Abstract
Using six Enterococcus faecalis and five Enterococcus faecium strains, the ketolide ABT-773 (ABT), now known as cethromycin, was found to have in vivo efficacy against both erythromycin (ERY)-susceptible (Erys) and -intermediate (Eryi) enterococci (ABT 50% protective doses [PD50s], 0.5 to 4.1 and 10.3 to 16.2 mg/kg of body weight, respectively). Against four highly Ery-resistant (Eryr) strains for which ABT MICs were low, ABT showed much greater activity (PD50, 6.3 to 32.5 mg/kg) than ERY (PD50, >200 mg/kg) but was not protective for strains for which ABT MICs were high. In conclusion, ABT-773 showed in vivo efficacy and considerably greater activity than ERY in a mouse peritonitis model.
Enterococci are important causes of nosocomial infections, including infective endocarditis, urinary tract infections, and bacteremia (7, 14), and are problematic because of increasing antibiotic resistance. Although two agents (quinupristin-dalfopristin and linezolid) have been approved for use against vancomycin-resistant (Vanr) enterococci since 1999, emergence of resistance to these agents among vancomycin-resistant enterococci and/or adverse events have continued the need for new antibiotics (5, 6). ABT-773 (cethromycin [ABT]) is a new semisynthetic ketolide that differs from the natural macrolide erythromycin (ERY), with an 11,12-position cyclic carbamate group in addition to the 3-keto group. ABT has a broad spectrum of activity against some gram-positive, gram-negative, and intracellular bacteria (1, 3, 4, 17, 18), but there is no published information regarding in vivo activity against enterococci. In the present study, we evaluated the activity of ABT against Enterococcus faecalis and Enterococcus faecium strains with various susceptibilities to ERY in a mouse peritonitis model and found that the in vivo efficacy of ABT was considerably greater than that of ERY.
This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy (S. R. Pai, K. V. Singh, and B. E. Murray, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1652, 2001).
Six E. faecalis isolates were selected for the present study based on their varied antibiotic susceptibility profiles (Table 1). E. faecalis OG1RF (ATCC 47077) is a commonly used strain that is plasmid free (9). E. faecalis TX0921 (HH22) is a β- lactamase-producing strain with high-level resistance to gentamicin (Genr) (8). E. faecalis TX0052 was isolated from the blood of an endocarditis patient and is resistant to ERY {Eryr [erm(B)]}. E. faecalis V583 (13) is Eryr [erm(B)] and Vanr (vanB). Both E. faecalis TX0860 and E. faecalis TX0641 are highly resistant to ERY but susceptible to ABT.
TABLE 1.
MICs, LD50s, and PD50s of ABT and ERY against enterococci in the mouse peritonitis modelc
| Organism (characteristics)d | MIC (μg/ml)
|
LD50 (CFU) | Treatment route | No. of doses | PD50 (mg/kg of body wt)
|
||
|---|---|---|---|---|---|---|---|
| ABT | ERY | ABT | ERY | ||||
| OG1RF (ATCC 47077) E. faecalis (Gel+, Fusr, Rifr) | 0.062 | 0.5 | 1.2 × 108 | s.c. | 1 | 4.1 | 31.5 |
| p.o. | 1 | 37.3 | >200 | ||||
| TX0921 (HH22) E. faecalis Gel+, βla+, Genr, Erys | 0.031 | 0.5 | 7.3 × 106-4.7 × 107 | s.c. | 1 | 0.5 | 15.7 |
| TX0052 E. faecalis {Gel+, Strr, Genr, Eryr [erm(B)], an endocarditis isolate} | ≥128 | 1,024 | 1.3 × 108 | s.c. | 1 | >100 | >200 |
| s.c. | 2 | —e | >200 | ||||
| V583 E. faecalis {Gel+, Strr, Genr, Eryr [erm(B)], VANr (vanB)} | ≥128 | 512-1,024a | <1.5 × 108 | s.c. | 1 | >100 | >200 |
| s.c. | 2 | — | >200 | ||||
| TX0860 (BE88) E. faecalis {Eryr [erm(B)]} | 0.062 | >512 | 2.3 × 108 | s.c. | 1 | 32.5 | >200 |
| TX0641 (CH25) E. faecalis {Eryr [erm(B)]} | 0.031 | >512 | 2.1 × 108 | s.c. | 1 | 6.3 | >200b |
| TX0016 (DO) E. faecium {Kanr, Strr, Eryr [erm(B)], Tetr, an endocarditis isolate} | ≥128 | 1,024 | 3.7 × 108 | s.c. | 1 | >100 | >200 |
| s.c. | 2 | >100 | >200 | ||||
| TX0016.01 (DO) E. faecium [erm(B)-cured Eryi (msrC), Strr, Tetr] | 0.062 | 2 | 3.7 × 108 | s.c. | 1 | 16.2 | 27.7 |
| p.o. | 1 | 16.6 | >200 | ||||
| TX2465 E. faecium [Eryi (msrC), Vanr (vanA)] | 0.062 | 2-4a | 2.5 × 108 | s.c. | 1 | 10.3 | 36.4 |
| TX2597 E. faecium [Eryr (msrC), Vanr (vanA)] | 0.016 | 16 | 1.1 × 109 | s.c. | 1 | 16.2 | >200 |
| TX4051 E. faecium [Eryr (msrC)] | 0.031 | 16 | 1.4 × 109 | s.c. | 1 | 9.1 | >200 |
Values represent results of different determinations.
