Abstract
We characterized the potent in vitro antimalarial activity and biologic assessment of 13 phospholipid polar head analogs on a comparative basis. There was a positive relationship between the abilities of the drugs to inhibit parasite growth in culture and their abilities to specifically inhibit phosphatidylcholine biosynthesis of Plasmodium falciparum-infected erythrocytes. Maximal activity of G25 was observed for the trophozoite stage of the 48-h erythrocytic cycle (50% inhibitory concentration, 0.75 nM), whereas the schizont and ring stages were 12- and 213-fold less susceptible. The compounds exerted a rapid nonreversible cytotoxic effect, with complete clearance of parasitemia after 5 h of contact with the mature stages. The compounds were highly specific against P. falciparum, with much lower toxicity against three other mammalian cell lines, and the in vitro therapeutic indices ranged from 300 to 2,500,000. Finally, the monoquaternary ammonium E10 and two bis-ammonium salts, G5 and G25, were similarly active against multiresistant strains and fresh isolates of P. falciparum. This impressive selective in vitro toxicity against P. falciparum strongly highlights the clinical potential of these quaternary ammonium salts for malarial chemotherapy.
The increasing polypharmacoresistance of Plasmodium falciparum to conventional antimalarials, combined with the resistance of mosquitoes to pesticides, partially accounts for the current dramatic resurgence of malaria (7, 16). Malaria has an enormous impact on world health, thus justifying the intensive research under way to design new therapeutic approaches to this disease and to develop a new range of drugs with original structures and modes of action (22, 37).
In the human host, the parasite undergoes cyclical development within red blood cells (RBC), which accounts for the clinical manifestations of this disease. The rate of multiplication within RBC, with 10 to 20 merozoites produced every 48 h, requires high metabolic activity, including a high membrane formation level. Phospholipids (PL) are essential lipids of Plasmodium membranes, and the erythrocyte PL content increases by as much as 500% after malarial infection (17, 27). Plasmodial PL biosynthesis, which is absent from normal mature human erythrocytes (29), was previously characterized as an ideal potential target for antimalarial chemotherapy due to its vital importance to the parasite (30, 32). Phosphatidylcholine (PC) is the major PL of infected erythrocytes, representing about 45% of total PL, much of which is provided by parasite-driven de novo biosynthesis from choline (4, 31). In the pathway, parasitic cholinephosphate cytidylyltransferase (38) and choline transport, which regulates the precursor supply, are a limiting and likely regulatory step (4, 33).
More than 100 analogs of PL polar heads have been synthesized and tested in vitro for their activity against the intraerythrocytic stage of P. falciparum (2, 8, 9). More particularly, 36 compounds which possess one (or two) quaternary ammonium ions, such as choline and long lipophilic alkyl chains, were found to be very potent against P. falciparum, with a 50% inhibitory concentration (IC50) within the micro- and picomolar range (8). A recently synthesized radioactive derivative of this class of compounds was shown to be specifically and highly accumulated in infected RBC. This property likely accounts for the potency and selectivity of the compounds (36). In the present study, we characterized their mechanism of action and in vitro biological activities by assessing the susceptibilities of various multidrug-resistant P. falciparum strains and isolates, their time- and concentration-dependent effects on parasite growth, and their specificities compared to mammalian cells.
MATERIALS AND METHODS
Chemicals.
The compounds were stock samples from LAPP (UMR 5810). Their synthesis has been described previously (8). Chloroquine and quinine were obtained from Sigma Chemical Co. (St. Louis, Mo.), mefloquine was from Hoffmann La Roche (Basel, Switzerland), halofantrine was from Smith Kline Beecham (Ware, Hertfordshire, United Kingdom), and artemether was from Aventis (Antony, France). G-[3H]hypoxanthine, [Me-3H]choline, and [1-3H]ethan-1-ol-2-amine were purchased from Amersham Corp. (Les Ulis, France), and [3H]thymidine was from ICN (Orsay, France). RPMI 1640 was obtained from GIBCO (Eragny, France). All other reagents were of analytical grade.
Biological materials.
The susceptibility of P. falciparum strains and isolates to ammonium compounds were tested by the following independent laboratories according to methods published by each of them: UMR 5539 (H. Vial) (2); London School of Hygiene and Tropical Medicine, London, United Kingdom (D. Warhurst) (35); Pasteur Institute, Antananarivo, Madagascar (R. Jambou) (18); Institut de Recherche pour le Développement, Yaoundé, Cameroon (P. Ringwald) (25); Museum National d'Histoire Naturelle, Paris, France (J. Schrevel) (1); Centre National de Référence de la Chimiosensibilité du Paludisme, Paris, France (J. Le Bras) (13); and Institut de Médecine Tropicale du Service de Santé des Armées, Marseille, France (J. C. Doury) (24).
P. falciparum isolates were collected by venipuncture from outpatients presenting at the Nlongkak dispensary (Yaoundé, Cameroon) with uncomplicated malaria infection and monospecific parasitemia (25). Other African P. falciparum isolates from six different countries collected at the Institut de Médecine Tropicale du Service de Santé des Armées were also used (J. C. Doury).
Parasites at 1 to 7% hematocrit were maintained in complete medium, which consisted of RPMI 1640 medium supplemented with 25 mM HEPES buffer (pH 7.4) and 10% AB+ serum from the local blood bank or Albumax (lipid-rich bovine serum albumin) (GIBCO), by using the petri dish-candle jar method (19, 28) or under a 5% CO2 and 5% O2 atmosphere with an N2 balance.
Splenectomized Macaca fascicularis monkeys (Centre de Recherches Primatologiques, Mahebourg, Mauritius) were infected with the cryopreserved Plasmodium knowlesi Washington strain, variant 1, as described previously (2).
Human Jurkat lymphoblasts and U937 macrophage cell lines were routinely cultured at 37°C in RPMI 1640 medium complemented with 50 μM β-mercaptoethanol, 1 mM fresh glutamine, and 10% fetal calf serum (GIBCO) (15). Megakaryoblastic cells (MEG-01) were suspended and grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 1 mM sodium pyruvate, and 0.4% nonessential amino acids (21). They were incubated at 37°C with a CO2 incubator.
In vitro screen for intrinsic antimalarial activity.
