Abstract
The in vivo antibacterial activity of S-3578, a new parental cephalosporin, was compared with those of cefepime, ceftriaxone, ceftazidime, imipenem-cilastatin, and vancomycin. The efficacy of S-3578 against systemic infections caused by methicillin-resistant Staphylococcus aureus (MRSA) SR3637 (50% effective dose [ED50], 7.21 mg/kg of body weight) was almost the same as that of vancomycin. In contrast, cefepime and imipenem-cilastatin were less active against this pathogen (ED50s, >100 and >100 mg/kg, respectively). S-3578 was the most effective compound against penicillin-resistant Streptococcus pneumoniae SR20946 (ED50, 1.98 mg/kg). S-3578 (10 mg/kg) induced a significant reduction in the numbers of viable MRSA SR17764 and Pseudomonas aeruginosa SR10396 organisms in polymicrobial pulmonary infections. The therapeutic efficacy of S-3578 was more potent than that of the combination of vancomycin and ceftazidime. High levels of S-3578 were detected in plasma in vivo, and its efficacy against experimentally induced infections in mice caused by MRSA and P. aeruginosa reflected its potent in vitro activity. We conclude that S-3578 is a promising new cephalosporin for the treatment of infections caused by gram-positive and -negative bacteria, including MRSA and P. aeruginosa.
Drug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae (PRSP), and vancomycin-resistant enterococci, are widespread throughout the world. MRSA constitutes 50 to 70% of all S. aureus isolates and are also prevalent in Japan (10). MRSA infections are becoming a serious clinical problem. Recently, new cephalosporins with potent activities against MRSA, such as Ro 63-9141 and BMS-247243, have been developed (5, 7). However, the clinical therapeutic options for MRSA infections are scarce or are limited to glycopeptide-type drugs, such as vancomycin or, more recently, oxazolidinones, such as linezolid.
To find a cephalosporin with higher levels of activity against MRSA, a program was launched at Shionogi Co., Ltd. (Osaka, Japan). It led to the discovery of S-3578, (6R, 7R)-7-[(Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-ethoxyiminoacetylamino]-3-{1-[3-(N-methylamino)propyl]}-1H-imidazo(4,5-b]pyridin-4-ium-4-yl)methyl-8-oxo-5-thia-l-azabicyclo[4.2.0]oct-2-ene-2-carboxylate monosulfate, an inner salt monosulfate and a novel parental cephalosporin that displays broad and potent activities in vitro against both MRSA and Pseudomonas aeruginosa (4, 20). In the present study, we compared the in vivo efficacy of S-3578 with those of cefepime (6), ceftazidime, imipenem-cilastatin, vancomycin, and linezolid using experimental mouse models.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
MATERIALS AND METHODS
Antibiotics.
S-3578 and vancomycin were supplied by Shionogi Co., Ltd. Cefepime was obtained from Bristol-Myers Squibb Co. (Tokyo, Japan), ceftriaxone was obtained from Hoechst Japan Co. (Tokyo, Japan), ceftazidime was obtained from Glaxo Wellcome Co., (Tokyo, Japan), ampicillin was obtained from Meiji Seika (Tokyo, Japan), imipenem-cilastatin was obtained from Banyu Pharmaceutical Co. (Tokyo, Japan), and linezolid was obtained from Pharmacia & Upjohn Co. (Kalamazoo, Mich.). S-3578 and the other antibiotics were formulated with saline (Otsuka Pharmaceutical, Tokyo, Japan).
Microorganisms.
S. aureus strains Smith, SR14 (a methicillin-susceptible, β-lactamase producing strain), SR3637 (a methicillin-resistant strain), SR17505 (a methicillin-resistant strain), SR20005 (a methicillin-resistant strain), and SR20045 (a methicillin-resistant strain) were used for the systemic infection and subcutaneous abscess models. S. pneumoniae strains SR20946 (a penicillin-resistant serotype 6 strain) and SR11031 (a penicillin-resistant serotype 6 strain) were used for the systemic and pulmonary infection models. P. aeruginosa strains SR24, SR10411, and SR10396 were used for the systemic and urinary tract infection models. These strains were clinically isolated and collected at Shionogi Discovery Research Laboratories. S. aureus and P. aeruginosa were incubated at 37°C in heart infusion agar (Eiken, Tokyo, Japan) and scraped from the plates. The bacterial suspension was stocked in 10% inactivated horse serum at −80°C until use. P. aeruginosa was stored at −80°C in 10% skim milk until use. For S. pneumoniae, Todd-Hewitt broth (Difco, Detroit, Mich.) supplemented with 30% heat-inactivated fetal bovine serum (Gibco, Introgen Japan K.K., Tokyo, Japan) was used as the growth medium. The bacteria were centrifuged at 9,200 × g for 15 min at 4°C and resuspended in 10% fetal bovine serum. For the in vivo study, these stock bacterial solutions were rapidly thawed and immediately diluted to the desired concentration in heart infusion broth (Eiken).
