Abstract
We previously showed that quaternary ammonium salts have potent antimalarial activities against the blood stage of drug-resistant Plasmodium falciparum. In the present study, 13 compounds of this series were comparatively assessed in murine in vivo malarial models. Mice infected with Plasmodium berghei were successfully treated with 11 quaternary ammonium salts in a 4-day suppressive test with a once-daily intraperitoneal administration. The dose required to decrease parasitemia by 50% (ED50) ranged from 0.04 to 4.5 mg/kg of body weight. For six mono- and three bis-quaternary ammonium salts, the therapeutic indices (i.e., 50% lethal dose and ED50) were higher than 5, and at best, around 20 to 30 for five of them (E6, E8, F4, G5, and G25), which is comparable to that of chloroquine under the same conditions. Plasmodium chabaudi was significantly more susceptible to G5, G15, and G25 compounds than P. berghei. Similar therapeutic indices were obtained, regardless of the administration mode or initial parasitemia (up to 11.2%). Parasitemia clearance was complete without recrudescence. Subcutaneously administered radioactive compounds had a short elimination half-life in mice (3.5 h) with low bioavailability (17.3%), which was likely due to the permanent cationic charge of the molecule. The high in vivo therapeutic index in the P. chabaudi-infected mouse model and the absence of recrudescence highlight the enormous potential of these quaternary ammonium salts for clinical malarial treatment.
In the absence of vaccines, and due to the widespread resistance to antimalarials in current use, new chemotherapies are urgently needed to help in the prevention and control of malaria. The most promising strategy is to strive to discover new chemically diverse entities directed towards novel biological targets. Potential chemotherapeutic targets in the malaria parasite can be broadly classified into three categories: those involved in processes occurring in the digestive vacuole, enzymes involved in macromolecular and metabolite synthesis, and those responsible for membrane processes and signaling. The potential of putative targets to be validated will be tapped to develop effective and safe drugs (14, 17, 29).
We previously characterized phospholipid (PL) metabolism as an attractive target for new malaria chemotherapy due to its vital importance to the parasite. PL metabolism is absent from normal mature human erythrocytes (19), but the erythrocyte PL content increases by as much as 500% after infection, specifically due to the metabolic machinery of the parasite (10, 18). Phosphatidylcholine is the major PL of infected erythrocytes, representing about 45% of the total PL. In this pathway, choline transport which regulates the supply of polar head precursors to the parasite is a regulatory rate-limiting step (3, 22, 23).
PL polar head analogs are able to interfere with PL biosynthesis and exhibit antimalarial activity against the intraerythrocytic stage of Plasmodium falciparum in vitro (24). More particularly, 36 compounds which possess one (or two) quaternary ammoniums such as choline were found to be very efficient against P. falciparum, with a 50% inhibitory concentration (IC50) ranging from the micromolar to picomolar range (6, 7), and also against multidrug-resistant P. falciparum malaria. Biochemical characterizations indicate a probable link between PL biosynthesis inhibition and antimalarial activity (1, 2, 4, 25).
The bis-quaternary ammonium salts showed the highest in vitro differential of activity compared to mammalian cell lines, and their selective accumulation in P. falciparum-infected red blood cells likely explain their specificity and potency (4, 26). Here, we conducted a detailed investigation of the in vivo antimalarial activities of the most active of these mono- and bis-quaternary ammonium compounds in various murine malaria-infected models while also assessing their tolerances and pharmacokinetic properties. We especially focused on characterizing G25 activity with respect to its capacity to cure very high parasitemia without recrudescence.
MATERIALS AND METHODS
Chemicals.
The compounds were stock samples from the Laboratoire des Aminoacides, Peptides et Protéines, UMR CNRS 5810. Their synthesis has been described previously (6). Chloroquine and other chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.). N-(2-Hydroxyethyl)-N,N-di[3H]Me-1-dodecanaminium iodide (F4) was synthesized and radiolabeled by Amersham Corp. (Les Ulis, France).
Biological materials.
Male Swiss albino mice, weighing 30 to 40 g, were obtained from C.E.R. Janvier (Le Genest, France). Plasmodium chabaudi chabaudi (864VD) and Plasmodium vinckei petteri (279BY) (8, 11) strains were provided by I. Landau (Paris, France). The Plasmodium berghei N strain (16) was donated by D. Camus (Lille, France). P. berghei NS strains (15) and the Plasmodium yoelii NS strain were from W. Peters (London, United Kingdom). Parasitemia was routinely monitored on blood smears with a 10% Giemsa azure type B stain in phosphate buffer (pH 7.2).
Assessment of acute and subacute toxicity in mouse.
Acute toxicity was determined after one single-drug injection into four noninfected random-bred Swiss mice and expressed as the 50% lethal dose (LD50), which corresponds to the dose leading to deaths of 50% of the subjects 10 days after the drug injection. Subacute toxicity was evaluated after a twice-daily drug injection for 4 consecutive days (eight doses) to four mice. Drugs were routinely dissolved in water. Drugs were administered intraperitoneally (i.p., 250 μl), subcutaneously (s.c., 250 μl), or orally (500 μl).
