Abstract
Pneumolysin, a virulence factor of Streptococcus pneumoniae with cytotoxic and proinflammatory activities, occurs at concentrations from 0.85 to 180 ng/ml in cerebrospinal fluid (CSF) of meningitis patients. In pneumococcal cultures and in a rabbit meningitis model, the concentrations of pneumolysin in supernatant and CSF were lower after addition of nonbacteriolytic bactericidal antibiotics (rifampin and clindamycin) than after incubation with ceftriaxone.
Streptococcus pneumoniae is the most common cause of bacterial meningitis in adults and even more frequently the causative agent of various bacterial infections, including pneumonia and otitis media. Pneumococcal meningitis frequently causes severe neurological sequelae and death (19).
The thiol-activated membrane-damaging toxin pneumolysin is one of the important virulence factors of pneumococci. It is a cytoplasmic protein (14) released during bacterial growth and autolysis: e.g., due to antibiotic treatment (2, 13). Pneumolysin-deficient mutants were less virulent in animal models of infection (3, 27), and immunization protected exposed mice (1, 22). Pneumolysin possesses cytolytic and immunomodulatory effects (17), including direct cytotoxicity on neurons (5, 25).
Pneumolysin is released in humans during respiratory infection (15, 28), but has not been demonstrated in cerebrospinal fluid (CSF) of patients with pneumococcal meningitis. We investigated CSF specimens from meningitis patients to address this question.
The current standard therapy of bacterial meningitis is based upon highly bactericidal β-lactam antibiotics, through which CSF sterilization is achieved quickly. However, β-lactam antibiotics act by bacterial lysis, and intracellular bacterial compounds with toxic effects will be released. Therefore, the influence of nonbacteriolytic bactericidal antibiotics in comparison to ceftriaxone on the release of pneumolysin from pneumococci in vitro and in vivo was examined by quantitative immunoblotting.
Patients' CSF.
Pneumolysin concentrations were measured in the CSF of diagnostic lumbar punctures from eight patients with culture-proven S. pneumoniae meningitis and one suspected case treated at the University Hospitals of Göttingen and Düsseldorf in 2002 (Table 1).
TABLE 1.
Characterization of patients whose CSF was analyzed
| Patient (sex, age)a | Concn of pneumolysin in CSF (ng/ml) | Antibiotic pretreatment before diagnostic LPb | No. of leukocytes/ μl of CSF | Concn in CSF
|
Clinical outcome | ||
|---|---|---|---|---|---|---|---|
| Proteind (mg/liter) | Glucose (mg/dl) | Lactate (mmol/ liter) | |||||
| I (M, 36 yr) | 0.85 | No | 10,117 | 3,514 | NDc | 16.5 | Bilateral deafness |
| II (F, 5 mo) | 1.2 | No | 833 | Pandy + | ND | ND | Focal seizures, hearing impairment |
| III (M, 68 yr) | 1.9 | Roxithromycin for 7 days | 3,413 | Pandy +++ | ND | ND | Incontinence, tetraparesis |
| IV (M, 71 yr) | |||||||
| 1st LP | 3.1 | Amoxicillin + clavulanate and ciprofloxacin for 4 h before LP | 897 | 14,410 | ND | 13.9 | Organic personality disorder, bilateral distal paresis |
| 2nd LP | 7.7 | Ceftriaxone and ampicillin for 13 h since 1st LP | 2,000 | 14,888 | ND | 17.5 | |
| V (F, 41 yr) | 7.0 | No | 1,493 | 2,236 | 14 | ND | No neurologic sequelae |
| VI (M, 73 yr) | 13 | Cefotaxime and gentamicin for 2 days | 558 | 5,715 | ND | 18.3 | Chaddock sign positive on both sides, no further neurologic sequelae |
| VII (M, 71 yr) | 73 | No | 453 | 3,520 | ND | 20.6 | Paraparesis |
| VIII (M, 42 yr) | 160 | No | 396 | 3,540 | <2 | 20.9 | Death 40 days after admission |
| IX (F, 27 yr) | 180 | No | 373 | 3,010 | ND | 14 | 1 wk after admission, altered coordination; at release, no neurologic sequelae |
M, male; F, female.
LP, lumbar puncture.
ND, not determined.
Pandy is a qualitative test for detection of elevated CSF protein content.
Bacterial strain.
