286, or thiacetazone at 40 mg/kg of body weight/day as oral monotherapy. In some experiments, SRI-286 was also used at 80 mg/kg. Each drug treatment group contained at least 19 animals. A control group of 10 animals received the drug vehicle only. In addition, an experimental group of five mice was harvested after 7 days of infection in order to establish a baseline level of infection in tissues. In a subsequent series, mice were treated with either SRI-286 (40 mg/kg/day), moxifloxacin (100 mg/kg/day), or SRI -286 and moxifloxacin in combination. Mice received treatment for 4 weeks and were then bled for quantitative culture 48 h after their last dose and euthanized. Livers and spleens of all mice were aseptically dissected, weighed, and homogenized in 5 ml of Middlebrook 7H9 broth (Difco) containing 20% glycerol. Tissue suspensions were serially diluted and plated onto Middlebrook 7H11 agar plates to determine the number of viable organisms. Plates were incubated at 37°C for 10 days.
Statistical analysis.
The statistical significance of the differences in tissue bacterial loads was assessed by analyses of variance. Differences between experimental groups were considered significant at P values of <0.05.
Activity in vitro.
The inhibitory concentration of thiacetazone for five M. avium strains ranged from 0.25 to 32 μg/ml (Table 1).
TABLE 1.
MICs of thiacetazone and thiacetazone analogs for M. avium strains
| Straina | MIC (μg/ml)
|
||
|---|---|---|---|
| Thiacetazone | SRI-224 | SRI-286 | |
| MAC 100 | 16 | 0.125 | 0.5 |
| MAC 101 | 0.25 | 0.125 | 0.5 |
| MAC 108 | 32 | 0.125 | 1 |
| MAC 109 | 0.25 | 0.125 | 0.5 |
| MAC 116 | 32 | 16 | 16 |
All strains are clinical isolates.
Efficacy of antimicrobials.
The efficacies of thiacetazone, SRI-224, and SRI-286 were examined at the concentration of 40 mg/kg/day, the mouse dose corresponding to the human dose of thiacetazone (6, 8). At 40 mg/kg/day, treatment with SRI-286 was associated with significant inhibition of bacterial growth in liver and spleen. In contrast, treatment with SRI-224 or thiacetazone did not result in any reduction in bacterial load compared with the number of bacteria in livers and spleens of untreated mice (Table 2). The effect of SRI-286 was bacteriostatic.
TABLE 2.
Activity of SRI-224, SRI-286, and thiacetazone against M. avium in mice
| Treatmenta | No. of mice | Mean no. of CFU/g of liver ± SEM | Mean no. of CFU/g of spleen ± SEM |
|---|---|---|---|
| SRI-224 | 19 | (8.5 ± 1.5) × 108b | (2.4 ± 0.4) × 109b |
| SRI-286 | 20 | (2.5 ± 0.6) × 108b,c | (5.6 ± 2.4) × 108b,c |
| Thiacetazone | 19 | (9.7 ± 1.7) × 108b | (1.4 ± 0.2) × 109b |
| None (4 wk) | 10 | (1.2 ± 0.3) × 109 | (2.2 ± 0.5) × 109 |
| None (1 wk) | 5 | (7.9 ± 2.1) × 107 | (1.7 ± 0.4) × 108 |
SRI-224, SRI-286, and thiacetazone were given at doses of 40 mg/kg/day.
P > 0.05 compared with untreated control at 1 week.
P < 0.05 compared with untreated control at 4 weeks.
Efficacy of SRI-286 at 40 and 80 mg/kg.
To determine whether the activity of SRI-286 could be enhanced by increasing the dose of the compound without toxicity, we treated mice with 40 and 80 mg/kg. Table 3 shows that SRI-286 was significantly more active at 80 mg/kg/day than at 40 mg/kg/day in liver, spleen, and blood. SRI-286 also had greater activity than ethambutol based on results of 62 complete experiments over the years (1; data not shown).
TABLE 3.