In vivo antimalarial activities against murine Plasmodium spp
In vivo antimalarial activity was determined against three different rodent strains P. berghei, P. chabaudi, or P. vinckei petteri according to a modified version of the 4-day suppressive test of Peters et al. (16). Mice were inoculated either i.p. or intravenously (i.v.) with 107 parasitized red blood cells (resuspended in 0.2 ml of saline medium). Thereafter, the drugs were administered to the animals once or twice daily in 0.9% sodium chloride for 4 consecutive days. Drug treatments were initiated either 1 or 24 h after parasite inoculation, when the curative properties were studied. When applicable, the second daily drug injection was performed 11 to 12 h later and then administration was continued for 3 days with the same frequency. Parasitemia levels were determined on the day following the last treatment. The 50% efficient dose (ED50), which is the dose leading to 50% parasite growth inhibition compared to the control (treated with an equal volume of vehicle), was evaluated from a plot of activity (expressed as a percentage of the control) versus the log dose. For each dose, at least three animals were treated while six control animals were considered. The results are means of at least two independent experiments. Treatment was considered curative when no parasites were detected after 60 days. In some cases, mice were again challenged at that time by i.v. inoculation of 107 infected erythrocytes to check whether they were fully susceptible to a new malarial infection.
Pharmacokinetics.
F4 was chosen for pharmacokinetic studies because it was judged to be representative of the monoammonium series due to its low IC50 and tolerance in the mouse. Uninfected Swiss mice were injected with 100 μl of [3H]F4 dissolved in saline either i.v. (17 μCi, 0.26 μCi/nmol) or s.c. (142 μCi, 27 μCi/mmol). The single injection contained the radioactive compound at a dose of 0.75 mg/kg of body weight (i.v.) or 60 mg/kg (s.c.). At times ranging from 5 to 480 min, 15 μl of blood was collected in heparinized polypropylene tubes from the tail vein. Tubes containing blood samples were immediately gently agitated to prevent coagulation and then centrifuged at 2,000 × g for 20 min. Plasma was removed and then stored at −20°C until assay. The radioactive content of 6 μl of each plasma sample was determined by liquid scintillation spectrometry. Radioactive material from the plasma fraction was shown to comigrate with standard F4 in a thin-layer chromatography silica gel developed in CH3OH (0.5%), NaCl (25%), and NH4OH (100/100/10, vol/vol/vol).
Pharmacokinetic parameters were calculated with the pK-fit software package (9). The area under the plasma concentration-time curve from time zero to infinity (AUC) was obtained by linear trapezoidal approximation with correction to time infinity by dividing the last observed data point by the elimination rate constant (λ2). The elimination half-life (t1/2) was determined from the slope of the log linear part curves. The volume of distribution in equilibrated tissues was evaluated as F × dose/(AUC × λ2), where F is the fraction of dose absorbed. The ratio of AUCs after s.c. and i.v. administration normalized to the same administered dose was used to calculate the fraction of drug that was absorbed after s.c. administration.
RESULTS
The in vitro effects of more than 100 different mono- and bis-quaternary ammonium salts against the intraerythrocytic stage of P. falciparum were previously reported (1, 6, 7). They included monoquaternary (series E and F) or bis-quaternary (series G and H) ammonium salts, which were N substituted (F and H) or not (E and G) by a hydroxyethyl group. The IC50 of 22 of the most active mono- and bis-quaternary ammonium salts are listed in Tables 1 and 2, respectively. Their activities are linked with the presence of a long alkyl chain (8 to 21 carbon atoms) on the nitrogen atom (for monoammoniums) or between the two nitrogen atoms (for bis-ammoniums). All compounds possessed high antimalarial activities, with IC50 ranging from 0.8 μM to 33 nM for monoquaternary ammonium salts and from 90 nM to 3 pM for bis-quaternary ammonium salts, including seven compounds that had an IC50 of less than 10 nM. The present study involved a thorough evaluation of their in vivo antimalarial activities to assess both their tolerances and activities in malaria-infected murine models. Optimal in vivo activity was also studied as a function of structural characteristics.
TABLE 1.
In vivo antimalarial activities and acute toxicity of monoquaternary ammonium salts in micea R1 | R (CH2)n—N+—R2, X− | R3
Antimalarial activities were determined after i.p. administration of the compounds once daily for 4 days to infected mice. The first drug administration began 1 h after i.p. inoculation with P. berghei strain NS. TI were calculated on the basis of the acute LD50/ED50 ratios. IC50s were already reported in references 1 and 6. ND, not determined; Ts, tosyl.
Values are for in vivo P. berghei per mouse.
TABLE 2.
In vivo antimalarial activities and acute toxicities of bisquaternary ammonium salts in mice
Acute and subacute toxicity in mice.