A penicillin-sensitive S. pneumoniae type 3 strain (kind gift of M. Täuber, University of Bern, Bern, Switzerland) was used with the antibiotics ceftriaxone (MIC, 0.03 μg/ml; minimum bactericidal concentration [MBC], 0.06 μg/ml), rifampin (MIC, 0.008 μg/ml; MBC, 0.06 μg/ml), and clindamycin (MIC, 0.06 μg/ml; MBC, 0.12 μg/ml).
In vitro experiments.
A culture of S. pneumoniae grown for 10 h at 37°C in tryptic soy broth was divided into fractions of 15 ml and cultivated after addition of antibiotics for another 12 h at 37°C. Control cultures were grown without antibiotics. The experiment was performed six times on separate days. Bacterial counts were determined at 0, 3, 6, 9, and 12 h by plating 10-μl samples of 10-fold dilutions of bacteria on blood agar plates. In the presence of antibacterial agents, the bactericidal rates (Δlog CFU per milliliter per hour) were determined by log-linear regression analysis of bacterial titers (CFU per milliliter) versus time. The concentrations of the antibiotics added to the cultures were chosen according to the concentrations attainable in CSF in vivo during treatment of central nervous system infections: 8 μg/ml for ceftriaxone (Hoffmann-LaRoche, Grenzach-Wyhlen, Germany) (16), 3 μg/ml for rifampin (Grünenthal, Stolberg, Germany) (18), and 1 μg/ml for clindamycin (Sigma-Aldrich, Taufkirchen, Germany) (21, 23). One group of cultures was first incubated with 3 μg of rifampin per ml for 6 h, and then 8 μg of ceftriaxone per ml was added. The bactericidal rates were compared by repeated-measures analysis of variance followed by the Tukey-Kramer multiple-comparisons test to correct for repeated testing.
Samples (1 ml) for the detection of pneumolysin were collected immediately after addition of the antibiotics and at 1, 2, 3, 6, 9, and 12 h. Supernatants were separated from bacterial cells by centrifugation, mixed with an equal volume of reducing sample buffer, boiled for 5 min, and stored at −20°C. Pneumolysin release was compared by two-sided parametric covariance analysis using the software SAS 8.01, procedure GLM.
Animal experiments.
CSF supernatants from a previous study were reevaluated (4). CSF was drawn by cisternal puncture at 16 (prior to antibiotic treatment), 18, and 21 h after infection. Pneumococcal CSF titers were measured by plating 10 μl of undiluted CSF and serial 10-fold dilutions. The logarithms of bacterial titers in the treatment groups at 16 h were compared by unpaired t test. CSF was immediately centrifuged (3,000 × g, 10 min), and supernatants were stored at −70°C. For pneumolysin quantification, all available specimens collected at 16, 18, and 21 h were used (at each time point, n = 6 to 7 for ceftriaxone and n = 5 to 6 for rifampin). After logarithmic transformation of the pneumolysin concentrations measured, an unpaired t test was performed. To assess pneumolysin concentrations in infected but untreated rabbits, CSF of two animals of another experimental series (CSF drawn at 15, 18, and 21 h after infection) was used.
Quantification of pneumolysin.
Sample proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Blots were blocked and successively incubated with monoclonal murine antibodies to recombinant pneumolysin (NCL-SPNm; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom) and peroxidase-conjugated goat anti-mouse antibodies (Jackson Immunoresearch Laboratories, West Grove, Pa.). The reaction was developed with the ECL+ enhanced chemiluminescence Western blotting detection reagents and exposed to Hyperfilm ECL (both from Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom). Pneumolysin was detected as a band at 53 kDa. For quantitation, a set of standards of recombinant pneumolysin was included on all immunoblots. Specimens with pneumolysin concentrations exceeding the linear range of the standard curve were diluted and reexamined. Densitometry of the films was performed with NIH Image 1.62 software.
Detection of pneumolysin in human CSF.
Pneumolysin was detected in all CSF specimens of eight patients with culturally proven S. pneumoniae meningitis (Table 1). In one suspected but not culture-proven case, the diagnosis was established by detecting pneumolysin at a concentration of 0.85 ng/ml in the patient's CSF. The pneumolysin concentrations in CSF ranged from 0.85 to 180 ng/ml (Table 1) at the first puncture. Five CSF specimens of patients suffering from meningeal inflammation due to other etiologies were tested as controls. In those specimens, no pneumolysin was detectable. In one patient (no. IV), the concentration of pneumolysin in CSF was measured repeatedly during antibiotic treatment. Initially, an increment from 3.1 ng/ml to 7.7 ng/ml was found; however, 32 days later, no pneumolysin was detectable in CSF.