Drug effects on in vitro P. falciparum growth were measured in microtiter plates according to Desjardins et al. (11) with the following modifications. After blood collection for the isolates or continuous culture for the strains, parasitized RBC were washed three times with RPMI 1640. When parasitemia was over 0.5%, cultures were diluted with nonparasitized RBC until 0.5% parasitemia. The final volume in each well was 200 μl, consisting of 50 μl of complete medium with or without (control) the drug and 150 μl of P. falciparum-infected RBC suspension (1 to 2% final hematocrit and 0.5% parasitemia). Generally, the compounds were water soluble, otherwise they were dissolved in ethanol or dimethyl sulfoxide (final concentration, ≤0.2%). After 48 h of incubation at 37°C, 30 μl of complete medium containing 0.8 μCi of [3H]hypoxanthine was added to each well and candle jar incubations were continued for 12 to 18 h. Reactions were then stopped by cell filtration (2). IC50s were evaluated from the plotted parasite growth (expressed as a percentage of the control) versus the log of the dose and are means of at least two independent experiments conducted in triplicate, with different culture batches and stock drug solutions. A nonparametric Mann-Whitney U test was used to compare IC50 means, and the Spearman rank test was applied to determine correlations.
Time course of in vitro parasite growth inhibition by choline analogs.
One-milliliter aliquots of suspensions of P. falciparum-infected RBC at 1% hematocrit and 0.3 to 0.5% parasitemia were injected at time zero into 24-well plates; 500 μl of complete medium without (control) or with the drug was added. After various incubation times at 37°C, culture media were removed twice and replaced by fresh medium without drug. Cell suspensions were then transferred to 96-well plates (200 μl/well), and [3H]hypoxanthine (1.5 μCi/well) was added either immediately after drug removal or at 52 h. Reactions were stopped by cell filtration at 76 h.
In some experiments, P. falciparum-infected RBC were synchronized by three successive treatments with 5% d-sorbitol (20) at −82, −43, and −1 h and then incubated with the drug as above for various times starting at 0 h for the ring stage, 24 h for the trophozoite stage, and 36 h for the schizont stage. At 52 h, cell suspensions were transferred to 96-well plates and [3H]hypoxanthine was added.
Differential susceptibility of parasite development stages to the compounds.
At time zero, 1-ml aliquots of suspensions of synchronized infected RBC at 1.5% hematocrit and 0.3% initial parasitemia (100% rings) were transferred to 24-well plates and 500 μl of complete medium containing drug at various concentrations was added when the parasites were in the ring (0 h), trophozoite (24 h), or schizont (36 h) stage. After 4 h of incubation in the presence of the drug, media were replaced twice by fresh complete medium and cell suspensions were further cultured for 20 h at 37°C. At 24, 48, and 60 h for ring, trophozoite, and schizont stages, respectively, cell suspensions were transferred to 96-well plates and [3H]hypoxanthine was added. Incubations were finally stopped at 76 h. IC50s were evaluated for each stage.
Assay of PL biosynthesis in Plasmodium-infected erythrocytes.
The effect of drugs on the incorporation of 10 μM [3H]choline (1.5 μCi/well) or 2 μM [3H]ethanolamine (0.8 μCi/well) into PC or phosphatidylethanolamine (PE) or [3H]hypoxanthine (0.7 μCi/well) into nucleic acids of P. falciparum-infected RBC (4 × 106 infected cells/assay) was assessed after 4 h of incubation in RPMI 1640 at 37°C. Cells were subsequently collected on glass fiber filters with a cell harvester, as described previously (2). The results are expressed as PL50 (PC50 or PE50) and NA50, corresponding to the drug concentrations that reduce the amount of synthesized PL (PC or PE) and nucleic acids by 50%, respectively.
In vitro toxicity against U937 macrophages, Jurkat lymphoblasts, or MEG-01 megakaryoblastic cells.
The effect of the drugs on cell viability was measured in microtiter plates following [3H]thymidine incorporation into DNA of the cell suspensions (6,000 cells/200 μl) as described previously (15). Cell suspensions were exposed to various drug concentrations for 24 h at 37°C, then 30 μl of [3H]thymidine (0.7 μCi/well) was added for an additional 6-h period, and the reactions were stopped by cell filtration.
As for IC50 determination, the results are expressed as the drug concentrations resulting in 50% inhibition of cell growth and are means of at least two independent experiments performed in triplicate with different stock drug solutions and culture batches. Within each experiment, the standard deviation was always lower than 20% of the mean (intraexperiment) and, when comparing the two mean values for each experiment (interexperiment), they did not differ significantly by the Student t test). In the few cases for which the two means differed by more than 50%, a third experiment was performed to ascertain the value.
RESULTS
Mono- and bis-quaternary ammonium salts have potent activity against the intraerythrocytic stage of P. falciparum (8). The IC50s of 13 of the most active mono and bis-quaternary ammonium salts are listed in Table 1. All compounds possessed high antimalarial activities, with IC50s ranging from 0.8 μM to 33 nM for monoquaternary ammonium salts and 0.54 μM to 3 pM for bis-quaternary ammonium salts. The present study concerns a thorough characterization of this new class of antimalarials with a comparative evaluation of their in vitro antimalarial activities and mechanisms of action.
TABLE 1.
In vitro activities of mono- and bis-quaternary ammonium salts against P. falciparum (Nigerian strain) and their specificities against PC biosynthesis
| Formula | Name | R1 | R2 | R3 | n | IC50 (μM) | PC50 (μM) | PE50 (μM) | NA50 (μM) |
|---|---|---|---|---|---|---|---|---|---|
| R1|R—(CH2)n—N+—R2| R3, Br− | E8a | CH3 | CH3 | CH3 | 16 | 0.8 | 21 | 23 | 44 |
| E10a | C2H5 | C2H5 | C2H5 | 12 | 0.064 | 1 | 22 | 3.6 | |
| E13a | C3H7 | C3H7 | C3H7 | 12 | 0.033 | 3 | 21 | 2 | |
| F4a | CH3 | CH3 | CH2— CH2— OH | 12 | 0.48 | 6.5 | 28 | 19 | |
| R1 R1 ||R2—N+—(CH2)n—N+—R2 || R3R3, 2Br− | G4a | CH3 | CH3 | CH3 | 12 | 0.090 | 4.8 | >20 | >200 |
| G5 | CH3 | CH3 | CH3 | 16 | 0.004 | 0.6 | ≥100 | 5 | |
| G12 | C2H5 | C2H5 | C2H5 | 8 | 0.540 | ||||
| G14 | C2H5 | C2H5 | C2H5 | 12 | 0.045 | 7.5 | >20 | 20 | |
| G15 | C2H5 | C2H5 | C2H5 | 16 | 0.0016 | 0.35 | 100 | 3 | |
| G19 | C2H5 | C2H5 | C2H5 | 21 | 0.000003 | ||||
| G24 | CH3 | —(CH2)4—
|
12 | 0.013 | 6.5 | >200 | >200 | ||
| G25 | CH3 | —(CH2)4—
|
16 | 0.00064 | 0.4 | ≥100 | 1.2 | ||
| H5 | C2H5 | C2H5 | CH2— CH2— OH | 16 | 0.0049 | 0.16 | 2 | 0.8 | |
Specificity of mechanism of action against P. falciparum.