The acute toxicity of the compounds (expressed as LD50) was first evaluated in mice after i.p. administration. Irrespective of the compound, very steep curves were obtained for toxicity as a function of dose (data not shown). For monoquaternary ammonium salts, the LD50 ranged between 4 and 50 mg/kg (Table 1), whereas bis-quaternary ammonium salts had lower LD50, i.e., between 0.15 and 7.5 mg/kg (Table 2). Both for mono- and bis-quaternary ammonium salts, increasing the bulk around the nitrogen (compare E6, E24, E10, E13 or G4, G94, G14 or G5, G25, and G15) increased the toxicity of the compounds. Toxicity was also slightly increased by increasing the length of the N-alkyl chain from 12 to 16 (or 21) methylene groups (compare E6 to E8, G4 and G5 or G14, G15, and G19). With equal N substituents and alkyl chain length, monoammonium salts were better tolerated (by 6- to 13-fold) than bis-quaternary ammonium salts (compare E6 and G4, E8 and G5, and E10 and G14).
One monoammonium quaternary ammonium salt (E10) and one bis-ammonium quaternary ammonium salt (G25) were also tested for toxicity after oral administration. Their LD50 were 230 and 110 mg/kg, compared to 13 and 1.4 mg/kg, respectively, after i.p. administration (data not shown). The difference between the i.p. and oral toxicities (18- and 80-fold, respectively) indicated that these compounds have low oral absorptions.
The LD50 of G25 was very similar after i.v., i.p., intramuscular, or s.c. administration (around 1.4 mg/kg), irrespective of the animal, i.e., mouse or rat (data not shown). For dogs or monkeys, only maximum tolerable doses were determined but they were in the same dosage range (around 1 mg/kg) (26). When administered twice daily for four consecutive days, the LD50 of G25 was decreased by less than 1.5-fold compared to acute conditions (one single dose), irrespective of the administration mode (i.p., s.c., or oral) (data not shown). The subacute toxicity was also measured for 12 compounds (E6, E8, E10, E13, E23, E50, F4, G5, G14, G15, G19, and H5) after one daily i.p. administration for 4 consecutive days. LD50 were similar or slightly lower (by less than 1.5-fold) than those obtained under acute toxicity conditions (data not shown).
In vivo antimalarial activities of mono- and bis-quaternary ammonium salts against P. berghei and P. chabaudi.
Seven mono- and six bis-ammonium salts were tested in mice infected with P. berghei or in some cases, P. chabaudi, according to a modified version of the 4-day suppressive test of Peters et al. (16). Compounds were i.p. administered once daily for 4 days, with the first drug administered 1 h after i.p. parasite inoculation. Parasitemia in control mice generally reached as high as 20 to 40% at day 5, with death occurring within the following 4 days.
All of the herein-reported compounds had significant in vivo antimalarial activities. These activities generally occurred over less than 2 log drug concentrations, indicating specific targeting, and parasitemia clearance was complete at the highest dose (data not shown). ED50 of monoammonium compounds ranged from 0.4 to 4.5 mg/kg, whereas the ED50 of bis-ammonium compounds were significantly lower, ranging from 0.04 to 0.3 mg/kg (against P. berghei). Of the seven monoammonium salts tested (against P. berghei), six had a therapeutic index (TI, i.e., ratio of LD50 to ED50) higher than 5 (Table 1). E6 and E8 exhibited tolerance patterns and in vivo antimalarial activities very close to chloroquine tested under the same conditions. Among the monoquaternary ammonium compounds substituted with a dodecyl chain, the in vivo selectivity increased slightly when the polar head volume decreased, with the most active being the trimethyl ammonium salt (E6, TI = 17), whereas the TI of the tripropyl-substituted compound was 10 (E13). For the trimethyl ammonium compound, increasing the chain length from 12 to 16 also improved the activity (TI of E8 = 34). By contrast, E50, which possesses a hexadecyl chain, was found to be weakly active, with the low TI (3.5) resulting from lower tolerance (LD50), possibly due to the pyridinium ring.
Three bis-quaternary ammonium compounds out of six had a TI of higher than 5 (G5, G15, G25) (Table 2). As for monoammonium salts, for the same long alkyl chain (hexadecyl), compounds with the highest TI against P. berghei were those with the smallest polar head (trimethyl, G5, TI = 27 > methyl pyrrolidinium, G25, TI = 7 ≥ triethyl, G15, TI = 5 > diethyl, hydroxyethyl, H5, TI = 2.5). Bis-ammonium compounds were always about two to three times more active against P. chabaudi than against P. berghei, but the extent of activity as a function of the polar head volume was similar (TI against P. chabaudi were 50, 18, and 9 for G5, G25, and G15, respectively). Irrespective of the polar head, substitution of N-hexadecyl appeared to be more efficient than substitution of an N-dodecyl group (compare G15 with G14), whereas G19, which possesses 21 methylene groups in the alkyl chain, was inactive. In summary, five compounds possessed a high TI, ranging from 15 to 50 (in P. berghei or P. chabaudi), three monoammonium salts, E6, E8, and F4, and two bis-ammonium salts, G5 and G25.
In vivo antimalarial activity as a function of parasite inoculation and murine strains.
