Abstract
We assessed the potential of lactoferrin (LF), a multifunctional milk protein, for treatment of oral candidiasis with immunosuppressed mice, which have local symptoms characteristic of oral thrush. Oral administration of bovine LF in drinking water starting 1 day before the infection significantly reduced the number of Candida albicans in the oral cavity and the score of lesions on the tongue on day 7 after the inoculation. The symptomatic effect of LF was confirmed by macroscopic and microscopic observations of the tongue's surface. Similar effects were also observed upon administration of LF pepsin hydrolysate, but not lactoferricin B, an antimicrobial peptide of LF. The anticandidal activity of LF was evident on administration either in drinking water or by intragastric intubation with a stomach tube. These results suggest that the effect of LF in this oral candidiasis model is not due to direct antifungal action. In conclusion, LF could have potential as a food component supporting antifungal drug treatment.
The opportunistic fungus Candida albicans is a major cause of oral and esophageal infections in immunocompromised patients such as human immunodeficiency virus (HIV)-infected individuals and the elderly (12, 13, 20). Other conditions predisposing individuals to oral C. albicans infection include hyposalivation (9, 16), diabetes mellitus, prolonged use of antibiotics or immunosuppressive drugs, use of dentures, and poor oral hygiene (2, 21). The clinical significance of the oral candidiasis, which is not life-threatening but causes significant morbidity in patients, is increasing with time (8). Some drugs such as azole antifungal agents are used for chemotherapy of this fungal infection (30). HIV-positive patients receiving the new therapy (highly active antiretroviral treatment) demonstrate significantly fewer episodes of oral candidiasis than those without highly active antiretroviral treatment (17). However, long-term treatment with antifungal drugs still causes the appearance of drug-resistant Candida or side effects (13, 30).
Lactoferrin (LF) is an iron-binding glycoprotein, which is present in milk, saliva, and other exocrine secretions as well as in neutrophil granules. This protein has a number of biological functions, including antimicrobial and immunomodulatory effects in vitro and in vivo (6, 26). It was reported that LF and an LF-derived antimicrobial peptide, lactoferricin B (LFcin B), inhibit the in vitro growth of C. albicans, including azole-resistant strains, not only in yeast form but also in hyphal form, which is important for the pathogenesis of this fungus (24, 28, 32). Furthermore, Masci reported that severe oral candidiasis of an HIV patient, which became resistant to conventional treatment by antifungals, was completely resolved by a treatment with mouthwash containing LF and lysozyme in combination with an antifungal agent, itraconazole (15). Recently, it has been reported that orally administered bovine LF improves the survival rate of the host or reduces the number of pathogenic organisms in the tissues of animals with bacterial infection (5, 7). It has also been shown that orally administered LF has antifungal activity against systemic C. albicans infection (1) and cutaneous Trichophyton mentagrophytes infection (29). From these findings, both the direct antifungal effect and a host-mediated protection by LF are expected for treatment of oral candidiasis. If bovine LF has beneficial effects against oral candidiasis, it may be utilized as a dietary supplement, supporting antifungal chemotherapy and improving the quality of life of oral candidiasis patients.
Very recently, a reproducible experimental oral candidiasis model with immunosuppressed mice, which has local symptoms characteristic of oral thrush in humans, was developed (25). Using this model, we demonstrate here the efficacy of LF and the pepsin hydrolysate of LF (LFH) against experimental oral C. albicans infection microbiologically and symptomatically.
MATERIALS AND METHODS
C. albicans.
C. albicans strain TIMM2640, a clinical isolate from a patient with cutaneous candidiasis (Teikyo University Institute of Medical Mycology, Tokyo, Japan) (14), was stored at −80°C in Sabouraud dextrose broth (Becton Dickinson, Sparks, Md.) containing 0.5% yeast extract (Becton Dickinson) and 10% glycerol until the experiment was performed. The strain was grown on Candida GS agar plates (Eiken Chemical Co., Tokyo, Japan) at 37°C for 24 h. Yeast cells were harvested by microspatula and suspended in RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo.) containing 2.5% fetal calf serum. The cell number of the suspension was adjusted to 2.5 × 107 cells/ml by hemacytometer in a setting for counting Candida conidia.
Experimental oral candidiasis of mice.
All animal experiments were performed according to the guidelines for the care and use of animals approved by Teikyo University. Six-week-old female ICR mice (Charles River Japan, Kanagawa, Japan) were used for all animal experiments. The animals were randomized, assigned to groups of 5 to 11 individuals, and given food and water ad libitum.