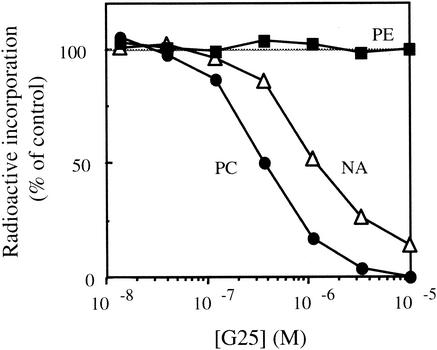
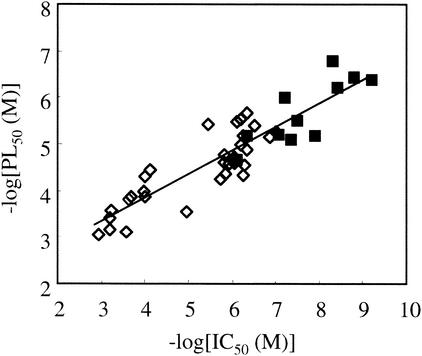
The effects of the test compounds on the de novo biosynthesis of PC and PE and on the biosynthesis of nucleic acids were compared to determine their specific effects (5). A compound was considered to be a specific inhibitor of de novo PC biosynthesis when it affected its biosynthesis without any simultaneous effect on PE or nucleic acid synthesis, i.e., when the PC50 was at least 2.5-fold lower than PE50 and NA50 (2). Figure 1 shows the typical dose-response curves obtained with the bis-quaternary ammonium G25, which quite specifically inhibited choline incorporation into PC (PC50 = 0.4 μM). Inhibition of nucleic acid (NA50 = 1.2 μM) and PE biosynthesis (PE50 > 100 μM) occurred at significantly higher concentrations. For two monoquaternary ammonium salts (E10 and F4) and all of the bis-quaternary ammonium salts (Table 1), the PC50s were 3- to >40-fold lower than the NA50, indicating specificity with respect to de novo PC biosynthesis. Figure 2 shows a significant correlation between PC biosynthesis inhibition (PC50) and parasite growth inhibition (IC50).
FIG. 1.
Effect of G25 on [3H]choline, [3H]ethanolamine, and [3H]hypoxanthine incorporation into PC, PE, and nucleic acids (NA) of P. falciparum-infected erythrocytes. Infected RBC (3.4% hematocrit, 10% parasitemia, Nigerian strain) were incubated for 4 h at 37°C in the absence (control) or presence of G25 and in the presence of 1.5 μCi of [3H]choline (at 10 μM) (•), 0.8 μCi of [3H]ethanolamine (2 μM) (▪), and 1 μCi of [3H]hypoxanthine (trace concentration) (▵).
FIG. 2.
Correlation between the inhibition of PL biosynthesis and the inhibition of parasite growth (▪). PC50 and IC50 were from Table 1. Some values (⋄) considered for this correlation have already been published (see text).
The effect of G25 on choline transport was also assessed as described previously (2) with pure P. knowlesi-infected RBC incubated in the presence of 9 μM choline, which corresponds to the KT of choline carriers in infected RBC (3). The inhibition pattern was sigmoidal, occurring over 1 to 2 orders of magnitude. The G25 concentration leading to 50% inhibition of choline influx was 0.8 μM, which corresponds (assuming that inhibition was competitive) to a calculated Ki of 0.4 μM (10), i.e., exactly the PC50 value (Table 1).
Time course of in vitro P. falciparum growth inhibition as a function of parasite development.
Compounds E10, G5, and G25 were added at 5 to 16 times the IC50 for pulse-inhibitory times to a nonsynchronized infected RBC suspension. Fifty percent inhibition required 15 h of contact with the drug for E10 but less than 5 h for G5 or G25, and after 24 h of contact, the inhibition was complete. A similar inhibition pattern was obtained whether [3H]hypoxanthine (used to monitor parasite viability) was added immediately after drug removal or only during the 52- to 76-h period after parasite culture in the absence of drug, which suggests a cytocidal rather than a cytostatic effect (data not shown).
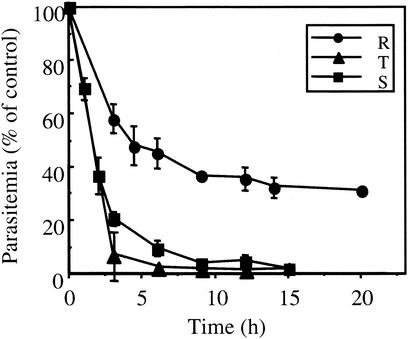
G25 was then added at 20 nM for pulse-inhibitory periods to synchronized P. falciparum-infected RBC suspensions, at the ring, trophozoite, or schizont stage (Fig. 3). Three successive sorbitol treatments at 35- to 40-h intervals allowed a tight synchronization window of around 5 h (20). After 3 h of incubation in the presence of G25, parasite growth was inhibited by 92 and 79% for the trophozoite and schizont stages, respectively, whereas the ring stage viability was only partly inhibited (by 40%). The time to completely kill mature parasites was less than 5 to 10 h. The drug treatment was also efficient on young ring forms but to lesser degree, with a longer incubation time required to obtain just a partial toxic effect (around 10 h). Beyond that time, a plateau was reached, indicating that a fraction of the parasites was not affected by 20 nM G25.