The conditions of the 4-day suppressive Peters test used in the experiments described above were more representative for the prophylactic properties of a drug than for its curative properties, since these drugs were administered about 1 h after infected cell inoculation. We then investigated the antimalarial activity of the compounds when administered 1 day after the inoculation of parasitized cells. Two infection routes were used for comparison, the i.p. or i.v. mode, the latter being less likely to generate an immune response before the infection is fatal (20). Irrespective of the strain, P. berghei, P. chabaudi, or P. vinckei, G25 antimalarial activities obtained when parasites were i.p. inoculated were 3.5-fold higher than when they were i.v. inoculated. The ED50 were in the same range (differing by less than 1.5-fold) when the drug was administered 1 h or 1 day after parasite inoculation, irrespective of the inoculation route (data not shown).
Regarding murine strain susceptibility, P. chabaudi and P. vinckei petteri showed very similar susceptibilities to the bis-quaternary G25 (ED50 = 0.03 mg/kg after a twice-daily i.p. administration) (data not shown). P. berghei appeared to be significantly less susceptible to G25 (by three- to eightfold, depending on the P. berghei strain, NS or N, respectively) compared to P. chabaudi (Fig. 1). With P. yoelii, the TI was lower than 2, suggesting a very low susceptibility of this species to G25 (data not shown). The lower susceptibility of P. berghei relative to P. chabaudi was also observed for G5 and G15 (Table 2).
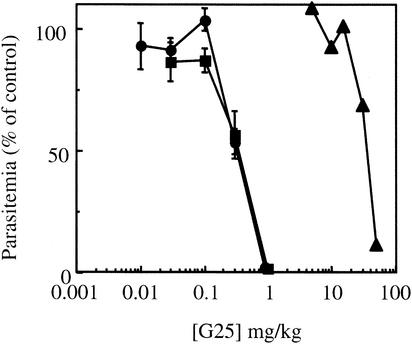
FIG. 1.
In vivo G25 antimalarial activity against three different rodent strains. Mice were i.p. inoculated with P. chabaudi (▪) or P. berghei strain N (•) or NS (○), as described in Materials and Methods. Animals were treated twice daily for 4 consecutive days; the first drug injection started 1 h after parasite inoculation. G25 was administered i.p. Parasitemia was determined on the day following the last treatment, and the ED50 was estimated from a plot of log dose against activity (expressed as a percentage of the control). The results are expressed as means ± standard errors of the means (n ≥ 3).
In vivo antimalarial activity as a function of the drug administration route.
G25 antimalarial activity was evaluated after i.p., s.c., or oral drug administration while using the i.v. route for parasite inoculation. G25 was administered twice daily for 4 days to P. chabaudi-infected mice. Irrespective of the administration route, very steep curves were obtained, with activity occurring over 1 to 2 orders of magnitude (Fig. 2). Similar activities were obtained after i.p. or s.c. administration. By contrast, the dose to obtain antimalarial activity after oral treatment was 100-fold higher than after s.c. or i.p. administration. The same TI was obtained irrespective of the administration route. This difference in ED50 between s.c. (or i.p.) and oral administration likely resulted from low intestinal absorption of this type of compounds, as also observed in the ratio of the LD50 in both modes.
FIG. 2.
In vivo G25 antimalarial activity against P. berghei as a function of the route of drug administration to mice. Mice were infected i.v. with 107 P. berghei (strain NS)-infected erythrocytes, and G25 was administered twice daily for 4 days either i.p. (•), s.c. (▪), or orally (▴). The first drug administration began 24 h after parasite inoculation.
The higher degree of synchronism of P. chabaudi than of P. berghei (10) allowed determination of parasite stage susceptibility to G25. The compound was administered once daily at 7, 15, or 23 h, i.e., when the parasites were predominantly in the trophozoite, schizont, and ring forms, respectively. G25 appeared to be slightly more active (by twofold) when the parasite was in the trophozoite form. When administered twice daily, the ED50 was 2.5-fold lower than after one daily administration. No significant improvement was obtained when the drug was given three times a day compared to twice a day (data not shown).
Curative G25 activity against P. chabaudi-infected mice at high parasitemia.
We assessed whether G25 could be curative at high parasitemia and determined the most efficient treatment doses and durations to achieve a definitive cure. G25 (from 0.01 to 0.9 mg/kg) was s.c. administered twice a day to P. chabaudi-infected mice on the day of infection at t0 + 2 h (P = 0.05%), at t0 + 2 days (P = 3.5%), or at t0 + 3 days (P = 11.2%). G25 was administered for 4 or 8 days, and parasitemia was monitored daily for 11 days and then at regular intervals for up to 60 days to ascertain a definitive curative effect without recrudescence.
After a 4-day treatment period, G25 exerted its antimalarial activity over a narrow concentration range of between 0.1 and 1 mg/kg (1 order of magnitude). Regardless of the initial parasitemia (0.05, 3.5, and 11.2%), the ED50 were close (0.35, 0.42, and 0.5 mg/kg, respectively), and there was no significant decrease in the TI when the initial parasitemia increased. Similar antimalarial activity was obtained after only 3 days of treatment with G25 (data not shown).
