Abstract
SM-197436, SM-232721, and SM-232724 are new 1β-methylcarbapenems with a unique 4-substituted thiazol-2-ylthio moiety at the C-2 side chain. In agar dilution susceptibility testing these novel carbapenems were active against methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus epidermidis (MRSE) with a MIC90 of ≤4 μg/ml. Furthermore, SM-232724 showed strong bactericidal activity against MRSA, in contrast to linezolid, which was bacteriostatic up to four times the MIC. SM-232724 showed good therapeutic efficacy comparable to those of vancomycin and linezolid against systemic infections of MRSA in cyclophosphamide-treated mice. The MICs of SM-197436, SM-232721, and SM-232724 for streptococci, including penicillin-intermediate and penicillin-resistant Streptococcus pneumoniae strains, ranged from ≤0.063 to 0.5 μg/ml. These drugs were the most active β-lactams tested against Enterococcus faecium, and the MIC90 s for ampicillin-resistant E. faecium ranged between 8 and 16 μg/ml, which were slightly higher than the value for linezolid. However, time-kill assays revealed the superior bactericidal activity of SM-232724 compared to those of quinupristin-dalfopristin and linezolid against an E. faecium strain with a 4-log reduction in CFU at four times the MIC after 24 h of exposure to antibiotics. In addition, SM-232724 significantly reduced the numbers of bacteria in a murine abscess model with the E. faecium strain: its efficacy was superior to that of linezolid, although the MICs (2 μg/ml) of these two agents are the same. Among gram-negative bacteria, these three carbapenems were highly active against Haemophilus influenzae (including ampicillin-resistant strains), Moraxella catarrhalis, and Bacteroides fragilis, and showed antibacterial activity equivalent to that of imipenem for Escherichia coli, Klebsiella pneumoniae, and Proteus spp. Thus, these new carbapenems are promising candidates for agents to treat nosocomial bacterial infections by gram-positive and gram-negative bacteria, especially multiresistant gram-positive cocci, including MRSA and vancomycin-resistant enterococci.
The emergence of multiresistant gram-positive cocci has become a serious problem worldwide, especially in nosocomial settings (5, 13, 17). Methicillin-resistant Staphylococcus aureus (MRSA) are resistant not only to available β-lactam antibiotics, but also to structurally unrelated classes of antibacterials, including quinolones and aminoglycosides (4). Enterococci, especially Enterococcus faecium, are intrinsically resistant to β-lactams and aminoglycosides and have acquired high-level resistance to many other antibacterials (18). Moreover, the multiple resistance of penicillin-intermediate and penicillin-resistant Streptococcus pneumoniae (PISP and PRSP, respectively) is of great concern (12). Vancomycin has been used to treat such resistant gram-positive bacterial infections for some time. However, the wide spread of vancomycin-resistant enterococci (VRE) and the emergence of glycopeptide-intermediate S. aureus reduce the clinical efficacy of vancomycin, and the lack of effective drugs against such multiresistant pathogens is also a serious medical problem (18, 22). Recently, new classes of antibacterial agents such as quinupristin-dalfopristin and linezolid have become available in several countries. Although their antibacterial spectra cover VRE, their pharmacological profiles, such as narrow spectra, side effects, and/or the rapid emergence of bacterial resistance, clearly limit their clinical usefulness (3, 9, 14, 15). Therefore, there is an urgent need for new antibacterial agents effective against resistant gram-positive bacteria.
The molecular mechanisms of resistance to β-lactams in the gram-positive bacteria mostly depend on various types of mutations in penicillin-binding proteins (PBPs), which are the targets of β-lactams (10). These mutations cause the PBPs to become desensitized to β-lactams, resulting in a reduction in β-lactam-susceptibility. Improving the affinity of existing β-lactams for such mutated PBPs by chemical modifications is one way to restore their activity against resistant bacteria. Carbapenems, a class of β-lactam antibiotics, bind with high affinity to a wide range of bacterial PBPs covering susceptible (not mutated) staphylococci, streptococci, and some enterococci. They exhibit a broader antibacterial spectrum compared to other β-lactams with a highly bactericidal activity. In addition, the carbapenem nucleus is very stable against hydrolysis by serine β-lactamases. Based on the high intrinsic potential of carbapenem nucleus, we have selected it as a prototype to design novel β-lactam antibiotics effective against multiresistant gram-positive pathogens.
We previously reported that the 2-(4-arylthiazol-2-ylthio)-1β-methylcarbapenem SM-17466 and its derivatives exhibited potent anti-MRSA activity corresponding to a high affinity for PBP2a (23, 25). Although SM-17466 showed good efficacy in a murine systemic infection model of MRSA and acceptable toxicological profiles in preliminary animal experiments, it did not show sufficient activity against E. faecium, prompting us to search for new carbapenems with potent activity against both MRSA and E. faecium, including VRE. Consequently, we found that a series of novel carbapenems having a five- or six-member cyclic amino group instead of the pyridium moiety of SM-17466 showed improved activity against E. faecium. In addition, the representative compounds SM-197436, SM-232721, and SM-232724 showed low neurotoxicity and acute toxicity in mice and were relatively resistant to hydrolysis by human renal dehydropeptidase I (DHP-I), making them promising candidates that warrant further evaluation (Fig. 1) (26). In this study, we primarily focused on the in vitro antibacterial activities of these compounds against various clinical isolates, including multiresistant gram-positive bacteria, compared with those of vancomycin, linezolid, and several β-lactams. Determination of minimum bactericidal concentrations (MBCs) and time-kill studies of SM-232724 for key resistant pathogens, such as MRSA, VRE, PRSP, and ampicillin-resistant Haemophilus influenzae, were also described. In addition, we investigated the efficacy of SM-232724 in systemic infections with methicillin-susceptible S. aureus (MSSA) and MRSA in cyclophosphamide-pretreated mice and experimental E. faecium subcutaneous abscesses in mice.
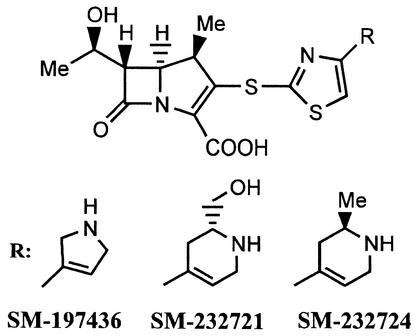
FIG. 1.
Chemical structure of 1β-methyl-2-(thiazol-2-ylthio)carbapenems, SM-197436, SM-232721, and SM-232724.
(Part of this work was presented in abstract form at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 16 to 19 December 2001, Chicago, Ill. [abstr. F-364, p. 208].)
MATERIALS AND METHODS
Organisms.
The clinical isolates used in this study were collected in Japan, the United States, and Europe. The β-lactamase-producing organisms were from our bacterial collections.
Antimicrobial agents.
SM-197436, SM-232721, SM-232724, meropenem, and linezolid were synthesized, and imipenem and cilastatin were prepared from Thienam (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan) in the laboratories of the Sumitomo Pharmaceuticals Research Division. [14C]benzylpenicillin (PCG) was purchased from Amersham International, Plc. (Buckinghamshire, United Kingdom). The other antimicrobial agents were purchased from commercial sources.
Susceptibility testing.
MICs were determined by the twofold serial agar dilution method, with Mueller-Hinton agar (MHA; Difco Laboratories, Detroit, Mich.) unless otherwise specified. Susceptibility testing was performed with MHA supplemented with 5% defibrinated horse blood for streptococci and with 5% Fildes enrichment (BBL Microbiology Systems, Cockeysville, Md.) for H. influenzae. Brucella HK agar (Kyokutoh Seiyaku, Tokyo, Japan) supplemented with 5% defibrinated horse blood was used for the culture of anaerobic bacteria. The final inocula comprised approximately 104 CFU per spot for aerobic bacteria and 106 CFU per spot for anaerobic bacteria. Agar plates were incubated at 35°C for 18 to 24 h. For anaerobes, incubation was carried out anaerobically in GasPak jars (BBL), and for streptococci, H. influenzae, and Moraxella catarrhalis, incubation was carried out in an atmosphere of 5% CO2. The MIC was defined as the lowest drug concentration that completely prevented visible growth.