FIG. 3.
Time dependence of G25-induced growth arrest at the ring, trophozoite, and schizont stages. Cells were synchronized with 5% d-sorbitol at −82, −43, and −1 h (0.5% initial parasitemia, 1.5% hematocrit, Nigerian strain). Ring, trophozoite, and schizont stage parasites, which were obtained at time zero and 24 and 36 h, respectively, were exposed to 20 nM G25 for the indicated period of time, and then the drug was removed, as described in Materials and Methods. Parasites were labeled with [3H]hypoxanthine at 52 h (the parasitemia in control experiments was 2.2%) until 72 h. Hypoxanthine incorporation is expressed as a percentage of the control (without drug) for the ring (R), trophozoite (T), and schizont (S) stage parasites.
Stage-specific susceptibility of P. falciparum to G25.
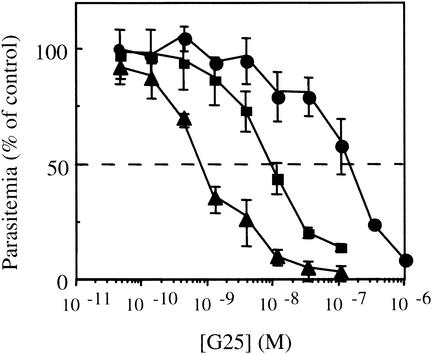
The partial inhibition obtained when G25 was incubated with ring forms (Fig. 3, plateau) led us to investigate the susceptibility of the parasite at different stages. Increasing drug concentrations were applied to synchronized P. falciparum-infected RBC suspensions for 4 h (Fig. 4). G25 was most efficient against the trophozoite stage, with an IC50 of 0.75 nM, i.e., more than 2 orders of magnitude lower than that against the ring stage (IC50 of 160 nM). Against schizonts, G25 was also very efficient, with an IC50 of 9 nM. Increasing the time of contact of the parasites with the drug from 4 to 9 h slightly decreased the IC50 for ring stages (160 ± 40 and 70 ± 28 nM, respectively), schizonts (from 9 ± 1 to 2 ± 0.3 nM) and trophozoites (from 0.75 ± 0.05 to 0.28 ± 0.02 nM).
FIG. 4.
G25 in vitro antimalarial activity as a function of the parasite stage. P. falciparum-infected RBC were synchronized with 5% d-sorbitol as described in the legend to Fig. 3. Drug was added for 4 h when the parasites were in the ring (•), trophozoite (▴), or schizont (▪) stage. At the end of the incubation, media were replaced by fresh complete medium, and the suspensions were further cultured for 20 h before the addition of [3H]hypoxanthine.
IC50 as a function of the incubation conditions.
We observed that the IC50s reported by some independent investigators were higher than those obtained in our laboratory (Table 2). Distinct assay conditions could account for this difference, e.g., times of drug contact with synchronized (young) parasite populations, shorter incubations with the compounds before hypoxanthine addition, and/or the initial parasitemia of the infected RBC suspension.
TABLE 2.
In vitro activities of E10, G5, and G25 against various P. falciparum clones and strainsa
| Strain and/or place of origin | Laboratory | IC50 (nM)
|
||||||
|---|---|---|---|---|---|---|---|---|
| CQ | Qui | MFQ | Pyr or Cyc | E10 | G5 | G25 | ||
| Nigerian strain | CNRS 5539 | 20 | S | S | 64 | 4 | 0.64 | |
| 3D7 | CNRS 5539 | 6 | 0.2 | |||||
| L-16, Sierra Leone | CNRCP | S | S | 103 (R) | 12.3* | 9.5* | ||
| FCR3, The Gambia | IMTSSA | 820 (R) | 1,680 (R) | 65 (R) | Cyc, 3,500 (R) | 129 | 12.7 | 3.5 |
| L1, Liberia | IMTSSA | 906 (R) | 2,300 (R) | 68 (R) | Cyc, 3,015 (R) | 77 | 5 | 1.4 |
| FCB-1, Colombia | MNHN | 420 (R) | 240 | 240* | 22* | 9.4* | ||
| Palo Alto, Calif. | Pasteur Institute | 50 | 4.9 | |||||
| F147, Nigeria | LSHTM | 19 | 2.3 | |||||
| H1, Honduras | LSHTM | 24 | Pyr (R) | 3.6 | ||||
| K1, Thailand | LSHTM | 288 (R) | (R) | M (R) | Pyr (R) | 15.3 | ||
| T996, Thailand | LSHTM | 27 | M (R) | Pyr (R) | 1.1 | |||
| 7G8, Brazil | LSHTM | 228 (R) | M (S) | 3.4 | ||||
| W2, Indochina | OCEAC | 510 (R) | 4.5 | |||||
| WRAIR | 191 (R) | 203 | 13 | Pyr, 212 (R) | 4.2 | |||
| D6, Sierra Leone | WRAIR | 9 | 37 | 20 | 5.3 | |||
| TM90C3B, Thailand | WRAIR | 114 (R) | 147 | 29 (R) | 1.9 | |||
IC50 were assessed after 48 h contact of infected RBC with the drug, except for cases in which the exposure time was 24 h (marked with an asterisk). S and R indicate susceptibility and resistance to the drug. The threshold IC50s for in vitro resistance to chloroquine (CQ), quinine (Qui), mefloquine (MFQ), pyrimethamine (Pyr), and cycloguanil (Cyc) were estimated to be >100, >800, >20, and >500 nM, respectively.
In this context, the IC50s of E10, G5, G25, and cycloguanil (for comparison) were evaluated after contact with the synchronized parasites only during the maturation of young trophozoites and/or schizonts or after longer incubations to allow schizogony to occur (data not shown). Regardless of the compounds, IC50s were significantly higher (by 4- to 20-fold, depending on the compound and the strain, L1 or FCR3) when the drug was administered during the maturation period than after a longer incubation. This was also observed, although to a lesser degree (IC50s differed by fourfold), with the antifolinic agent cycloguanil, which is also a metabolic inhibitor.