The study of experimental oral candidiasis of mice was performed according to the method described in a previous report (25). Mice were immunosuppressed with two subcutaneous injections of prednisolone (Mitaka Pharmaceutical Co., Tokyo, Japan) at a dose of 100 mg/kg of body weight 1 day prior to and 3 days after the infection with Candida. Tetracycline hydrochloride (Takeda Shering Purau Animal Health Co., Osaka, Japan) in drinking water at a concentration of 0.83 mg/ml was given to mice beginning 1 day before the infection. Animals were anesthetized by an intramuscular injection with 50 μl of 2-mg/ml chlorpromazine chloride (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in each femur. Small cotton pads (baby cotton buds; Johnson & Johnson Co., Tokyo, Japan) were soaked in a C. albicans cell suspension (2.5 × 107 cells/ml), with which the entire oral cavity of the anesthetized mice was swabbed to produce oral infections.
Bovine LF (Morinaga Milk Industry Co., Tokyo, Japan) or LFH (3), at a concentration of 0.3% solution in drinking water (equivalent to 0.5 g/kg/day), or LFcin B purified from LFH (3) at a concentration of 0.01% (equivalent to 0.02 g/kg/day) was consecutively administered from 1 day before the infection. Alternatively, LF at a dose of 0.05, 0.5, or 2.5 g/kg/day was daily administered by intragastric intubation with a stomach tube.
Evaluation of severity of infections.
The end point of infection evaluation was day 7 in this model (25). Mice were sacrificed under anesthesia on days 1, 3, 5, and 7, and the severities of tongue lesions were evaluated. Macroscopic evaluation of the infection was indicated by a lesion score from 0 to 4 on the basis of the extent and severity of whitish, curd-like patches on the tongue surface as follows: 0, normal; 1, white patches over less than 20%; 2, white patches over less than 90% but more than 21%; 3, white patches over more than 91%; 4, thick white patches like pseudomembranes over more than 91% of the tongue.
Microbiological evaluation of progression of the infection was carried out as follows. The whole oral cavity, including the buccal mucosa, the tongue, the soft palate, and other oral mucosal surfaces, was swabbed with a cotton pad. The end of the cotton pad was then cut off and placed in a tube containing 5 ml of sterile physiological saline. After mixing with a vortex mixer to release Candida cells from the swab into the saline, 100 μl of undiluted cell suspension or its 100-fold dilutions were plated and incubated on Candida GS plates at 37°C for 20 h. Then, the CFU of Candida cells were counted. The detection limit was 50 CFU/mouse.
Histopathological study.
Tongues were taken from the sacrificed animals, fixed in 20% formalin solution, and embedded in paraffin. Five-micron-thick sections were cut from paraffin blocks and stained with periodic acid-Schiff stain for histopathological examination and fungal detection.
Statistical analysis.
The log10 CFU of C. albicans isolated from the mouths of infected mice was analyzed by using Student's t test, and the lesion scores were analyzed by using the Mann-Whitney U test for comparison of two groups. P values of <0.05 were considered significant. All analysis was performed by using a statistical software program (Stat View; SAS Institute Inc., Cary, N.C.). Data are indicated as means ± standard errors of the means (SEM).
RESULTS
Effect of LF administered ad libitum.
In a preliminary experiment in vitro, we confirmed that the MIC of LF is 200 μg/ml for C. albicans TIMM2640 in culture medium at pH 7.2 by the NCCLS method (19), and this activity is similar to that reported before against other strains of C. albicans (27). Here, the antifungal efficacy of LF against the oral candidiasis was investigated by using a murine model infected with C. albicans TIMM2640, microbiologically and symptomatically. No Candida cell was detectable microbiologically in the oral cavity cultures of mice before the infection (data not shown).