Animals.
Five-week-old female SLC/ICR mice (body weight, 20 to 25 g) were obtained from Japan SLC, Inc. (Shizuoka, Japan), and 5-week-old male or female JCL/ICR mice (body weight, 20 to 25 g) were obtained from Clea Japan, Inc. (Tokyo, Japan). The SLC/ICR mice were used in the systemic, subcutaneous, and pulmonary infection models, and the JCL/ICR female mice were used in the ascending urinary tract infection model. Seven mice per group were used in all experimental infection models. The JCL/ICR male mice were used for the pharmacokinetic study. All studies with animals were approved by the Experimental Animal Committee of Shionogi Co., Ltd.
MIC determination.
The MIC of each compound was determined by the broth microdilution method according to the guidelines of the National Committee for Clinical Laboratory Standards (12, 13). Cation-adjusted Mueller-Hinton broth (Difco) was used for nonfastidious aerobic bacteria. For streptococci, the medium was supplemented with 5% lysed horse blood, 5 mg of yeast extract (Difco) per ml, and 15 μg of NAD (Sigma-Aldrich, St. Louis, Mo.) per ml. The plates were incubated at 35°C in ambient air. The MIC was defined as the lowest antibiotic concentration that inhibited visible growth after 18 to 24 h of incubation.
Systemic infection models.
The in vivo potency of S-3578 was determined by using a mouse model of septicemia. The mice were injected intraperitoneally with 0.5 or 1.0 ml of a bacterial suspension (approximately 20 to 2,000 times the 50% lethal dose). All strains with exception of S. pneumoniae SR20946 were injected as a suspension with 5% mucin (ICN, Cleveland, Ohio). S-3578 and the reference compounds were administered subcutaneously 1 and 5 h after infection (19). Mortality was recorded over 7 days to estimate the 50% effective dose (ED50) and 95% confidence limits, which were determined by the logit method (2).
Subcutaneous abscess models.
The therapeutic efficacy of S-3578 was tested with a mouse model of subcutaneous infection caused by methicillin-susceptible S. aureus (MSSA) SR14 (a β-lactamase-producing strain) or MRSA SR20045. Dextran-based microcarrier beads (Cytodex 1; Sigma-Aldrich, Steinheim, Germany) were suspended in Dulbecco's phosphate-buffered saline (Nissui, Tokyo, Japan) and autoclaved. Freshly thawed bacterial suspensions were diluted in heart infusion broth and added to the microcarrier beads. Before use, this suspension was mixed well. The mice were infected subcutaneously with 0.2 ml of the bacterial suspension containing the microcarrier beads (3). The challenge dose of MSSA SR14 was 5.6 × 105 CFU per mouse, and that of MRSA SR20045 was 8.4 × 105 CFU per mouse. The compounds were administered subcutaneously 3, 7, 24, and 30 h after infection. The site for subcutaneous drug administration and the site for administration of the bacterial suspension were anatomically distinct. The mice were killed 48 h after infection. The abscesses were excised, added to 2 ml of heart infusion broth, and homogenized in a Physcotron handy homogenizer (Microtec Ltd., Chiba, Japan). The number of viable organisms in the abscess (number of CFU per abscess) was determined by a standard plate procedure with mannitol salt agar (Eiken).
Pulmonary infection models. (i) PRSP infection.
The therapeutic efficacies of S-3578 and the reference compounds were tested with a mouse model of pulmonary infection caused by penicillin-resistant S. pneumoniae SR11031 (penicillin G MIC, 2 μg/ml). The mice were anesthetized by intramuscular injection of a mixture of ketamine (6 mg/kg of body weight; Sankyo Co., Tokyo, Japan) and xylazine (1 mg/kg; Bayer Co., Tokyo, Japan) (17), and 0.04 ml of a 1% formaldehyde solution (Kanto Chemical Co., Tokyo) was administered intranasally to injure the trachea. After 3 days, the mice were infected by instillation of 0.07 ml of a bacterial suspension. The challenge dose was 8.2 × 105 CFU/mouse. The compounds were administered subcutaneously 4, 7, 24, 30, 48, 54, 72, and 78 h after infection. The mice were killed 96 h after infection. The lungs were then removed and homogenized in heart infusion broth, the homogenates were serially diluted 10-fold, and 1.0-ml aliquots were incorporated into brain heart infusion agar (Difco) supplemented with 0.5% Bacto yeast extract (Difco) and 2% defibrinated horse blood. The plates were incubated at 37°C for 72 h, and the number of viable organisms in the lungs (number of CFU per gram) was determined.
(ii) Polymicrobial pulmonary infection caused by both MRSA and P. aeruginosa.