The daily parasitemia pattern in mice treated with 0.9 mg of G25/kg is shown in Fig. 3. At the lowest initial parasitemia (0.05%), after 4 days of treatment, the parasitemia was low (0.3% ± 0.06% compared to 31.7% ± 0.9% in the control) (Fig. 3A). At higher initial parasitemia, 3.5 or 11.2%, 2 days were necessary to obtain a decrease in parasitemia compared to controls, and after this time a marked reduction in parasitemia was observed (Fig. 3B and C).
FIG. 3.
Curative G25 (0.9 mg/kg) antimalarial activity against P. chabaudi-infected mice as a function of parasitemia and the treatment window. Mice were inoculated i.v. with 107 P. chabaudi-infected erythrocytes at time zero. Animals were then s.c. treated with G25 either for 8 (filled symbols) or 4 (open symbols) consecutive days in comparison with untreated controls (x). The treatment began either on the day of infection (parasitemia = 0.05%) (A), on day 2 (parasitemia = 3.5%) (B), or on day 4 (parasitemia = 11.2%) (C). The parasitemia level was determined daily between time zero and time zero plus 11 days. The points at 100% parasitemia corresponded to the death of control mice. Black arrows correspond to the beginning (upward direction) and end (downward direction) of the 8-day treatment period; white arrows correspond to the end of the 4-day treatment period.
When the treatment lasted for only 4 days, recrudescence was observed even at low parasitemia (see the progressive rise in parasitemia Fig. 3A and B). By contrast, a definite curative effect without recrudescence after 60 days was obtained when the treatment was applied for 8 days, irrespective of the initial parasitemia. An especially dramatic decrease in parasitemia was observed at the very high parasitemia of 11.2%, and there was also no recrudescence after 60 days. G25 thus fully succeeded in inhibiting parasitemia as high as 11.2% without any decrease in its TI.
Pharmacokinetics.
Radioactive F4 was administered either i.v. (0.75 mg/kg) or s.c. (60 mg/kg) to four mice. Semilogarithmic plots of plasma concentration-time curves are shown in Fig. 4. After i.v. administration, the drug concentration versus time curve had a biexponential decay pattern. The distribution was rapid (t1/2 = 13 min), and the t1/2 of the terminal part of the curve was 3.8 h. The area under the plasma concentration-time curve from 0 h to infinity was 3.1 μg·h/ml. The low distribution volume at steady state (1.3 liters/kg) suggested a weak distribution of this compound in the body. After s.c. administration, the maximal drug concentration (8.4 μg/ml) occurred about 20 min after drug intake. The elimination t1/2 (3.3 h) was of the same order of magnitude as that computed after i.v. administration. The bioavailability was 17.3%.
FIG. 4.
Plasma concentration-time profile of tritiated F4 following i.v. (⧫) and s.c. (◊) administration to uninfected mice. Uninfected Swiss mice (male, 34 g) were injected either i.v. (⧫) with 100 μl of [3H]F4 (0.26 μCi/nmol) dissolved in saline or s.c. (◊) (27 μCi/mmol). The single injection contained the radioactive compound at a dose of 0.75 mg/kg (i.v.) or 60 mg/kg (s.c.). The figure shows the results for one typical experiment.
DISCUSSION
Plasmodial PL biosynthesis inside infected red blood cells may provide potential targets for novel antimalarial weapons. The most effective interference involves blockage of the choline carrier, a limiting step which supplies the parasite with the precursor for synthesis of phosphatidylcholine, the major PL of Plasmodium (22). Compounds which were designed for their capacities to mimic choline structure and optimized through in vitro antimalarial activity have a substantial potential against multidrug-resistant malaria. These new and chemically diverse molecules, directed towards this novel biological target, were found to be highly active and selective in vitro against P. falciparum, with IC50 ranging from 10−6 to 10−12 M. They are believed to act as anti-PL effectors, with an additional unique property of being specifically accumulated by Plasmodium-infected red blood cells (4, 24, 26).
The compounds consist of mono- and bis-quaternary ammonium salts whose cationic charges and the presence of a long lipophilic chain on the nitrogen atom are crucial for in vitro antimalarial activity against P. falciparum (6, 7). Bis-quaternary ammonium salts exhibited higher activities than monoammonium salts, especially those with an intercationic chain of more than 12 methylene groups (IC50 of 10−9 to 10−12 M). More specifically, G5 and G25 were very potent (in the nanomolar range) against strains and fresh human isolates of P. falciparum, including those resistant or multidrug-resistant to current antimalarials (4). G25 also had very potent in vivo activity against P. falciparum in curing infected Aotus monkeys without recrudescence and with a TI higher than 30 (26).