The G25 antimalarial activity was also assayed after 12, 24, and 48 h of contact of the drug with the unsynchronized infected suspension before adding hypoxanthine. The respective IC50s were 6.5 ± 1.2, 2.6 ± 0.9, and 0.4 ± 0.1 nM. By contrast, the IC50 of G25 remained unchanged irrespective of the initial parasitemia (a range of 0.1 to 1.2% at 1.2% hematocrit) or of the serum concentration (a range of 2 to 20%, at 1.5% hematocrit and 0.6% parasitemia).
Effect of G25 pretreatment of erythrocytes on ability to support parasite growth.
Normal RBC were preincubated for 48 h at 1.5% final hematocrit in complete medium in the absence (control) or in the presence of 20 nM G25. After being washed with fresh medium and then infected at 0.5% initial parasitemia with a highly parasitized suspension, they were further cultured for 48 h. Parasite growth was finally monitored by the incorporation of [3H]hypoxanthine over a 48- to 72-h period. Compared to untreated RBC, no change in parasitemia was observed, indicating that 48 h of pretreatment of RBC with a lethal dose of G25 did not affect the ability of RBC to support parasite growth.
Antimalarial activities against chemoresistant strains of P. falciparum.
The susceptibility of laboratory strains or clones and of field isolates to this new class of compounds was studied by independent laboratories with broad experience in malariology by using representative compounds of both ammonium series. Irrespective of the P. falciparum strains, the IC50 of the monoquaternary E10 ranged from 64 to 240 nM and that of the bis-quaternary G5 ranged from 4 to 22 nM (Table 2). There was remarkably high activity of these compounds against the L1 and FCR3 strains, which are highly polypharmacoresistant to chloroquine, quinine, mefloquine, and cycloguanil. The antimalarial activity of the lead bis-quaternary compound, G25, has been assessed by eight independent laboratories against 15 P. falciparum clones showing distinct susceptibilities to current antimalarials and that are often multidrug-resistant (Table 2). The G25 IC50 ranged from 0.2 to 15.3 nM.
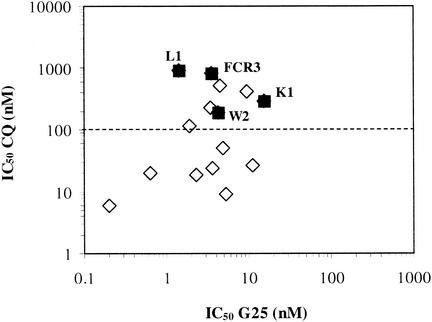
Note that the highest IC50s obtained by some investigators did not correspond to lower strain susceptibility to the compounds but rather to distinct assay conditions, i.e., shorter incubations or synchronized parasite (ring) populations (see above). G25 had the same activity against the strains most resistant to chloroquine and to other current antimalarials (L1, FCR3, K1, and W2) as against some of the most susceptible strains (F147, H1, D6, and 3D7). This is also clearly indicated by the total absence of correlation between chloroquine and G25 susceptibility (Fig. 5).
FIG. 5.
Susceptibility to G25 of 15 P. falciparum strains as a function of susceptibility to chloroquine. The results are replotted from Table 2. The open diamonds correspond to susceptible strains, and the filled squares correspond to multichemoresistant strains. The dotted line indicates the chloroquine susceptibility threshold.
Antimalarial activity against P. falciparum field isolates.
The in vitro antimalarial activity of E10, G5 and G25 against nine chloroquine-resistant P. falciparum isolates originating from six distinct countries was determined after 24 h of incubation with the drugs. Some of these isolates were also quinine or cycloguanil resistant (Table 3). E10, G5, and G25 showed antimalarial activity against all of the isolates. Irrespective of the compounds, the IC50 varied to a minor extent between the various isolates (by less than 3.5-fold). As already observed, monoquaternary ammonium E10 was less active than the bis-quaternary ammonium compounds G5 or G25, by 1 and 2 orders of magnitude, respectively. G25 was the most active, with an IC50 ranging between 7.7 and 20 nM. There was no correlation between the isolate's susceptibility to quaternary ammonium salts and its susceptibility to current antimalarials.
TABLE 3.
Effects of the selected compounds on pharmacoresistant P. falciparum isolatesa
| Patient's country of origin | IC50 (nM)
|
|||||
|---|---|---|---|---|---|---|
| CQ | Qui | Cyc | E10 | G5 | G25 | |
| Comoros | 147 (R) | 123 | 24 | 41 | 7.7 | |
| Comoros | 615 (R) | 146 | 9 | |||
| Senegal | 165 (R) | 193 | 36 | 785 | ||
| Gabon | 250 (R) | 13,100 (R) | 460 | 31 | ||
| Côte d'Ivoire | 270 (R) | 153 | 31,700 (R) | 743 | 111 | 20 |
| Côte d'Ivoire | 656 (R) | 357 | 1,057 | |||
| Côte d'Ivoire | 1,260 (R) | 277 | 300 | |||
| Guinea | 725 (R) | 1,250 (R) | 729 | 66 | 12 | |
| Burkina Faso | 730 (R) | 300 | 3,083 (R) | 1,530 | ||
Suspensions of P. falciparum isolates were incubated for 24 h at an initial parasitemia of 0.1 to 1% and at 2.5% hematocrit in the presence of the indicated drugs. See footnote a to Table 2 for abbreviations.
Finally, G25 was tested against a large number of P. falciparum isolates collected from patients at the Nlongkak dispensary. The in vitro susceptibilities of 106 isolates were determined in comparison with chloroquine, quinine, mefloquine halofantrine, and artemether (Table 4). According to the in vitro resistance criteria, 40 isolates were susceptible to chloroquine and 66 were resistant (with an IC50 > 100 nM). Susceptibility to G25 generally occurred within the same concentration range as susceptibility to mefloquine. The IC50 varied to a minor extent between the different isolates, as reflected by the 95% confidence interval. The geometric mean of IC50 for G25 did not differ significantly between chloroquine-resistant and chloroquine-susceptible isolates. There was no cross-resistance between G25 and the other drugs in vitro.
TABLE 4.
