Abstract
This study was designed to test the efficacy of antiviral treatment with entecavir (ETV) in combination with DNA vaccines expressing duck hepatitis B virus (DHBV) antigens as a therapy for persistent DHBV infection in ducks. Ducks were inoculated with 109 DHBV genomes at 7 days of age, leading to widespread infection of the liver and viremia within 7 days, and were then treated orally with either ETV (0.1 mg/kg of body weight/day) or distilled water from 21 days posthatch for 244 days. Treatment with ETV caused a 4-log drop in serum DHBV DNA levels within 80 days and a slower 2- to 3-log drop in serum DHBV surface antigen (DHBsAg) levels within 120 days. Following withdrawal of ETV, levels of serum DHBV DNA and DHBsAg rebounded to match those in the water-treated animals within 40 days. Sequential liver biopsy samples collected throughout the study showed that ETV treatment reduced DHBV DNA replicative intermediates 70-fold in the liver, while the level of the stable, template form, covalently closed circular DNA decreased only 4-fold. ETV treatment reduced both the intensity of antigen staining and the percentage of antigen-positive hepatocytes in the liver, but the intensity of antigen staining in bile duct cells appeared not to be effected. Intramuscular administration of five doses of a DNA vaccine expressing the DHBV presurface, surface, precore, and core antigens, both alone and concurrently with ETV treatment, on days 50, 64, 78, 127, and 141 did not result in any significant effect on viral markers.
Hepatitis B virus (HBV) is a noncytopathic virus that replicates primarily in hepatocytes. HBV infection of humans can result in either a transient infection with development of neutralizing antibodies and immunity to reinfection or a persistence of viral infection for many years. Patients with either acute or persistent HBV infection often have extensive infection of the liver with high titers of infectious virus and noninfectious surface antigen particles circulating in the bloodstream. Immune responses to HBV are responsible for clearance of virus-infected cells from the liver and for the liver damage seen in both acute and persistent infection. Models of hepadnavirus pathogenesis usually propose that effective humoral and cell-mediated immune responses lead to recovery from infection with elimination of virally infected cells or replicative intermediates, while ineffective immune responses allow extensive viral replication with or without hepatocyte damage (reviewed in references 2 and 3). Thus, in individuals destined to become persistently infected, the high viral load may overwhelm the capacity of the immune system or favor the induction of ineffective antibody responses.
Persistent HBV infection occurs in 5 to 10% of adults with acute infections and in >90% of neonates. Current approaches to treatment for persistent HBV infection include long-term alpha interferon administration and antiviral drug therapy aimed at inhibiting viral replication. Partial suppression of levels of circulating virus can be achieved during therapy, but rebound following cessation of therapy is common. Immunotherapy using DNA vaccines has been proposed as a way to improve viral clearance via the induction by DNA vaccination of effective immune responses. Also, because the type and level of immune response induced are antigen dose dependent, treatment of chronically infected individuals with antiviral drugs with the aim of suppressing HBV replication and antigen expression prior to DNA vaccination is another approach that needs further investigation. The present study was performed using ducks inoculated with a high dose of the duck hepatitis B virus (DHBV). Like HBV-infected humans, DHBV-infected ducks exhibit age-related outcomes of infection, with the development of persistent infection in young ducks and transient infection in adults (11). These different outcomes are dose dependent, with persistent infection developing in young ducks inoculated with higher doses of virus (11).
The present study was designed to test the efficacy of both a potent inhibitor of the hepadnaviral polymerases, entecavir (ETV; formerly known as BMS-200475), and DHBV DNA vaccines expressing the DHBV surface (S), precore S/S, (C), and pre-C/C antigen genes. The possibility that reducing the antigen load in persistently infected ducks using antiviral therapy might enhance the effectiveness of the response to DNA vaccines was also examined. ETV is a cyclopentyl 2′-deoxyguanosine nucleoside that is highly inhibitory against hepadnavirus replication in cell culture, animal models, and human clinical trials (4, 7, 17, 21) and is currently in phase III development. ETV triphosphate directly inhibits all three functional activities of hepadnaviral polymerases and has been shown to inhibit HBV replication in vitro and woodchuck hepatitis virus (WHV) replication in woodchucks (4, 7). The ability of ETV to inhibit DHBV replication has been examined in short-term studies (21) which showed that the antiviral activity of ETV (50% effective concentration [EC50], 0.13 nM) was >1,000-fold greater than that of lamivudine (EC50, 138 nM) in DHBV-infected primary duck hepatocyte cultures. A 21-day in vivo treatment of ducks with 1 mg of ETV/kg of body weight/day resulted in a mean reduction of log10 3.1 in serum DHBV DNA levels, while treatment with 0.1 mg/kg/day resulted in an average viral DNA decrease of log10 2.1.
In the present study, 20 7-day-old ducks were inoculated with a dose of DHBV that is known from previous work (11) to result in persistent DHBV infection. The efficacy of ETV treatment against DHBV was then assessed by treating the ducks orally with either 0.1 mg of ETV/kg/day or distilled water for 244 days. Liver and serum samples were monitored for decreases in DHBV DNA and antigen expression, and by day 50 of treatment, levels of DHBV DNA and DHBV surface antigen (DHBsAg) in the liver and serum of the ETV-treated ducks were significantly reduced. The ducks then received five doses of either DHBV DNA vaccines or the vector only. ETV therapy was withdrawn after 244 days, and all ducks were monitored for a further 70 days to assess the sustained nature of the responses seen.
MATERIALS AND METHODS
Animals.
Twenty-five 1-day-old DHBV-negative Pekin Aylesbury ducks (Anas domesticus platyrhyncos) were obtained from a commercial hatchery and were held in the animal house facilities of the Institute of Medical and Veterinary Science (IMVS), Adelaide, Australia. The 25 ducks were divided into five treatment groups (groups 1 to 5), each containing five ducks as described below and in the legend to Fig. 1. All animal handling procedures were approved by both the IMVS and University of Adelaide animal ethics committees and followed the guidelines of the National Health and Medical Research Committee of Australia.
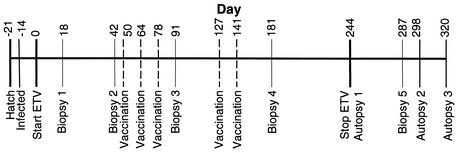
FIG. 1.
Time line of the experiments performed in this study. Twenty-five ducks were divided into five treatment groups (1 to 5) containing five ducks each. The group 1 to 4 ducks were infected with DHBV at 7 days posthatch (day −14), while the group 5 ducks were used as uninfected controls. The group 1 and 2 ducks were treated daily with ETV for 244 days, while the group 3, 4, and 5 ducks were treated at the same time with distilled water. The group 1 and 3 ducks received DHBV vaccine, while the group 2 and 4 ducks received DNA vector on days 50, 64, 78, 127, and 141. All ducks underwent surgical liver biopsy on days 18, 42, 91, and 181. Two ducks from groups 1 to 4 (Table 1) were autopsied on day 243 (the day before drug was withdrawn), while the remaining ducks in groups 1 to 4 were biopsied on day 287 and then autopsied on either day 298 or day 320. The group 5 ducks were autopsied on day 320.
Construction of DNA vaccines.