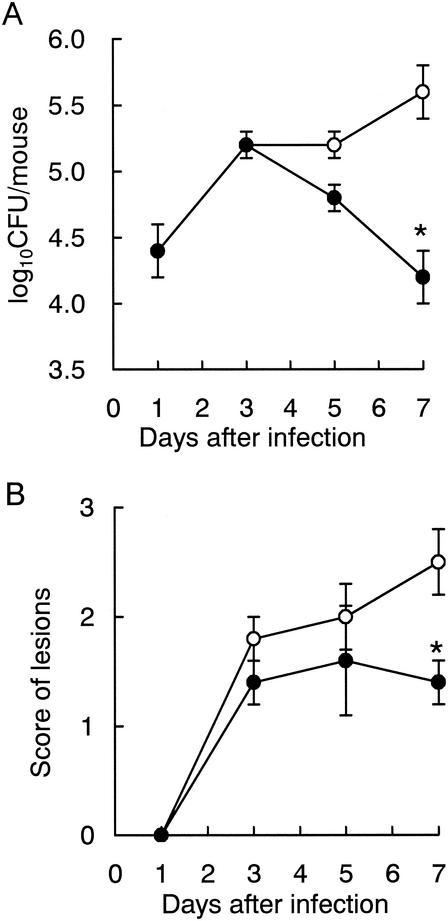
The pathological progression was kinetically examined in Candida-infected mice treated with or without LF administration. LF-treated mice were consecutively given 0.3% LF in drinking water (equivalent to 0.5 g/kg/day) from 1 day before the Candida infection, and control mice were given conventional water. Figure 1 shows typical data, and similar results were obtained at least three times in other experiments. In control mice from days 3 to 7 after the infection, the number of CFU of viable Candida cells in the oral cavity was between 105 and 106 (Fig. 1A), and the lesions on the tongue were scored at about 2 to 3 (Fig. 1B). Until day 3, there were no differences in the severity of Candida infection between LF-treated and untreated mice. In LF-treated mice, the number of viable Candida cells in the oral cavity and the score of the lesions on the tongue started to decrease from day 5. On day 7, the reductions of these values by LF administration were significant in comparison with those of the untreated control. In another examination, we confirmed that 0.3% bovine serum albumin in drinking water had no effect in this model; on day 7, the numbers of oral CFU were 105.16 ± 100.3 and 105.16 ± 100.5 and the lesion scores were 2.4 ± 0.2 and 2.2 ± 0.6 in untreated control (n = 5) and bovine serum albumin-administered mice (n = 5), respectively.
FIG. 1.
Time course of the therapeutic effects of ad libitum administration of LF on oral candidiasis in mice. (A) Numbers of viable C. albicans in the oral cavity; (B) scores of tongue lesions. Water (open circles) or a 0.3% LF solution (closed circles) was administered to mice as drinking water from day −1 throughout the experiment. The data shown are means ± SEM for 5 to 8 mice. *, P < 0.05.
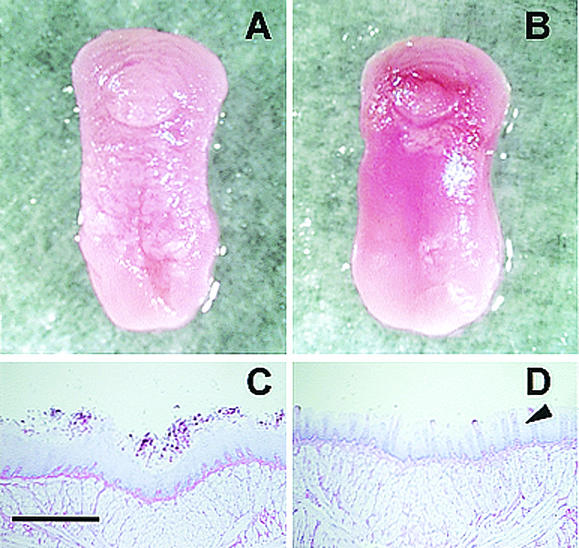
Figure 2 shows the macroscopic and microscopic appearance of typical lesions observed on the dorsal surface of the tongue on day 6, when the score of the lesions was 2.4 ± 0.5 in untreated mice and 1.2 ± 0.4 in LF-treated mice. Macroscopically, we observed that the tongues of untreated mice were covered with a lot of white patches (score, 3) (Fig. 2A). On the other hand, there was less area occupied with white patches on the tongues of LF-treated mice (score, 1) (Fig. 2B). In the histopathological study, periodic acid-Schiff stain-positive fungi could be observed attached to the oral epithelium (Fig. 2C and D). The untreated animals showed extensive colonization of the epithelium of the dorsal surface of the tongue by numerous hyphae (Fig. 2C). In contrast, the tongue's surface in mice treated with LF showed only very limited colonization of hyphae and the structure of lingual papillae, which is seen on the healthy tongue (Fig. 2D).