The in vivo efficacy of S-3578 was tested with a mouse model of a polymicrobial pulmonary infection caused by both MRSA SR17764 and P. aeruginosa SR10396. Immunosuppression was caused by intraperitoneal administration of cyclophosphamide (Shionogi Co., Ltd.), which was dissolved in distilled water at doses of 150 mg/kg 4 days before infection and 100 mg/kg 1 day before infection. After the neutropenic mice were anesthetized with ketamine and xylazine, the mice were also given 0.04 ml of a 1% formaldehyde solution, which injured the trachea, intranasally 4 days before infection. After 4 days, the mice were anesthetized and infected by instillation of 0.08 ml of the mixed bacterial suspension. The challenge dose of MRSA SR17764 was 3.2 × 105 CFU per mouse, and that of P. aeruginosa SR10396 was 7.9 × 101 CFU per mouse. The compounds were administered subcutaneously 3, 5, 7, 24, 30, 48, and 54 h after infection. The mice were killed 72 h after infection. The lungs were then removed and homogenized in heart infusion broth, and the homogenates were serially diluted 10-fold. For MRSA isolates, 1.0-ml aliquots were incorporated into mannitol salt agar (Eiken). For P. aeruginosa isolates, heart infusion agar supplemented with 20 μg of vancomycin (Sigma-Aldrich) per ml and 0.15% potassium nitrate (Nacalai Tesque, Kyoto, Japan) was used. The plates were incubated at 37°C for 72 h, and the number of viable organisms in the lungs (number of CFU per gram) was determined.
Ascending urinary tract infection model.
The therapeutic efficacy of S-3578 was tested with a mouse model of ascending urinary tract infection caused by P. aeruginosa SR10396. The mice were subjected to water restriction for 20 to 24 h prior to infection. Under pentobarbital anesthesia, a round-point needle was inserted transurethrally for injection of 0.1 ml of the bacterial suspension into the bladder. The urethral needle was removed immediately after inoculation, and the external urethral meatus was clamped for 4 h. The challenge dose was 4.7 × 104 CFU/mouse. The compounds were administered subcutaneously 6, 24, 30, 48, 54, 72, and 78 h after infection.
The mice were killed 96 h after infection. The kidneys were then removed and homogenized in heart infusion broth, the homogenates were serially diluted 10-fold, and 1.0-ml aliquots were incorporated into heart infusion agar supplemented with 0.1% potassium nitrate. The plates were incubated at 37°C for 72 h, and the number of viable organisms in the kidneys (number of CFU per gram) was determined.
Pharmacokinetic study.
Each compound was injected intravenously at a dose of 20 mg/kg. Five mice were used for each time point. Samples of heart blood was obtained 0.25, 0.5, 1, and 2 h after drug administration. Samples of urine and bile were collected 2 h after drug administration. The concentration of biologically active antibiotic in the samples was determined by the bioassay method with Escherichia coli 7437 as the indicator organism (9). Active metabolites in samples of plasma, urine, and bile were examined by use of autobiograms obtained by thin-layer chromatography (15). The pharmacokinetic parameter of the concentration in plasma was calculated by one-compartment analysis with the WinNonlin program (Scientific Consulting, Inc.).
Statistical analysis.
Differences in viable counts between the drug-untreated and -treated groups were analyzed by Dunnett's multiple-comparison procedure. The comparison between S-3578 and reference compounds at the same dose was performed by Student's t test. P values below 0.05 were considered statistically significant.
RESULTS
Systemic infection.
The ED50s, 95% confidence limits, and MICs of S-3578 and the reference compounds for the strains tested are shown in detail in Table 1. The ED50 of S-3578 against MSSA Smith was 1.37 mg/kg. S-3578 was less effective than imipenem-cilastatin but three times more effective than vancomycin against this pathogen. The ED50 of S-3578 against MSSA SR14, a penicillinase-producing strain, was 1.38 mg/kg. S-3578 was less effective than imipenem-cilastatin but was as effective as cefepime and vancomycin against MSSA SR14. S-3578 had almost the same effectiveness (ED50, 7.21 mg/kg) as that of vancomycin against MRSA SR3637. However, cefepime and imipenem-cilastatin were not effective. The ED50 of S-3578 against MRSA SR20005 was 8.91 mg/kg, which was similar to that of vancomycin. The ED50s of imipenem-cilastatin and cefepime against these two MRSA strains were greater than 100 mg/kg. The in vivo activities of S-3578 against these two MRSA strains were similar to those of vancomycin.
TABLE 1.