The presurface (pre-S/S) and S antigen genes of an Australian strain of DHBV (AusDHBV) (30) were previously cloned downstream of a cytomegalovirus promoter in pcDNA1.1Amp (31) and are referred to as pcDNA I-PreS/S and pcDNA I-S, respectively. The precore (pre-C/C) and C antigen genes of AusDHBV were similarly cloned by PCR amplification using a covalently closed circular DNA (cccDNA) template isolated from AusDHBV-infected duck liver. Directional cloning of the amplified PCR products was facilitated by the introduction of BamHI and XhoI extensions (underlined) to the 5′ end of each primer. Cloning of the pre-C/C and C genes involved the use of two upstream primers, pre-C/C primer 5′GGAGGATCCATTGGACGGCTCTTACA3′ and C primer 5′GGAGGATCCGTCTACATTGCTGTTGTCA3′, and a common downstream primer, 5′CCGCTCGAGTTACCTACTATTGCCTCA3′. The final coordinates of the AusDHBV PCR products were pre-C/C 5′ 2466-675 3′ and C 5′ 2583-675 3′. The PCR products and vector, pcDNA-1.1/Amp (Invitrogen, San Diego, Calif.), were digested with BamHI and XhoI, ligated with T4 DNA ligase, and transformed into the Escherichia coli strain Top 10 F′. Clones were identified by restriction enzyme digestion, sequencing of the pre-C/C and C genes, and detection of DHBcAg by immunofluorescence of transfected COS7 and HepG2 cells (data not shown). Identified plasmid clones were then grown in large-scale cultures (BRESApure Maxi Plasmid) and dissolved in phosphate-buffered saline (PBS) at a concentration of 1 mg/ml and are referred to as pcDNA I-PreC/C and pcDNA I-C.
Drug source, preparation, and uptake.
ETV was synthesized and supplied in powdered form by Bristol-Myers Squibb. A 0.1-mg/ml stock solution of ETV was prepared by adding 1 mg of ETV to 10 ml of distilled water, followed by sonication in a 65°C water bath. Stock solutions of ETV were stored at 4°C for up to 3 days and allowed to reach room temperature before use. ETV or distilled water was administered by gavage using a cuffless 2.0 Oral/Nasal tube attached to a 3-ml syringe.
Preliminary studies were performed to determine the rate of uptake and the half-life of ETV in the plasma of ducks. A single dose of 0.5 mg of ETV/kg was administered orally to two uninfected adult ducks that were bled regularly over 48 h. ETV concentrations in duck plasma were assayed using a liquid chromatography-mass spectrometry method previously validated for analysis of ETV in rat plasma. Peak plasma levels of ETV (198.05 ng/ml) occurred 30 min after dosing, and the mean half-life of ETV in duck plasma was found to be 8.73 h (J.-H. Yan, S. Pang, and V. Mummaneni, Bristol-Myers Squibb, personal communication).
DHBV infection and DNA vaccination protocols.
The virus inoculum used in the experiments was derived from pooled serum collected from ducks congenitally infected with AusDHBV containing 9.5 × 109 DHBV genomes/ml and 50 μg of DHBsAg/ml (13). Twenty of the 25 ducks were inoculated intravenously at 7 days of age via the jugular vein with 100 μl of pooled serum containing 9.5 × 108 AusDHBV virions. These ducks were then divided into four groups: group 1 (ETV plus DHBV vaccine), group 2 (ETV plus vector), group 3 (water plus DHBV vaccine), and group 4 (water plus vector). The remaining five ducks were used as uninfected control animals (group 5).
DNA vaccines consisted of 250 μg of each of the four constructs (pcDNA I-PreS/S, pcDNA I-S, pcDNA I-PreC/C, and pcDNA I-C) for a total of 1 mg of DNA or 250 μg of pcDNA1.1 A DNA (vector) injected intramuscularly into the quadriceps anterior muscle 3 days after an injection of 100 μl of bupivacaine HCl (Marcain; Astra Pharmaceuticals) into the same site. Vaccines were given on days 50, 64, 78, 127, and 141 of ETV treatment, and the schedule of infection, vaccination, and biopsy is shown in Fig. 1.
Drug administration.
Ducks from groups 1 and 2 were weighed three times a week, and ETV at a dose of 0.1 mg/kg/day was administered by gavage to these ducks each day for 244 days. Control ducks (groups 3, 4, and 5) were weighed weekly and given 2 ml of distilled water by gavage daily.
Analysis of serum samples.
Blood samples were taken from all ducks twice weekly for the first month after treatment commenced and for the first month after treatment was withdrawn, with weekly blood sampling for the remaining time. Serum samples were tested on the day of collection for levels of gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) by using an automatic analyzer in the Diagnostic Services Laboratories of the IMVS. Serum samples were also tested for the presence of DHBV DNA by spot blot hybridization (12) and were extracted for DNA using a High Pure viral nucleic acid kit (Roche Molecular Biochemicals). Viral DNA levels were then quantitated by real-time PCR using a Roche LightCycler. Extracts of serum from congenitally DHBV-infected ducks were diluted with extracts of uninfected duck serum and water to produce standards containing 108, 107, 106, 105, 104, 103, and 102 genomes, and a negative control of water alone was also included. Samples consisted of 2.5 μl of duck serum extract (equivalent to 10 μl of duck serum) and 7.5 μl of water plus 10 μl of reaction mix. The reaction mix contained 2 μl of FastStart Master SYBR Green 1, 2.4 μl of MgCl2 (final concentration of 4 mM), 0.5 μl of 10 μM primers, and 5.1 μl of water. The primers used were P3 (5′AGCTGGCCTAATCGGATTAC3′) and P4 (5′TGTCCGTCAGATACAGCAAG3′). The protocol required an initial denaturation for 10 min at 95°C and then 40 cycles of 5 s at 95°C, 10 s at 55°C, and 15 s at 72°C, with a ramping rate of 20°C/s. DNA concentration was calculated by comparing the fluorescence of SYBR Green from samples, in the log-linear phase of amplification, to the known standards.
Samples were also analyzed for DHBsAg levels by quantitative enzyme-linked immunosorbent assay (ELISA). Microtiter plates (96 well) were coated and incubated at 37°C for 1 h and then overnight at 4°C with rabbit serum containing anti-DHBs antibodies diluted 1/500 in 0.1 M NaHCO3 (pH 9.6). Plates were washed three times with 0.05% Tween 20 in PBS between all steps of the assay. Plates were blocked with 5% skim milk-0.05% Tween 20 in PBS for 1 h at 37°C before the serum samples diluted in PBS to 1/100, 1/1,000, or 1/4,000 (depending on the DHBsAg load) were added and incubated for 1 h at 37°C and then overnight at 4°C. Standard curves were determined using a pool of DHBV-positive serum from congenitally infected ducks that contained 50 μg of DHBsAg/ml (13). The pooled serum was diluted from 1/1,000 to 1/128,000 in PBS containing uninfected duck serum at the same dilution (1/100, 1/1,000, or 1/4,000) as that of the original samples being tested. Bound DHBsAg was detected using anti-DHBV pre-S1 monoclonal antibodies (1H.1) (28) diluted 1/5,000 in 5% skim milk-0.05% Tween 20 in PBS by incubating the plates for 1 h at 37°C, followed by the addition of sheep anti-mouse horseradish peroxidase (HRP) (NXA931; Amersham). The concentration of sheep anti-mouse HRP varied depending on the dilution of original serum. For example, serum samples diluted 1/100 were detected with sheep anti-mouse HRP diluted 1/500 in 5% skim milk, 0.05% Tween 20, 5% normal sheep serum, and 5% normal rabbit serum in PBS for 1 h at 37°C, while serum samples diluted 1/1,000 and 1/4,000 were detected using sheep anti-mouse HRP diluted 1/5,000 in 5% skim milk-0.05% Tween 20 in PBS for 1 h at 37°C. Finally, plates were washed three times in PBS, and HRP was detected by the addition of an o-phenylene diamine substrate and incubation for 15 min at room temperature in the dark. The reaction was stopped by the addition of 2.5 M H2SO4, and optical densities (OD) at 490 nm were measured.
The specificity of the assay was determined using samples of uninfected duck serum, and the cutoff for DHBsAg-positive samples was set at 2 standard deviations above the average background obtained using uninfected duck serum. Levels of DHBsAg in all test samples were quantitated using the standard curves described above. Accurate quantitation of DHBsAg was only possible within the linear range of the standard curves at DHBsAg levels of 0.3 μg/ml and above. Below this level, accurate quantitation was not possible but samples containing levels of DHBsAg above the positive cutoff (greater than 2 standard deviations above the average background obtained using uninfected duck serum) have been indicated in Fig. 2B.