FIG. 2.
Macroscopic and microscopic observations of typical lesions on the tongues of mice with oral candidiasis on day 6 after the infection. Macroscopic observations show the tongue of an untreated mouse covered with abundant white patches (score, 3) (A) and the tongue of an LF-administered mouse with fewer white patches (score, 1) (B). Microscopic observations show extensive colonization by numerous Candida hyphae on the epithelium of the dorsal surface of the tongue in an untreated mouse (C) and the tongue's surface of an LF-treated mouse with fewer Candida hyphae and normal lingual papillae (arrowhead) (D). Bar, 0.5 mm.
Effects of LFH and LFcin B administered ad libitum.
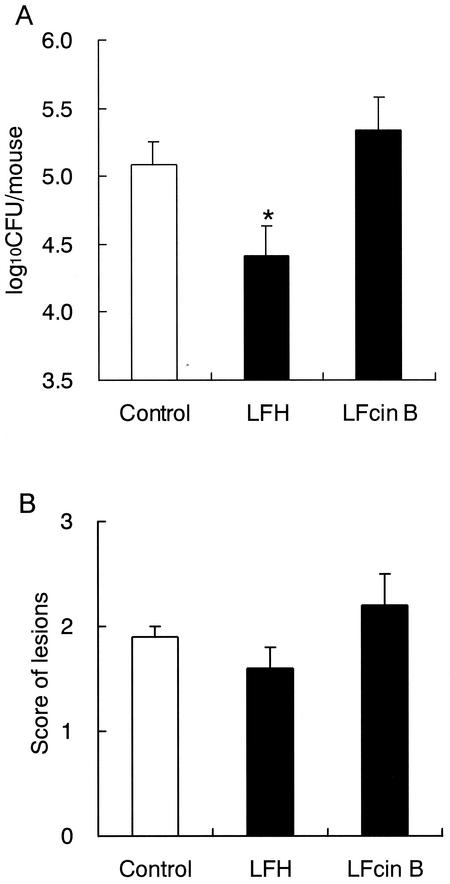
The infected animals were given 0.3% LFH in drinking water (equivalent to 0.5 g/kg/day), given 0.01% LFcin B (equivalent to 0.02 g/kg/day, which is the amount contained in 0.3% LFH), or not given any test agents (untreated control). Administration of LFH and LFcin B was started 1 day before the infection and was continued throughout the experiment. On day 7 after the infection, LFH administration significantly reduced the count of Candida in the oral cavity (Fig. 3A), but the therapeutic effect of LFH on the tongue's lesions was weaker than that of LF at the same dose (Fig. 3B). On the other hand, LFcin B, an antimicrobial peptide of LF, showed no effect in this model microbiologically or symptomatically.
FIG. 3.
Effect of LFH and LFcin B administered ad libitum on oral candidiasis in mice. (A) Numbers of viable C. albicans in the oral cavity; (B) scores of tongue lesions on day 7 after inoculation. LFH (0.3%) or LFcin B (0.01%) solution was administered to mice as drinking water from day −1 to day 7. The data shown are means ± SEM for 5 to 11 mice. *, P < 0.05.
Effects of various doses of LF administered by intragastric intubation.
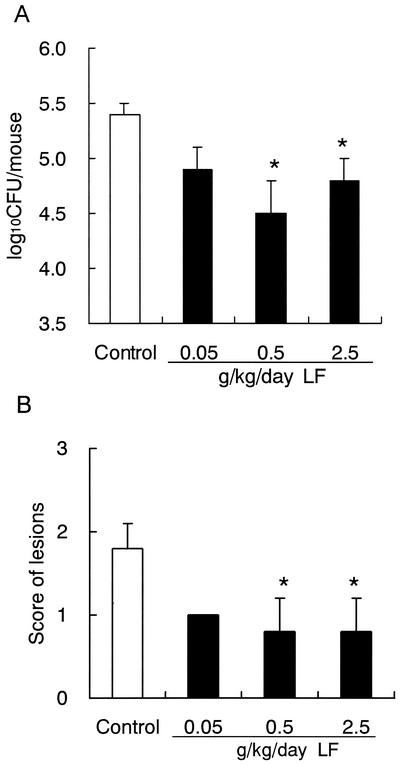
We examined the anticandidal efficacy of LF against the oral candidiasis under conditions where LF does not make direct contact with Candida cells in the oral cavity. In this experiment, LF was administered by intragastric intubation with a stomach tube. In addition, the influence of the dosage of LF was examined. Administration of LF was started 1 day before the infection and was continued daily during the experiment. LF at 0.5 g/kg/day, which is an equivalent dose to that used for the ad libitum administration of 0.3% LF in drinking water, reduced the fungal counts and improved the symptoms significantly (Fig. 4). The higher dose of LF (2.5 g/kg/day) also had significant effects. But the lower dose of LF (0.05 g/kg/day) showed weak effects on the number of Candida cells in the oral cavity and the lesion score.