Bactericidal activities.
The bactericidal activities of the drugs against each of six strains of S. aureus, E. faecium, S. pneumoniae, and H. influenzae were assessed by a broth microdilution technique (20). The test organisms (105 to 106 CFU/ml) in 0.1 ml of cation-adjusted Mueller-Hinton broth with the supplements described above was treated with the drugs at 35°C for 16 to 24 h and inspected for turbidity. A 10-μl sample of the medium from growth-negative cultures was removed and spread onto drug-free plates, which were then incubated at 35°C for 48 h for colony formation. The minimum bactericidal concentration (MBC) was defined as the minimum drug concentration that killed >99.9% of bacteria in the initial inoculum.
For time-kill assays, the test organisms were precultured at 35°C for 1 h and consequently treated with the drugs at one-fourth, one, and four times the MIC with shaking at 35°C. Viable cells were counted at 1, 2, 4, 6, 8, and 24 h after drug addition.
Affinity for PBP2a of MRSA.
Affinities for PBP2a of two MRSA strains were determined by a competition assay with [14C]PCG (24, 25). Briefly, membrane fractions prepared from S. aureus SP9099-9H and MS-9408-6H (penicillinase-free, homologous resistant strains), were pretreated with drugs at 30°C for 30 min and then incubated with [14C]PCG at 30°C for another 30 min. Binding affinity was quantified with a BAS 2000 Image Analyzer (Fuji Photo Film, Tokyo, Japan) and expressed as the 50% inhibitory concentration (IC50): the concentration (in micrograms per milliliter) that inhibited radiolabeling with [14C]PCG by 50% compared with the control value.
Stability against mouse renal DHP-I.
The stability of SM-232724 against renal DHP-I was determined with purified mouse renal DHP-I, as reported previously (7). The activity of DHP-I was spectrophotometrically determined by measuring the hydrolysis of glycyldehydrophenylalanine as a substrate. The relative rate of hydrolysis was expressed as a ratio against the hydrolysis rate for imipenem, which was assigned a value of 1.00.
In vivo experiments.
Three-week-old male slc:ICR mice weighing 11 to13 g were purchased from Japan SLC, Inc. (Shizuoka, Japan), and adapted to standardized environmental conditions (temperature, 23 ± 2°C; humidity, 55% ± 10%) for 1 week before beginning the experiments. All animal procedures were performed in accordance with the institution's guidelines for the humane handling, care, and treatment of research animals. Because the hydrolyzing activities of DHP-I for a carbapenem differ greatly among animal species, all carbapenems were used as a mixture with an equal dose of cilastatin, a DHP-I inhibitor, in the animal experiments unless specified otherwise. All statistical analyses described below were performed with the Statistical Analysis System (SAS) version 8.1 for Windows (SAS Institute, Inc., Cary, N.C.).
(i) Experimental S. aureus septicemia in immunosuppressed mice.
Mice were administered 200 mg of cyclophosphamide per kg of body weight subcutaneously 4 days before infection. Overnight cultures of S. aureus Smith (MSSA) and SP-12249 (MRSA) were harvested and suspended with 8% gastric mucin (Difco) in phosphate-buffered saline. A 0.2-ml aliquot of the bacterial suspension was administered intraperitoneally to each of the cyclophosphamide-pretreated mice. Two hours after infection, groups of 10 mice were subcutaneously injected with a single dose of antibiotics. The 50% effective dose (ED50) (in milligrams per kilogram of body weight) and 95% confidence limits were calculated by the probit method from survival rates 7 days after infection.
(ii) Experimental E. faecium subcutaneous abscesses in mice.
Each mouse was injected beneath the loose skin of the left groin with 0.5 ml of bacterial diluent of E. faecium TL-3273 (approximately 108 CFU). SM-232724 and linezolid were administered subcutaneously 1 and 3 h after infection (six mice per group). The abscesses were excised 72 h after infection, at which time abscess formation could be visibly confirmed, and visible cell counts of the number of bacteria per abscess were made in duplicate by standard plating procedures. Statistical analysis of the difference between drug-treated groups and a control group was performed by Dunnett's test for multiple comparisons of significance.
RESULTS
Antimicrobial activity against clinical isolates of gram-positive bacteria.
The antimicrobial activities of SM-197436, SM-232721, and SM-232724 against clinical isolates of various gram-positive bacterial strains were compared with those of imipenem, meropenem, cefepime, vancomycin, and linezolid. For S. aureus and S. epidermidis, methicillin and oxacillin were used as references, respectively; as was penicillin G for streptococci; ampicillin and gentamicin instead of cefepime for enterococci; and clindamycin instead of vancomycin and linezolid for peptostreptococci. The results are presented in Table 1. SM-197436, SM-232721, and SM-232724 showed potent activity against both β-lactam-susceptible and β-lactam-resistant staphylococci. The MICs of the three carbapenems at which 90% of the 112 isolates of MRSA were inhibited were 2 to 4 μg/ml and were comparable to those of vancomycin and linezolid. The 34 strains of methicillin-resistant Staphylococcus epidermidis (MRSE) were also susceptible to these new carbapenems, with MICs ranging from 1 to 4 μg/ml.
TABLE 1.