Nevertheless, to demonstrate the full potential of the compounds, their antimalarial properties need to be compared in vivo. We thus used murine malaria-infected models, which are more convenient for large-scale screening of compounds but not necessarily representative of P. falciparum human malaria (12, 21) (see below). Comparative studies were carried out to select the best compounds for further studies, e.g., against human malaria in monkeys. Tolerance after administration to mice was first investigated. After i.p. administration, the acute LD50 generally ranged from 0.15 to 50 mg/kg. For both mono- and bis-quaternary ammonium salts, toxicity was boosted by increasing the bulk around the nitrogen or increasing the length of the N-alkyl chain between 12 and 16 (or 21) methylene groups. At equal N substituents and alkyl chain lengths, monoammonium salts were better tolerated (by 6- to 13-fold) than bis-quaternary ammonium salts.
These quaternary ammonium salts were then tested in vivo for their antimalarial activities against three distinct murine species, i.e., P. berghei-, P. chabaudi-, or P. vinckei-infected mice. The two latter murine strains differ from the former by their marked preferences for invading mature erythrocytes and their high degrees of synchronization (5, 11). Most of the tested compounds were able to clear parasitemia, which is evidence of their antimalarial potencies. Against P. berghei, the ED50 after i.p. administration ranged from 0.4 to 5 mg/kg for monoammonium compounds. Bis-ammonium compounds were much more potent, with ED50 ranging from 0.04 to 0.3 mg/kg (Tables 1 and 2). For both mono- and bis-quaternary ammonium salts, the in vivo selectivity (TI) increased slightly as the volume of the polar head decreased, with the trimethyl ammonium salts (E6, E8, or G5, TI = 17 or 27, respectively) being the most active. Increasing the chain length from 12 to 16 also slightly improved the activity. For seven compounds (of 13 tested in vivo), the TI achieved after i.p. administration was higher than 10. Three compounds possessed a high TI, ranging from 18 to 50 (against P. berghei or P. chabaudi), one monoammonium (E8) and two bis-ammonium (G5 and G25) salts, all of which were N substituted with a hexadecyl chain.
Similar TI were obtained when the in vivo antimalarial activity of G25 was investigated after i.p., s.c., and oral administrations. There was a 90-fold difference in LD50 (or ED50) between the i.p. and oral modes, which indicates that this compound has very low oral absorption. This was likely due to the presence of two cationic permanent charges in the molecule. A difference in LD50 was also observed, but to a slighter degree (18-fold), for E10, which possesses only one quaternary ammonium.
Finally, it was noteworthy that G25 successfully cured high parasitemia in P. chabaudi-infected mice (up to 11.2% parasitemia) without modification of the TI, which indicates that there is no need to increase the dose, even for very severe infections. This was also observed in the P. falciparum-infected monkey model. Moreover, the potency of the compounds might be due to the high and selective accumulation of these quaternary ammonium compounds inside Plasmodium-infected erythrocytes (26).
Results obtained in vivo with the various Plasmodium murine species indicated variations in the ED50 according to the Plasmodium species invading the rodents. The ED50 of compounds G5, G15, and G25 were two- to threefold lower against P. chabaudi than against P. berghei (Table 2). G25 was similarly active against P. vinckei petteri and P. chabaudi but inactive against P. yoelii (data not shown). These rodent species notably differed in their abilities to invade mature or immature erythrocytes and in their degrees of synchronism. Interestingly, the intrinsically lower susceptibility of P. berghei to G25 was also demonstrated in vitro, with IC50 differing by roughly 2 orders of magnitude relative to P. falciparum (C. J. Janse and H. J. Vial, unpublished data). Nevertheless, it was not clear whether or not the antimalarial activity could be much higher on Plasmodium species invading mature erythrocytes than on species invading immature erythrocytes. Indeed, the in vitro IC50 of G25 against Plasmodium vivax or Plasmodium cynomolgi occur in the same nanomolar range as against P. falciparum, indicating high susceptibility of reticulocyte-restricted parasites to this class of drugs. Moreover, recent studies have shown very potent in vivo activity of G25 against human P. falciparum malaria in Aotus monkeys (26). Cure was obtained at a very low dose (as low as 0.03 mg/kg), indicating a very high TI (higher than 30). Presently, there is no clear explanation for this difference, but it could involve an intrinsically lower susceptibility of P. berghei or P. yoelii species to anti-PL effectors.
Finally, the intrinsically lower susceptibility of P. berghei or P. yoelii strains to G25 also evidences that these murine models could not be appropriate biological models to predict the antimalarial activity of quaternary ammonium compounds against P. falciparum in vivo (as already reported for other unrelated compounds) (12, 21).
The pharmacokinetic characteristics of these compounds should also be discussed. The results obtained after i.v. administration were consistent with a two-compartment model. After s.c. administration into mice, F4 was readily diffused in the systemic circulation, with a peak concentration reached in less than 30 min, and the bioavailability was 17.3%. The elimination t1/2 was in the same order of magnitude after i.v. and s.c. administrations (around 3.5 h). A relatively short elimination t1/2 is also suggested by the absence of increased toxicity under semichronic administration conditions or by the moderate decrease in ED50 obtained after twice-daily compared to once-daily i.p. administration. Such elimination t1/2 values are in the same range as those reported for quinine, primaquine, or artemisinin (13, 27).