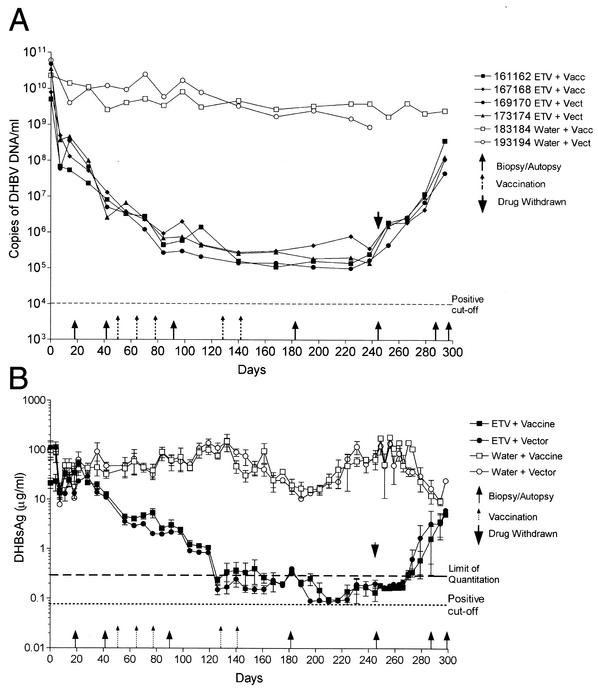
FIG. 2.
Detection of DHBV DNA (A) and DHBsAg (B) in the serum of ducks in groups 1 (ETV plus DHBV vaccine), 2 (ETV plus vector), 3 (water plus DHBV vaccine), and 4 (water plus vector). DHBV DNA was detected by real-time PCR using a Roche LightCycler in ducks 161162 and 167168 from group 1, 169170 and 173174 from group 2, 183184 from group 3, and 193194 from group 4. DHBsAg was detected by quantitative ELISA as described in the text, and the results for each group are shown as means ± standard deviations. Six ducks (one or two from each of groups 1 to 4 as described in the text) that had highly lipemic serum were excluded from analysis. The limit of quantitation (0.3 μg/ml) indicates the lower limit of the linear range of the standard curves, below which samples contained levels of DHBsAg that were still detectable but too low for accurate quantitation. The cutoff for DHBsAg-positive samples was set at 2 standard deviations above the average background obtained using uninfected duck serum.
Serum samples collected from a total of six ducks (ducks 163164 and 165166 from group 1, 39172 from group 2, 181182 and 185186 from group 3, and 100200 from group 4) contained high levels of visible lipid from day 140 (23 weeks of age). The presence of this lipid resulted in 5- to 10-fold increases in the OD values obtained in the DHBsAg ELISA. Efforts to remove the lipid without also removing DHBsAg were not successful, so all samples from the six affected ducks were excluded from the analysis of DHBsAg. Five of the six ducks were female, and the increased levels of lipids in the serum coincided with the commencement of egg laying. Similar increases in lipid levels in serum have been seen in egg-laying mallards (5).
Analysis of liver tissue.
Liver samples from biopsy and autopsy (Fig. 1) were snap-frozen in liquid nitrogen, and 300 mg of each sample was later extracted to obtain both total DNA and protein-free DNA (that includes the viral cccDNA forms) and analyzed using Southern blot hybridization exactly as described previously (14). Levels of total and cccDNA were compared in each treatment group by extracting identical amounts of liver tissue and analyzing the same proportion of each sample by Southern blot hybridization followed by comparison to a plasmid DNA standard and quantitation using a Molecular Dynamics PhosphorImager. Biopsy samples of liver and autopsy samples of liver, kidney, pancreas, and spleen were fixed in ethanol-acetic acid, wax embedded, sectioned, and then examined for DHBsAg by immunoperoxidase staining (11) using anti-pre-S 1H.1 monoclonal antibodies (28). Quantitation of the percentage of DHBsAg-positive cells was performed using a microscope eyepiece grid at a magnification of ×400. DHBsAg-positive cells (including both hepatocytes and bile duct cells) were counted in 5 to 10 representative grids and expressed as a percentage of total liver cell nuclei in the same area. Biopsy and autopsy tissues were also fixed in 10% formaldehyde in PBS, wax embedded, sectioned, and stained with hematoxylin and eosin for histological analysis and Congo red for detection of amyloid deposits. Sections of formalin-fixed liver tissue were examined under code for levels of inflammation and amyloid.
RESULTS
Effect of ETV treatment on serum DHBV DNA and DHBsAg levels.
All 20 ducks inoculated intravenously on day 7 posthatch with 109 DHBV genomes had DHBV DNA (>5 × 109 copies/ml) and DHBsAg (10 to 100 μg/ml) in the serum by day 7 postinoculation. This confirmed the results of a previous infection study with 7-day-old ducks where the rapid development of widespread DHBV replication in the liver and high levels of persistent DHBV DNA and DHBsAg in the serum were observed (11).
Within 4 days of the beginning of ETV treatment (0.1 mg/kg/day), serum DHBV DNA levels became undetectable by spot blot hybridization in the ETV-treated, infected ducks (groups 1 and 2) but remained detectable in the water-treated, infected ducks (groups 3 and 4 [data not shown]). DHBV DNA levels in the serum were then determined by quantitative PCR using a Roche LightCycler; in all of the ETV-treated ducks, DHBV DNA levels were found to have decreased by ∼2 logs on day 10, ∼3 logs on day 40, and ∼4 logs to <106 genomes/ml by day 80 of treatment (Fig. 2A). These levels of DHBV DNA were then seen to persist during the remainder of the ETV treatment. At no time did serum DHBV DNA levels fall to undetectable levels (assay limit of detection, 104 genomes per ml). DHBV DNA levels in the serum of water-treated control ducks (groups 3 and 4) decreased slightly over the 244 days but remained within the range of 109 to 1010 genomes per ml (Fig. 2A).
In contrast to the marked decline in the levels of serum DHBV DNA in the ETV-treated ducks (∼4 logs by day 80 of treatment), serum DHBsAg levels decreased at a lower rate (Fig. 2B). When tested by quantitative ELISA, serum DHBsAg levels in the ETV-treated ducks (groups 1 and 2), which were initially 10 to 100 μg/ml, had decreased by only ∼1 log by day 77 and ∼2 logs by day 120 and remained at levels of <0.3 μg/ml for the rest of the drug treatment course, while in the water-treated infected ducks (groups 3 and 4), DHBsAg levels remained within the 10- to 100-μg/ml range. The continued production of DHBsAg particles reflects the lack of a direct effect of ETV treatment on DHBsAg production (from the transcriptional template cccDNA), while decreased DHBV DNA levels reflect the direct inhibition of viral DNA replication by ETV, leading to reduced secretion of virions.
The differences in decay rates of DHBV DNA and DHBsAg can also be thought of in terms of the relative ratio of circulating virions and DHBsAg particles in each treatment group. For example, Jilbert et al. (13) have previously shown that pooled serum collected from congenitally AusDHBV-infected ducks contains 9.5 × 109 DHBV genomes/ml and 50 μg of DHBsAg/ml, representing a ∼103-fold excess of DHBsAg particles over virions. This calculation was based on the assumption that DHBV virions and DHBsAg particles both contain ∼100 surface antigen molecules per particle and that each particle type contains a similar ratio of pre-S/S and S proteins (16). In the present study, serum samples collected from the water-treated ducks (groups 3 and 4) had similar ratios, with a 1 × 101- to 2 × 103-fold excess of DHBsAg particles over virions, while the ETV-treated ducks (groups 1 and 2) had a 9 × 102- to 1 × 106-fold excess of DHBsAg particles over virions.
Despite the length of antiviral treatment, no “breakthrough” increases in DHBV DNA or DHBsAg (Fig. 2) were observed. Following withdrawal of ETV, serum DHBsAg levels initially remained at <0.3 μg/ml, but within 40 days of drug withdrawal, levels had rebounded to match those of the group 3 and 4 water-treated control ducks (Fig. 2B). Serum DHBV DNA levels did not show a similar initial plateau after ETV withdrawal; increases within a week after treatment was withdrawn were seen, and levels continued to rise, reaching almost the same level as that of the group 3 and 4 ducks by the end of the experiment, 40 days after drug withdrawal (Fig. 2A).