Activities of SM-197436, SM-232721, and SM-232724 against gram-positive clinical isolates
| Organism (no. of isolates) and antimicrobial agent | MIC
(μg/ml)a
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Staphylococcs aureus | |||
| Methicillin susceptible (20) | |||
| SM-197436 | 0.016 | 0.016 | 0.016 |
| SM-232721 | 0.016-0.031 | 0.016 | 0.031 |
| SM-232724 | 0.016-0.031 | 0.031 | 0.031 |
| Imipenem | 0.016-0.031 | 0.016 | 0.031 |
| Meropenem | 0.063-0.125 | 0.063 | 0.125 |
| Methicillin | 1-2 | 2 | 2 |
| Cefepime | 1-2 | 2 | 2 |
| Vancomycin | 0.5-1 | 1 | 1 |
| Linezolid | 1-2 | 1 | 2 |
| Methicillin resistant (112) | |||
| SM-197436 | ≤0.25-4 | 1 | 2 |
| SM-232721 | ≤0.25-4 | 2 | 4 |
| SM-232724 | ≤0.25-4 | 1 | 2 |
| Imipenem | ≤0.25-128 | 32 | 64 |
| Meropenem | 1-128 | 32 | 64 |
| Methicillin | 16->512 | >512 | >512 |
| Cefepime | 32->512 | 256 | 256 |
| Vancomycin | 0.5-4 | 1 | 2 |
| Linezolid | 0.5-4 | 2 | 2 |
| Staphylococcus epidermidis | |||
| Methicillin susceptible (22) | |||
| SM-197436 | ≤0.008-0.016 | 0.016 | 0.016 |
| SM-232721 | ≤0.008-0.031 | 0.016 | 0.016 |
| SM-232724 | 0.063-0.25 | 0.016 | 0.031 |
| Imipenem | 0.063-0.125 | 0.016 | 0.016 |
| Meropenem | 0.25-1 | 0.063 | 0.125 |
| Oxacillin | 0.016-0.31 | 0.125 | 0.25 |
| Cefepime | 0.25-1 | 0.5 | 0.5 |
| Vancomycin | 1-2 | 1 | 2 |
| Linezolid | 0.5-1 | 1 | 1 |
| Methicillin resistant (34) | |||
| SM-197436 | ≤0.063-2 | 0.125 | 1 |
| SM-232721 | ≤0.063-4 | 0.25 | 2 |
| SM-232724 | ≤0.063-2 | 0.125 | 1 |
| Imipenem | 0.125-64 | 0.25 | 32 |
| Meropenem | 0.25-64 | 2 | 32 |
| Oxacillin | 1->128 | 4 | 128 |
| Cefepime | 1-128 | 4 | 64 |
| Vancomycin | 0.5-2 | 2 | 2 |
| Linezolid | 0.25-4 | 1 | 4 |
| Streptococcus pyogenes (20) | |||
| SM-197436 | ≤0.008 | ≤0.008 | ≤0.008 |
| SM-232721 | ≤0.008-0.016 | 0.016 | 0.016 |
| SM-232724 | ≤0.008 | ≤0.008 | ≤0.008 |
| Imipenem | ≤0.008-0.016 | ≤0.008 | ≤0.008 |
| Meropenem | 0.016 | 0.016 | 0.016 |
| Penicillin G | ≤0.008-0.016 | 0.016 | 0.016 |
| Cefepime | 0.016-0.031 | 0.031 | 0.031 |
| Vancomycin | 0.5 | 0.5 | 0.5 |
| Linezolid | 1-2 | 1 | 1 |
| Streptococcus agalactiae (20) | |||
| SM-197436 | ≤0.008-0.016 | 0.016 | 0.016 |
| SM-232721 | ≤0.008-0.016 | 0.016 | 0.016 |
| SM-232724 | ≤0.008-0.016 | 0.016 | 0.016 |
| Imipenem | 0.016-0.031 | 0.016 | 0.031 |
| Meropenem | 0.031-0.063 | 0.063 | 0.063 |
| Penicillin G | 0.031-0.063 | 0.063 | 0.063 |
| Cefepime | 0.063-0.125 | 0.125 | 0.125 |
| Vancomycin | 0.5 | 0.5 | 0.5 |
| Linezolid | 1 | 1 | 1 |
| Streptococcus pneumoniae | |||
| Penicillin susceptible (20) | |||
| SM-197436 | ≤0.008-0.016 | 0.016 | 0.016 |
| SM-232721 | ≤0.008-0.016 | 0.016 | 0.016 |
| SM-232724 | ≤0.008-0.016 | 0.016 | 0.016 |
| Imipenem | ≤0.008-0.031 | 0.016 | 0.016 |
| Meropenem | ≤0.008-0.063 | 0.031 | 0.063 |
| Penicillin G | 0.016-0.063 | 0.063 | 0.063 |
| Cefepime | 0.016-1 | 0.25 | 1 |
| Vancomycin | 0.125-0.5 | 0.5 | 0.5 |
| Linezolid | 0.25-1 | 0.5 | 1 |
| Penicillin intermediate (30) | |||
| SM-197436 | ≤0.008-0.125 | 0.063 | 0.125 |
| SM-232721 | 0.016-0.125 | 0.032 | 0.063 |
| SM-232724 | ≤0.008-0.125 | 0.063 | 0.125 |
| Imipenem | ≤0.008-0.125 | 0.063 | 0.125 |
| Meropenem | 0.031-0.5 | 0.25 | 0.5 |
| Penicillin G | 0.125-1 | 1 | 1 |
| Cefepime | 0.125-1 | 0.5 | 1 |
| Vancomycin | 0.125-2 | 0.5 | 0.5 |
| Linezolid | 0.25-1 | 0.5 | 1 |
| Penicillin resistant (30) | |||
| SM-197436 | ≤0.031-0.5 | 0.125 | 0.25 |
| SM-232721 | 0.031-1 | 0.125 | 0.25 |
| SM-232724 | 0.031-0.25 | 0.125 | 0.25 |
| Imipenem | 0.063-0.5 | 0.125 | 0.25 |
| Meropenem | 0.125-2 | 0.5 | 1 |
| Penicillin G | 2->16 | 2 | >16 |
| Cefepime | ≤0.031-4 | 1 | 2 |
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 |
| Linezolid | 0.25-2 | 0.5 | 1 |
| Enterococcus faecalis | |||
| Vancomycin susceptible (24) | |||
| SM-197436 | 1-4 | 2 | 2 |
| SM-232721 | 1-4 | 2 | 2 |
| SM-232724 | 1-4 | 2 | 2 |
| Imipenem | 1-4 | 2 | 2 |
| Meropenem | 4-16 | 8 | 8 |
| Ampicillin | 1-4 | 1 | 2 |
| Gentamicin | 2->512 | 8 | 256 |
| Vancomycin | 1-2 | 1 | 2 |
| Linezolid | 1-2 | 2 | 2 |
| Vancomycin resistant (10) | |||
| SM-197436 | 2 | 2 | 2 |
| SM-232721 | 2-4 | 4 | 4 |
| SM-232724 | 2 | 2 | 2 |
| Imipenem | 4 | 4 | 4 |
| Meropenem | 16 | 16 | 16 |
| Ampicillin | 2-4 | 4 | 4 |
| Vancomycin | >512 | >512 | >512 |
| Linezolid | 2 | 2 | 2 |
| Enterococcus faecium | |||
| Ampicillin susceptible, vancomycin susceptible (17) | |||
| SM-197436 | 0.5-2 | 0.5 | 2 |
| SM-232721 | 0.25-2 | 0.5 | 1 |
| SM-232724 | 0.25-2 | 0.5 | 2 |
| Imipenem | 2-32 | 8 | 16 |
| Meropenem | 8-64 | 32 | 64 |
| Ampicillin | 1-8 | 4 | 4 |
| Gentamicin | 4-16 | 8 | 16 |
| Vancomycin | 0.5-2 | 0.5 | 2 |
| Linezolid | 2 | 2 | 2 |
| Ampicillin susceptible, vancomycin resistant (9) | |||
| SM-197436 | 0.5-2 | 1 | |
| SM-232721 | 1-2 | 1 | |
| SM-232724 | 0.5-2 | 0.5 | |
| Imipenem | 4-16 | 16 | |
| Meropenem | 16-64 | 64 | |
| Ampicillin | 4-8 | 4 | |
| Gentamicin | 4->512 | 8 | |
| Vancomycin | 512->512 | 512 | |
| Linezolid | 2 | 2 | |
| Ampicillin resistant, vancomycin susceptible (21) | |||
| SM-197436 | 2-16 | 8 | 16 |
| SM-232721 | 2-16 | 8 | 8 |
| SM-232724 | 2-16 | 4 | 8 |
| Imipenem | 64-512 | 256 | 512 |
| Meropenem | 256->512 | 512 | >512 |
| Ampicillin | 32-256 | 64 | 128 |
| Gentamicin | 4->512 | 16 | >512 |
| Vancomycin | 0.25-1 | 1 | 1 |
| Linezolid | 2-4 | 2 | 4 |
| Ampicillin resistant, vancomycin resistant (24) | |||
| SM-197436 | 2-16 | 4 | 16 |
| SM-232721 | 2-16 | 4 | 16 |
| SM-232724 | 1-8 | 2 | 8 |
| Imipenem | 64->512 | 256 | >512 |
| Meropenem | 128->512 | 512 | >512 |
| Ampicillin | 32->512 | 128 | >512 |
| Gentamicin | 4->512 | >512 | >512 |
| Vancomycin | 128->512 | 512 | >512 |
| Linezolid | 2 | 2 | 2 |
| Peptostreptococcus spp. (20)b | |||
| SM-197436 | ≤0.016-4 | 0.25 | 2 |
| SM-232721 | ≤0.016-4 | 0.25 | 4 |
| SM-232724 | ≤0.016-2 | 0.25 | 2 |
| Imipenem | ≤0.016-4 | 0.25 | 4 |
| Meropenem | ≤0.016-4 | 1 | 2 |
| Cefepime | 0.125->128 | 8 | >128 |
| Clindamycin | ≤0.063-32 | 0.25 | 0.5 |
50% and 90%, MIC50 and MIC90, respectively.