Comparison of susceptibilities to chloroquine, quinine, mefloquine, halofantrine, artemether, and G25 of 40 P. falciparum chloroquine-susceptible isolates and 66 chloroquine-resistant isolatesa
| Drug | IC50 (nM)
|
Pb | |||
|---|---|---|---|---|---|
| Chloroquine-susceptible isolates (n = 40)
|
Chloroquine-resistant isolates (n = 66)
|
||||
| Geometric mean | Confidence interval | Geometric mean | Confidence interval | ||
| Chloroquine | 35 | 29.5-41.5 | 271.5 | 240.8-306.7 | 0.0001 |
| Quinine | 124.7 | 99.5-162.9 | 244.2 | 205.2-290.6 | 0.0001 |
| Mefloquine | 5.92 | 4.67-7.51 | 5.96 | 5.04-7.04 | NS |
| Halofantrine | 1.15 | 0.88-1.5 | 0.98 | 0.84-1.14 | NS |
| Artemether | 2.37 | 1.8-3.08 | 1.6 | 1.3-1.96 | 0.03 |
| G25 | 7.25 | 5.83-9.02 | 6.11 | 5.11-7.29 | NS |
Suspensions of P. falciparum isolates were incubated at 1.5% hematocrit and 0.5% initial parasitemia in the presence of drugs as described in Materials and Methods. The resistance threshold for chloroquine is 100 nM (25).
Nonparametric Mann-Whitney U test was used for comparison of IC50. NS, not significant.
Differential effects of quaternary ammonium salts on three mammalian cell lines.
The toxicities of some of the most active compounds, three monoquaternary ammonium salts (E8, E13, and F4) and four bis-quaternary ammonium salts (G4, G12, G19, and H5), were determined against three human cell lines, U937 macrophages, Jurkat lymphoblasts, and MEG-01 megakaryoblasts which, like Plasmodium, have rapid cell division. The in vitro inhibitory effects were expressed as the Ma50, Ly50, and Mg50, i.e., concentrations leading to 50% inhibition of macrophage, lymphoblast, and megakaryoblast growth, respectively, as measured by thymidine incorporation.
For all of the compounds, the toxicity against mammalian cell lines occurred in the micromolar range, with maximal toxicity obtained for E13 and G19. Note also that each compound had a very similar toxicity against the three mammalian cell lines, since the Ly50, Ma50, and Mg50 differed by less than fourfold (Table 5). By contrast, all compounds clearly inhibited the intracellular growth of P. falciparum at concentrations significantly lower than those required to inhibit mammalian cell proliferation. The monoquaternary ammonium salts E8, E13, and F4 exhibited the lowest toxicity differential between mammalian cells and Plasmodium, differing by roughly 1 order of magnitude (4- to 36-fold for E13, depending on the cell type, 18-fold for E8, and 8-fold for F4). Bis-quaternary ammonium compounds were much less toxic against mammalian cells than against Plasmodium, with an activity differential ranging from 300 (G12 against macrophages) to as high as 2.5 × 106 (G19 against megakaryoblasts). This indicates a substantial difference in the respective susceptibility thresholds of both kinds of cells and highlights the selectivity of these compounds against Plasmodium-infected RBC.
TABLE 5.
Comparative effects of seven quaternary ammonium compounds on the growth of P. falciparum (IC50), and lymphoblastoid cells (Ly50), macrophages (Ma50), and megakaryoblasts (Mg50)a
| Compound | IC50 (μM) | Ly50 (μM) | Ma50 (μM) | Mg50 (μM) |
|---|---|---|---|---|
| E8 | 0.8 | 14*b | ND | ND |
| E13 | 0.033 | 0.3 | 0.13 | 1.2 |
| F4 | 0.48 | 3.7*b | ND | ND |
| G4 | 0.09 | 95b | ND | ND |
| G12 | 0.54 | 220 | 160 | >300 |
| G19 | 3 × 10−6 | 0.5 | 0.7 | 1.5 |
| H5 | 0.0049 | 20 | 10 | 19 |
IC50 against P. falciparum-infected RBC are from Table 1. Ly50, Ma50, and Mg50 were measured after 24 h of drug contact with 6,000 cells/well, as described in Materials and Methods. ND, not determined.
These values have already been reported in reference 2.
DISCUSSION
Our extensive fundamental research on Plasmodium PL metabolism revealed that choline is essential for the intraerythrocytic parasite—this nutrient is required for its synthesis of PC, the major Plasmodium PL. Choline entry in the parasite is a rate-limiting step and a potential new target for chemotherapeutic interference (30, 32). This pharmacological interference was previously validated with the design and in vitro screening of 77 molecules against P. falciparum (2, 9).
New compounds were then designed on the basis of a quantitative structure-activity relationship analysis and our targeted chemical synthesis led to eight novel compounds whose IC50s were lower than 10 nM (8). Some essential parameters, e.g., electronegativity and lipophilicity distribution on nitrogen substituents, required for polar head analogs to inhibit P. falciparum PL metabolism and growth were identified. The structural requirements of mono- and bis-quaternary ammonium salts for antimalarial activity were very similar, except that this activity was dramatically increased by 2 orders of magnitude when duplicating the quaternary ammonium, provided that the alkyl chain between the two nitrogen atoms contained at least 12 methylene groups. Most of these duplicated molecules had an activity of around 1 nM, and the most lipophilic compound, i.e., G19 (21 methylene groups), exhibited an IC50 as low as 3 pM. These compounds are thus more active than most current antifolic, antifolinic, or lysosomotropic antimalarials. Only halofantrine and artemisinin analogs exhibit IC50s in the low nanomolar range (23, 26).
The present study demonstrated that the selected compounds had a similar potent in vitro antimalarial effect on different strains and fresh isolates of P. falciparum with various degrees of multidrug resistance, which also indicates an original mechanism of action. Representative compounds in the monoammonium series (E10) and bis-ammonium series (G5 and G25) showed the same high activity against multidrug-resistant strains as against susceptible strains. More specifically, G25 remained very potent (nanomolar range) against various chloroquine-, quinine-, cycloguanil-, or mefloquine-resistant strains and also against fresh human isolates with various degrees of resistance. This indicates that these classes of compounds (mono- or bis-ammonium salts) constitute promising antimalarial candidates against multidrug-resistant malaria.
The in vitro antimalarial activity of these highly potent compounds was then characterized to determine the possible effects on host erythrocytes and the susceptibility of the different parasite developmental stages to the compounds. The influence of parameters such as parasitemia and serum quantities on their antimalarial activities was also investigated to assess the potential in vivo antimalarial activity.