In vivo efficacies of S-3578 and reference compounds in murine systemic infection
| Organism (challenge dose [no. of CFU/mouse]) | Compound | ED50 (mg/kg/dose) | 95% confidence interval (mg/kg/dose) | MIC (μg/ml) |
|---|---|---|---|---|
| S. aureus Smitha (3.6 × 107) | S-3578 | 1.37 | 1.12-1.61 | 1 |
| Cefepime | 2.18 | 1.62-2.93 | 2 | |
| Imipenem-cilastatin | 0.05 | 0.04-0.06 | ≤0.063 | |
| Vancomycin | 4.17 | NCb | 1 | |
| S. aureus SR14a (2.2 × 107) | S-3578 | 1.38 | 1.07-1.79 | 1 |
| Cefepime | 2.19 | 1.69-2.81 | 4 | |
| Imipenem-cilastatin | 0.04 | NC | ≤0.063 | |
| Vancomycin | 2.60 | 2.06-3.37 | 1 | |
| S. aureus SR3637a (MRSA) (2.4 × 107) | S-3578 | 7.21 | 6.11-8.36 | 4 |
| Cefepime | >100 | NC | >64 | |
| Imipenem-cilastatin | >100 | NC | 64 | |
| Vancomycin | 7.21 | 6.23-8.51 | 1 | |
| S. aureus SR20005a (MRSA) (3.4 × 107) | S-3578 | 8.91 | 7.87-10.0 | 4 |
| Cefepime | >100 | NC | >64 | |
| Imipenem-cilastatin | >100 | NC | 64 | |
| Vancomycin | 6.31 | 4.46-7.40 | 1 | |
| S. pneumoniae SR20946 (PRSP) (1.8 × 102) | S-3578 | 1.98 | 1.69-2.34 | 1 |
| Cefepime | 3.63 | 3.13-4.08 | 2 | |
| Ceftriaxone | 4.00 | 3.61-4.54 | 1 | |
| P. aeruginosa SR24a (2.8 × 104) | S-3578 | 5.28 | 3.38-8.47 | 4 |
| Cefepime | 3.27 | 2.17-5.71 | 1 | |
| Ceftazidime | 2.09 | 1.45-3.08 | 1 | |
| P. aeruginosa SR10411a (6.4 × 104) | S-3578 | 7.00 | 4.79-10.4 | 8 |
| Cefepime | 4.26 | 2.18-7.61 | 4 | |
| Ceftazidime | 3.35 | 2.13-5.00 | 4 |
The bacterial suspension were mixed with 5% mucin (ICN).
NC, not calculated.
Imipenem-cilastatin and cefepime were active against MSSA but not MRSA. S-3578 exhibited potent efficacy against both MSSA and MRSA. S-3578 was the most effective compound against PRSP SR20946 (ED50, 1.98 mg/kg). In P. aeruginosa infection models, the in vivo efficacies of S-3578 (ED50s, 5.28 to 7.00 mg/kg) against strains SR24 and SR10411 were similar. S-3578 was slightly less effective than cefepime and ceftazidime.
Subcutaneous infection caused by MSSA and MRSA.
The results of treatment of experimental subcutaneous infections caused by MSSA SR14 or MRSA SR20045 with S-3578, cefepime, and vancomycin are presented in Table 2. The number of viable organisms in abscesses caused by MSSA SR14 was approximately 107 CFU per abscess in the untreated group. S-3578 at doses of >3 mg/kg induced about a 3-log10 decrease in the number of bacteria in the abscesses. The reductions in bacterial numbers in mice treated with S-3578 at >3 mg/kg were significantly higher than those in the control group. Although the MIC of S-3578 for MSSA SR14 was similar to that of vancomycin, the in vivo antibacterial activity of S-3578 at 3 mg/kg was significantly higher than those of vancomycin and cefepime.
TABLE 2.
Therapeutic effects of S-3578, cefepime, and vancomycin against subcutaneous infection with S. aureus SR14 or SR20045 in micea
| Organism | Compound | Dose (mg/kg/dose) | MIC (μg/ml) | Log CFU/abscess (mean ± SD)b |
|---|---|---|---|---|
| S. aureus SR14 (MSSA) | Control | 7.24 ± 0.58 | ||
| S-3578 | 10 | 1 | 3.03 ± 0.53c | |
| 3 | 3.30 ± 0.25c | |||
| 1 | 5.64 ± 1.09 | |||
| Cefepime | 10 | 4 | 3.35 ± 0.39c | |
| 3 | 5.47 ± 1.25d | |||
| 1 | 6.90 ± 0.63 | |||
| Vancomycin | 10 | 1 | 3.85 ± 1.52c | |
| 3 | 6.04 ± 1.24d | |||
| 1 | 7.08 ± 0.94 | |||
| S. aureus SR20045 (MRSA) | Control | 6.60 ± 0.58 | ||
| S-3578 | 30 | 4 | 4.61 ± 0.47c | |
| 10 | 4.78 ± 0.69c | |||
| 3 | 6.56 ± 0.68 | |||
| Cefepime | 30 | >64 | 6.29 ± 1.15d | |
| Vancomycin | 30 | 1 | 4.16 ± 0.37c | |
| 10 | 4.76 ± 1.00c | |||
| 3 | 5.96 ± 1.05 |
The challenge dose of methicillin-susceptible S. aureus SR14 was 5.6 × 105 CFU per mouse, and that of methicillin-resistant S. aureus SR20045 was 8.4 × 105 CFU per mouse. The drugs were subcutaneously administered 3, 7, 24, and 30 h after infection. The number of viable organisms in the abscess (number of CFU abscess) was determined with mannitol salt agar.
n = 7.