In vitro experiments.
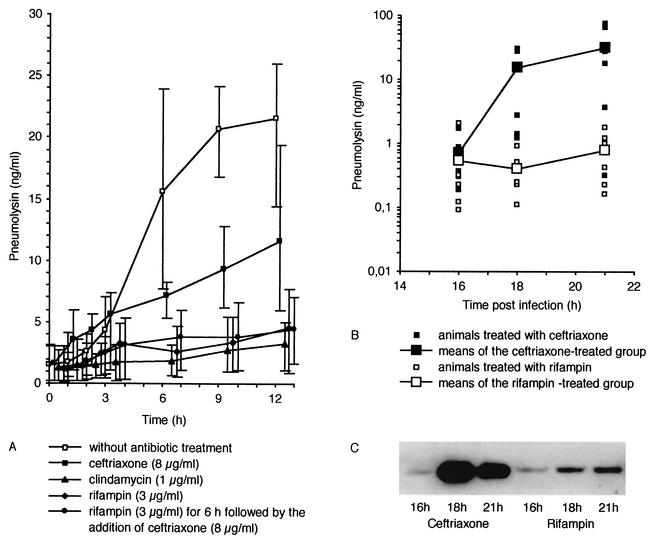
In the absence of antibiotics, the cultures displayed logarithmic growth for another 6 h after the beginning of the experiment (mean ± standard deviation, 0.18 ± 0.03 Δlog CFU ml−1 h−1) (P < 0.001 versus all antibiotic treatments). At the end of the logarithmic phase, bacterial titers exceeded 108 CFU/ml. The bactericidal activity of 8 μg of ceftriaxone per ml (−0.44 ± 0.03 Δlog CFU ml−1 h−1) was higher than the activity of 1 μg of clindamycin per ml (−0.28 ± 0.04 Δlog CFU ml−1 h−1) (P < 0.05). The differences between the effects of ceftriaxone and 3 μg of rifampin per ml (−0.33 ± 0.02 Δlog CFU ml−1 h−1) and those of the sequential combination of 3 μg of rifampin per ml for 6 h followed by the addition of 8 μg of ceftriaxone per ml (−0.41 ± 0.03 Δlog CFU ml−1 h−1) failed to reach statistical significance.
Overall release of pneumolysin was highest in control cultures. At the end of logarithmic growth (6 h), spontaneous autolysis of the bacterial cells occurred. Pneumolysin concentrations in the culture supernatants increased earlier (3 to 6 h), suggesting that the release of toxin may not be solely due to autolysis. Type 3 pneumococci can secrete the toxin independent of autolysis (2), possibly by a mechanism requiring protein synthesis. Overall release of pneumolysin was lower in cultures incubated with ceftriaxone. However, during the first 3 h after addition of antibiotics, the concentrations of pneumolysin in cultures incubated with ceftriaxone tended to be higher than those in control cultures, most likely due to the ceftriaxone-induced bacteriolysis of pneumococci. The release of pneumolysin in cultures incubated with clindamycin, rifampin, and sequential rifampin and ceftriaxone was lower than that in cultures exposed to ceftriaxone (Fig. 1A; P < 0.0001 for all groups versus ceftriaxone). There was no significant difference in the release of pneumolysin between cultures incubated with rifampin and clindamycin (P = 0.13) and between cultures exposed to rifampin versus those with sequential addition of rifampin and ceftriaxone (P = 0.67).
FIG. 1.