Reduction in viral replication and antigen expression in the liver.
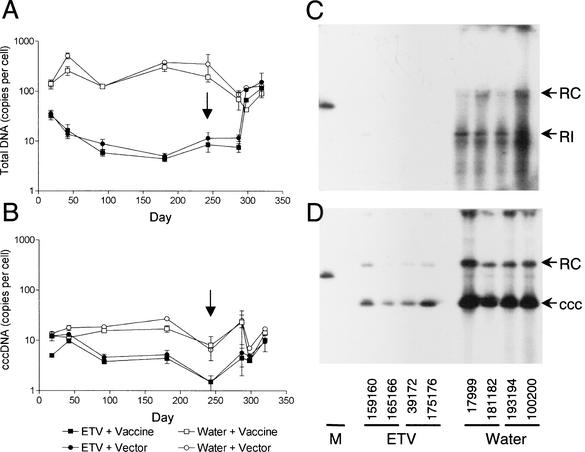
Liver tissues collected on days 18, 42, 91, 181, 243, 287, 298, and 320 were examined by Southern blot hybridization for levels of total and cccDNA and by immunoperoxidase staining for DHBsAg. As shown in Fig. 3A and Table 1, the levels of total DHBV DNA in the ETV-treated ducks (groups 1 and 2), when compared with levels in the infected control ducks (groups 3 and 4), were reduced by 0.6, 1.4, 1.2, 1.8, and 1.6 logs by days 18, 42, 91, 181, and 243, respectively. No significant differences were seen between the results for the DHBV-vaccinated groups (groups 1 and 3) and those for their respective vector-vaccinated controls (groups 2 and 4). In contrast, the transcriptional template cccDNA was found to have decreased only fourfold in the ETV-treated groups (groups 1 and 2 [Fig. 3B] [Table 1]). Interestingly, as shown in Fig. 3B and Table 1, the copy number of cccDNA per liver cell had decreased fourfold by day 91 and was maintained at this level until after the withdrawal of ETV therapy on day 244. The maintenance of levels of cccDNA suggests replenishment of cccDNA despite the presence of antiviral drug or an inherent stability of cccDNA and/or the cells harboring these molecules.
FIG. 3.
Liver tissue was extracted for total and cccDNA and then subjected to Southern blot hybridization using a 32P-labeled genome-length DHBV DNA probe. (A and B) Graphs show the average total DHBV DNA (A) and DHBV cccDNA (B) levels per liver cell for all ducks in each group ± standard deviation. Arrows indicate when ETV treatment was withdrawn. (C and D) Liver tissue was collected from two ducks in each of groups 1 to 4 on day 243 after extraction for total DHBV DNA (C) and cccDNA (D) and Southern blot hybridization. Individual duck numbers are shown. Relaxed circular (RC) and cccDNA (ccc) were the predominant forms of DHBV DNA detected in the liver of the ETV-treated ducks on day 243. RI, replicative intermediates; M, 200 pg of plasmid DNA containing the 3-kb DHBV genome.
TABLE 1.
Analysis of liver tissue from each of the group 1 to 4 DHBV-infected ducks
| Group (treatment) | Duckg | Daya | % of DHBsAg-positive liver cellsb | cccDNA copy no. perc:
|
Total DHBV DNAd | Fatty changese | Presence of amyloidf | |
|---|---|---|---|---|---|---|---|---|
| Liver cell | DHBsAg-positive cell | |||||||
| 1 (ETV plus vaccine) | 159160 (M) | 18 | >83 | 5 | 6 | 45 | − | NT |
| 42 | >83 | 10 | 12 | 34 | − | NT | ||
| 91 | 39 | 4 | 10 | 7 | − | NT | ||
| 181 | 23 | 7 | 30 | 6 | − | − | ||
| 244 | 19 | 2 | 11 | 11 | − | − | ||
| 161162 (M) | 18 | >83 | 6 | 7 | 28 | − | NT | |
| 42 | >83 | 13 | 16 | 15 | − | NT | ||
| 91 | 33 | 3 | 9 | 5 | − | NT | ||
| 181 | 15 | 2 | 14 | 3 | − | − | ||
| 287 | 28 | 7 | 25 | 6 | +++ | + | ||
| 298 | >83 | 4 | 5 | 67 | + | + | ||
| 165166 (F) | 18 | >83 | 4 | 5 | 52 | − | NT | |
| 42 | >83 | 10 | 12 | 20 | − | NT | ||
| 91 | 32 | 5 | 16 | 7 | − | NT | ||
| 181 | 8 | 3 | 40 | 4 | + | − | ||
| 244 | 6 | 1 | 17 | 6 | − | − | ||
| 163164 (F) | 18 | >83 | 5 | 6 | 16 | − | NT | |
| 42 | >83 | 7 | 8 | 7 | − | NT | ||
| 91 | 37 | 3 | 8 | 3 | − | NT | ||
| 181 | 26 | 4 | 15 | 4 | ++ | − | ||
| 167168 (M) | 18 | >83 | 5 | 6 | 26 | − | NT | |
| 42 | >83 | 9 | 11 | 6 | − | NT | ||
| 91 | 23 | 4 | 17 | 7 | − | NT | ||
| 181 | 4 | 6 | 158 | 5 | − | − | ||
| 287 | 1 | 2 | 222 | 9 | − | + | ||
| 320 | >83 | 10 | 12 | 116 | − | + | ||
| 2 (ETV plus vector) | 169170 (M) | 18 | >83 | 6 | 7 | 29 | − | NT |
| 42 | >83 | 14 | 17 | 13 | − | NT | ||
| 91 | 39 | 4 | 10 | 4 | − | NT | ||
| 181 | 14 | 8 | 58 | 7 | − | − | ||
| 287 | 10 | 3 | 30 | 11 | ++ | − | ||
| 320 | >83 | 13 | 16 | 233 | − | − | ||
| 39172 (F) | 18 | >83 | 8 | 10 | 17 | − | NT | |
| 42 | >83 | 14 | 17 | 10 | − | NT | ||
| 91 | 43 | 5 | 12 | 6 | − | NT | ||
| 181 | 14 | 7 | 49 | 6 | − | − | ||
| 244 | 26 | 1 | 4 | 15 | ++ | + | ||
| 175176 (M) | 18 | >83 | 12 | 14 | 33 | − | NT | |
| 42 | >83 | 8 | 10 | 13 | − | NT | ||
| 91 | 26 | 4 | 15 | 7 | − | NT | ||
| 181 | 3 | 3 | 103 | 5 | − | − | ||
| 244 | 2 | 2 | 87 | 8 | − | − | ||
| 177178 (M) | 18 | >83 | 17 | 20 | 44 | − | NT | |
| 42 | >83 | 14 | 17 | 20 | − | NT | ||
| 91 | 37 | 7 | 19 | 15 | − | NT | ||
| 181 | 3 | 2 | 71 | 3 | ++ | − | ||
| 287 | >83 | 11 | 13 | 15 | − | + | ||
| 298 | >83 | 5 | 6 | 107 | + | + | ||
| 173174 (M) | 18 | >83 | 18 | 22 | 54 | − | NT | |
| 42 | >83 | 16 | 19 | 13 | − | NT | ||
| 91 | 19 | 3 | 16 | 12 | − | NT | ||
| 181 | 1 | 6 | 461 | 4 | − | − | ||
| 287 | 5 | 3 | 56 | 9 | − | + | ||
| 320 | >83 | 6 | 7 | 75 | +++ | − | ||
| 3 (water plus vaccine) | 17999 (M) | 18 | >83 | 10 | 12 | 180 | − | NT |
| 42 | >83 | 11 | 13 | 93 | − | NT | ||
| 91 | >83 | 18 | 22 | 156 | − | NT | ||
| 181 | >83 | 15 | 18 | 118 | − | − | ||