Comparison of the anti-M. avium activities of SRI-286 in mice when administered at 40 and 80 mg/kg/day
| Regimen | No. of mice | Mean no. of CFU/g of liver ± SEM | Mean no. of CFU/g of spleen ± SEM | Log change in mean no. of CFU/ml of blood ± SEM |
|---|---|---|---|---|
| SRI-286 (40 mg/kg) | 12 | (8.9 ± 1.4) × 108 | (1.7 ± 2) × 109 | −0.25 ± 0.17 |
| SRI-286 (80 mg/kg) | 11 | (6.4 ± 8) × 108a | (9.1 ± 1.6) × 108a | −0.65 ± 0.13a |
| Control (4 wk) | 10 | (5.1 ± 5) × 109 | (1.0 ± 0.07) × 1010 | +0.39 ± 2.8 |
| None (1 wk)b | 5 | (5.8 ± 0.3) × 108 | (8.2 ± 0.9) × 108 |
P < 0.05 compared to SRI-286 at 40 mg/kg. Comparison between the mean bacterial loads in the experimental group after treatment and at 1 week (before treatment) determines if the drug is bactericidal or bacteriostatic. Comparison between the experimental group and the control group at 4 weeks determines the significance of the inhibitory effect.
Treatment was initiated after 1 week of infection.
Efficacy of SRI-286 in combination with moxifloxacin.
Moxifloxacin is active against M. avium (1). Therefore, we investigated whether the combination of moxifloxacin and SRI-286 was more efficacious than each drug alone. SRI-286 was administered at 40 mg/kg. This dose was chosen because of the diminished chance of side effects from this dose compared with that from the dose of 80 mg/kg. The association of moxifloxacin and SRI-286 was significantly more active against M. avium than each drug by itself. Although the combination was bacteriostatic in the liver, bactericidal activity was observed in the spleen (Table 4).
TABLE 4.
Activity of SRI-286 and moxifloxacin against M. avium in mice
| Treatment | No. of mice | Mean no. of CFU/g of liver ± SEM | Mean no. of CFU/g of spleen ± SEM | Log change in mean no. of CFU/ml of blood ± SEM |
|---|---|---|---|---|
| SRI-286 (40 mg/kg) | 17 | (2.1 ± 0.5) × 108a | (4.9 ± 0.7) × 108a | (−)0.81 ± 0.27a |
| Moxifloxacin (100 mg/kg) | 18 | (2.4 ± 0.4) × 108a | (6.0 ± 0.9) × 108a | (−)0.71 ± 0.25a |
| SRI-286 and moxifloxacin | 16 | (1.2 ± 0.2) × 108a | (2.2 ± 0.3) × 108a | (−)2.55 ± 0.42a |
| None (4 wk) | 17 | (1.2 ± 0.2) × 109 | (4.0 ± 0.7) × 109 | (+)0.29 ± 0.22 |
| None (1 wk) | 10 | (1.1 ± 0.1) × 108 | (3.7 ± 0.6) × 108 |
P < 0.05 compared with untreated control at 4 weeks.
Summary.
M. avium infection is common in individuals with chronic lung diseases (such as emphysema and chronic bronchitis) and in patients with AIDS with CD4 T-cell counts lower than 100/mm3 (9, 12, 15, 16). The introduction of new macrolides had a remarkable positive impact on the management of M. avium infection (5, 17). However, the emergence of resistance to clarithromycin and azithromycin during treatment represents a challenge for the clinician, since no oral drug with efficacy comparable to that of these macrolides is available. Therefore, there is a need to establish alternative regimens without macrolides.
We describe the first thiosemicarbazole with activity against M. avium in vivo. Although the MICs of thiacetazone and SRI-224 are comparable for the MAC 101 strain of M. avium in vitro, treatment with those compounds had no effect on the infection. The activity of SRI-286 was comparable to the activity of moxifloxacin, the most potent quinolone against M. avium in vivo, and better than that of ethambutol, a drug used in patients with M. avium infection (1). The combination of SRI-286 and moxifloxacin had additive effect.
A previous study indicated that thiacetazone was active against M. avium in vitro (7), a result that was equivalent to our results for two of five strains tested. However, it is interesting that the MICs of all three compounds that we evaluated (thiacetazone, SRI-224, and SRI-286) were below 1 μg/ml for strain MAC 101 while only SRI-286 showed activity in vivo. Since the structures of the three compounds are very similar, it would be interesting to investigate what modification in the side chains is responsible for the activity in vivo. The possibility that differences in pharmacokinetics would explain the activity in vivo is certainly not strong, because of the activity of thiacetazone in humans (8). Therefore, two plausible hypotheses are that SRI-286 has the ability to maintain activity in the environment where M. avium is in vivo and that it is active against the phenotype of M. avium in the host. A recent study showed that M. avium within cells can develop a phenotype of resistance to drugs that are active in vitro (13).