(A) Release of pneumolysin (means and minimum/maximum) from S. pneumoniae type 3 in culture without addition of antibiotics; during incubation with ceftriaxone, clindamycin, or rifampin; or with sequential addition of rifampin and ceftriaxone (delay of 6 h). Control cultures showed a strong increase of extracellular pneumolysin when reaching the end of logarithmic growth (at 6 h). The release of pneumolysin was lower during exposure to nonbacteriolytic antibiotics than during exposure to ceftriaxone (P < 0.0001, two-sided covariance analysis). (B) Pneumolysin concentrations in the CSF of rabbits (S. pneumoniae meningitis model) during antibiotic treatment with rifampin versus ceftriaxone before and 2 and 5 h after onset of treatment. Small symbols represent all individual measurements. Large symbols represent the means of each group. Bacterial titers (means ± standard deviations, ceftriaxone-treated group, 8.15 ± 0.95 log CFU/ml; rifampin-treated group, 7.91 ± 1.66 log CFU/ml) and pneumolysin concentrations in rabbit CSF before onset of antibiotic treatment (16 h after infection) did not differ significantly (P = 0.74 and 0.18, respectively). Two and 5 h after antibiotic treatment, pneumolysin concentrations in the rifampin-treated group were lower than those in the ceftriaxone-treated group (P = 0.001 and 0.018, respectively). In the CSF of two infected but untreated animals, pneumolysin concentrations were 0.26 and 0.45 μg/ml at 15 h after infection, 0.57 and 1.1 μg/ml at 18 h after infection, and 5 and 60 μg/ml at 21 h after infection. In comparison to release in the untreated rabbits, the release of pneumolysin in ceftriaxone-treated animals appeared to be accelerated. (C) Representative immunoblot comparing the pneumolysin concentration in the CSF of a ceftriaxone-treated animal with that in a rifampin-treated animal.
In vivo experiments.
Bacterial titers and pneumolysin concentrations in rabbit CSF before onset of antibiotic treatment (16 h postinfection) did not differ significantly (P = 0.74 and 0.18, respectively). Two and 5 h after onset of treatment, pneumolysin concentrations in the CSF of rifampin-treated rabbits were lower than in the CSF of ceftriaxone-treated animals (P = 0.001 and 0.018, respectively) (Fig. 1B).
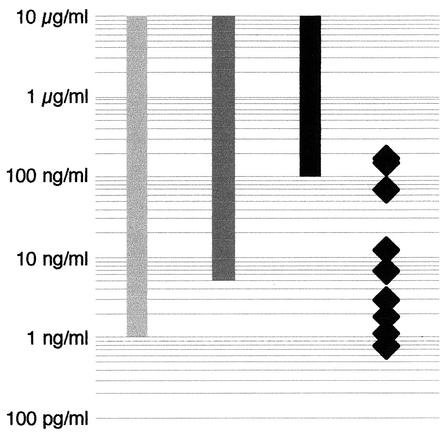
Pneumolysin can act directly as a toxin on different eukaryotic cells or indirectly by stimulating the host's immune response. In general, the concentrations necessary for a direct cytotoxic effect of pneumolysin are higher than those causing immunomodulatory or functional interference (Fig. 2). In concentrations above 0.1 μg/ml, pneumolysin is directly cytotoxic for neuronal cells (5, 25). Furthermore, pneumolysin acts in a cytotoxic manner on other cells within the central nervous system, such as ependymal cells or microglia and ganglion cells within the inner ear (5, 29). In sublytic concentrations, pneumolysin causes cellular malfunction or disturbance of immune cell function (17): At concentrations ≥5 ng/ml, pneumolysin reduces the ciliary beat frequency of the respiratory (8) or ependymal (11) epithelium. From 1 ng/ml upward, pneumolysin stimulates neutrophilic granulocytes (increased production of superoxide [7], prostaglandin E2 and leukotriene B4 [6]), monocytes (increased release and production of tumor necrosis factor alpha and interleukin-1β [12]), activates phospholipase A (24), and inhibits the capacity of stimulated lymphocytes to produce lymphokines and immunoglobulins (9).
FIG. 2.
Comparative illustration of selected toxic effects of pneumolysin with respect to the pneumolysin concentrations detected in human CSF during pneumococcal meningitis. Solid bar, cytotoxicity to neuronal cells (5, 25); darkly shaded bar, reduction of ciliary beat frequency of respiratory and ependymal epithelium (8, 11); lightly shaded bar, release of proinflammatory cytokines (6, 13); solid diamonds, pneumolysin concentrations in CSF of patients with S. pneumoniae meningitis.
In human CSF, pneumolysin was detected at concentrations up to 180 ng/ml (Table 1 and Fig. 2). These data suggest that during bacterial meningitis, usually immunomodulatory and proinflammatory CSF concentrations are reached. In the CSF of some patients, directly cytotoxic pneumolysin concentrations occur. The concentrations measured in CSF after centrifugation are possibly underestimates of the amounts released. An unknown portion of the toxin liberated from bacteria probably binds quickly to the cells present and may either remain in the cell pellet after centrifugation of CSF specimens or be associated with the tissue in the patient or experimental animal.