P < 0.05 compared with the results for the control group.
P < 0.05 compared with the results obtained with S-3578 at the same dose.
The number of viable MRSA SR20045 bacteria in abscesses was approximately 106 CFU per abscess in the control group. S-3578 also showed potent activity, comparable to that of vancomycin, against MRSA SR20045. Although the MIC of S-3578 for MRSA SR20045 was fourfold less than that of vancomycin, the in vivo antibacterial activity of S-3578 was similar to that of vancomycin. Treatment with cefepime (30 mg/kg) reduced the viable counts only slightly compared to the number of viable bacteria in the control group. S-3578 was potent against both MSSA SR14 and MRSA SR20045 in the subcutaneous abscess model. The in vivo efficacy of S-3578 against this model of localized infection was superior to that expected from the in vitro activities of the reference compounds.
Pulmonary infection caused by PRSP.
The results of treatment of experimental pulmonary infection with S-3578, cefepime, ceftriaxone, and ampicillin are presented in Table 3. Control mice (two of seven) died between 3 and 4 days after infection, while the survival rates for all mice treated with S-3578, cefepime, ceftriaxone, and ampicillin were 100%. The control mice that survived maintained S. pneumoniae SR11031 at a level of approximately 108 CFU/g of lung. Compared with the untreated group, treatment with ampicillin at 30 mg/kg achieved a reduction of 1 log10 CFU/g of lung for strain SR11031. S-3578 produced a dose-response decrease in bacterial numbers throughout the dose range tested. The reduction in bacterial numbers achieved in mice treated with S-3578 at a dose of 10 mg/kg was significantly greater than that achieved in the control group (P < 0.05) but was not greater than that achieved in mice treated with the other agents tested. At 3 mg/kg, the reduction in bacterial numbers following treatment with S-3578 was more marked than that following treatment with cefepime and cefotaxime. The efficacy of S-3578 was superior to those of cefepime and ceftriaxone in this localized PRSP infection model.
TABLE 3.
Therapeutic effects of S-3578, cefepime, ceftriaxone, and ampicillin against pulmonary infection caused by PRSP SR11031 in micea
| Compound | Dose (mg/kg/dose) | MIC (μg/ml) | Log CFU/g (lung) (mean ± SD)b |
|---|---|---|---|
| Control | 7.79 ± 0.52 | ||
| Ampicillin | 30 | 2 | 6.58 ± 1.55 |
| S-3578 | 10 | 1 | 1.78 ± 0.17c |
| 3 | 3.20 ± 1.31c | ||
| 1 | 7.20 ± 1.37 | ||
| Cefepime | 10 | 1 | 3.44 ± 1.97c |
| 3 | 7.75 ± 0.61d | ||
| 1 | 7.93 ± 0.74 | ||
| Ceftriaxone | 10 | 1 | 2.22 ± 0.78c |
| 3 | 6.52 ± 0.90c,d | ||
| 1 | 6.79 ± 1.32 |
The inoculum was 8.2 × 105 CFU per mice. The drugs were subcutaneously administered 4, 7, 24, 30, 48, 54, 72, and 78 h after infection. The number of viable organisms in the lungs (number of CFU per gram) was determined by using brain heart infusion agar supplemented with 0.5% Bacto yeast extract and 2% defibrinated horse blood.
n = 7.
P < 0.05 compared with the results for the control group.
P < 0.05 compared with the results obtained with S-3578 at the same dose.
Urinary tract infection caused by P. aeruginosa.
The results of treatment of an experimental urinary tract infection with S-3578, cefepime, and ceftazidime are shown in Table 4. S-3578 exhibited a high degree of efficacy against P. aeruginosa SR10396 infection. The antibacterial activity of S-3578 at >10 mg/kg was significantly higher than that of no treatment (P < 0.05) but was not significantly higher than those of the other agents tested. Although the MIC of S-3578 for P. aeruginosa SR10396 was fourfold less than those of cefepime and ceftazidime, the in vivo efficacy of S-3578 against pseudomonal urinary tract infection was similar to those of cefepime and ceftazidime.
TABLE 4.