Conventionally, to maintain a suitable seric level, a drug should be given at intervals roughly corresponding to its biological t1/2 (28). However, if there is a concentration-dependent toxic effect, biological effects could persist well after the drug has declined below the therapeutic level, and the drug can then be given less frequently than predicted from pharmacokinetic studies. For G25, once-daily administration was sufficient to cure high parasitemia, with no great improvement in ED50 when the drug was given two or three times a day; this was likely related to the specific capacity of the parasite to highly and selectively concentrate this class of compounds (26).
In summary, with these quaternary ammonium compounds, a suitable very high level of activity has now been reached in vitro against P. falciparum (seven molecules possess an IC50 lower than 10 nM), but the capacity of the compounds to exert potent in vivo antimalarial activity is even more important. Indeed, mice infected with P. berghei were successfully treated with 11 of these compounds with ED50 ranging from 0.04 to 4.5 mg/kg (after a once-daily i.p. administration for 4 days). Lower ED50 were even obtained against P. chabaudi. For six mono- and three bis-quaternary ammonium salts, the TI were higher than 5 and, at best, around 20 to 30 for five of them. Hence, in the murine model, the tolerance levels and satisfactory TI obtained for several compounds of these structurally related chemical series confirm the potential of this class of compounds as antimalarials. The complete clearance of parasitemia, the absence of recrudescence, the capacity of curing highly infected animals with a moderate dose, and the absence of mutagenic activity highlight that these PL biosynthesis inhibitors have a very promising clinical malaria treatment potential.
Finally, it should be added that although similar therapeutic indices were obtained regardless of the administration mode (including per os), these compounds have not been optimized for high oral absorption. In fact, oral uptake was expected to be weak due to the presence of positive charge(s) of the molecule. Nevertheless, this study indicates that there were no inappropriate pharmacokinetic properties for this new class of antimalarials. One of our major current efforts for the development of this class of anti-PL effectors into antimalarial drugs is to improve oral bioavailability by designing uncharged prodrugs of these quaternary ammonium salts. Our recent experiments with these molecules have shown much higher oral bioavailabilities, and we are currently evaluating their in vivo antimalarial activities.
In summary, with these quaternary ammonium compounds, a suitable very high level of activity is now reached in vitro against P. falciparum (seven molecules possess an IC50 lower than 10 nM), but the capacity of the compounds to exert potent in vivo antimalarial activity is even more important. In the murine model, the tolerance levels and satisfactory TI obtained for several compounds of these structurally related chemical series make realistic this class of compounds as antimalarials. The complete clearance of parasitemia, the absence of recrudescence, and the capacity of curing highly infected animals at a moderate dose highlight that these PL biosynthesis inhibitors have a very promising clinical malaria treatment potential.
Acknowledgments
We thank W. Peters for assistance in testing the compounds in his laboratory and for expertise and skillful work. We thank F. Bressolle for help in pharmacokinetics experiments and X. Canron for mouse and murine strain care and skillful assistance in the studies.
This work was supported by the European Union (INCO-DEV, IC18-CT960056, QLK2-CT-2000-01166), CNRS/DGA (GDR 1077), MENRT (PRFMMIP and VIHPAL), the UNDP/World Bank/WHO special program for Research and Training in Tropical Diseases, and VIRBAC Laboratories.
REFERENCES
- 1.Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. J. Vial. 1998. Antimalarial activity of 77 phospholipid polar head analogs: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. [PubMed] [Google Scholar]
- 2.Ancelin, M. L., and H. J. Vial. 1986. Quaternary ammonium compounds efficiently inhibit Plasmodium falciparum growth in vitro by impairment of choline transport. Antimicrob. Agents Chemother. 29:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancelin, M. L., and H. J. Vial. 1989. Regulation of phosphatidylcholine biosynthesis in Plasmodium-infected erythrocytes. Biochim. Biophys. Acta 1001:82-89. [DOI] [PubMed] [Google Scholar]
- 4.Ancelin, M. L., M. Calas, V. Vidal-Sailhan, S. Herbuté, P. Ringwald, and H. J. Vial. 2003. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob. Agents Chemother. 47:2590-2597. [DOI] [PMC free article] [PubMed]