| 244 | >83 | 12 | 14 | 192 | ++ | −/PICK> | ||
| Liver cell | DHBsAg-positive cell | |||||||
| 183184 (M) | 18 | >83 | 15 | 18 | 89 | − | NT | |
| 42 | >83 | 9 | 11 | 274 | − | NT | ||
| 91 | >83 | 15 | 18 | 125 | + | NT | ||
| 181 | >83 | 19 | 22 | 229 | − | − | ||
| 287 | >83 | 7 | 8 | 42 | + | + | ||
| 298 | >83 | 4 | 5 | 43 | +++ | + | ||
| 181182 (F) | 18 | >83 | 13 | 16 | 100 | − | NT | |
| 42 | >83 | 11 | 13 | 209 | − | NT | ||
| 91 | >83 | 14 | 17 | 126 | − | NT | ||
| 181 | >83 | 10 | 12 | 183 | ++ | − | ||
| 244 | >83 | 4 | 5 | 195 | − | + | ||
| 3797 | 18 | >83 | 10 | 12 | 95 | − | NT | |
| 42 | >83 | 10 | 12 | 320 | − | NT | ||
| 91 | >83 | 15 | 18 | 91 | − | NT | ||
| 181 | >83 | 25 | 30 | 292 | − | − | ||
| 185186 (M) | 18 | >83 | 13 | 16 | 236 | − | NT | |
| 42 | >83 | 16 | 19 | 393 | − | NT | ||
| 91 | >83 | 16 | 19 | 122 | − | NT | ||
| 181 | >83 | 16 | 19 | 353 | ++ | − | ||
| 287 | >83 | 39 | 46 | 96 | − | + | ||
| 320 | >83 | 14 | 17 | 90 | + | + | ||
| 4 (water plus vector) | 195196 (M) | 18 | >83 | 10 | 12 | 115 | − | NT |
| 42 | >83 | 16 | 19 | 448 | − | NT | ||
| 91 | >83 | 20 | 24 | 124 | − | NT | ||
| 181 | >83 | 32 | 39 | 372 | − | − | ||
| 287 | >83 | 32 | 39 | 104 | ++ | − | ||
| 320 | >83 | 17 | 20 | 128 | − | + | ||
| 193194 (M) | 18 | >83 | 13 | 16 | 119 | − | NT | |
| 42 | >83 | 14 | 17 | 575 | − | NT | ||
| 91 | >83 | 19 | 23 | 136 | − | NT | ||
| 181 | >83 | 27 | 33 | 317 | + | − | ||
| 244 | >83 | 6 | 7 | 154 | +++ | − | ||
| 100200 (F) | 18 | >83 | 16 | 19 | 198 | − | NT | |
| 42 | >83 | 17 | 20 | 364 | − | NT | ||
| 91 | >83 | 16 | 19 | 130 | + | NT | ||
| 181 | >83 | 20 | 24 | 528 | +++ | − | ||
| 244 | >83 | 7 | 8 | 548 | +++ | − | ||
| 197198 (M) | 18 | >83 | 15 | 18 | 131 | − | NT | |
| 42 | >83 | 24 | 29 | 666 | − | NT | ||
| 91 | >83 | 19 | 23 | 105 | − | NT | ||
| 181 | >83 | 29 | 35 | 396 | ++ | − | ||
| 287 | >83 | 18 | 22 | 68 | ++ | + | ||
| 298 | >83 | 7 | 8 | 119 | +++ | + | ||
All ducks underwent surgical liver biopsy on days 18, 42, 91, and 181. Two of the five ducks from each of groups 1 to 4 were autopsied on day 243 (the day before drug was withdrawn), and the remaining ducks in groups 1 to 4 were biopsied on day 287 and then autopsied on either day 298 or day 320.
The percentage of DHBsAg-positive liver cells was determined from cell counts by using a microscope eyepiece grid at a magnification of ×400. DHBsAg-positive cells were counted in 5 to 10 representative grids and expressed as a percentage of total liver cell nuclei in the same area. The group 3 and 4 ducks had detectable DHBsAg in all hepatocytes at each time point. Hepatocytes comprise ∼83% of total liver cells (13).
Levels of cccDNA are expressed as the average copy number per liver cell based on liver weight as previously described (14) and as the calculated average copy number per DHBsAg-positive liver cell.
Levels of total DHBV DNA are expressed as the average copy number per liver cell based on liver weight as previously described (14).
Fatty changes were detected in formalin-fixed liver tissue stained by hematoxylin and eosin. −, not tested; +, mild; ++, moderate; +++, marked.
Amyloid was detected in sections of formalin-fixed liver tissue using Congo red staining. NT, not tested. Liver samples collected from day 181 onwards only were tested for the presence of amyloid.
The sex of each duck was determined to be female (F) or male (M). Ducks 163164 and 165166 from group 1, 39172 from group 2, 181182 and 185186 from group 3, and 100200 from group 4 contained high levels of visible lipid from day 140 (23 weeks of age) that resulted in 5 to 10-fold increases in the OD values obtained in the DHBsAg ELISA, so all samples from these ducks were excluded from the analysis of DHBsAg. Ducks 163164 (group 1) and 3797 (group 3) were euthanatized due to poor health on day 200. No tissues were available from these ducks after the liver biopsy samples collected on day 181.
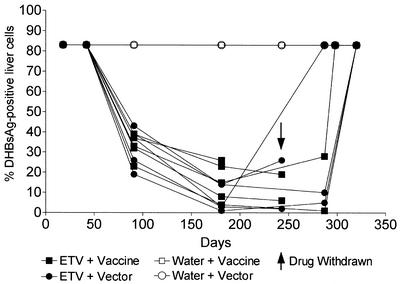
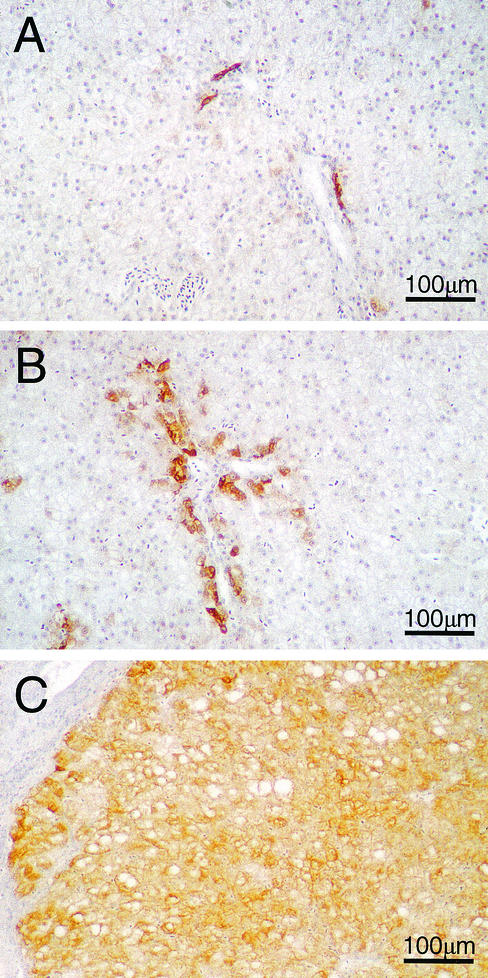
When DHBsAg in liver tissue was measured by immunoperoxidase staining, the ETV-treated ducks (groups 1 and 2) showed reductions in the percentage of DHBsAg-positive cells (Table 1) (Fig. 4) compared with water-treated controls (groups 3 and 4). This reduction occurred in the percentage of hepatocytes containing detectable DHBsAg and was also accompanied by a reduction in the intensity of antigen staining in these cells (Fig. 5A and B). Reduction in the percentage of DHBsAg-positive hepatocytes suggests that cells are being cured of DHBV infection, either by dilution of cccDNA copy number through cell division or by natural breakdown due to the half-life of cccDNA. Alternatively, if cccDNA is still present in the DHBsAg-negative hepatocytes, then DHBsAg production may be falling to undetectable levels due to transcriptional silencing.