Therapeutic effects of S-3578, cefepime, and ceftazidime against urinary tract infection caused by P. aeruginosa SR10396 in micea
| Compound | Dose (mg/kg/dose)a | MIC (μg/ml) | Log CFU/g (kidney) (mean ± SD)b |
|---|---|---|---|
| Control | 7.73 ± 0.42 | ||
| S-3578 | 30 | 8 | 3.31 ± 2.06c |
| 10 | 4.04 ± 2.30c | ||
| 3 | 5.86 ± 2.17 | ||
| Cefepime | 30 | 2 | 3.27 ± 2.03c |
| 10 | 4.51 ± 2.65c | ||
| 3 | 6.50 ± 1.85 | ||
| Ceftazidime | 30 | 2 | 3.34 ± 2.38c |
| 10 | 4.90 ± 2.06c | ||
| 3 | 4.20 ± 2.70c |
The inoculum was 4.7 × 104 CFU per mouse. The drugs were subcutaneously administered 6, 24, 30, 48, 54, 72, and 78 h after infection. The number of viable organisms in the kidneys (number of CFU per gram was determined by using heart infusion agar supplemented with 0.1% potassium nitrate.
n = 7.
P < 0.05 compared with the results for the control group.
Polymicrobial infections caused by both MRSA and P. aeruginosa. (i) Systemic infection.
The ED50, 95% confidence limits, and MICs for the strains tested are shown in Table 5. S-3578 exhibited good activity against MRSA and P. aeruginosa isolates, with an ED50 of 10.9 mg/kg. However, cefepime, ceftazidime, linezolid, and vancomycin were not effective. The ED50 of S-3578 was less than the total ED50 of vancomycin and ceftazidime, and therapy with S-3578 was more effective than combination therapy with vancomycin and ceftazidime in the polymicrobial systemic infection model.
TABLE 5.
In vivo efficacies of S-3578 and other agents against murine polymicrobial systemic infection caused by methicillin-resistant S. aureus SR17505 and P. aeruginosa SR24a
| Compound | ED50 (mg/kg/dose) | 95% confidence limit (mg/kg/dose) | MICb (μg/ml) |
|---|---|---|---|
| S-3578 | 10.9 | 8.57-13.8 | 4/4 |
| Cefepime | >100 | NCc | >64/1 |
| Ceftazidime | >100 | NC | >64/1 |
| Linezolid | >30 | NC | 2/>128 |
| Vancomycin | >100 | NC | 1/>128 |
| Ceftazidime + vancomycind | 15.1 | 9.98-19.7 |
The challenge dose of MRSA SR17505 was 4.9 × 107, and that of P. aeruginosa SR24 was 1.1 × 102.
MIC for MRSA/MIC for P. aeruginosa.
NC, not calculated.
Ceftazidime and vancomycin were used in combination at a ratio of 1:1.
(ii) Pulmonary infection.
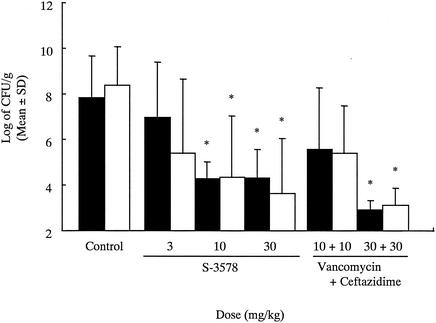
The results of treatment of experimental pulmonary infection with S-3578 and the combination of vancomycin and ceftazidime are shown in Fig. 1. In the untreated group, the number of viable cells was approximately 108 CFU/g of lung for both MRSA SR17764 and P. aeruginosa SR10396. The MICs of S-3578, ceftazidime, and vancomycin for MRSA SR17764 were 4, >64, and 1 μg/ml, respectively. The MICs of S-3578, cefepime, and vancomycin for P. aeruginosa SR10396 were 8, 2, and > 128 μg/ml, respectively. Monotherapy with vancomycin at 30 mg/kg reduced the numbers of viable MRSA SR17764 only and was not effective in reducing the numbers of viable P. aeruginosa (data not shown). Monotherapy with ceftazidime at 30 mg/kg reduced the numbers of viable P. aeruginosa SR10396 only (data not shown). However, S-3578 at >10 mg/kg induced a significant reduction in the numbers of both viable MRSA SR17764 and viable P. aeruginosa SR10396. Combination therapy with ceftazidime and vancomycin (each at 30 mg/kg) significantly reduced the number of bacteria in the lungs compared with the number in the lungs in the untreated control group. The therapeutic efficacy of S-3578 was more potent than that of combination therapy with vancomycin and ceftazidime.
FIG. 1.
In vivo effects of S-3578, ceftazidime, and vancomycin alone and in combination against pulmonary infections caused by both methicillin-resistant S. aureus SR17764 (▪) and P. aeruginosa SR10396 (□) in mice. The challenge dose of MRSA SR17764 was 3.2 × 105 CFU per mouse, and that of P. aeruginosa SR10396 was 7.9 × 101 CFU per mouse. The compounds tested were given subcutaneously 3, 5, 7, 24, 30, 48, and 54 h after challenge. For MRSA, the number of viable organisms in the lungs (CFU per gram) was determined by using mannitol salt agar. For P. aeruginosa, heart infusion agar supplemented with 20 μg of vancomycin per ml and 0.15% potassium nitrate was used. ∗, P < 0.05 compared with the results for the control group.