Including five strains of P. anaerobius, a strain of P. magnus, five strains of P. micros, six strains of P. prevotii, and three strains of P. asaccharolyticus.
SM-197436, SM-232721, and SM-232724 showed potent activity against penicillin-susceptible streptococci, with the highest MIC being 0.016 μg/ml. On the basis of the MIC90s, of which the highest value was 0.25 μg/ml, the potency of three compounds against a total of 60 isolates of PISP and PRSP was comparable to that of imipenem, and these compounds were >64-fold and 8-fold more potent than penicillin G and cefepime, respectively.
As we expected, the new carbapenems proved to be highly active β-lactam antibiotics for various enterococci. The MICs of three compounds against 34 isolates of E. faecalis, including 10 vancomycin-resistant strains, ranged from 1 to 4 μg/ml, which were similar to those of imipenem, ampicillin, and linezolid and superior to that of meropenem. The new carbapenems were 2- to 8-fold more active than ampicillin and 8- to 16-fold more active than gentamicin on the basis of the MIC90 for 26 isolates of ampicillin-susceptible E. faecium, including 9 vancomycin-resistant strains. Interestingly, three compounds retained intermediate activity against multiresistant E. faecium, which exhibited no susceptibility to ampicillin, imipenem, meropenem, and gentamicin. The MIC90s of SM-197436, SM-232721, and SM-232724 were 16, 8, and 8 μg/ml for 21 isolates of vancomycin-susceptible E. faecium and 16, 16, and 8 μg/ml for 24 isolates of vancomycin-resistant E. faecium, respectively, suggesting that SM-232724 was the most active of the three carbapenems.
In terms of the potency against anaerobic gram-positive bacteria, the new carbapenems showed strong activity against peptostreptococci, similar in potency to imipenem and meropenem.
Antimicrobial activity against clinical isolates of gram-negative bacteria.
The antimicrobial activities of SM-197436, SM-232721, and SM-232724 for clinical isolates of various gram-negative bacterial strains were compared with those of imipenem, meropenem, cefepime, and cefotaxime. For H. influenzae, ampicillin was used as a reference, as was clindamycin for B. fragilis. The results are presented in Table 2. The new carbapenems exhibited high levels of activity against E. coli, Klebsiella pneumoniae, H. influenzae, M. catarrhalis, and members of the Proteus family and moderate levels of activity against other gram-negative bacteria, except for Pseudomonas aeruginosa and Stenotrophomonas maltophilia. The highest activities of the three compounds against ampicillin-resistant H. influenzae were particularly notable in comparison with those of the other β-lactams tested. In addition, the new carbapenems showed strong activity against B. fragilis: all three carbapenems were similar in potency to the older carbapenems tested. The activity of SM-197436 against gram-negative bacteria was superior to those of SM-232721 and SM-232724.
TABLE 2.
Activities of SM-197436, SM-232721, and SM-232724 against gram-negative clinical isolates
| Organism (no. of isolates) and antimicrobial agent | MIC
(μg/ml)a
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Haemophilus influenzae | |||
| Ampicillin susceptible (13) | |||
| SM-197436 | ≤0.031-0.063 | ≤0.031 | 0.063 |
| SM-232721 | ≤0.031-0.063 | ≤0.031 | 0.063 |
| SM-232724 | ≤0.031-0.063 | 0.063 | 0.063 |
| Imipenem | 0.25-8 | 2 | 4 |
| Meropenem | ≤0.031-0.5 | 0.125 | 0.25 |
| Ampicillin | 0.25-2 | 0.5 | 2 |
| Cefepime | ≤0.031-2 | 0.125 | 2 |
| Cefotaxime | ≤0.031-0.5 | ≤0.031 | 0.5 |
| Ampicillin resistant (28) | |||
| SM-197436 | 0.063-1 | 0.125 | 0.5 |
| SM-232721 | 0.063-1 | 0.125 | 0.5 |
| SM-232724 | 0.063-1 | 0.125 | 0.5 |
| Imipenem | 1-64 | 8 | 64 |
| Meropenem | 0.25-4 | 1 | 4 |
| Ampicillin | 4-64 | 32 | 64 |
| Cefepime | 0.25-2 | 2 | 2 |
| Cefotaxime | 0.063-4 | 1 | 4 |
| Moraxella catarrhalis (13) | |||
| SM-197436 | ≤0.016-0.125 | 0.031 | 0.063 |
| SM-232721 | ≤0.016-0.125 | 0.031 | 0.125 |
| SM-232724 | ≤0.016-0.125 | 0.063 | 0.125 |
| Imipenem | ≤0.016-0.25 | 0.125 | 0.125 |
| Meropenem | ≤0.016 | ≤0.016 | ≤0.016 |
| Cefepime | 0.25-4 | 1 | 2 |
| Cefotaxime | 0.125-1 | 1 | 1 |
| Escherichia coli (13) | |||
| SM-197436 | 0.063-0.5 | 0.125 | 0.25 |
| SM-232721 | 0.125-1 | 0.25 | 1 |
| SM-232724 | 0.125-1 | 0.5 | 0.5 |
| Imipenem | 0.063-0.25 | 0.125 | 0.125 |
| Meropenem | ≤0.016 | ≤0.016 | ≤0.016 |
| Cefepime | ≤0.016-0.25 | 0.031 | 0.063 |
| Cefotaxime | ≤0.016-0.5 | 0.031 | 0.25 |
| Klebsiella pneumoniae (13) | |||
| SM-197436 | 0.063-0.25 | 0.125 | 0.125 |
| SM-232721 | 0.25-0.5 | 0.25 | 0.5 |
| SM-232724 | 0.25-0.5 | 0.5 | 0.5 |
| Imipenem | 0.125-0.25 | 0.125 | 0.125 |
| Meropenem | ≤0.016 | ≤0.016 | ≤0.016 |
| Cefepime | ≤0.016-0.31 | 0.031 | 0.031 |
| Cefotaxime | ≤0.016-0.031 | ≤0.016 | 0.031 |
| Enterobacter cloacae (10) | |||
| SM-197436 | ≤0.063-8 | 2 | 8 |
| SM-232721 | 0.125-32 | 4 | 16 |
| SM-232724 | 0.25-32 | 4 | 16 |
| Imipenem | 0.125-1 | 0.25 | 0.25 |
| Meropenem | ≤0.016-0.125 | 0.031 | 0.125 |
| Cefepime | ≤0.063-2 | ≤0.063 | 0.5 |
| Cefotaxime | ≤0.063-128 | 0.125 | 64 |
| Serratia marcescens (13) | |||
| SM-197436 | 0.5-4 | 2 | 4 |
| SM-232721 | 2-8 | 4 | 8 |
| SM-232724 | 2-16 | 8 | 16 |