Quaternary ammonium salts had time- and concentration-dependent effects, with a higher specificity of action against mature parasites. Monoquaternary (E10) and bis-quaternary (G5 and G25) ammonium salts had a clear cytocidal rather than cytostatic effect. Bis-quaternary ammonium salts acted more quickly than monoammonium salts, i.e., 50% growth inhibition occurring after 5 or 15 h contact, respectively (Fig. 3 and data not shown).
Although these compounds showed broad stage-specific action, they were found to be especially toxic against mature intraerythrocytic Plasmodium stages. The trophozoite stage was the most susceptible stage to G25 (IC50 = 0.75 nM), i.e., more than 200- and 10-fold-more susceptible than the ring and schizont stages, respectively. This stage specificity is similar to trends noted with many other antimalarials, especially antifolates (12, 14).
A consequence of this distinct parasite stage susceptibility is that the IC50s depend on the assay conditions, e.g., whether incubations are performed with synchronized parasite suspensions, the duration of incubation with the drugs, and the time of hypoxanthine addition. By contrast, the same IC50 was obtained irrespective of the initial parasitemia or concentration in serum, which suggests that the in vivo antimalarial activity will be satisfactory even at high parasitemia (6, 36).
Quaternary ammonium salts with a long alkyl chain are lipophilic molecules which could interact with membranes. However, pretreatment of healthy RBC with parasite-lethal concentrations of G25 did not change the susceptibility of RBC to P. falciparum invasion and their ability to support parasite growth, indicating that the G25 toxic effect did not occur at the host cell level. In fact, potential modifications of the host properties (if any) would be reversible after drug removal or have no effect on parasite growth.
Bis-quaternary ammonium compounds likely interfere with in vitro P. falciparum growth by affecting its essential de novo PC biosynthesis. G25 impaired choline transport into infected RBC, and most of the compounds selectively blocked de novo PC biosynthesis (Table 1; Fig. 1). They were highly specific to mature parasite stages (Fig. 4), which are the most metabolically active stages with respect to PL biosynthesis (34). There was a close correlation between impairment of PL biosynthesis and inhibition of in vitro malaria parasite growth (Fig. 2). By contrast, the most active of these compounds showed a much lower toxicity (by 3 to 5 orders of magnitude) against rapidly dividing lymphoblastoid, macrophage, or megakaryoblastic cells at concentrations affecting parasite viability as well as a total absence of correlation between parasite growth inhibition and the growth inhibition of other human cell lines (Table 5). This selectivity might be explained by the inability of mammalian cells to accumulate these compounds as noted for P. falciparum (36). The novelty of the target was also shown by the fact that the structure activity relationships of these compounds for antimalarial activity were not applicable for toxicity against mammalian cells. Altogether, the specific antimalarial effects of choline analogs are likely mediated by their alteration of parasite PL metabolism, indicating that de novo PC biosynthesis from choline is a very realistic target for new malaria chemotherapy.
In summary, a suitable very high level of activity is now reached in vitro against P. falciparum with these quaternary ammonium salts that mimic choline structure (8, 32) and act by inhibiting PC biosynthesis. We observed lower in vitro mammalian cell cytotoxicities and higher antiparasitic activities, i.e., the possible innocuousness for the animal of curative doses for malaria, thus boosting our confidence in future in vivo therapeutic indices. The rapid activity of the compounds and their different stage-specific profiles warrant further in vivo investigation. Subsequent tests on their tolerance and activity in vivo in the malaria-infected murine model also gave promising results since cures without recrudescence were obtained with a satisfactory safety margin (6). The next step that we are currently investigating before preclinical studies is to optimize the oral bioavailability of these very active antimalarial compounds.
Acknowledgments
We thank J. C. Doury, J. Le Bras, D. Warhurst, R. Jambou, J. Schrevel, the Walter Reed Institute, and the W.H.O. for assistance in testing the compounds in their laboratories and for valuable expertise and skillful work. We also thank X. Canron and A. Bonhoure for assistance in the studies.
This work was supported by the European Union (INCO-DEV, IC18-CT960056, QLK2-CT-2000-01166), CNRS/DGA (GDR 1077), MENRT (PRFMMIP and VIHPAL), the UNDP/World Bank/WHO special program for Research and Training in Tropical Diseases, and VIRBAC Laboratories.
REFERENCES
- 1.Adovelande, J., J. Deleze, and J. Schrevel. 1998. Synergy between two calcium channel blockers, verapamil and fantofarone (SR33557), in reversing chloroquine resistance in Plasmodium falciparum. Biochem. Pharmacol. 55:433-440. [DOI] [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. J. Vial. 1998. Antimalarial activity of 77 phospholipid polar head analogs: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. [PubMed] [Google Scholar]
- 3.Ancelin, M. L., M. Parant, M. J. Thuet, J. R. Philippot, and H. J. Vial. 1991. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem. J. 273:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ancelin, M. L., and H. J. Vial. 1989. Regulation of phosphatidylcholine biosynthesis in Plasmodium-infected erythrocytes. Biochim. Biophys. Acta 1001:82-89. [DOI] [PubMed] [Google Scholar]
- 5.Ancelin, M. L., F. Vialettes, and H. J. Vial. 1991. An original method for rapid serial determination of phospholipid biosynthesis. Applications to mammalian lymphocytic cells and a lower eucaryote, Plasmodium falciparum. Anal. Biochem. 199:203-209. [DOI] [PubMed] [Google Scholar]
- 6.Ancelin, M. L., M. Calas, A. Bonhoure, S. Herbute, and H. J. Vial. 2003. In vivo antimalarial activities of mono- and bis quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 47:2598-2605. [DOI] [PMC free article] [PubMed]