Another aspect to be considered is the potential side effects of this class of compounds. Phototoxicity has been reported in patients receiving thiacetazone, although it did not prevent the extensive use of this drug in Africa and South America. Since we do not know what portion of the molecule is associated with toxicity, it is possible that analogs may not have similar side effects.
Thiacetazone has been found to be a very effective drug for the treatment of tuberculosis when administered in combination with isoniazid (8). In fact, the World Health Organization uses a preparation in both Africa and South America which contains both compounds in the same tablet. The results obtained with the combination of moxifloxacin and SRI-286 suggest that SRI-286 may also be useful when used in a similar strategy against M. avium.
In summary, we report the identification of the first thiosemicarbazole with activity against M. avium in vivo. Further investigation will specifically address some of the issues raised by this study.
Acknowledgments
We thank Karen Allen for preparing the manuscript.
This work was supported by the contract NO1-AI-25140 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Petrofsky, P. Aralar, M. Wu, and L. S. Young. 2001. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium. Antimicrob. Agents Chemother. 45:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Wu, P. Aralar, and L. S. Young. 2001. Telithromycin is active against Mycobacterium avium in mice despite lacking significant activity in standard in vitro and macrophage assays and is associated with low frequency of resistance during treatment. Antimicrob. Agents Chemother. 45:2210-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., P. Kolonoski, and L. S. Young. 1996. Roxithromycin alone and in combination with either ethambutol or levofloxacin for disseminated Mycobacterium avium infections in beige mice. Antimicrob. Agents Chemother. 40:1033-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertram, M. A., C. B. Inderlied, S. Yadegar, P. Kolanoski, J. K. Yamada, and L. S. Young. 1986. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J. Infect. Dis. 154:194-195. [DOI] [PubMed] [Google Scholar]
- 5.Chaisson, R. E., C. A. Benson, M. P. Dube, L. B. Heifets, J. A. Korvick, S. Elkin, T. Smith, J. C. Craft, F. R. Sattler, et al. 1994. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. A randomized, double-blind, dose-ranging study in patients with AIDS. Ann. Intern. Med. 121:905-911. [DOI] [PubMed] [Google Scholar]
- 6.Council, E. A. B. M. R. 1963. Thiacetazone investigation: isoniazid with thiacetazone in the treatment of pulmonary tuberculosis in East Africa—second investigation. Tubercle 44:301-333. [Google Scholar]
- 7.Heifets, L. B., P. J. Lindholm-Levy, and M. Flory. 1990. Thiacetazone: in vitro activity against Mycobacterium avium and M. tuberculosis. Tubercle 71:287-291. [DOI] [PubMed] [Google Scholar]
- 8.Houston, S., and A. Fanning. 1994. Current and potential treatment of tuberculosis. Drugs 48:689-708. [DOI] [PubMed] [Google Scholar]
- 9.Inderlied, C. B., C. A. Kemper, and L. E. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inderlied, C. B., L. S. Young, and J. K. Yamada. 1987. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob. Agents Chemother. 31:1697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koletar, S. L. 1997. Treatment of Mycobacterium avium in human immunodeficiency virus-infected individuals. Am. J. Med. 102:16-21. [Google Scholar]
- 12.McGarvey, J., and L. E. Bermudez. 2002. Pathogenesis. In A. Catanzaro and C. Daley (ed.), Lung disease due to mycobacteria other than tuberculosis. Clinics in Chest Medicine, New York, N.Y.
- 13.Miltner, E. C., and L. E. Bermudez. 2000. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 44:1990-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prince, D. S., D. D. Peterson, R. M. Steiner, J. E. Gottlieb, R. Scott, H. L. Israel, W. G. Figueroa, and J. E. Fish. 1989. Infection with Mycobacterium avium complex in patients without predisposing conditions. N. Engl. J. Med. 321:863-868. [DOI] [PubMed] [Google Scholar]
- 15.Rosenweig, D. Y. 1979. Pulmonary mycobacterial infections due to Mycobacterium intracellulare-avium complex. Chest 75:115-119. [DOI] [PubMed] [Google Scholar]
- 16.Wolinsky, E. 1979. Nontuberculous mycobacteria and associated diseases. Am. Rev. Respir. Dis. 119:107-159. [DOI] [PubMed] [Google Scholar]
- 17.Young, L. S., L. Wiviott, M. Wu, P. Kolonoski, R. Bolan, and C. B. Inderlied. 1991. Azithromycin for treatment of Mycobacterium avium-intracellulare complex infection in patients with AIDS. Lancet 338:1107-1109. [DOI] [PubMed] [Google Scholar]