The PD50 of erythromycin for this strain was inadvertently determined using an inoculum of more than 10 times the LD50.
vanA and vanB results were derived on the basis of PCR or hybridization to PCR products.
Fus, fusidic acid; Gen, gentamicin; Kan, kanamycin; Rif, rifampin; Str, streptomycin; Tet, tetracycline; βla+, β-lactamase producer.
—, not tested.
The E. faecium strains studied included TX0016 (also known as DO) (for a partial sequence, see http://www.hgsc.bcm.tmc.edu/microbial/efaecium/) (2), an endocarditis isolate that is Eryr [erm(B)]; E. faecium TX0016.01 (DO cured of ERY resistance by novobiocin) (DOc) (2, 15); E. faecium TX2465, a vanA-containing clinical isolate showing intermediate resistance to ERY (Eryi, Vanr); E. faecium TX2597, a Vanr (vanA) isolate; and E. faecium TX4051(1464-74), showing moderate resistance to ERY but susceptibility to ABT. Both ERY (Erytrocin I.V [erythromycin lactobionate]) and ABT were obtained from Abbott Laboratories, Chicago, Ill. The antibiotics were appropriately reconstituted and the stocks were stored according to the manufacturer's instructions. MIC tests were performed according to the recommended guidelines for susceptibility testing of the National Committee for Clinical Laboratory Standards (NCCLS) (9, 10) by agar dilution with Mueller-Hinton agar II (Becton Dickinson and Company, Cockeysville, Md.) and using E. faecalis ATCC 29212 as a control strain. Enterococci were considered susceptible (Erys) when the MIC of ERY was ≤0.5 μg/ml, intermediate when the MIC was between 1 and 4 μg/ml, and resistant when the MIC was ≥8 μg/ml (10, 11).
Female, 4- to 6-week-old, outbred ICR mice (Harlan Sprague Dawley, Houston, Tex.) with a mean weight of 25 g were used in the study. The 50% lethal dose (LD50) of enterococci for mice was determined as described earlier (15), with a 12.5% concentration of sterile rat fecal extract (SRFE). SRFE was prepared using crushed, dried rat feces by mixing with 2 volumes of 0.9% (wt/vol) saline and autoclaving at 121°C and 15 lb of pressure for 15 min. The autoclaved sample was centrifuged at ∼1,543 × g at a temperature of 4°C, and the supernatant (100% SRFE) was reautoclaved under the conditions described above. Table 1 shows the LD50s observed. To determine the 50% protective doses (PD50s), both ABT and ERY were administered by subcutaneous (s.c.) injection immediately following intraperitoneal inoculation of 10 × the LD50 of enterococci in SRFE, except in the initial stages, in which ABT was also administered orally (p.o.) by gavage. When two doses were used, they were administered at 0 and 4 h after infection. The dose ranges studied were between 3.12 and 100 mg/kg of body weight. The PD50s of ABT and ERY were determined by the method of Reed and Muench (12). Six mice/dose/drug were used to generate LD50 and PD50 values and dose response curves. In both the LD50 and PD50 experiments, mouse spleen homogenates were used to recover and confirm the identity of the lethal organism either by phenotypic characteristics or by using pulsed-field gel electrophoresis.