In meningitis therapy, it appears advantageous to eradicate the pathogen quickly, but also with a minimal release of bacterial products, which act toxic on nervous tissue either directly or by stimulating immune defense mechanisms (19). Ceftriaxone, the standard therapeutic agent in pneumococcal meningitis, acts by bacterial lysis. Sterilization is achieved quickly during treatment. Nevertheless, pneumolysin is released during incubation of pneumococcal cultures or treatment of rabbits suffering from meningitis with ceftriaxone. In contrast, nonbacteriolytic bactericidal antibiotics effectively inhibited the release of pneumolysin from S. pneumoniae in cultures (rifampin and clindamycin) and in rabbit CSF (rifampin). Similarly, rifampin released smaller quantities of teichoic and lipoteichoic acids and bacterial DNA from S. pneumoniae than ceftriaxone (10, 26), decreased mortality in a mouse model of S. pneumoniae meningitis (20), reduced the densities of apoptotic neurons in the dentate gyrus of the hippocampal formation (P = 0.005) (4), and decreased the production of reactive oxygen species by CSF phagocytes (4) in experimental rabbits.
Because of the high risk of development of antibiotic resistance (rifampin) and because of the relatively high prevalence of resistant pneumococci in the community (clindamycin), minimizing the release of toxic bacterial products cannot be done by using rifampin or clindamycin as a single antibiotic agent in treatment of bacterial meningitis. For this reason, the influence of an initial therapy with rifampin followed by the combination of rifampin with the standard antibiotic ceftriaxone after 6 h was studied: Rifampin pretreatment prevented the release of pneumolysin after addition of ceftriaxone.
In conclusion, during pneumococcal meningitis, the toxin pneumolysin is present in human CSF in concentrations able to exert pathophysiological effects. The release of pneumolysin by S. pneumoniae during therapy is reduced during treatment with nonbacteriolytic antibiotics in comparison to standard therapy with ceftriaxone in vitro and in vivo. We hypothesize that particularly those patients with a high bacterial load in the central nervous system would benefit from treatment directed at the reduction of noxious bacterial products in CSF. For this reason, antibiotic regimens relying on bactericidal protein synthesis inhibitors should be evaluated for meningitis therapy.
Acknowledgments
This work was supported by a grant to T. Böttcher and R. Nau from the Else Kröner-Fresenius Foundation and by grant NA 165/4-2 to R. Nau from the Deutsche Forschungsgemeinschaft.
We thank Utz Reichard for many thoughtful discussions and suggestions and James Merle Thomas for critical reading of the manuscript.
REFERENCES
- 1.Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böttcher, T., J. Gerber, A. Wellmer, A. V. Smirnov, F. Fakhrjanali, E. Mix, J. Pilz, U. K. Zettl, and R. Nau. 2000. Rifampin reduces production of reactive oxygen species of cerebrospinal fluid phagocytes and hippocampal neuronal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 181:2095-2098. [DOI] [PubMed] [Google Scholar]
- 5.Braun, J. S., J. E. Sublett, D. Freyer, T. Mitchell, J. Cleveland, E. Tuomanen, and J. Weber. 2002. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockeran, R., H. C. Steel, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Pneumolysin potentiates production of prostaglandin E2 and leukotriene B4 by human neutrophils. Infect. Immun. 69:3494-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183:604-611. [DOI] [PubMed] [Google Scholar]
- 8.Feldman, C., T. J. Mitchell, P. W. Andrew, G. J. Boulnois, R. C. Read, H. C. Todd, P. J. Cole, and R. Wilson. 1990. The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb. Pathog. 9:275-284. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante, A., B. Rowan-Kelly, and J. C. Paton. 1984. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect. Immun. 46:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber, J., H. Eiffert, H. Fleischer, A. Wellmer, U. Munzel, and R. Nau. 2001. Reduced release of DNA from Streptococcus pneumoniae after treatment with rifampin in comparison to spontaneous growth and ceftriaxone treatment. Eur. J. Clin. Microbiol. Infect. Dis. 20:490-493. [DOI] [PubMed] [Google Scholar]