FIG. 4.
ETV treatment reduced the percentage of liver cells that contained detectable levels of DHBsAg. DHBsAg was detected in ethanol-acetic acid-fixed liver tissue from all ducks in groups 1 to 4 by using anti-DHBV pre-S monoclonal antibodies (1H.1 [28]) as described in the text. The percentage of liver cells expressing detectable levels of DHBsAg was determined by cell counts using a microscope grid as described in the text. Results from individual ducks are shown.
FIG. 5.
Detection of DHBsAg-positive cells in liver by indirect immunoperoxidase staining of ethanol-acetic acid-fixed liver tissue counterstained with hematoxylin from an ETV-treated duck from group 1 (167168) on day 287 (A), an ETV-treated duck from group 2 (173174) on day 287 in which DHBsAg detection in bile duct cells was largely unaffected by ETV treatment (B), and a water-treated DHBV-infected duck from group 4 (193194) on day 243 (C). Final magnification, ×114.
In addition to hepatocytes, bile duct cells also contained DHBsAg. It was interesting that decreases in the intensity of DHBsAg staining were not observed in the bile duct cells found in the ETV-treated, group 1 and 2 ducks (Fig. 5A and B) compared with the water-treated, group 3 and 4 controls (Fig. 5C).
The copy number of cccDNA per DHBsAg-positive liver cell was also calculated (Table 1) and found to range from 5 to 46 copies in the group 3 and 4 ducks. Similarly, in the group 1 and 2 ducks, in which >5% of liver cells still expressed detectable levels of DHBsAg, the cccDNA copy number ranged from 4 to 58 copies per DHBsAg-positive cell. In contrast, when the percentage of liver cells expressing detectable levels of DHBsAg dropped below 5% (on days 181, 244, and 287 after extended ETV treatment), the calculated levels of cccDNA increased to 71 to 461 copies per DHBsAg-positive cell. Since elevated levels of cccDNA (>50 copies per infected cell) have previously been found only in cells infected with cytopathic strains of DHBV (19) and not in wild-type DHBV infection (13, 14), this result suggests either that cccDNA is present in hepatocytes with undetectable levels of DHBsAg, i.e., cccDNA is transcriptionally inactive, or that cccDNA is present at higher copy numbers in nonhepatocyte cell types (e.g., bile duct cells). Bile duct cells have been reported to contain increased levels of DHBsAg and DHBV DNA (14, 18, 26), but quantitation of cccDNA copy number per infected bile duct cell in vivo has not been performed.
In autopsy tissues collected from half of the ducks from groups 1 and 2 at the end of ETV therapy (day 243), the numbers of cells with and intensity of DHBsAg staining were also lower in the acinar and islet cells of the pancreas and in the glomerular cells of the kidney compared with what was seen with the water-treated group 3 and 4 ducks. In the group 1 and 2, ETV-treated ducks autopsied at the end of the experiment (day 298 or 320), the levels and intensity of staining in the liver, pancreas, spleen, and kidney were all comparable to those in the group 3 and 4 ducks, suggesting that viral replication had also rebounded in these sites after the withdrawal of drug treatment. No difference in antigen staining was seen when comparing the DHBV DNA-vaccinated (groups 1 and 3) and vector control (groups 2 and 4) groups. No significant differences were seen in the extent of cellular inflammation in tissue sections of ducks from groups 1 to 5. The appearance of the liver tissue ranged from normal to mild portal hepatitis with occasional pockets of inflammatory cells and lymphoid follicles within the portal tracts. Some minor variation in inflammation between biopsy samples was noted, but there was no consistent increase or decrease seen for any of the treatment groups. Amyloid deposits were detected in autopsy liver samples collected on day 244, 298, or 320 in five of nine ETV-treated ducks (groups 1 and 2), five of eight water-treated controls (groups 3 and 4), and two of four uninfected control ducks (group 5) (Table 1). The detection of amyloid was unrelated to the DHBV infection status of the ducks, to ETV treatment, and to vaccination with constructs expressing the DHBV antigens and was not seen in biopsy samples at any earlier time points. Amyloidosis in ducks has been previously reported to occur in a high percentage of ducks used in experimental studies and is thought to be related to stress, genetic factors, and immune stimulation (8).
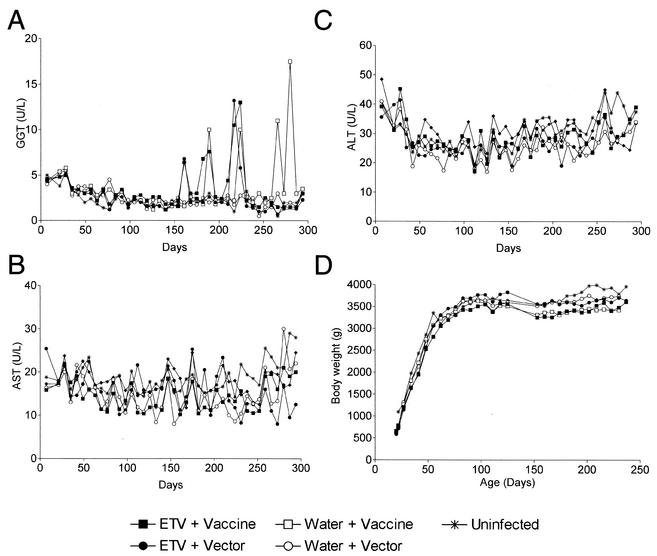
Serum samples collected weekly from all ducks (groups 1 to 5) were tested for levels of the liver function enzymes GGT, AST, and ALT. The normal range for each enzyme (mean ± standard deviation) was determined to be 2.3 ± 1.2 U/liter for GGT, 15.9 ± 5.9 U/liter for AST, and 26.6 ± 7.7 U/liter for ALT. No significant differences were observed in any of the groups (Fig. 6A to C). Some ducks from each treatment group showed occasional large increases of GGT, up to 10-fold above the normal range. Similar increases have also been seen in normal mallard female ducks when they begin egg laying (5), and it is likely that the flares seen in this study were related to egg laying as they were seen only in female ducks after the onset of egg laying at 23 weeks of age. No major changes in inflammation were seen in liver biopsy samples collected after the GGT flares in any of the ducks. No ducks receiving ETV treatment, vaccination, or both (groups 1, 3, and 2, respectively) showed significant differences in body weight compared with the age-matched, infected (group 4) or uninfected (group 5) control ducks (Fig. 6D). It is interesting that the presence of widespread DHBV infection of the liver did not lead to any measurable differences in levels of liver function enzymes or body weight.
FIG. 6.
(A to C) Average levels of the liver function enzymes GGT (A), AST (B), and ALT (C) in serum collected at weekly intervals for all ducks in groups 1 to 5. (D) Average body weights for all ducks in each group are shown at weekly intervals.
DNA vaccination did not lead to significant effects on viral replication.
Intramuscular vaccination of ducks with DNA vaccines expressing the pre-S/S and S genes and pre-C/C and C genes of AusDHBV (groups 1 and 3) on days 50, 64, 78, 127, and 141 did not result in significant differences in the levels of DHBsAg and DNA in liver and serum compared with those of the vector-vaccinated ducks in groups 2 and 4. ELISAs were also used to test for the presence of anti-DHBs and anti-DHBc antibodies (results not shown), but ducks in all groups showed low but highly variable levels of antibodies with no significant differences between any of the four groups.
DISCUSSION
Long-term ETV treatment led to significant decreases in levels of DHBV DNA and DHBsAg in the serum and liver of DHBV-infected ducks, although the levels eventually plateaued. However, ETV treatment alone or in conjunction with DNA vaccination did not lead to the clearance of DHBV infection from any of the treated animals, in contrast to what was observed in ETV-treated woodchucks (3). That DHBV DNA in the serum declined more rapidly than DHBsAg is consistent with the direct effect of ETV on reducing viral DNA replication and progeny virus production. The effects on antigen production would be expected to be more indirect. Also consistent was the observation that in the liver of the ETV-treated ducks, replicative intermediates (measured as total DHBV DNA) declined more rapidly than levels of cccDNA. This finding was also consistent with the results of a previous study of ETV in the duck model (18), where 21 days of treatment with 1.0 mg of ETV/kg/day led to a 96% reduction in total DHBV DNA and a threefold reduction in cccDNA.