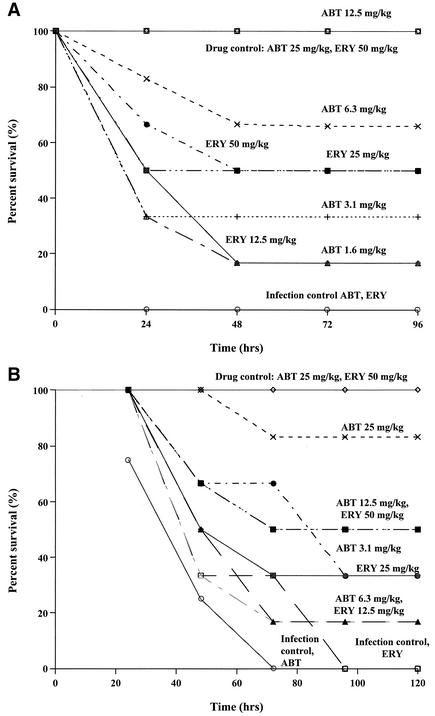
MICs of the antibiotics are shown in Table 1 along with the LD50 and PD50 values, and dose-response curves are shown in Fig. 1. Strains with erm(B) that were highly resistant to ABT and ERY were classified as cMLSB, whereas those highly resistant to ERY only were considered to be iMLSB.
FIG. 1.
Dose-response curves. (A) Drug-only (no bacteria) control animals showed 100% survival, and animals in the infection control (no antibiotic) group that were infected with E. faecalis strain OG1RF showed 0% survival. All of the mice received s.c. therapy with ABT or ERY for peritonitis caused by E. faecalis strain OG1RF. (B) Drug-only (no bacteria) control animals showed 100% survival, and animals in the infection control (no antibiotic) group that were infected with E. faecium strain TX2465 showed 0% survival. All the mice received s.c. therapy with ABT or ERY for peritonitis caused by E. faecium strain TX2465.
For determination of PD50s, both ABT and ERY were first tested by the s.c. and the p.o. routes against E. faecalis OG1RF-infected and E. faecium TX0016.01 (DOc, Eryi)-infected mice. The PD50 of ABT for mice infected with OG1RF via the p.o. route was nine times higher than the PD50 for those infected via the s.c. route. For E. faecium TX0016.01 (DOc, Eryi), the PD50s of ABT administered by the p.o. (16.6 mg/kg of body weight) and s.c. (16.2 mg/kg) routes were similar.
ERY administered s.c. demonstrated a PD50 of 31.5 mg/kg against OG1RF and 27.7 mg/kg against E. faecium TX0016.01 (DOc, Eryi), while orally administered ERY for OG1RF-infected and E. faecium TX0016.01 (DOc, Eryi)-infected mice showed no protection even at the highest dose of ERY (200 mg/kg). For this reason, the remainder of the study was performed using injections.
For OG1RF mice inoculated by the s.c. route, ABT displayed a PD50 value 7.7 times lower than the PD50 value found for ERY (ABT PD50, 4.1 mg/kg; ERY PD50, 31.5 mg/kg). For E. faecalis TX0921-infected mice, ABT displayed a PD50 value (0.5 mg/kg) that was 31 times lower than the PD50 value found for ERY (PD50, 15.7 mg/kg). ABT displayed MICs of 0.062 and 0.031 μg/ml for OG1RF and TX0921, respectively, and ERY displayed MICs of 0.5 μg/ml for both strains.
Both ABT and ERY displayed high PD50 values for mice infected with the highly ERY- and ABT-resistant E. faecalis strains TX0052 and V583 and E. faecium TX0016 (DO). The two-dose regimen for infected mice, consisting of one dose of 50 mg of ABT/kg of body weight at time zero and a second dose after a 4-h interval, presented better protection and survival than the 100-mg/kg regimen, suggesting that the higher level of mortality at 100 mg/kg was due in part to drug toxicity.
Unlike the nonprotective effect observed against strains highly resistant to ABT and ERY, an in vivo protective effect of ABT was seen against E. faecalis strains highly resistant to ERY but for which ABT MICs were low, with an ABT PD50 of 32.5 mg/kg for E. faecalis TX0860 (ERY MIC, >512 μg/ml; ABT MIC, 0.062 μg/ml)-infected mice and an ABT PD50 value of 6.25 mg/kg for E. faecalis TX0641 (ERY MIC, >512 μg/ml; ABT MIC, 0.031 μg/ml)-infected mice. Protective effects were also seen with ABT for E. faecium TX2597 (ABT MIC, 0.016 μg/ml; ERY MIC, 16 μg/ml)-infected mice and E. faecium TX4051 (ABT MIC, 0.031 μg/ml; ERY MIC, 16 μg/ml)-infected mice, with ABT PD50s of 16.2 and 9.1 mg/kg, respectively.