- 5.Beaute-Lafitte, A., V. Altemayer-Caillard, F. Gonnet-Gonzalez, L. Ramiaramanana, A. G. Chabaud, and I. Landau. 1994. The chemosensitivity of the rodent malarias-relationships with the biology of merozoites. Int. J. Parasitol. 24:981-986. [DOI] [PubMed] [Google Scholar]
- 6.Calas, M., M. L. Ancelin, G. Cordina, P. Portefaix, G. Piquet, V. Vidal-Sailhan, and H. Vial. 2000. Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J. Med. Chem. 43:505-516. [DOI] [PubMed] [Google Scholar]
- 7.Calas, M., G. Cordina, J. Bompart, M. Ben Bari, T. Jei, M. L. Ancelin, and H. Vial. 1997. Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism. Structure-activity relationship analysis. J. Med. Chem. 40:3557-3566. [DOI] [PubMed] [Google Scholar]
- 8.Carter, R., and D. Walliker. 1975. New observations on the malaria parasites of rodents of the Central African Republic-Plasmodium vinckei petteri subsp. nov. and Plasmodium chabaudi Landau, 1965. Ann. Trop. Med. Parasitol. 69:187-196. [DOI] [PubMed] [Google Scholar]
- 9.Farenc, C., J. R. Fabreguette, and F. Bressolle. 2000. Pk-fit: a pharmacokinetic/pharmacodynamic and statistical data analysis software. Comput. Biomed. Res. 33:315-329. [DOI] [PubMed] [Google Scholar]
- 10.Holz, G. G. 1977. Lipids and the malaria parasite. Bull. W. H. O. 55:237-248. [PMC free article] [PubMed] [Google Scholar]
- 11.Landau, I., and P. Gautret. 1998. Animal models: rodents, p. 401-417. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 12.McCall, D. L. C., J. Alexander, J. Barber, R. G. Jaouhari, A. Satoskar, and R. D. Waigh. 1994. the first protoberberine alkaloid analogue with in vivo antimalarial activity. Bioorg. Med. Chem. Lett. 4:1663-1666. [Google Scholar]
- 13.Navaratnam, V., S. M. Mansor, N. W. Sit, J. Grace, Q. Li, and P. Olliar. 1993. Pharmacokinetics of artemisinin-type compounds. Clin. Pharmacokinet. 39:255-270. [DOI] [PubMed] [Google Scholar]
- 14.Olliaro, P. L., and Y. Yuthavong. 1999. An overview of chemotherapeutic targets for antimalarial drug discovery. Pharmacol. Ther. 81:91-110. [DOI] [PubMed] [Google Scholar]
- 15.Peters, W., M. L. Chance, R. Lissner, H. Momen, and D. C. Warhurst. 1978. The chemotherapy of rodent malaria. XXX. The enigmas of the ‘NS lines’ of P. berghei. Ann. Trop. Med. Parasitol. 72:23-36. [DOI] [PubMed] [Google Scholar]
- 16.Peters, W., J. H. Portus, and B. L. Robinson. 1975. The chemotherapy of rodent malaria. XXII. The value of drug-resistant strains of P. berghei in screening for blood schizonticidal activity. Ann. Trop. Med. Parasitol. 69:155-171. [PubMed] [Google Scholar]
- 17.Ridley, R. G. 2002. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415:686-693. [DOI] [PubMed] [Google Scholar]
- 18.Sherman, L. 1979. Biochemistry of Plasmodium (malarial parasites). Microbiol. Rev. 43:453-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Deenen, L. L. M., and J. De Gier. 1975. Lipids of the red cell membrane, p. 147-211. In G. Surgenor (ed.), The red blood cell. Academic Press, New York, N.Y.
- 20.Van Vianen, P. H., D. L. Klayman, A. J. Lin, C. B. Lugt, A. L. Van Engen, H. J. Van der Kaay, and B. Mons. 1990. Plasmodium berghei: the antimalarial action of artemisinin and sodium artelinate in vivo and in vitro, studied by flow cytometry. Exp. Parasitol. 70:115-123. [DOI] [PubMed] [Google Scholar]
- 21.Vennerstrom, J. L., and D. L. Klayman. 1988. Protoberberine alkaloids as antimalarials. J. Med. Chem. 31:1084-1087. [DOI] [PubMed] [Google Scholar]
- 22.Vial, H., and M. Ancelin. 1998. Malarial lipids, p. 159-175. In I. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 23.Vial, H. J., and M. L. Ancelin. 1992. Malarial lipids. An overview. Subcell. Biochem. 18:259-306. [PubMed] [Google Scholar]
- 24.Vial, H. J., and M. Calas. 2001. Inhibitors of phospholipid metabolism, p. 347-365. In P. Rosenthal (ed.), Antimalarial chemotherapy, mechanisms of action, modes of resistance, and new directions in drug development. Humana Press, Totowa, N.J.
- 25.Vial, H. J., M. J. Thuet, M. L. Ancelin, J. R. Philippot, and C. Chavis. 1984. Phospholipid metabolism as a new target for malaria chemotherapy. Mechanism of action of D-2-amino-1-butanol. Biochem. Pharmacol. 33:2761-2770. [DOI] [PubMed] [Google Scholar]
- 26.Wengelnik, K., V. Vidal, M. L. Ancelin, A. M. Cathiard, J. L. Morgat, C. H. Kocken, M. Calas, S. Herrera, A. W. Thomas, and H. J. Vial. 2002. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 295:1311-1314. [DOI] [PubMed] [Google Scholar]
- 27.White, N. J. 1992. Antimalarial pharmacokinetics and treatment regimens. Br. J. Clin. Pharmacol. 34:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winstanley, P. A. 2000. Chemotherapy for falciparum malaria: the armoury, the problems and the prospects. Parasitol. Today 16:146-153. [DOI] [PubMed] [Google Scholar]