Pharmacokinetic studies.
The pharmacokinetic properties of S-3578 administered intravenously at a dose of 20 mg/kg were compared with those of cefepime and ceftazidime. The levels of S-3578, cefepime, and ceftazidime at 0.25 h were 21.6, 26.9, and 19.1 μg/ml, respectively. The values of the pharmacokinetic parameters for S-3578 and the reference compounds are summarized in Table 6. The area under the plasma concentration-time curve for S-3578 was 14.7 μg · h/ml, whereas those for cefepime and ceftazidime were 11.5 μg · h/ml and 11.4 μg · h/ml, respectively. The elimination half-life of S-3578 was also similar to those of the reference compounds and ranged from 0.25 to 0.28 h. S-3578, cefepime, and ceftazidime were mainly eliminated by renal excretion. No active metabolites were found in the plasma or urine of animals given these compounds.
TABLE 6.
Pharmakokinetic parameters for S-3578 and reference drugs in micea
| Compound | C15 (μg/ml) | AUC0-∞ (μg · h/ml) | t1/2 (h) | Excretion (% at 0 to 2 h)
|
|
|---|---|---|---|---|---|
| Urine | Bile | ||||
| S-3578 | 21.6 | 14.7 | 0.26 | 73.2 | 2.9 |
| Cefepime | 26.9 | 11.5 | 0.28 | 80.8 | 1.7 |
| Ceftazidime | 19.1 | 11.4 | 0.25 | 50.4 | 3.1 |
Abbreviations: t1/2, half-life; AUC0-∞, area under the concentration-time curve from time zero to infinity; C15, concentration of drug in serum at 15 min. The concentration in plasma was calculated by one-compartment analysis with the WinNonlin program. The plasma and urine were negative for the active metabolite.
DISCUSSION
S-3578 is a novel cephalosporin with an imidazopyridinium group in the side chain at position 3. The major finding of the present study was that this new compound exhibits potent efficacy against MRSA and P. aeruginosa in vivo. In particular, S-3578 was highly effective against MRSA. The in vivo activity of S-3578 against two MRSA strains was equal to that of vancomycin. Although the in vitro activity of S-3578 against MRSA was fourfold less than that of vancomycin, the in vivo efficacy of S-3578 against systemic and subcutaneous infections caused by MRSA was superior to that predicted from the in vitro activity of vancomycin. One possible explanation of these results may be that S-3578 possesses better bactericidal activity than vancomycin. The level of binding of S-3578 to serum proteins in mice was 22%. On the other hand, the level of binding of vancomycin to serum proteins in mice was 47% (data not shown). Thus, a second possible reason for the difference in the in vivo efficacies between S-3578 and vancomycin may be related to the levels of free drug.
Our results also indicated that S-3578 was more effective than cefepime against various MRSA infection models. S-3578 has an aminothiadiazol group in the side chain at position 7. Cefepime has an aminothiazole group in the side chain at position 7 (16). Although cefozopran and cefclidin, like S-3578, have the same side chain at position 7, these compounds have no anti-MRSA activities (8, 18). S-3578 has the imidazopyridinium group in the side chain at position 3 (20). Cefepime has an N-methylpyrrolidinium group in the side chain at position 3 (16). The anti-MRSA efficacy of S-3578 may be related to structural aspects, such as the new side chain at position 3 rather than the side chain at position 7 on the cephem nucleus.
Other gram-negative bacteria such as P. aeruginosa are often simultaneously isolated from patients with MRSA infections (1, 10). Therefore, monotherapy with agents with activities against gram-positive bacteria, such as vancomycin, is not effective against polymicrobial infections caused by both MRSA and P. aeruginosa (14). New cephalosporins such as cefepime are active against P. aeruginosa and less active against MRSA. Thus, combination therapy with an anti-MRSA drug such as vancomycin and an antipseudomonal drug such as ceftazidime is usually used for the treatment of polymicrobial infections caused by MRSA and P. aeruginosa (11). A better approach to the development of new parenteral cephalosporins would be to aim for drugs with broad and potent activities against a wide range of gram-positive and -negative bacteria, including MRSA and P. aeruginosa. Our results demonstrated that S-3578 was more effective than combination therapy with vancomycin and ceftazidime against polymicrobial infections caused by both MRSA and P. aeruginosa. Our results, together with those of pharmacokinetic studies with mice, suggest that S-3578 is a promising new cephalosporin for the treatment of polymicrobial infections or superinfection caused by gram-positive and -negative bacteria, including MRSA and P. aeruginosa.