| Imipenem | 0.25-1 | 0.25 | 0.5 |
| Meropenem | 0.025-0.5 | 0.031 | 0.25 |
| Cefepime | ≤0.063-64 | 0.125 | 8 |
| Cefotaxime | 0.125-128 | 0.5 | 128 |
| Proteus mirabilis (13) | |||
| SM-197436 | 0.063-0.25 | 0.125 | 0.25 |
| SM-232721 | 0.125-1 | 0.25 | 0.5 |
| SM-232724 | 0.25-1 | 0.25 | 0.5 |
| Imipenem | 0.25-2 | 0.5 | 2 |
| Meropenem | ≤0.016-0.063 | 0.031 | 0.031 |
| Cefepime | 0.031-4 | 0.063 | 0.125 |
| Cefotaxime | ≤0.016-32 | ≤0.016 | 0.063 |
| Proteus vulgaris (13) | |||
| SM-197436 | 0.125-1 | 0.25 | 0.5 |
| SM-232721 | 0.25-4 | 0.5 | 2 |
| SM-232724 | 0.25-4 | 0.5 | 2 |
| Imipenem | 0.25-2 | 0.5 | 2 |
| Meropenem | ≤0.016-0.063 | 0.031 | 0.063 |
| Cefepime | ≤0.016-0.125 | 0.063 | 0.125 |
| Cefotaxime | ≤0.016-0.063 | 0.031 | 0.063 |
| Providencia rettgeri (13) | |||
| SM-197436 | 0.063-4 | 2 | 4 |
| SM-232721 | 0.25-16 | 8 | 8 |
| SM-232724 | 0.25-16 | 4 | 8 |
| Imipenem | 0.5-2 | 1 | 1 |
| Meropenem | ≤0.016-0.25 | 0.031 | 0.063 |
| Cefepime | ≤0.016-0.125 | ≤0.016 | 0.063 |
| Cefotaxime | ≤0.016-0.063 | ≤0.016 | 0.063 |
| Morganella morganii (13) | |||
| SM-197436 | 0.25-2 | 0.5 | 2 |
| SM-232721 | 1-8 | 2 | 8 |
| SM-232724 | 1-8 | 2 | 4 |
| Imipenem | 1-4 | 2 | 4 |
| Meropenem | 0.063-0.125 | 0.063 | 0.125 |
| Cefepime | ≤0.063 | ≤0.063 | ≤0.063 |
| Cefotaxime | ≤0.063-2 | 0.125 | 0.25 |
| Citrobacter freundii (13) | |||
| SM-197436 | 0.25-4 | 2 | 2 |
| SM-232721 | 0.5-8 | 4 | 8 |
| SM-232724 | 1-8 | 4 | 8 |
| Imipenem | 0.125-0.5 | 0.25 | 0.25 |
| Meropenem | ≤0.016-0.031 | 0.015 | 0.031 |
| Cefepime | ≤0.063 | ≤0.063 | ≤0.063 |
| Cefotaxime | ≤0.063-0.25 | 0.125 | 0.25 |
| Pseudomonas aeruginosa (13) | |||
| SM-197436 | 4-16 | 16 | 16 |
| SM-232721 | 8-32 | 32 | 32 |
| SM-232724 | 8-32 | 16 | 32 |
| Imipenem | 0.5-8 | 1 | 2 |
| Meropenem | 0.125-4 | 0.25 | 4 |
| Cefepime | 1-16 | 2 | 8 |
| Cefotaxime | 4-128 | 16 | 64 |
| Stenotrophomonas maltophilia (13) | |||
| SM-197436 | >128 | >128 | >128 |
| SM-232721 | >128 | >128 | >128 |
| SM-232724 | >128 | >128 | >128 |
| Imipenem | >128 | >128 | >128 |
| Meropenem | 64->128 | 128 | >128 |
| Cefepime | 16-128 | 32 | 64 |
| Cefotaxime | 16-128 | 128 | >128 |
| Acinetobacter spp. (13)b | |||
| SM-197436 | 0.25-0.5 | 0.5 | 0.5 |
| SM-232721 | 0.5-2 | 1 | 1 |
| SM-232724 | 0.5-2 | 1 | 2 |
| Imipenem | 0.063-0.25 | 0.25 | 0.25 |
| Meropenem | 0.125-0.5 | 0.25 | 0.5 |
| Cefepime | 0.25-32 | 2 | 8 |
| Cefotaxime | 1-32 | 8 | 32 |
| Bacteroides fragilis (20) | |||
| SM-197436 | ≤0.063-16 | 0.5 | 4 |
| SM-232721 | ≤0.063-32 | 0.5 | 4 |
| SM-232724 | 0.125-16 | 1 | 4 |
| Imipenem | 0.125-8 | 0.25 | 4 |
| Meropenem | 0.031-4 | 0.5 | 2 |
| Cefepime | 8->128 | 64 | >128 |
| Clindamycin | 0.125->128 | 2 | >128 |
50% and 90%, MIC50 and MIC90, respectively.
Including 10 strains of A. baumannii and 2 strains of A. lwoffii.
Antimicrobial activity for β-lactamase-producing bacteria.
SM-232724 was highly active against gram-negative bacteria producing various types of penicillinase, including extended-spectrum β-lactamases (ESBLs). The MICs of SM-232724 and imipenem for cephalosporinase-producing strains were slightly high, but were still lower than those of cefotaxime, suggesting high stability of the carbapenems against hydrolysis by this type of β-lactamase. There was no activity against carbapenemase-producers, consistent with the results obtained with imipenem (Table 3). SM-197436 and SM-232721 also showed similar patterns of susceptibility for these β-lactamase-producing strains (data not shown).
TABLE 3.
Antimicrobial activities of SM-232724 against various β-lactamase producers
| Organism | β-Lactamase | MIC
(μg/ml)
|
||||
|---|---|---|---|---|---|---|
| SM-232724 | Imipenem | Cefotaxime | Ampicillin | Ampicillin-sulbactam | ||
| Penicillinase | ||||||
| E. coli ML 1410/Rms213 | 1 | 0.125 | 0.5 | >128 | 64 | |
| E. coli ML 1410/Rte16 | 0.5 | 0.125 | 0.5 | 16 | 8 | |
| E. coli ML 1410/Rms149 | 0.5 | 0.5 | 0.5 | 16 | 8 | |
| E. coli TL-3203 | TEM | 1 | 0.125 | 0.5 | >128 | 64 |
| K. pneumoniae TL-3207 | SHV | 0.5 | 0.25 | 0.5 | 32 | 8 |
| K. oxytoca TL-3212 | SHV | 0.5 | 0.125 | 0.5 | 64 | 32 |
| ESBL | ||||||
| E. coli TL-3138 | Toho-I | 0.5 | 0.063 | 128 | >128 | 32 |
| E. coli TL-3135 | Toho-II | 2 | 0.125 | 8 | >128 | 32 |
| K. pneumoniae TL-3139 | MEN-I | 0.5 | 0.125 | 8 | >128 | 32 |
| E. coli TL-3141 | MEN-I | 1 | 0.125 | 32 | >128 | 32 |
| E. coli TL-3180 | SHV-12 | 0.25 | 0.06 | 1 | >128 | 16 |
| Cephalosporinase | ||||||
| E. cloacae TL-3227 | AmpC | 8 | 0.5 | 32 | >128 | 128 |
| S. marcescens TL-3228 | AmpC | 64 | 16 | >128 | >128 | >128 |
| P. aeruginosa TL-3231 | AmpC | 128 | 16 | >128 | >128 | >128 |
| C. freundii TL-3137 | AmpC | 64 | 1 | 128 | >128 | 128 |
| M. morganii SP 7341 | 16 | 1 | 64 | >128 | 128 | |
| P. aeruginosa GN 918 | 64 | 2 | >128 | >128 | >128 | |
| P. vulgaris GN 7919 | 2 | 1 | 4 | >128 | 16 | |
| B. cepacia IID 1340a | 16 | 8 | 32 | >128 | >128 | |
| B. cepacia SP 9849a | 32 | 8 | 16 | >128 | >128 | |
| Carbapenemase | ||||||
| S. maltophilia IID 1275 | L-1 | >128 | >128 | >128 | >128 | >128 |
| S. marcescens TL-3230 | IMP-I | >128 | >128 | >128 | >128 | >128 |
| P. aeruginosa TL-3232 | IMP-I | >128 | >128 | >128 | >128 | >128 |
Burkholderia cepacia.
Bactericidal activity.