Similar effects have also been observed in DHBV-infected ducks undergoing long-term antiviral treatment with penciclovir (20) and 9-(2-phosphonylmethoxyethyl) adenine (PMEA) (25). In these studies, drug treatment for 1 to 6 months with penciclovir and PMEA led to marked decreases in viral replicative intermediates in the liver but to less dramatic changes in levels of cccDNA, viral RNA, pre-S1, and core antigen expression. Taken together, these findings reflect the inability of the reverse transcriptase inhibitors to markedly affect DHBV cccDNA levels.
One of the surprising findings of the present study was that over the 244-day treatment period, the levels of cccDNA, although initially declining fourfold within 91 days of the start of drug treatment, remained relatively constant thereafter, while the levels of DHBsAg released into the bloodstream and the numbers of hepatocytes expressing detectable levels of DHBsAg continued to decline, reaching the lowest plateau between days 125 and 244. This finding suggests (i) that the residual pool of cccDNA is relatively stable or is replenished through a mechanism insensitive to ETV, (ii) that increased levels of cccDNA may be present in cell types other than hepatocytes, and (iii) that the rate of transcription and antigen expression from the residual cccDNA may have decreased throughout the study, perhaps reflecting differences in nucleosome binding of the cccDNA molecules. DHBV cccDNA has been shown to be present in the nucleus of infected cells as a heterogeneous population of minichromosomes (1, 24) that can bind up to 20 nucleosomes per 3-kb molecule (24).
The persistence of cccDNA may also be related to the persistence of viral antigen expression in bile duct cells in the liver. Bile duct cells have been shown previously to support DHBV replication in vivo (14) and in vitro (18) and to be resistant to therapy with nucleoside analogues (25). The possibility exists that at least some of the residual cccDNA might be present in bile duct cells, highlighting the importance of these cells as a reservoir for HBV and DHBV infection (26). In contrast to what was seen with the other extrahepatic sites that were examined in this study (kidney and pancreas), DHBsAg staining in bile duct cells was relatively unaffected by treatment with ETV.
Following withdrawal of ETV therapy, all markers of viral replication rebounded to levels comparable to those of water-treated control ducks, with delays in rebound of up to 40 days but an average lag phase of 10 to 30 days. Delays in rebound of 1 week have also been observed in the 2.2.15 human hepatoma cell line treated for 7 days with 0.1 μM ETV (10) and from 1 to 8 weeks with some animals not rebounding, i.e., in WHV-infected woodchucks treated with a range of doses of ETV (4, 7). The half-life of intracellular ETV triphosphate was previously determined to be 15 h and was reported to be similar to the half-life of the triphosphate forms of lamivudine and penciclovir (32), so it is difficult to ascribe this delay to intracellular drug half-life alone.
Significant delays in the rebound of DHBV replication have also been observed in ducks treated with 2′-carbodeoxyguanosine (CDG) (27) where infection rebounded to include the whole hepatocyte population within a few weeks to several months after therapy commenced. The chemical structure of ETV is similar to that of 2′-CDG (23), i.e., a guanine-based nucleoside in which the natural furanose oxygen is also replaced by a carbon. However, ETV is more potent than 2′-CDG when tested in 2.2.15 cells (EC50, 0.00375 μM compared to 0.05 μM [10]). Interestingly, antiviral therapy of ducks with 100 μg of 2′-CDG/kg for 5 weeks led to at least a 10-fold reduction in the number of infected hepatocytes expressing DHBV (6, 23), but these decreases were linked to increased hepatocyte turnover due to 2′-CDG therapy. Similar rapid decreases in the percentage of cells expressing DHBV were not observed in this study, and although ETV may affect cell turnover rates, this was not specifically examined and was not suggested by changes in liver function enzymes.
In other long-term experimental antiviral studies, lamivudine-resistant mutants developed within a year of therapy, which led to a rebound in levels of viremia (22, 33). However, ETV-resistant strains were not found in long-term studies lasting up to 3 years with WHV-infected woodchucks (3). No rebounds in viremia or virus replication in the liver were observed in this study. However, sequencing of low-level residual virus circulating during treatment for ETV resistance was not performed.
Previous studies have demonstrated antigen expression and immunogenicity of the pre-S/S and S genes of DHBV (31), and vaccination of young ducks with the pre-S/S and S vaccines has been shown to protect young ducks against the development of persistent DHBV infection. However, the protective value of the vaccines was dose dependent and could be overcome by high challenge doses of DHBV (Darren Miller, unpublished data). Vaccination of young ducks with DNA vaccines containing the DHBV C and pre-C/C constructs did not protect ducks against the development of persistent DHBV infection (Darren Miller, unpublished data). Administration of DNA vaccines expressing the pre-S/S and S (31) and the pre-C/C and antigen genes of DHBV to ETV-treated infected ducks did not lead to further decreases in viral replication or antigen expression.
The failure of the DNA vaccination protocol used in this study to reduce levels of markers of DHBV infection in ducks with established DHBV infection is in contrast to the results of a previous study by Rollier et al. (29), where vaccination of experimentally DHBV-infected ducks with DHBV pre-S/S DNA vaccines (without concomitant antiviral treatment) led to significant decreases in serum and liver DHBV DNA (fivefold reduction in replicative intermediates) in all DHBV DNA-vaccinated ducks compared with vector-vaccinated controls. The differences in results seen between the two studies may be due to differences in the infection protocol, the strain of virus, or vaccination protocols used.
This study highlights a number of significant gaps in our understanding of hepadnavirus persistence during treatment. What is the mechanism of continued virus production during treatment? Are some producer cell types (e.g., bile duct cells [18, 26]) less sensitive to ETV, or is there ongoing breakthrough of suppression of replication? Have the DHBsAg-negative cells in the ETV-treated ducks cleared their infection, or do they still contain low levels of cccDNA? If they still contain viral DNA, what are the mechanisms suppressing antigen expression? If not, are they now fully susceptible to infection, and if so, why do they not become infected by the continuing viremia? In the stable state of reduced production of virus during ETV treatment, how are the clearance mechanisms recalibrated to balance production and result in steady serum levels of HBV DNA and DHBsAg?
Antiviral drug treatment alone was not able to clear DHBV infection, suggesting that additional strategies designed to stimulate effective immune responses against viral antigens may be needed, although it is unclear whether the DHBV results are predictive of what would occur in humans. Future studies using DNA vaccines expressing duck alpha interferon and gamma interferon (9) and DNA vaccination in conjunction with fowlpox virus prime-boost protocols that have shown promise in human immunodeficiency virus vaccine development (15) are planned.
In summary, ETV is a potent, safe, rapid-acting, and long-term suppressor of DHBV replication, the mechanism which allows low-level infection to persist during ETV treatment requires further study, and the DNA vaccination protocols used above did not result in any additional reduction in viral load, whether used alone or in conjunction with ETV treatment. This study has opened the way for further studies, including those using higher doses of ETV (1.0 mg/kg/day) in conjunction with other therapeutic protocols.
Acknowledgments
This research was supported by the National Health and Medical Research Council of Australia and by a research grant from Bristol-Myers Squibb Pharmaceutical Research Institute.
We appreciate the work of the IMVS animal care staff for the day-to-day care of the ducks involved in this study. We are also grateful to Catherine Swanson for technical support; Miriam Triyatni for supplying the DHBV pre-S/S- and S-containing DNA vaccines; J.-H. Yan, S. Pang, and V. Mummaneni for determination of levels of ETV in duck plasma; and Christopher Burrell and William Mason for helpful suggestions and critical reading of the manuscript.