FIG. 4.
Effects of various doses of LF administered by intragastric intubation on oral candidiasis in mice. (A) Numbers of viable C. albicans in the oral cavity; (B) scores of tongue lesions on day 7 after inoculation. Water or LF at each dose was administered by stomach tube once a day from day −1 to day 7. The data shown are means ± SEM for 5 mice. *, P < 0.05.
DISCUSSION
We showed here the protective activities of LF and LFH administered orally against oral candidiasis with an immunosuppressed-mouse model. In this model, the severity of Candida infection was estimated both by measuring the number of viable Candida cells in the oral cavity and by manifestation of a score of white patches on the tongue. Compared with the untreated control, LF facilitated recovery from oral candidiasis between days 5 and 7 after the infection not only microbiologically but also symptomatically. These symptomatic effects of LF were also confirmed by microscopic observation of the tongue's surface.
In the dose-response examination, LF at 0.5 or 2.5 g/kg/day similarly reduced the fungal counts in the oral cavity and improved the symptoms, but the lower dose, 0.05 g/kg/day, had weaker effects. This result indicates that LF at a dose of more than 0.5 g/kg/day exerts therapeutic activity microbiologically and symptomatically in our candidiasis model. Dose-dependent efficacy of LF at 0.5 and 2.5 g/kg/day was not observed, suggesting that LF may act in a different manner from the conventional antifungal agents that clearly show dose dependency. It has been reported that orally administered LF at the dosage of 2.5 g/kg/day facilitated the clinical improvement of skin lesions of cutaneous T. mentagrophytes infection in guinea pigs (29). The effective doses of LF against these two experimental infections are not contradictory.
Bhimani et al. (5) have reported that LF was most effective against systemic staphylococcal infections in mice when given 1 day prior to the bacterial injection. Following this experimental schedule, we also administered LF to mice from 1 day prior to Candida inoculation in our study. By this administration schedule, the severity of Candida infection in LF-treated animals started to improve on day 5, and this effect became evident on day 7 compared with the untreated control. This retarded elicitation of protective activity of LF contrasted with the quick effects of the antifungals such as fluconazole and amphotericin B, which cured the oral candidiasis on day 2 or 3 in this model (25). These results suggest that the protective effects of LF against oral candidiasis are exhibited in ways different from those of the chemotherapeutic agents with direct antifungal activities.
In addition to LF, we showed here the effect of orally administered LFH, an enzymatic hydrolysate of LF with pepsin, which lacks the iron-chelating ability. The microbiological efficacy of LFH was similar to that of LF, but LFH was not significantly effective in the symptomatic evaluation. On the other hand, orally administered LFcin B, which was a cationic bactericidal peptide purified from LFH and has more-potent anti-Candida activity than LF in vitro (27, 28), was not effective in our model. It is known that the direct antimicrobial functions of LF are based on several biochemical activities, such as an ability to deprive microorganisms of iron (31) and the perturbation of the microbial cell membrane by LFcin (27, 33). These findings suggest that the anticandidal effect of LF-related compounds observed in this model is not due to the direct anti-Candida activities described previously (4). Recently, Kuipers et al. (11) reported that LF in RPMI 1640 medium showed weaker anti-Candida activity at an acidic pH. In our animal model, LF and its derivatives were added to drinking water in which the pH was around 4 because of coadministered tetracycline hydrochloride. If the pH of the drinking water is raised, a direct anti-Candida activity of LF-related compounds in the oral cavity may increase in this model. However, a further investigation is necessary to elucidate this possibility. In the present study, we also investigated the antifungal effect of LF under conditions in which LF does not come into contact directly with Candida cells in the oral cavity by using a stomach tube for the administration. By this route, LF displayed protective activities similar to those by ad libitum administration. Taken together, it is further suggested that the effect of LF in this oral candidiasis model is mediated by a mechanism other than the direct antifungal activity.