REFERENCES
- 1.Crane, L. R., D. P. Levine, M. J. Zervos, and G. Cummings. 1986. Bacteremia in narcotic addicts at the Detroit Medical Center. I. Microbiology, epidemiology, risk factors, and empiric therapy. Rev. Infect. Dis. 8:364-373. [DOI] [PubMed] [Google Scholar]
- 2.Finney, D. J. 1978. Statistical methods in biological assays, 3rd ed. Charles Griffin & Co. Ltd., London, England.
- 3.Ford, C. W., J. C. Hamel, D. Stapert, and R. J. Yancey. 1989. Establishment of an experimental model of a Staphylococcus aureus abscess in mice by use of dextran and gelatin microcarriers. J. Med. Microbiol. 28:259-266. [DOI] [PubMed] [Google Scholar]
- 4.Fujimura, T., Y. Yamano, I. Yoshida, J. Shimada, and S. Kuwahara. 2003. In vitro activity of S-3578, a new broad-spectrum cephalosporin active against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 47:923-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung-Tomc, J. C., J. Clark, B. Minassian, M. Pucci, Y. H. Tsai, E. Gradelski, L. Lamb, I. Medina, E. Huczko, B. Kolek, S. Chaniewski, C. Ferraro, T. Washo, and D. P. Bonner. 2002. In vitro and in vivo activities of a novel cephalosporin, BMS-247243, against methicillin-resistant and -susceptible staphylococci. Antimicrob. Agents Chemother. 46:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grassi, G. G., and C. Grassi. 1993. Cefepime: overview of activity in vitro and in vivo. J. Antimicrob. Chemother. 32(Suppl. B):87-94. [DOI] [PubMed] [Google Scholar]
- 7.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwahi, T., K. Okonogi, T. Yamazaki, S. Shiki, M. Kondo, A. Miyake, and A. Imada. 1992. In vitro and in vivo activities of SCE-2787, a new parenteral cephalosporin with a broad antibacterial spectrum. Antimicrob. Agents Chemother. 36:1358-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura, Y., M. Nakano, and T. Yoshida. 1987. Microbiological assay methods for 6315-S (flomoxef) concentration in body fluid. Chemotherapy (Tokyo) 35(Suppl. 1):129-136. [Google Scholar]
- 10.Konno, M. 1995. Nosocomial infections caused by methicillin-resistant S. aureus in Japan. J. Infect. Chemother. 1:30-39. [Google Scholar]
- 11.Matsuoka, K., Y. Nagatomi, K. Imanishi, M. Matsubara, I. Nakanishi, and H. Watanabe. 1994. A study on MRSA infections and replacement of bacteria. Efficacy of vancomycin-ceftazidime combination therapy. Jpn. J. Antibiot. 47:1363-1368. [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Method for dilution antibacterial susceptibility tests for bacteria that grow aerobically, 5th ed.; approved standard M-7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing; eleventh informational supplement. M-100-S-11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Shimizu, K., M. Orizu, H. Kanno, S. Kitamura, T. Konishi, K. Soma, H. Nishitani, Y. Noguchi, S. Hasegawa, H. Hasegawa, and K. Wada. 1996. Clinical studies on vancomycin in the treatment of MRSA infection. Jpn. J. Antibiot. 49:782-799. [PubMed] [Google Scholar]
- 15.Takeda, M., and K. Maeda. 1993. Microbiological assay method for cefozopran in biological specimens. Chemotherapy (Tokyo) 41(Suppl. 4):135-141. [Google Scholar]
- 16.Tsuji, A., A. Maniatis, M. A. Bertram, and L. S. Young. 1985. In vitro activity of BMY-28142 in comparison with those of other beta-lactam antimicrobial agents. Antimicrob. Agents Chemother. 27:515-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji, M., Y. Ishii, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1998. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob. Agents Chemother. 42:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe, N., K. Katsu, M. Moriyama, and K. Kitoh. 1988. In vitro evaluation of E1040, a new cephalosporin with potent antipseudomonal activity. Antimicrob. Agents Chemother. 32:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida, T., S. Matsuura, M. Mayama, Y. Kameda, and S. Kuwahara. 1980. Moxalactam (6509-S), a novel 1-oxa-β-lactam with an expanded antibacterial spectrum: laboratory evaluation. Antimicrob. Agents Chemother. 17:302-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshizawa, H., H. Itani, K. Ishikura, T. Irie, K. Yokoo, T. Kubota, K. Minami, T. Iwaki, H. Miwa, and Y. Nishitani. 2002. S-3578, a new broad spectrum parenteral cephalosporin exhibiting potent activity against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. Synthesis and structure-activity relationships. J. Antibiot. (Tokyo) 55:975-992. [DOI] [PubMed] [Google Scholar]