- 11.Hirst, R. A., A. Rutman, K. Sikand, P. W. Andrew, T. J. Mitchell, and C. O'Callaghan. 2000. Effect of pneumolysin on rat brain ciliary function: comparison of brain slices with cultured ependymal cells. Pediatr. Res. 47:381-384. [DOI] [PubMed] [Google Scholar]
- 12.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, M. K. 1977. Cellular location of pneumolysin. FEMS Microbiol. Lett. 2:243-245. [Google Scholar]
- 15.Kalin, M., K. Kanclerski, M. Granstrom, and R. Mollby. 1987. Diagnosis of pneumococcal pneumonia by enzyme-linked immunosorbent assay of antibodies to pneumococcal hemolysin (pneumolysin). J. Clin. Microbiol. 25:226-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klugman, K. P., I. R. Friedland, and J. S. Bradley. 1995. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob. Agents Chemother. 39:1988-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell, T. J., and P. W. Andrew. 1997. Biological properties of pneumolysin. Microb. Drug Resist. 3:19-26. [DOI] [PubMed] [Google Scholar]
- 18.Nahata, M. C., P. Fan-Havard, W. J. Barson, H. M. Bartkowski, and E. J. Kosnik. 1990. Pharmacokinetics, cerebrospinal fluid concentration, and safety of intravenous rifampin in pediatric patients undergoing shunt placement. Eur. J. Clin. Pharmacol. 38:515-517. [DOI] [PubMed] [Google Scholar]
- 19.Nau, R., and W. Brück. 2002. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 25:38-45. [DOI] [PubMed] [Google Scholar]
- 20.Nau, R., A. Wellmer, A. Soto, K. Koch, O. Schneider, H. Schmidt, J. Gerber, U. Michel, and W. Brück. 1999. Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 179:1557-1560. [DOI] [PubMed] [Google Scholar]
- 21.Paris, M. M., S. Shelton, M. Trujillo, S. M. Hickey, and G. H. McCracken, Jr. 1996. Clindamycin therapy of experimental meningitis caused by penicillin- and cephalosporin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton, J. C., R. A. Lock, and D. J. Hansman. 1983. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 40:548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picardi, J. L., H. P. Lewis, J. L. Tan, and J. P. Phair. 1975. Clindamycin concentrations in the central nervous system of primates before and after head trauma. J. Neurosurg. 43:717-720. [DOI] [PubMed] [Google Scholar]
- 24.Rubins, J. B., T. J. Mitchell, P. W. Andrew, and D. E. Niewoehner. 1994. Pneumolysin activates phospholipase A in pulmonary artery endothelial cells. Infect. Immun. 62:3829-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stringaris, A. K., J. Geisenhainer, F. Bergmann, C. Balshüsemann, U. Lee, G. Zysk, T. J. Mitchell, B. U. Keller, U. Kuhnt, J. Gerber, A. Spreer, M. Bähr, U. Michel, and R. Nau. 2002. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen-activated protein kinase. Neurobiol. Dis. 11:355-368. [DOI] [PubMed] [Google Scholar]
- 26.Stuertz, K., H. Schmidt, F. Trostdorf, H. Eiffert, M. Mäder, and R. Nau. 1999. Lower lipoteichoic and teichoic acid CSF concentrations during treatment of pneumococcal meningitis with non-bacteriolytic antibiotics than with ceftriaxone. Scand. J. Infect. Dis. 31:367-370. [DOI] [PubMed] [Google Scholar]
- 27.Wellmer, A., G. Zysk, J. Gerber, T. Kunst, M. von Mering, S. Bunkowski, H. Eiffert, and R. Nau. 2002. Decreased virulence of a pneumolysin-deficient strain of Streptococcus pneumoniae in murine meningitis. Infect. Immun. 70:6504-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheeler, J., R. Freeman, M. Steward, K. Henderson, M. J. S. Lee, N. H. Piggott, G. J. A. Eltringham, and A. Galloway. 1999. Detection of pneumolysin in sputum. J. Med. Microbiol. 48:863-866. [DOI] [PubMed] [Google Scholar]
- 29.Winter, A. J., S. D. Comis, M. P. Osborne, M. J. Tarlow, J. Stephen, P. W. Andrew, J. Hill, and T. J. Mitchell. 1997. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 65:4411-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]