Recently, it has been reported that orally administered bovine LF reduces the number of pathogenic organisms in tissues distant from the gastrointestinal tract in several infectious animal models (1, 5, 7, 29). However, very little is known about the mechanism responsible for these protective effects. In the past few years, several immunomodulatory effects of ingesting LF on cancer and infection, or in healthy individuals, have been reported in vivo, for example, the enhancement of natural killer (NK) activity (23) and cytokine production by spleen cells in response to mitogen (18), an increase in the number of NK cells and T cells in peripheral blood (10), and the enhancement of the phagocytic activity of blood neutrophils (22). It is possible that these immunological effects of LF are related to the protective action against oral candidiasis because NK cells and T cells are considered the predominant host defense against mucosal Candida infections, such as oropharyngeal candidiasis. Now we are investigating the immunological effects of ingesting LF in this candidiasis model.
Masci (15) reported that a mouthwash containing LF and lysozyme was effective against oral candidiasis in an HIV patient. In our study, the efficacy of LF against oral candidiasis was obtained by supplementation as a food. Therefore, these results suggest that bovine LF from cow's milk could be used as a dietary supplement to support antifungal chemotherapy without side effects. In the future, we would like to conduct a clinical trial of LF in patients with oral candidiasis.
Acknowledgments
We thank Kentaro Ninomiya and Yoshie Abe (Teikyo University) for technical help.
REFERENCES
- 1.Abe, S., T. Okutomi, S. Tansho, H. Ishibashi, H. Wakabayashi, S. Teraguchi, H. Hayasawa, and H. Yamaguchi. 2000. Augmentation by lactoferrin of host defense against Candida infection in mice, p. 195-201. In K. Shimazaki, H. Tsuda, M. Tomita, T. Kuwata, and J. P. Perraudin (ed.), Proceedings of the 4th International Conference on Lactoferrin: structure, function and applications. Elsevier Science B.V., Amsterdam, The Netherlands.
- 2.Allen, C. M. 1994. Animal models of oral candidiasis. A review. Oral Surg. Oral Med. Oral Pathol. 78:216-221. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy, W., M. Takase, K. Yamauchi, H. Wakabayashi, K. Kawase, and M. Tomita. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121:130-136. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy, W., H. Wakabayashi, M. Takase, K. Kawase, S. Shimamura, and M. Tomita. 1993. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. (Berlin) 182:97-105. [DOI] [PubMed] [Google Scholar]
- 5.Bhimani, R. S., Y. Vendrov, and P. Furmanski. 1999. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J. Appl. Microbiol. 86:135-144. [DOI] [PubMed] [Google Scholar]
- 6.Brock, J. 1995. Lactoferrin: a multifunctional immunoregulatory protein? Immunol. Today 16:417-419. [DOI] [PubMed] [Google Scholar]
- 7.Haversen, L. A., I. Engberg, L. Baltzer, G. Dolphin, L. A. Hanson, and I. Mattsby-Baltzer. 2000. Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 68:5816-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermann, P., Z. Berek, G. Nagy, K. Kamotsay, and F. Rozgonyi. 2001. Pathogenesis, microbiological and clinical aspects of oral candidiasis (candidosis). Acta Microbiol. Immunol. Hung. 48:479-495. [DOI] [PubMed] [Google Scholar]
- 9.Jorge, A. O., M. A. Totti, O. P. de Almeida, and C. Scully. 1993. Effect of sialoadenectomy on the carriage of Candida albicans in the mouths of rats. J. Oral Pathol. Med. 22:138-140. [DOI] [PubMed] [Google Scholar]
- 10.Kuhara, T., M. Iigo, T. Itoh, Y. Ushida, K. Sekine, N. Terada, H. Okamura, and H. Tsuda. 2000. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr. Cancer 38:192-199. [DOI] [PubMed] [Google Scholar]
- 11.Kuipers, M. E., L. Beljaars, N. Van Beek, H. G. De Vries, J. Heegsma, J. J. Van Den Berg, D. K. Meijer, and P. J. Swart. 2002. Conditions influencing the in vitro antifungal activity of lactoferrin combined with antimycotics against clinical isolates of Candida. Impact on the development of buccal preparations of lactoferrin. APMIS 110:290-298. [DOI] [PubMed] [Google Scholar]