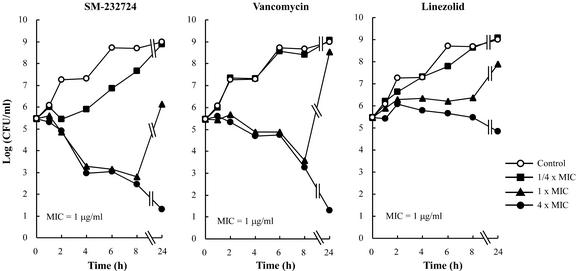
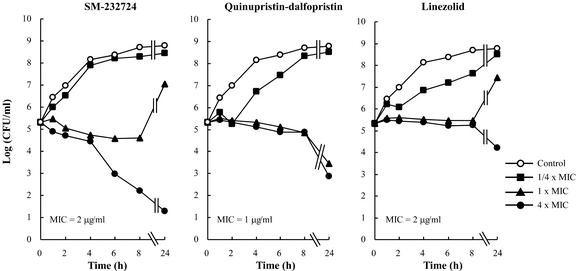
The activities of SM-197436, SM-232721, and SM-232724 were bactericidal. The MBCs were two- to fourfold higher than the MICs for MRSA, E. faecium, PRSP, and ampicillin-resistant H. influenzae (Table 4). In time-kill assays, SM-232724 showed a maximum 4-log CFU reduction against MRSA at four times the MIC (4 μg/ml) after 24 h of incubation. Although the bacterial cell numbers after 8 and 24 h of exposure to one and four times the MIC of SM-232724 were similar to those of vancomycin, the carbapenem caused a >2-log CFU reduction until 4 h, whereas vancomycin did not. Meanwhile, linezolid showed no bactericidal activity against MRSA at any concentrations tested (Fig. 2). Furthermore, 8 μg of SM-232724 per ml caused a 4-log CFU reduction for vancomycin- and ampicillin-resistant E. faecium after 24 h; this killing activity was the greatest among the compounds tested (Fig. 3). SM-197436 and SM-232721 showed levels of killing activity similar to those of SM-232724 (data not shown).
TABLE 4.
Bactericidal activities of SM-197436, SM-232721, and SM-232724 against drug-resistant pathogens
| Antimicrobial agent | MIC range (μg/ml) | No. of strains with MBC/MIC
ratio of:
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | ||
| S. aureus | |||||
| SM-197436 | 0.12-1 | 2 | 2 | 2 | 0 |
| SM-232721 | 0.12-1 | 4 | 1 | 1 | 0 |
| SM-232724 | 0.12-1 | 2 | 3 | 1 | 0 |
| Vancomycin | 1 | 5 | 1 | 0 | 0 |
| Linezolid | 1 | 0 | 0 | 0 | 6 |
| Quinupristin-dalfopristin | 0.5-1 | 0 | 0 | 0 | 6 |
| E. faecium | |||||
| SM-197436 | 0.5-4 | 2 | 4 | 0 | 0 |
| SM-232721 | 0.5-2 | 1 | 5 | 0 | 0 |
| SM-232724 | 0.5-2 | 2 | 4 | 0 | 0 |
| Linezolid | 1-2 | 0 | 0 | 0 | 6 |
| Quinupristin-dalfopristin | 1-4 | 0 | 0 | 0 | 6 |
| S. pneumoniae | |||||
| SM-197436 | 0.25 | 4 | 2 | 0 | 0 |
| SM-232721 | 0.25-0.5 | 4 | 2 | 0 | 0 |
| SM-232724 | 0.25-0.5 | 5 | 1 | 0 | 0 |
| Vancomycin | 0.5 | 5 | 1 | 0 | 0 |
| Linezolid | 1 | 0 | 4 | 2 | 0 |
| Ampicillin | 4-8 | 5 | 1 | 0 | 0 |
| Imipenem | 0.06-0.5 | 6 | 0 | 0 | 0 |
| Meropenem | 0.25-1 | 5 | 1 | 0 | 0 |
| H. influenzae | |||||
| SM-197436 | 0.06-0.12 | 4 | 2 | 0 | 0 |
| SM-232721 | 0.06-0.12 | 2 | 4 | 0 | 0 |
| SM-232724 | 0.06-0.12 | 4 | 2 | 0 | 0 |
| Cefotaxime | 0.5-1 | 3 | 3 | 0 | 0 |
| Ampicillin | 1-4 | 3 | 3 | 0 | 0 |
| Imipenem | 0.25-1 | 1 | 5 | 0 | 0 |
| Meropenem | 0.12-0.5 | 2 | 2 | 2 | 0 |
FIG. 2.
Time-kill curves of SM-232724 against S. aureus SP-12249 (MRSA).
FIG. 3.
Time-kill curves of SM-232724 against E. faecium TL-3273 (VRE).
Affinity for PBP2a of MRSA.
Table 5 shows IC50s of SM-197436, SM-232721, SM-232724, and imipenem, determined with [14C]PCG for PBP2a of two MRSA strains, SP-9099-9H and MS9408-6H. The three compounds showed a similar affinity for the PBP2a of both strains. Their IC50s ranged from 1.1 to 2.4 μg/ml and were more than 50-fold lower than those of imipenem.
TABLE 5.
Binding affinities of SM-197436, SM-232721, and SM-232724 for PBP2a of MRSA
| Antibiotic | MIC (μg/ml) | IC50 (μg/ml) |
|---|---|---|
| SP-9099-9H | ||
| SM-197436 | 2 | 1.3 |
| SM-232721 | 4 | 1.6 |
| SM-232724 | 2 | 1.1 |
| Imipenem | 64 | 166.2 |
| MS-9408-6H | ||
| SM-197436 | 2 | 2.4 |
| SM-232721 | 2 | 1.9 |
| SM-232724 | 2 | 1.3 |
| Imipenem | 128 | 145.9 |
In vivo efficacy against S. aureus and vancomycin-resistant E. faecium.
For further evaluations, we chose SM-232724 among three carbapenems, because of its favored antibacterial activity for E. faecium, as shown in Table 1, and low neurotoxic potential in mice, which has been reported previously (26). The therapeutic effect of SM-232724 (with cilastatin) on MSSA and MRSA systemic infections in immunosuppressed mice was determined (Table 6). Although SM-232724 was much more resistant to hydrolysis by human DHP-I than imipenem (26), mouse DHP-I preferentially hydrolyzed SM-232724 compared with imipenem: the relative hydrolysis rate of SM-232724 was 2.39, compared to which the rate of imipenem was assigned a value of 1.00. Therefore, we decided to coadminister equal amounts of cilastatin with SM-232724 and imipenem to avoid the negative impact of mouse DHP-I on the pharmacodynamics of these carbapenems in mice. The MICs of SM-232724, vancomycin, linezolid, and imipenem for the S. aureus Smith strain were 0.016, 1, 4, and 0.016 μg/ml, respectively. The ED50 of SM-232724 for protection against infection with the strain was 0.16 mg/kg, which was comparable to that of imipenem and much lower than those of vancomycin and linezolid. Meanwhile, SM-232724 showed in vitro activity against MRSA strain SP-12249 comparable to those exhibited by vancomycin and linezolid, with a MIC of 1 μg/ml. The ED50 of SM-232724 against MRSA infection was 2.78 mg/kg, equivalent to those of vancomycin and linezolid. In this model, imipenem showed weak protective activity (ED50 of 25.9 mg/kg), which seemed to be consistent with its low level of in vitro activity. These results suggest that the efficacy of SM-232724 against these MSSA and MRSA strains in vivo was consistent with the in vitro activity.
TABLE 6.