- 7.Breman, J. G., A. Egan, and G. T. Keusch. 2001. The intolerable burden of malaria: a new look at the numbers. Am. J. Trop. Med. Hyg. 64:iv-vii. [DOI] [PubMed]
- 8.Calas, M., M. L. Ancelin, G. Cordina, P. Portefaix, G. Piquet, V. Vidal-Sailhan, and H. Vial. 2000. Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J. Med. Chem. 43:505-516. [DOI] [PubMed] [Google Scholar]
- 9.Calas, M., G. Cordina, J. Bompart, M. Ben Bari, T. Jei, M. L. Ancelin, and H. Vial. 1997. Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism. Structure-activity relationship analysis. J. Med. Chem. 40:3557-3566. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, Y., and W. H. Prusoff. 1973. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099-3108. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieckmann, A., and A. Jung. 1986. Stage-specific sensitivity of Plasmodium falciparum to antifolates. Z. Parasitenkd. 72:591-594. [DOI] [PubMed] [Google Scholar]
- 13.Durand, R., E. Gabbett, J. P. Di Piazza, J. F. Delabre, and J. Le Bras. 1999. Analysis of kappa and omega repeats of the cg2 gene and chloroquine susceptibility in isolates of Plasmodium falciparum from sub-Saharan Africa. Mol. Biochem. Parasitol. 101:185-197. [DOI] [PubMed] [Google Scholar]
- 14.Geary, T. G., A. A. Divo, and J. B. Jensen. 1989. Stage specific actions of antimalarial drugs on Plasmodium falciparum in culture. Am. J. Trop. Med. Hyg. 40:240-244. [DOI] [PubMed] [Google Scholar]
- 15.Gumila, C., M. L. Ancelin, G. Jeminet, A. M. Delort, G. Miquel, and H. J. Vial. 1996. Differential in vitro activities of ionophore compounds against Plasmodium falciparum and mammalian cells. Antimicrob. Agents Chemother. 40:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastings, I. M., and U. D'Alessandro. 2000. Modelling a predictable disaster: the rise and spread of drug-resistant malaria. Parasitol. Today 16:340-347. [DOI] [PubMed] [Google Scholar]
- 17.Holz, G. G. 1977. Lipids and the malaria parasite. Bull. W. H. O. 55:237-248. [PMC free article] [PubMed] [Google Scholar]
- 18.Jambou, R., N. A. Ghogomu, D. Kouka-Bemba, and C. Hengy. 1992. Activity of chloroquine and desferrioxamine in vitro against newly isolated Plasmodium falciparum and their antagonism in combination. Trans. R. Soc. Trop. Med. Hyg. 86:11.. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, J. B., and W. Trager. 1977. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle jar method. J. Parasitol. 63:883-886. [PubMed] [Google Scholar]
- 20.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 21.Ogura, M., Y. Morishima, R. Ohno, Y. Kato, N. Hirabayashi, H. Nagura, and H. Saito. 1985. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood 66:1384-1392. [PubMed] [Google Scholar]
- 22.Olliaro, P. L., and Y. Yuthavong. 1999. An overview of chemotherapeutic targets for antimalarial drug discovery. Pharmacol. Ther. 81:91-110. [DOI] [PubMed] [Google Scholar]
- 23.Peters, W. 1984. Antimalarial drugs I, vol. 68. Springer-Verlag, Berlin, Germany.
- 24.Pradines, B., C. Rogier, T. Fusai, A. Tall, J. F. Trape, and J. C. Doury. 1998. In vitro activity of artemether against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial drugs. Am. J. Trop. Med. Hyg. 58:354-357. [DOI] [PubMed] [Google Scholar]
- 25.Ringwald, P., J. Bickii, and L. K. Basco. 1996. In vitro activity of antimalarials against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 55:254-258. [DOI] [PubMed] [Google Scholar]
- 26.Ringwald, P., F. S. Meche, J. Bickii, and L. K. Basco. 1999. In vitro culture and drug sensitivity assay of Plasmodium falciparum with nonserum substitute and acute-phase sera. J. Clin. Microbiol. 37:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman, L. 1979. Biochemistry of Plasmodium (malarial parasites). Microbiol. Rev. 43:453-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 29.Van Deenen, L. L. M., and J. De Gier. 1975. Lipids of the red cell membrane, p. 147-211. In G. Surgenor (ed.), The red blood cell. Academic Press, New York, N.Y.
- 30.Vial, H., and M. Ancelin. 1998. Malarial lipids, p. 159-175. In I. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 31.Vial, H. J., and M. L. Ancelin. 1992. Malarial lipids. An overview. Subcell. Biochem. 18:259-306. [PubMed] [Google Scholar]
- 32.Vial, H. J., and M. Calas. 2001. Inhibitors of phospholipid metabolism, p. 347-365. In P. Rosenthal (ed.), Antimalarial chemotherapy, mechanisms of action, modes of resistance, and new directions in drug development. Humana Press, Totowa, N.J.
- 33.Vial, H. J., P. Eldin, D. Martin, L. Gannoun, M. Calas, and M. L. Ancelin. 1999. Transport of phospholipid synthesis precursors and lipid trafficking into malaria-infected erythrocytes. Novartis Found. Symp. 226:74-88. [DOI] [PubMed] [Google Scholar]
- 34.Vial, H. J., M. J. Thuet, and J. R. Philippot. 1982. Phospholipid biosynthesis in synchronous Plasmodium falciparum cultures. J. Protozool. 29:258-263. [DOI] [PubMed] [Google Scholar]
- 35.von Seidlein, L., P. Milligan, M. Pinder, K. Bojang, C. Anyalebechi, R. Gosling, R. Coleman, J. I. Ude, A. Sadiq, M. Duraisingh, D. Warhurst, A. Alloueche, G. Targett, K. McAdam, B. Greenwood, G. Walraven, P. Olliaro, and T. Doherty. 2000. Efficacy of artesunate plus pyrimethamine-sulphadoxine for uncomplicated malaria in Gambian children: a double-blind, randomised, controlled trial. Lancet 355:352-357. [DOI] [PubMed] [Google Scholar]
- 36.Wengelnik, K., V. Vidal, M. L. Ancelin, A. M. Cathiard, J. L. Morgat, C. H. Kocken, M. Calas, S. Herrera, A. W. Thomas, and H. J. Vial. 2002. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 295:1311-1314. [DOI] [PubMed] [Google Scholar]
- 37.Winstanley, P. A. 2000. Chemotherapy for falciparum malaria: the armoury, the problems and the prospects. Parasitol. Today 16:146-153. [DOI] [PubMed] [Google Scholar]
- 38.Yeo, H. J., M. P. Larvor, M. L. Ancelin, and H. J. Vial. 1997. Plasmodium falciparum CTP: phosphocholine cytidylyltransferase expressed in Escherichia coli: purification, characterization and lipid regulation. Biochem. J. 324:903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]