- 12.Lockhart, S. R., S. Joly, K. Vargas, J. Swails-Wenger, L. Enger, and D. R. Soll. 1999. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J. Dent. Res. 78:857-868. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Ribot, J. L., R. K. McAtee, S. Perea, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 1999. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maebashi, K., T. Itoyama, K. Uchida, N. Suegara, and H. Yamaguchi. 1994. A novel model of cutaneous candidiasis produced in prednisolone-treated guinea-pigs. J. Med. Vet. Mycol. 32:349-359. [DOI] [PubMed] [Google Scholar]
- 15.Masci, J. R. 2000. Complete response of severe, refractory oral candidiasis to mouthwash containing lactoferrin and lysozyme. AIDS 14:2403-2404. [DOI] [PubMed] [Google Scholar]
- 16.Meitner, S. W., W. H. Bowen, and C. G. Haidaris. 1990. Oral and esophageal Candida albicans infection in hyposalivatory rats. Infect. Immun. 58:2228-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munro, C. A., and B. Hube. 2002. Anti-fungal therapy at the HAART of viral therapy. Trends Microbiol. 10:173-177. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima, M. 1999. Oral administration of lactoferrin enhances the productions of IFN-γ and IL-10 in spleen cells cultured with concanavalin A or lipopolysaccharide. Biomed. Res. 20:27-33. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1992. Reference method for broth dilution antifungal susceptibility testing for yeasts. Proposed standard. Document M27-P. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 20.Odds, F. C. 1988. Candidiosis of the oropharynx, p. 117-123. In Candida and candidosis: a review and bibliography. Bailliere Tindale, London, United Kingdom.
- 21.Samaranayake, Y. H., and L. P. Samaranayake. 2001. Experimental oral candidiasis in animal models. Clin. Microbiol. Rev. 14:398-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato, R., O. Inanami, Y. Tanaka, M. Takase, and Y. Naito. 1996. Oral administration of bovine lactoferrin for treatment of intractable stomatitis in feline immunodeficiency virus (FIV)-positive and FIV-negative cats. Am. J. Vet. Res. 57:1443-1446. [PubMed] [Google Scholar]
- 23.Sekine, K., Y. Ushida, T. Kuhara, M. Iigo, H. Baba-Toriyama, M. A. Moore, M. Murakoshi, Y. Satomi, H. Nishino, T. Kakizoe, and H. Tsuda. 1997. Inhibition of initiation and early stage development of aberrant crypt foci and enhanced natural killer activity in male rats administered bovine lactoferrin concomitantly with azoxymethane. Cancer Lett. 121:211-216. [DOI] [PubMed] [Google Scholar]
- 24.Soukka, T., J. Tenovuo, and M. Lenander-Lumikari. 1992. Fungicidal effect of human lactoferrin against Candida albicans. FEMS Microbiol. Lett. 69:223-228. [DOI] [PubMed] [Google Scholar]
- 25.Takakura, N., Y. Sato, H. Ishibashi, H. Oshima, K. Uchida, H. Yamaguchi, and S. Abe. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol. Immunol. 47:321-326. [DOI] [PubMed]
- 26.Vorland, L. H. 1999. Lactoferrin: a multifunctional glycoprotein. APMIS 107:971-981. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi, H., S. Abe, T. Okutomi, S. Tansho, K. Kawase, and H. Yamaguchi. 1996. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 40:821-825. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi, H., S. Abe, S. Teraguchi, H. Hayasawa, and H. Yamaguchi. 1998. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob. Agents Chemother. 42:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakabayashi, H., K. Uchida, K. Yamauchi, S. Teraguchi, H. Hayasawa, and H. Yamaguchi. 2000. Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J. Antimicrob. Chemother. 46:595-602. [DOI] [PubMed] [Google Scholar]
- 30.Walsh, T. J., C. E. Gonzalez, S. Piscitelli, J. D. Bacher, J. Peter, R. Torres, D. Shetti, V. Katsov, K. Kligys, and C. A. Lyman. 2000. Correlation between in vitro and in vivo antifungal activities in experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. J. Clin. Microbiol. 38:2369-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg, E. D. 1978. Iron and infection. Microbiol. Rev. 42:45-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu, Y. Y., Y. H. Samaranayake, L. P. Samaranayake, and H. Nikawa. 1999. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 37:35-41. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi, K., M. Tomita, T. J. Giehl, and R. T. Ellison, III. 1993. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 61:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]