Activity of SM-232724 against S. aureus systemic infection in immunosuppressed micea
| S. aureus strain | No. of CFU/mouse (multiple of LD50)b | Antimicrobial agent | MIC (μg/ml) | ED50 in mg/kg (95% confidence limit) |
|---|---|---|---|---|
| Smith (MSSA) | 1.99 × 103 (87.4) | SM-232724 | 0.016 | 0.16 (0.09-0.27) |
| Vancomycin | 1 | 1.93 (0.80-3.61) | ||
| Linezolid | 4 | 10.0 (6.11-16.3) | ||
| Imipenem | 0.016 | 0.08 (0.03-0.15) | ||
| SP-12249 (MRSA) | 1.15 × 106 (17.0) | SM-232724 | 1 | 2.78 (1.71-4.37) |
| Vancomycin | 1 | 1.64 (0.97-3.07) | ||
| Linezolid | 1 | 6.67 (3.18-23.0) | ||
| Imipenem | 32 | 25.9 (11.7-84.9) |
Ten cyclophosphamide-pretreated mice in each group received a single subcutaneous dose of the agent 2 h after bacterial challenge.
LD50, 50% lethal dose.
We next determined the efficacy of SM-232724 against E. faecium by using a subcutaneous abscess model in mice (Table 7). The strain of E. faecium TL-3273 used in this model exhibited reduced susceptibility to ampicillin and vancomycin (MICs of 128 and 256 μg/ml, respectively), but was susceptible to SM-232724 and linezolid. (Both MICs were 2 μg/ml.) This strain formed visible abscesses in mice 3 days after subcutaneous inoculation of approximately 108 CFU of bacteria, and the number of organisms in abscesses of nontreated mice ranged from 107 to 108 CFU in multiple experiments. SM-232724 dose dependently reduced bacterial numbers in abscesses by two subcutaneous injections each of 10, 20, and 40 mg/kg and caused a >3-log reduction at the highest dose. Linezolid also exhibited a 0.5-log CFU reduction of bacterial numbers at both 20 and 40 mg/kg. Linezolid's activity at 40 mg/kg was weaker than even that of SM-232724 at 10 mg/kg.
TABLE 7.
Activity of SM-232724 against experimental E. faecium TL-3273 subcutaneous abscesses in micea
| Antimicrobial agent | Dose (mg/kg) | Mean log CFU at site of infection | Decrease in log CFU vs control | P valueb |
|---|---|---|---|---|
| Control | 7.57 ± 0.32 | |||
| SM-232724 | 10 | 5.56 ± 0.53 | −2.01 | <0.0001 |
| 20 | 4.67 ± 0.16 | −2.90 | <0.0001 | |
| 40 | 4.50 ± 0.16 | −3.07 | <0.0001 | |
| Linezolid | 20 | 7.02 ± 0.39 | −0.55 | 0.0268 |
| 40 | 6.90 ± 0.18 | −0.67 | 0.0055 |
Six mice in each group received two subcutaneous doses of SM-232724 (MIC, 2 μg/ml) or linezolid (MIC, 2 μg/ml) at 1 and 3 h after subcutaneous inoculation of E. faecium TL-3272. Visible cell counts of the number of bacteria per abscess were determined at 3 days after infection.
Dunnett's test for multiple comparisons of significance versus control.
DISCUSSION
Multiresistant gram-positive cocci, including MRSA and VRE, show a reduced susceptibility to most of the β-lactam antibiotics that have been used in the clinic. As an approach to combat these resistant pathogens, chemical modifications of β-lactams targeting the resistance mechanism have been conceived, and to date, a number of reports have appeared that describe new β-lactams with improved activity against MRSA. These include different classes of β-lactams, especially cephalosporins and carbapenems (1, 2, 8, 11, 16, 19, 25). However, no agent has shown a measurable advantage over others in the late stages of clinical development.
We previously reported that a new 1β-methylcarbapenem, SM-17466, and its analogs, which have a unique thiazole side chain at the C-2 position, exhibited improved activity against MRSA. We continued to investigate new analogs in this series of 1β-methylcarbapenems, focusing on improvements of antienterococcal activity, and SM-197436, SM-232721, and SM-232724 were found to have potent antibacterial activity for both MRSA and E. faecium, helping to increase in depth these agents' microbiological and pharmacological characterization.
In the present study, susceptibility testing using a large number of recent clinical isolates revealed that SM-197436, SM-232721, and SM-232724 had excellent in vitro activity against gram-positive organisms in comparison with other β-lactams tested. The spectrum includes MSSA and MRSA, PISP and PRSP, and vancomycin-susceptible and -resistant enterococci. The MICs of these carbapenems ranged from <0.25 to 4 μg/ml for all 112 clinical isolates of MRSA tested, including highly resistant strains, while the MIC90s of methicillin and imipenem were >512 and 64 μg/ml, respectively. In addition, these carbapenems are also 16- to 128-fold more active against MRSE than the other β-lactam antibiotics tested. The good correlation between the IC50 of PBP2a and the MIC of each carbapenem suggested that the strong antibacterial activity of these compounds for MRSA could be ascribed to the inhibition of PBP2a. In time-kill experiments, SM-232724 more rapidly reduced the bacterial number of MRSA than vancomycin, although both antibiotics exhibited potent bactericidal activities against MRSA. In addition, the bactericidal activity of SM-232724 was superior to that of linezolid, which apparently showed bacteriostatic action against the same strain. Consistent with in vitro activities, SM-232724 showed good therapeutic efficacy in murine septicemia models against both MSSA and MRSA after subcutaneous administration. The activities of the new carbapenems against PISP and PRSP (30 isolates for each) were similar to that of imipenem and superior to those of the other agents tested.
SM-197436, SM-232721, and SM-232724 were highly active against both ampicillin-susceptible E. faecalis and E. faecium. The activity for E. faecalis was comparable to those of imipenem, ampicillin, and linezolid and better than that of meropenem. There was no difference in the pattern of susceptibility to three carbapenems between the vancomycin-susceptible and -resistant E. faecalis strains tested. The susceptibility of E. faecium to the new carbapenems was much higher than that to the old carbapenems. Against ampicillin-resistant E. faecium, three carbapenems still retained intermediate activity: the MIC90 of the most potent compound, SM-232724, for a total of 46 isolates of ampicillin-resistant E. faecium was 8 μg/ml, which was >64-fold more active than ampicillin and gentamicin. As with E. faecalis, vancomycin-susceptible and -resistant E. faecium strains revealed similar susceptibilities to the new carbapenems. In addition, the new carbapenems were effective against E. faecium harboring both the VanA and VanB types of resistance (data not shown).
SM-232724 showed good bactericidal activity (MIC/MBC ratio) against six strains of ampicillin-resistant, vancomycin-resistant E. faecium compared with quinupristin-dalfopristin and linezolid. The time-kill study suggested that SM-232724, but not quinupristin-dalfopristin and linezolid, possessed significant bactericidal activity against ampicillin-resistant, vancomycin-resistant E. faecium TL-3273, albeit the killing kinetics were relatively slow and weak in comparison with those against MRSA. The activity of SM-232724 was significantly stronger than that of linezolid in a murine subcutaneous abscess model against E. faecium TL-3273, which had the same susceptibility to both SM-232724 and linezolid in vitro. The bactericidal activity of SM-232724 against the strain might contribute to such a high in vivo efficacy, although a detailed pharmacodynamic study is necessary in mice. On the basis of the observations described above, we consider SM-197436, SM-232721, and SM-232724 to be the most active agents against E. faecalis and E. faecium, including the drug-resistant isolates in this study.
The resistance of enterococci to β-lactam antibiotics is considered to be well correlated to the overproduction of low-affinity PBP (PBP5), which is a natural component of the PBPs of enterococcal species and which is able to substitute for the functions of susceptible PBPs when they are inhibited by β-lactams (6). SM-197436, SM-232721, and SM-232724 inhibit the growth of ampicillin-resistant E. faecium quite effectively, which might suggest a significant increase in the affinity of these compounds for PBP5 of E. faecium.