It is of interest that while the ABT MICs were similar for the more ABT-sensitive (MICs, ≤0.062 μg/ml) E. faecalis and E. faecium strains, the PD50s were lower against three of the four E. faecalis strains than those against the four E. faecium strains; the reason for this in vivo-in vitro difference is not known. Two of these E. faecalis strains were Erys, and the other two were erm(B)+ with the iMLSB phenotype. None of the E. faecium strains for which the ABT MICs were very low were erm(B)+, but for each strain, the ERY MIC was 2 to 16 μg/ml, a result perhaps related in part to the presence of msrC (16). The in vivo activity (both intra- and interspecies) of ABT against ERY-susceptible and -intermediate resistance enterococci was also observed to be similar to that of telithromycin (HMR 3647) in the mouse peritonitis model, which also showed some efficacy when two doses were given s.c., indicating that it might be possible to achieve an effect even against more resistant organisms (15).
In conclusion, ABT showed in vivo efficacy against ERY-susceptible and ERY-intermediate enterococci and against some highly ERY-resistant enterococci that were inhibited by low concentrations of ABT. As with in vitro results, ABT was found to be more potent than ERY in the mouse peritonitis model but was not protective against cMLSB strains.
Acknowledgments
This study was supported by a grant from Abbott Laboratories.
REFERENCES
- 1.Andrews, J. M., T. M. A. Weller, J. P. Ashby, R. M. Walker, and R. Wise. 2000. The in vitro activity of ABT-773, a new ketolide antimicrobial agent. J. Antimicrob. Chemother. 46:1017-1022. [DOI] [PubMed] [Google Scholar]
- 2.Arduino, R. C., K. Jacques-Palaz, B. E. Murray, and R. M. Rakita. 1994. Resistance of Enterococcus faecium to neutrophil-mediated phagocytosis. Infect. Immun. 62:5587-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueggemann, A. B., G. V. Doern, H. K. Huynh, E. M. Wingert, and P. R. Rhomberg. 2000. In vitro activity of ABT-773, a new ketolide, against recent clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob. Agents Chemother. 44:447-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim, M. K., W. Zhou, P. R. Tessier, D. Xuan, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Bactericidal effect and pharmacodynamics of cethromycin (ABT-773) in a murine pneumococcal pneumonia model. Antimicrob. Agents Chemother. 46:3185-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 6.Murray, B. E. 1997. Vancomycin-resistant enterococci. Am. J. Med. 102:284-293. [DOI] [PubMed] [Google Scholar]
- 7.Murray, B. E. 1990. The life and times of the enterococci. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray, B. E., and B. Mederski-Samoraj. 1983. Transferable β-lactamase: a new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J. Clin. Investig. 72:1168-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically—fifth edition; approved standard. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Committee for Clinical Laboratory Standards. 2000. Performance standard for antimicrobial susceptibility testing; tenth informational supplement (aerobic dilution). NCCLS document M100—S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent end points. Am. J. Hygiene 27:493-497. [Google Scholar]
- 13.Sahm, D. F., J. Kissinger, M. S. Gilmore, P. R. Murray, R. Mulder, J. Soliday, and B. Clarke. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaberg, D. R., and D. H. Culver. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91:72S-75S. [DOI] [PubMed]
- 15.Singh, K. V., K. K. Zscheck, and B. E. Murray. 2000. Efficacy of telithromycin (HMR 3647) against enterococci in a mouse peritonitis model. Antimicrob. Agents Chemother. 44:3434-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh, K. V., K. Malathum, and B. E. Murray. 2001. Disruption of an Enterococcus faecium species-specific gene, a homologue of acquired macrolide resistance genes of staphylococci, is associated with an increase in macrolide susceptibility. Antimicrob. Agents Chemother. 45:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh, K. V., K. Malathum, and B. E. Murray. 2001. In vitro activities of a new ketolide, ABT-773, against multidrug-resistant gram-positive cocci. Antimicrob. Agents Chemother. 45:3640-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Eiff, C., and G. Peters. 2002. Comparative in vitro activity of ABT-773 and two macrolides against staphylococci. J. Antimicrob. Chemother. 49:189-192. [DOI] [PubMed] [Google Scholar]