REFERENCES
- 1.Bock, C. T., P. Schranz, C. H. Schroder, and H. Zentgraf. 1994. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes 8:215-229. [DOI] [PubMed] [Google Scholar]
- 2.Chang, K. M., and F. V. Chisari. 1999. Immunopathogenesis of hepatitis B virus infection. Clin. Liver Dis. 3:221-239. [DOI] [PubMed] [Google Scholar]
- 3.Chisari, F. V., and C. Ferrari. 1997. Viral hepatitis, p. 745-778. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 4.Colonno, R. J., E. V. Genovesi, I. Medina, L. Lamb, S. Durham, M. L. Huang, L. Corey, M. Littlejohn, S. Locarnini, B. C. Tennant, B. Rose, and J. M. Clark. 2001. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J. Infect. Dis. 184:1236-1245. [DOI] [PubMed] [Google Scholar]
- 5.Fairbrother, A., M. A. Craig, K. Walker, and D. O'Loughlin. 1990. Changes in mallard (Anas platyrhynchos) serum chemistry due to age, sex, and reproductive condition. J. Wildl. Dis. 26:67-77. [DOI] [PubMed] [Google Scholar]
- 6.Fourel, I., J. M. Cullen, J. Saputelli, C. E. Aldrich, P. Schaffer, D. R. Averett, J. Pugh, and W. S. Mason. 1994. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J. Virol. 68:8321-8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovesi, E. V., L. Lamb, I. Medina, D. Taylor, M. Seifer, S. Innaimo, R. J. Colonno, D. N. Standring, and J. M. Clark. 1998. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob. Agents Chemother. 42:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo, J. T., C. E. Aldrich, W. S. Mason, and J. C. Pugh. 1996. Characterization of serum amyloid A protein mRNA expression and secondary amyloidosis in the domestic duck. Proc. Natl. Acad. Sci. USA 93:14548-14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, A., C. A. Scougall, J. W. Lowenthal, A. R. Jilbert, and I. Kotlarski. 2001. Structural and functional homology between duck and chicken interferon-gamma. Dev. Comp. Immunol. 25:55-68. [DOI] [PubMed] [Google Scholar]
- 10.Innaimo, S. F., M. Seifer, G. S. Bisacchi, D. N. Standring, R. Zahler, and R. J. Colonno. 1997. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob. Agents Chemother. 41:1444-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jilbert, A. R., J. A. Botten, D. S. Miller, E. M. Bertram, P. Hall, I. Kotlarski, and C. J. Burrell. 1998. Characterization of age- and dose-related outcomes of duck hepatitis B virus infection. Virology 243:273-282. [DOI] [PubMed] [Google Scholar]
- 12.Jilbert, A. R., J. S. Freiman, E. J. Gowans, M. Holmes, Y. E. Cossart, and C. J. Burrell. 1987. Duck hepatitis B virus DNA in liver, spleen and pancreas: analysis by in situ and Southern blot hybridization. Virology 158:330-338. [DOI] [PubMed] [Google Scholar]
- 13.Jilbert, A. R., D. S. Miller, C. A. Scougall, H. Turnbull, and C. J. Burrell. 1996. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226:338-345. [DOI] [PubMed] [Google Scholar]
- 14.Jilbert, A. R., T. T. Wu, J. M. England, P. M. Hall, N. Z. Carp, A. P. O'Connell, and W. S. Mason. 1992. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J. Virol. 66:1377-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klingmuller, U., and H. Schaller. 1993. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J. Virol. 67:7414-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai, C., M. Rosmawati, J. Lao, H. Vlierberghe, F. Anderson, N. Thomas, and D. De Hertogh. 2001. A phase II study of entecavir vs. lamivudine in adults with chronic hepatitis B. J. Hepatol. 34:24. [Google Scholar]
- 18.Lee, J.-Y., J. G. Culvenor, P. Angus, R. Smallwood, A. Nicoll, and S. A. Locarnini. 2001. Duck hepatitis B virus replication in primary bile duct epithelial cells. J. Virol. 75:7651-7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenhoff, R. J., and J. Summers. 1994. Construction of avian hepadnavirus variants with enhanced replication and cytopathicity in primary hepatocytes. J. Virol. 68:5706-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, E., C. Luscombe, D. Colledge, Y. Y. Wang, and S. A. Locarnini. 1998. Long-term therapy with the guanine nucleoside analog penciclovir controls chronic duck hepatitis B virus infection in vivo. Antimicrob. Agents Chemother. 42:2132-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marion, P. L., F. H. Salazar, M. A. Winters, and R. J. Colonno. 2002. Potent efficacy observed with entecavir (BMS-200475) in a duck model of hepatitis B virus replication. Antimicrob. Agents Chemother. 46:82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason, W. S., J. Cullen, G. Moraleda, J. Saputelli, C. E. Aldrich, D. S. Miller, B. C. Tennant, L. Frick, D. Averett, L. D. Condreay, and A. R. Jilbert. 1998. Lamivudine therapy of WHV infected woodchucks. Virology 245:18-32. [DOI] [PubMed] [Google Scholar]
- 23.Mason, W. S., J. Cullen, J. Saputelli, T. T. Wu, C. Liu, W. T. London, E. Lustbader, P. Schaffer, A. P. O'Connell, I. Fourel, C. E. Aldrich, and A. R. Jilbert. 1994. Characterization of the antiviral effects of 2′ carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology 19:398-411. [PubMed] [Google Scholar]
- 24.Newbold, J. E., H. Xin, M. Tencza, G. Sherman, J. Dean, D. S. Bowden, and S. A. Locarnini. 1995. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J. Virol. 69:3350-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicoll, A. J., D. L. Colledge, J. J. Toole, P. W. Angus, R. A. Smallwood, and S. A. Locarnini. 1998. Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl) adenine, an acyclic phosphonate nucleoside analogue. Antimicrob. Agents Chemother. 42:3130-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoll, A., S. A. Locarnini, S. T. Chou, R. Smallwood, and P. Angus. 2000. Effect of nucleoside analogue therapy on duck hepatitis B viral replication in hepatocytes and bile duct epithelial cells in vivo. J. Gastroenterol. Hepatol. 15:304-310. [DOI] [PubMed] [Google Scholar]
- 27.Price, P. M., R. Banerjee, and G. Acs. 1992. The mechanism of inhibition of hepatitis B virus replication by the carbocyclic analog of 2′-deoxyguanosine. Hepatology 16:8-12. [DOI] [PubMed] [Google Scholar]
- 28.Pugh, J. C., Q. Di, W. S. Mason, and H. Simmons. 1995. Susceptibility to duck hepatitis B virus infection is associated with the presence of cell surface receptor sites that efficiently bind viral particles. J. Virol. 69:4814-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rollier, C., C. Sunyach, L. Barraud, N. Madani, C. Jamard, C. Trepo, and L. Cova. 1999. Protective and therapeutic effect of DNA-based immunization against hepadnavirus large envelope protein. Gastroenterology 116:658-665. [DOI] [PubMed] [Google Scholar]
- 30.Triyatni, M., P. L. Ey, H. Tran, M. F. LeMire, M. Qiao, C. J. Burrell, and A. R. Jilbert. 2001. Sequence comparison of an Australian duck hepatitis B virus strain with other avian hepadnaviruses. J. Gen. Virol. 82:373-378. [DOI] [PubMed] [Google Scholar]
- 31.Triyatni, M., A. R. Jilbert, M. Qiao, D. S. Miller, and C. J. Burrell. 1998. Protective efficacy of DNA vaccines against duck hepatitis B virus infection. J. Virol. 72:84-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka, G., T. Wilson, S. Innaimo, G. S. Bisacchi, P. Egli, J. K. Rinehart, R. Zahler, and R. J. Colonno. 1999. Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus. Antimicrob. Agents Chemother. 43:190-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou, T., J. Saputelli, C. E. Aldrich, M. Deslauriers, L. D. Condreay, and W. S. Mason. 1999. Emergence of drug-resistant populations of woodchuck hepatitis virus in woodchucks treated with the antiviral nucleoside lamivudine. Antimicrob. Agents Chemother. 43:1947-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]