To date, few β-lactam antibiotics have had a significant activity against ampicillin-resistant E. faecium. A novel 1β-methylcarbapenem, L-786,392, is reported to be active in vitro against multiresistant gram-positive cocci, including MRSA and vancomycin- and ampicillin-resistant E. faecium (21). L-786,392 has an antibacterial spectrum similar to those of SM-197436, SM-232721, and SM-232724, but differs structurally from these and existing carbapenems, such as imipenem and meropenem, in which it has a unique aromatic and dicationic C-2 side chain connected via a C-C bond to the 1β-methylcarbapenem nucleus. Further detailed study of structure-activity relationships should facilitate the development of unique carbapenem antibiotics with improved efficacy against highly resistant enterococci, especially E. faecium.
These three compounds, especially SM-197436, retained substantial antibacterial activity for gram-negative bacteria, such as E. coli, K. pneumoniae, H. influenzae, M. catarrhalis, Proteus spp., and Acinetobacter spp., relative to the other carbapenems and cephalosporins tested. However, they had relatively low activity against other members of the family Enterobacteriaceae and other glucose-nonfermenting gram-negative rods. As with most carbapenems, the new carbapenems were very active against anaerobes. Taken together, their antibacterial spectra against clinically important multiresistant gram-positive bacteria, such as PRSP and MRSA, and gram-negative bacteria, such as E. coli, K. pneumoniae, H. influenzae, M. catarrhalis, etc., suggest their clinical usefulness, especially for the treatment of serious infections in nosocomial settings.
In conclusion, the novel 1β-methlcarbapenems SM-197436, SM-232721, and SM-232724 are promising new parenteral carbapenems for the treatment of nosocomial infections by gram-positive and gram-negative bacteria, especially multiresistant gram-positive cocci, including MRSA and VRE. Further study of their pharmacological, toxicological, and physicochemical characteristics is warranted.
Acknowledgments
We greatly appreciate R. Then and colleagues at F. Hoffmann-La Roche, Ltd., for providing some of the bacterial isolates and for critical discussions. We thank K. Kanazawa, K. Takemoto, I. Eriguchi, and Y. Sumita for experimental support and helpful discussions. We also acknowledge excellent technical assistance by Y. Hirai and Y. Kanemitsu.
REFERENCES
- 1.Adachi, Y., K. Nakamura, Y. Kato, N. Hazumi, T. Hashizume, and S. Nakagawa. 1997. In vitro evaluation of BO-3482, a novel dithiocarbamate carbapenem with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 41:2282-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamberland, S., J. Blais, M. Hoang, C. Dinh, D. Cotter, E. Bond, C. Gannon, C. Park, F. Malouin, and M. N. Dudley. 2001. In vitro activities of RWJ-54428 (MC-02,479) against multiresistant gram-positive bacteria. Antimicrob. Agents Chemother. 45:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diekema, D. J., and R. N. Jones. 2001. Oxazolidinone antibiotics. Lancet 358:1975-1982. [DOI] [PubMed] [Google Scholar]
- 4.Diekema, D. J., M. A. Pfaller, F. J. Schmits, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, et al. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific Region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. l2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 5.Emori, T. G., and R. P. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana, R., M. Aldegheri, M. Ligozzi, H. Lopez, A. Sucari, and G. Satta. 1994. Overproduction of a low-affinity penicillin-binding protein and high-level ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 38:1980-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukasawa, M., Y. Sumita, E. T. Harabe, T. Tanio, H. Nouda, T. Kohzuki, T. Okuda, H. Matsumura, and M. Sunagawa. 1992. Stability of meropenem and effect of 1β-methyl substitution on its stability in the presence of renal dehydropeptidase I. Antimicrob. Agents Chemother. 36:1577-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung-Tomic, J. C., J. Clark, B. Minassian, M. Pucci, Y.-H. Tsai, E. Gradelski, L. Lamb, I. Medina, E. Huczko, B. Kolek, S. Chaniewski, C. Ferraro, T. Washo, and D. P. Bonner. 2002. In vitro and in vivo activities of a novel cephalosporin, BMS-247243, against methicillin-resistant and -susceptible staphylococci. Antimicrob. Agents Chemother. 46:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzales, R. D., P. C. Schreckenbergar, M. B. Graham, S. Kelkar, K. Denbesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1979.. [DOI] [PubMed] [Google Scholar]
- 10.Hakenbeck, R., and J. Coyette. 1998. Resistant penicillin-binding proteins. Cell. Mol. Life Sci. 54:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. P. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs, M. R. 1999. Drug-resistant Streptococcus pneumoniae: rational antibiotic choice. Am. J. Med. 106(Suppl. 5A):19S-25S. [DOI] [PubMed]
- 13.Jones, R. N. 2001. Resistance patterns among nosocomial pathogens. Trends over the past few years. Chest 119:397S-404S. [DOI] [PubMed]
- 14.Lamb, H. M., D. P. Figgitt, and D. Faulds. 1999. Quinupristin/dalfopristin. A review of its use in the management of serious gram-positive infections. Drugs 58:1061-1097. [DOI] [PubMed] [Google Scholar]
- 15.Livermore, D. M. 2000. Quinupristin/dalfopristin and linezolid: where, when, which and whether to use? J. Antimicrob. Chemother. 46:347-350. [DOI] [PubMed] [Google Scholar]
- 16.Malanoski, G., L. Collins, C. T. Eliopoulos, R. C. Moellering, Jr., and G. M. Elipoulos. 2000. Comparative in vitro activities of L-695, 256, a novel carbapenem, against gram-positive bacteria. Antimicrob. Agents Chemother. 39:990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan, J. E., Jr. 2000. The impact of changing pathogens of serious infections in hospitalized patients. Clin. Infect. Dis. 31(Suppl. 4):S124-S130. [DOI] [PubMed] [Google Scholar]
- 18.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 19.Nagano, R., K. Shibata, Y. Adachi, H. Imamura, T. Hashizume, and H. Morishima. 2000. In vitro activities of novel trans-3, 5-disubstituted pyrrolidinylthio-1β-methylcarbapenems with potent activities against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline M26-A. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 21.Ratcliffe, R. W., R. R. Wilkening, K. J. Wildonger, S. T. Waddell, G. M. Santorelli, D. L. Parker, Jr., J. D. Morgan, T. A. Blizzard, M. L. Hammond, J. V. Heck, J. Huber, J. Kohler, K. L. Dorso, E. S. Rose, J. G. Sundelof, W. J. May, and G. G. Hammond. 1999. Synthesis and properties of 2-(naphthosultamyl)methylcarbapenems with potent anti-MRSA activity: discovery of L-786,392. Bioorg. Med. Chem. 9:679-684. [DOI] [PubMed] [Google Scholar]
- 22.Rybak, M. J., and R. L. Akins. 2001. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: clinical significance and treatment options. Drugs 61:1-7. [DOI] [PubMed] [Google Scholar]
- 23.Shinagawa, H., H. Yamaga, H. Houchigai, Y. Sumita, and M. Sunagawa. 1997. Synthesis and biological properties of a new series of anti-MRSA beta-lactams: 2-(thiazol-2-ylthio) carbapenems. Bioorg. Med. Chem. 5:601-621. [DOI] [PubMed] [Google Scholar]
- 24.Sumita, Y., M. Fukasawa, S. Mitsuhashi, and M. Inoue. 1995. Binding affinities of β-lactam antibiotics for penicillin-binding protein 2′ in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 35:473-481. [DOI] [PubMed] [Google Scholar]
- 25.Sumita, Y., N. Nouda, K. Kanazawa, and M. Fukasawa. 1995. Antimicrobial activity of SM-17466, a novel carbapenem antibiotic with potent activity against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 39:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunagawa, M., M. Itoh, K. Kubota, A. Sasaki, Y. Ueda, P. Angehrn, A. Bourson, E. Goetschi, P. Hebeisen, and R. L. Then. 2002. New anti-MRSA and VRE carbapenems: synthesis and structure-activity relationships of 1 β-methyl-2-(thio-2-ylthio)carbapenems. J. Antibiot. (Tokyo) 55:722-757. [DOI] [PubMed] [Google Scholar]